Abstract
ATP-binding cassette (ABC) proteins include the best known mediators of resistance to anticancer drugs. In particular, ABCB1 (MDR1/P-gp) extrudes many types of drugs from cancer cells, thereby conferring resistance to those agents. Attempts to overcome P-gp-mediated drug resistance using specific inhibitors of P-gp has had limited success, and has faced many therapeutic challenges. As an alternative approach to using P-gp inhibitors, we characterize a thiosemicarbazone derivative (NSC73306) identified in a generic screen as a compound that exploits, rather than suppresses, P-gp function to induce cytotoxicity. Cytotoxic activity of NSC73306 was evaluated in vitro using human epidermoid, ovarian, and colon cancer cell lines expressing various levels of P-gp. Our findings suggest that cells become hypersensitive to NSC73306 in proportion to the increased P-gp function and multidrug resistance (MDR). Abrogation of both sensitivity to NSC73306 and resistance to P-gp substrate anticancer agents occurred with specific inhibition of P-gp function using either a P-gp inhibitor (PSC833, XR9576) or RNA interference (RNAi), suggesting that cytotoxicity was linked to MDR1 function, not to other, nonspecific factors arising during the generation of resistant or transfected cells. Molecular characterization of cells selected for resistance to NSC73306 revealed loss of P-gp expression and consequent loss of the MDR phenotype. Although hypersensitivity to NSC73306 required functional expression of P-gp, biochemical assays revealed no direct interaction between NSC73306 and P-gp. This work demonstrates that NSC73306 kills cells with intrinsic or acquired P-gp-induced MDR and indirectly acts to eliminate resistance to MDR1 substrates.
Keywords: multidrug resistance (MDR), P-glycoprotein, inhibitors, cancer, hypersensitivity, thiosemicarbazones, NCI 60, targeted therapy
Introduction
Surgery and targeted radiotherapy usually offer the greatest chance of a cure for localized malignancies. Treatment for patients with metastatic tumors, however, relies principally on chemotherapy, with some improvement in response in select cases by addition of immunotherapy or biological response modifiers. Despite considerable advances in drug discovery, with very few exceptions, metastatic solid malignancies remain incurable because of resistance to chemotherapy. Mechanisms of resistance extrinsic to cancer cells include altered pharmacokinetics, poor drug penetration through the extracellular matrix (1, 2), three dimensional-tumor geometry (3), cell adhesion (4), and increased intra-tumoral hydrostatic pressure (5). Intrinsic cellular resistance, studied extensively using cell lines selected in cytotoxic agents, further decreases the effectiveness of chemotherapy. Cancer cells can become resistant to a single drug or to a family of drugs with identical mechanisms of action. They may also acquire broad cross-resistance to mechanistically and structurally unrelated drugs, a phenomenon known as ‘classical’ multidrug resistance (MDR).
Of the 48 human ABC (ATP binding cassette) transport proteins (6), P-gp (a product of the MDR1/ABCB1 gene) is the best-known and most important mediator of MDR (7, 8). The first mechanistic glimpse of P-gp-induced resistance came from the cloning of MDR1 and from sequence homology between P-gp and bacterial ABC transport proteins (8, 9). Since then, P-gp structure and function have been extensively characterized; its 12 transmembrane domains form a transmembrane pore, and the two ATP-binding sites function to promote the promiscuous efflux of neutral to slightly cationic hydrophobic xenobiotics from cancer cells (10). Known substrates of P-gp include natural-product antineoplastics such as anthracyclines, vinca alkaloids, taxanes, and epipodophyllotoxins. Innate or acquired expression of P-gp, therefore, is a major problem in cancer chemotherapy.
P-gp expression, frequently detected in human solid and hematological cancers (11), is a marker of chemoresistance or decreased survival in leukemias (12), lymphomas (11, 13), osteosarcomas (14), small cell lung cancers (15), and breast cancers (16), among other malignancies. Although current chemotherapy regimens can achieve complete response in some patients with solid tumors, recurrence is the norm. The recurrent tumors have often acquired MDR, either by adaptation of previously P-gp negative cells or by selection of drug resistant P-gp positive clones. Elimination of such cells during initial treatment or at the time of recurrence is necessary to achieve cancer ‘cures’. Several P-gp inhibitors, such as verapamil and PSC833, have successfully antagonized Pgp function both in vitro and in vivo. However, phase III clinical trials have been disappointing, and no survival benefit of P-gp inhibition has yet been achieved (17, 18).
Recently, we used a bioinformatic approach to identify ABC transporter substrates (those with strong negative correlations between ABC transporter expression and growth inhibitory profiles of 1429 chemical compounds screened for anticancer activity in the NCI cancer cell panel (the NCI-60; ref. 19). Unexpectedly, several compounds, including NSC73306 (Fig. 1), showed a positive correlation between MDR1 expression and drug efficacy, suggesting that their toxicity was potentiated, rather than antagonized by P-gp. In the present study, we examined the phenomenon of P-gp-mediated hypersensitivity to NSC73306, using several cancer cell lines expressing various amounts of P-gp, combined with a variety of methods that modulate P-gp function. Molecular characterization of cells selected for resistance to NSC73306 revealed loss of P-gp expression and consequent loss of the MDR phenotype. Taken together, our results suggest that NSC73306 (and other ‘MDR1-potentiated’ agents) are candidate agents for treatment of multidrug resistant cancers expressing P-gp.
Figure 1.

Chemical structure of NSC73306
Materials and Methods
Drugs and chemicals
NSC73306 was initially obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program (DTP), Division of Cancer Treatment and Diagnosis, National Cancer Institute (19). APCI mass spectrometry showed that the compound supplied was in fact unchlorinated with an (M+H)+ ion at m/z 327. Furthermore, the proton NMR spectrum was consistent with the unchlorinated compound (data not shown). Hence, the chemical structure for NSC73306, shown in Fig. 1, is different from that in the DTP database (http://dtp.nci.nih.gov/) (19). All experiments were conducted with this unchlorinated version of NSC73306. Verapamil and dimethyl sulphoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). PSC833 and XR9576 were kindly provided by Dr. Susan Bates (CCR, NCI).
Growth of cells and analysis of drug sensitivity
Cell lines used included KB-3-1 and its MDR derivatives (KB-8-5, KB-8-5-11, KB-V1) (20, 21), HCT-15 (colon cancer), and NCI/ADR-RES (NCI/ADR-RES was initially thought to be a selected resistant variant of MCF7 and, therefore, called MCF7/ADR (22)). The designation was later changed to NCI/ADR-RES when the cell line was found to be unrelated to MCF-7 (23). Spectral karyotyping and other evidence have since shown it to likely be of ovarian origin (24). Prior to sensitivity assays, cell lines were maintained in DMEM supplemented with 10% FCS (BioWhittaker, Walkersville, MA), penicillin (50 units/ml), streptomycin (50 μg/ml), L-glutamine (BioWhittaker), and colchicine or vinblastine (KBV1 cell lines only) (25). NCI/ADR-RES cells used for RNA interference (RNAi) experiments were first maintained in doxorubicin (1 μM); this resulted in an increased doxorubicin IC50 value for this cell line when used for siRNA experiments compared with the unselected cells used for MTT assays (Table 1). Cell survival was measured by the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) assay (26). Cells were seeded in 100 μl medium at a density of 5000 cells/well in 96-well plates, and serially diluted drugs (with or without P-gp inhibitor) were added the following day in an additional 100 μl medium to give the indicated final concentrations. The Pgp inhibitors PSC833 and XR9576 were used at nontoxic concentrations (1 uM and 50 nM, respectively) as determined by cytotoxicity assays (not shown). Cells were then incubated for 72 h at 37°C in humidified 5% CO2, and the MTT assay was performed following the manufacturer's instructions (Molecular Probes, Eugene, OR).
Table 1.
MDR1/P-gp expression and relative drug resistance of select KB cell lines
| Cell Line | mRNA Expressiona | P-gp Expression (total)b | P-gp function (Calcein accumulation)c | Fold Resistance to Doxorubicind | Fold sensitivity to NSC73306d |
|---|---|---|---|---|---|
| KB-3-1 | 1 | Undetectable | 1.0 | 1 | 1 |
| KB-8-5 | 258 | + | 4.5 | 3.2 | 2.0 |
| KB-8-5-11 | 11013 | ++ | 29.0 | 62 | 3.8 |
| KB-V1 |
29738 |
+++ |
120.0 |
1090 |
7.3 |
| HCT-15 | 2795 | + | 3.5 | 6.9 | 4.0 |
| NCI/ADR-RES | 15826 | ++ | 11.0 | 115 | 4.6 |
mRNA expression measured by RT-PCR relative to KB-3-1 (fold change)
P-gp expression measured by Western blot (C219 Antibody)
P-gp mediated Calcein AM efflux relative to KB-3-1 (fold change)
Fold resistance / sensitivity of P-gp-positive cells compared to KB-3-1; numbers for HCT-15 and NCI/ADR-RES represent comparisons with those for the same cells treated with PSC-833.
Western blots
Crude membrane fractions from the cells (40 μg protein/lane) were separated on 4-12% gradient SDS–polyacrylamide gels (Invitrogen, Carlsbad, CA) and immunoblotted overnight onto PVDF membranes (Invitrogen). The membranes were incubated sequentially with mouse monoclonal anti-P-gp C219 (27) primary antibody at 1:2000 dilution and then HRP-conjugated goat anti-mouse IgG at 1:2,000 dilution. Proteins were visualized using the SuperSignal® protein detection kit.
Immuno-flow cytometry
Surface protein levels were analyzed by staining with the MRK16 antibody (28). Briefly, cells were harvested, and 250,000 cells were suspended in 200 μL of DMEM (10% FBS) containing 2.5 μg of MRK16 for 30 min at 4°C. The cells were pelleted, washed once with 200 μL of DMEM, resuspended in 200 μL of DMEM (10% FBS) containing 2.5 μg of FITC labeled anti-mouse IgG2a and incubated for 30 min at 4°C. The cells were again pelleted, washed once with 200 μL of DMEM (10% FBS), and resuspended in 300 μL PBS containing 0.1% BSA for FACS analysis. Cells incubated with mouse IgG2a kappa isotype control, instead of MRK16, followed by incubation with FITC labeled antimouse IgG2a were used to assess nonspecific labeling. For RNAi analysis the reported reduction in P-gp expression, assayed by MRK16, reflects the average and standard deviation of three independent experiments, as measured by comparing the background corrected median fluorescence of cells exposed to either MDR1-siRNA or negative control short interfering RNA (siRNA). The P-gp expression of HCT-15 cells selected in NSC73306 was compared with that of unselected cells.
Design of siRNAs
The siRNAs employed in these studies were obtained from Qiagen Inc. (Germantown, MD). The siRNA directed against MDR1 (ABCB1) corresponds to nucleotides 3484-3504 of the reference sequence (NM_000927). The siRNA duplex consists of 5′-r(CGGAAGGCCUAAUGCCGAA)dTdT (sense) and 5′-r(UUCGGCAUUAGGCCUUCCG)dTdG (antisense) strands. The negative siRNA duplex consists of 5′-r(ACGUGACACGUUCGGAGAA)dTdT and 5′-r(UUCUCCGAACGUGUCACGU)dTdT strands.
Evaluation of the effect of MDR1-targeted siRNA on drug sensitivity
Cytotoxicity experiments were performed in 96 well plates. For transfections, siRNA (5 pmol) was added to individual plate wells in 25 μL of serum-free DMEM. siLentFect lipid reagent (Bio-Rad Laboratories, Hercules, CA) was subsequently added to siRNA-containing wells in 25 μL of serum-free DMEM to provide a final lipid:siRNA ratio of 2:1 (w:w). The resulting mixture was allowed to complex for 30 min at ambient temperature. Cells (3,500) were then added in 50 μL of DMEM containing 20% FBS to yield transfection mixtures consisting of 50 nM siRNA in DMEM containing 10% FBS. The final mixture was incubated at ambient temperature for 45 min before being placed at 37°C in a humidified atmosphere containing 5% CO2. Experiments conducted in growth medium only were performed as described in the steps above except that no siRNA or lipid was added. After 24 h, the medium was replaced with 100 μl of fresh DMEM containing 10% FBS. The medium was replaced 72 h after transfection with medium containing various concentrations of doxorubicin or NSC73306 in DMEM (200 μL, 10% FBS, 1 % DMSO). For experiments using PSC833, the medium was replaced with the same concentration of doxorubicin or NSC73306 in the presence of 2 μM PSC833 (200 μL, 10% FBS, 1 % DMSO). After 72 h, the medium was removed and cells were washed once with 100 μL DMEM containing 10% FBS, before adding 100 μL of fresh DMEM containing 10% FBS. Cell viability was then assayed with the CellTiter 96 Aqueous One Solution Proliferation Assay reagent (Promega, Madison, WI). Absorbance at 490 nM was measured 90 minutes after addition of the reagent on a Wallac 1420 plate reader (PerkinElmer, Wellesley, MA). IC50 values were calculated using GraphPad Prism software version 4.0a (GraphPad Software, San Diego, CA). RNAi-mediated knockdown of MDR1 in KB-8-5-11 cells required two changes to the above protocol: 1) the lipid:siRNA ratio was increased to 4:1 (w:w) and 2) doxorubicin or NSC73306 was added 48 h after siRNA to account for faster growth kinetics.
Measurement of ATPase activity
High Five insect cells (Invitrogen) were infected with recombinant baculovirus carrying the human MDR1 cDNA with a 6-histidine tag at the C-terminal end (BV-MDR1(H6)). The cells were harvested, their membranes were isolated, and the membrane protein concentrations were determined as described previously (29). Membranes were kept at −80°C and used within 6 months of preparation. Drug-stimulated ATPase activity of the isolated membranes was measured as described elsewhere (30).
Calcein AM assay
Trypsinized cells were washed twice in phosphate-buffered saline (PBS). 5 × 105 cells were then preincubated for 5-30 min at 37°C in Iscove's Modified Dulbecco's Medium (Quality Biologicals, Gaithersburg, MD) with various concentrations of NSC73306. Calcein-AM was added to a final concentration of 0.25 μM, and the cells were incubated for 10 min at 37°C, then sedimented by centrifugation, and resuspended in PBS. Green fluorescence intensity was measured using a FacsCalibur flow cytometer equipped with a 488 nm argon laser (Becton Dickinson Biosciences, San Jose, CA, USA). Acquisition of events was stopped at 10,000.
Statistical analysis
Data are the means +/− S.D. from duplicate or triplicate samples of at least three independent experiments. Differences between the mean values were analyzed by two-sided Student's t-test and results were considered statistically significant at p< 0.05.
Results
NSC73306 is more potent in high-P-gp expressing cells
Well-characterized human KB epidermoid carcinoma cell lines originating from a single clone, KB-3-1, were chosen to evaluate the toxicity of NSC73306 (20, 21). These nearly isogenic cell lines were previously selected with increasing concentrations of either colchicine (KB-8-5 and KB-8-5-11) or vinblastine (KB-V1). The cell lines exhibit varying degrees of resistance to P-gp substrate anticancer agents, ranging from the relative sensitivity of KB-3-1 cells (IC50 doxorubicin = 0.13 μM) to the extreme resistance of KB-V1 cells (IC50 doxorubicin = 142 μM) shown in Figure 2A. Since the increasing drug resistance of these cells is due to increasing levels of P-gp expression, the KB cell panel is an ideal in vitro model of acquired clinical drug resistance, in which the complete spectrum of P-gp expression could be assessed.
Figure 2.

Growth inhibition of KB cell lines treated with either doxorubicin (A) or NSC73306 (B); data points reflect an average value of at least 5 independent experiments with fitted nonlinear dose-response curves displayed (diamond, red line: KB-V-1; down-triangle, orange line: KB-8-5-11; up-triangle, green line: KB-8-5; square, blue line: KB-3-1 cells). Chemical inhibition of P-gp, PSC833 (gray bars) and XR9576 (white bars), eliminated resistance to doxorubicin (C). In contrast, inhibition of P-gp rendered multidrug resistant KB-derivatives more resistant to NSC73306 (D). Bars represent mean IC50 values from three replicate experiments. Asterisks indicate a statistically significant difference as compared to the IC50 values observed in the KB-3-1 cell line (p<0.05).
Doxorubicin was used to characterize the drug-resistance phenotype, and PSC833 was added to estimate the P-gp-mediated component of resistance. In accord with P-gp expression, the KB cell lines demonstrated marked resistance (3.2- to 1090-fold) to known P-gp substrates, including doxorubicin (Table 1 and Fig. 2A). In contrast, NSC73306 was 2.0- to 7.3-fold more cytotoxic in the KB gradient cell lines in proportion to P-gp function (Table 1 and Fig. 2B). KB-8-5 cells express P-gp at modest levels typical of clinically resistant cancers. Still, they were 3.2-fold more resistant to doxorubicin and 2-fold more sensitive to NSC73306 (p=.016) than were the P-gp-negative KB-3-1 cell cells.
Potentiation of NSC73306 toxicity requires functional P-gp
PSC833, a cyclosporine D analog, is known to inhibit P-gp function in vitro at a concentration of 1 μM (31). That concentration was effective in inhibiting P-gp function in all KB gradient cell lines with no direct toxicity. As expected, inhibition of P-gp with PSC833 eliminated the resistance of MDR KB cell lines to doxorubicin (Fig. 2C). To test if the paradoxical hypersensitivity of P-gp expressing cells required functional P-gp, NSC73306 was coadministered with PSC833. In that setting, P-gp-positive KB cells were not significantly more sensitive to NSC73306 than were P-gp-negative KB-3-1 cells, suggesting that functional P-gp was required to induce sensitivity (Fig. 2D). To confirm that P-gp function was required for the potentiation of NSC73306 toxicity, a highly specific inhibitor, XR9576, was also evaluated in the KB-series (32). XR9576 (50 nM) was comparable in effect to PSC833 (1 μM) in reversing NSC73306 sensitivity and doxorubicin resistance (Fig. 2). Figures 2E and 2F display the IC50 values and 95% confidence intervals for cells exposed to either doxorubicin or NSC73306 (+/− P-gp inhibitors), respectively.
P-gp-mediated sensitivity to NSC73306 occurs in cells with intrinsic or acquired MDR
To test if the observed potentiation of cytotoxicity was restricted to MDR KB cells, we characterized other cell lines known to express P-gp. HCT15 colon cancer cells, which constitutively express high levels of P-gp, were used to profile the toxicity of NSC73306 in a non-selected cell line. The P-gp inhibitor PSC833 was used to establish the extent of P-gp-mediated sensitization or resistance. In MTT assays, HCT15 cells were 4-fold more sensitive to NSC73306 than were HCT15 cells co-treated with NSC73306 and PSC833 (Table 1). Similarly, PSC833 decreased the sensitivity of NCI/ADR-RES cells known to express high levels of P-gp (Table 1).
siRNA knockdown of MDR1 decreases sensitivity to NSC73306
PSC833 and XR9576 are relatively specific P-gp inhibitors. However, each can potentially interact with other molecular targets. To determine whether non-pharmacological inhibition of P-gp would also reverse sensitivity to NSC73306, RNAi was directed against MDR1 mRNA in drug resistant NCI/ADR-RES and KB-8-5-11 cells. Gene silencing was achieved by transfection of a 21-nt synthetic siRNA (MDR1-siRNA) and the efficacy of down-regulation was assayed 72 h after transfection. MDR1 mRNA levels were reduced by an average of 74% (± 3.5%) in NCI/ADR-RES cells treated with MDR1-siRNA, as compared with the levels in cells transfected with a negative control siRNA (data not shown). Concomitantly, cell surface associated P-gp was assayed by flow cytometry through binding of the MRK16 antibody; P-gp was reduced by an average of 68% (± 6.1%) for NCI/ADR-RES cells following treatment with MDR1-siRNA, as compared with cells transfected with negative control siRNA (data not shown). A similar reduction in P-gp levels was noted for KB-8-5-11 cells (54% ± 2.5%).
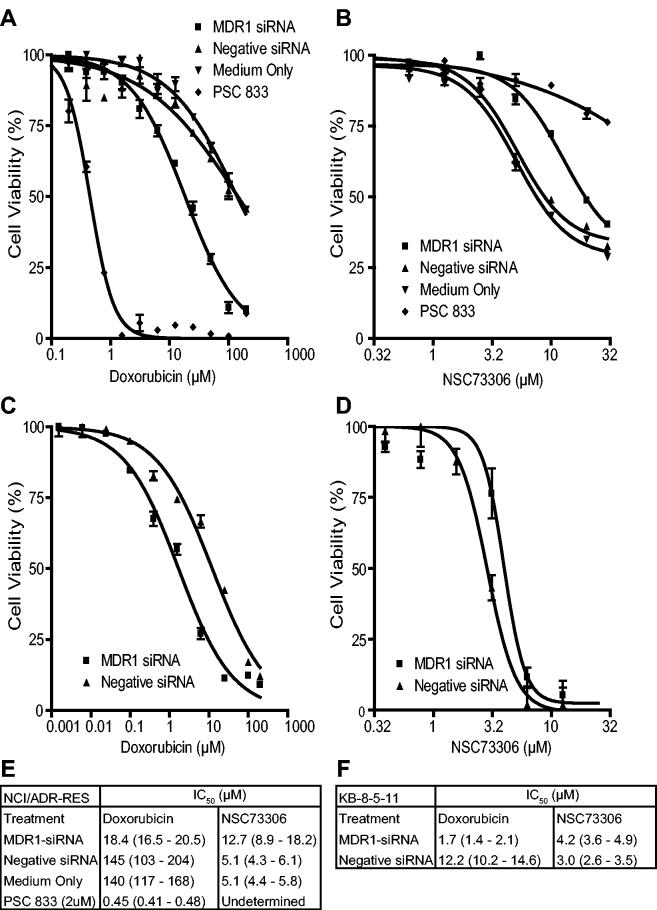
After establishing experimental conditions for siRNA knockdown of P-gp expression, we assayed the effect of P-gp-mediated resistance and sensitivity to doxorubicin and NSC73306, respectively in NCI/ADR-RES cells. For these experiments, cells were transiently transfected with MDR1-siRNA or negative control siRNA; cells grown in parallel in the absence of transfection reagents served as an additional negative control. 72 h after transfection, either doxorubicin or NSC73306 were added (+/− PSC833) to a separate set of cells treated with growth medium only. Growth inhibition was analyzed 72 h after drug addition by MTS assay. As shown in Fig. 3A, NCI/ADR-RES cells treated with MDR1-siRNA were 7-fold more sensitive to doxorubicin than cells exposed to negative control siRNA. The observed modulation of growth inhibition by doxorubicin is consistent with previously reported data (33). There was no difference between cells transfected with the negative control siRNA and cells not exposed to transfection reagent, indicating that transfection itself did not appreciably affect the cytotoxicity of doxorubicin. In contrast, treatment with MDR1-siRNA reduced the sensitivity of NCI/ADR-RES cells to NSC73306 by 2.5-fold, relative to cells exposed to control siRNA or medium alone (Fig. 3B). Analogous results were obtained in KB-8-5-11 cells where resistance to doxorubicin (Fig. 3C) and sensitivity to NSC73306 was slightly reduced following RNAi-mediated P-gp knockdown (Fig. 3D). Statistical analysis of three independent experiments indicated a modest, but significant protection of KB-8-5-11 against NSC73306 (p=0.014; 2-sided paired t-test of IC50 values). Figures 3E and 3F display the IC50 values and 95% confidence intervals for experiments performed using NCI/ADR-RES and KB-8-5-11 cells, respectively.
Figure 3.
Effect of siRNA targeted against MDR1 mRNA on P-gp positive NCI/ADR-RES ovarian carcinoma cells treated with doxorubicin or NSC73306. Dose response curves show NCI/ADR-RES cells treated with doxorubicin (A) or NSC73306 (B) for 72 hours following RNA interference (squares), negative siRNA (black triangles), growth medium only (circle), or PSC833 (white triangle). Dose response curves of KB-8-5-11 cells treated with doxorubicin (C) or NSC73306 (D) after either RNA interference (squares) or negative siRNA (black triangles). Error bars indicate 95% confidence intervals. IC50 values of NCI/ADR-RES (E) and KB-8-5-11 (F) are listed with 95% confidence intervals shown in parentheses.
Long-term exposure to NSC73306 results in loss of P-gp expression
To understand the mechanism by which NSC73306 induces growth inhibition, we exposed a clonal isolate of HCT15 cells (denoted HCT15-2A) to NSC73306 (1 μg/ml) for three weeks. The resulting HCT15-2A-R cells were 8- to 10-fold more resistant to NSC73306 than were the parental HCT15-2A cells (Fig. 4A). Resistance of HCT15-2A-R cells to NSC73306 was accompanied by increased sensitivity to the MDR1 substrate doxorubicin (Fig. 4B). The increased resistance to NSC73306, and renewed sensitivity to doxorubicin, was explained, at least in part, by loss of plasma membrane expression (Fig. 4C) and function of P-gp (Fig. 4D). Additionally, Western blots revealed the complete loss of P-gp following selection (Fig. 4E). Down-regulation of P-gp also occurred in NSC73306-resistant KB-V1 and NCI/ADR-RES cells selected for a similar period of time (Fig. 4E). That resistance to NSC73306 occurred through loss of P-gp expression provided further evidence for a central role of P-gp in mediating sensitivity to NSC73306.
Figure 4.
Effect of selecting HCT15-2A cells in NSC73306. Following selection in 1 μg/ml NSC73306, HCT15-2A-R cells demonstrate resistance to NSC73306 (A). Selection with NSC73306 for three weeks reversed resistance to P-gp substrates (B) (blue bars represent pre-selection drug resistance set to 100%, red bars represent post-selection effect, yellow bars represent elimination of P-gp function by PSC833). Loss of surface P-gp expression, as measured by MRK-16 FACS analysis (C) and function, as measured by Calcein accumulation (D), occurred following a three week selection with NSC73306. Immunoblots showing expression of Pgp in crude membranes (E) or whole cell lysates (F). The effect of NSC73306 treatment on Pgp expression is shown with GAPDH as a control (F).
Resistance to NSC73306 induces sensitivity to chemotherapeutic P-gp substrates
Given the loss of P-gp induced by selection in NSC73306 and its effect on doxorubicin sensitivity, we hypothesized that pre-treatment of MDR1 positive cells with NSC73306 would similarly resensitize MDR HCT15 colon cancer cells to additional MDR1 chemotherapeutic substrates. HCT15-2A-R cells were cultured in drug-free medium for a period of 2 days prior to MTT assays. HCT15-2A cells were used as a control. As shown in Fig. 4B, selection in NSC73306 resulted in almost complete elimination of drug resistance to all P-gp substrates evaluated. In contrast, drug sensitivity was unchanged for two non-P-gp substrates, cisplatin and methotrexate. This suggested that resensitization occurred through loss of P-gp, not by other non-specific mechanisms such as altered cell growth kinetics or metabolism.
NSC73306 is not a ‘classic’ P-gp inhibitor
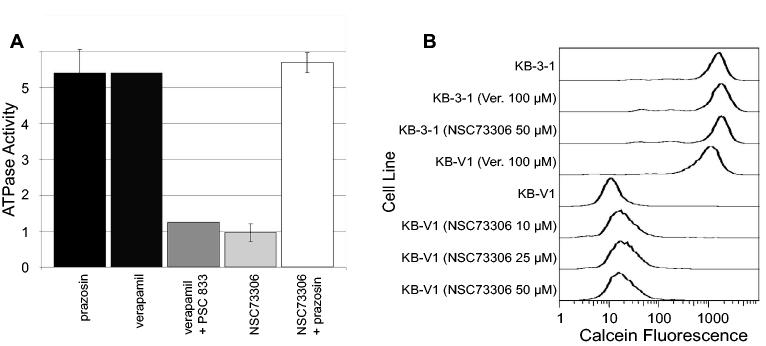
Since P-gp function seemed to be a prerequisite for the enhanced toxicity of NSC73306, we evaluated the interaction of P-gp and NSC73306 using various biochemical assays. To test if NSC73306 has a direct effect on the transporter, we conducted ATPase activity measurements using crude membranes purified from insect cells expressing human P-gp (34). The ATPase activity of P-gp is often stimulated in the presence of transported substrates, such as prazosin or verapamil, and this activation is prevented by ‘classical’ P-gp inhibitors, such as PSC833. In contrast to verapamil or prazosin, NSC73306 did not stimulate the basal catalytic activity, and unlike PSC833, it did not prevent the stimulation of the ATPase suggesting that it doesn't interact with P-gp (Fig. 5A). Consistent with this notion, NSC73306 did not alter the P-gp-mediated Calcein-AM efflux of KB-V1 cells (Fig. 5B).
Figure 5.
Effect of NSC73306 on P-gp function. (A) Drug stimulated ATPase activity was measured in the presence of Prazosin (50 μM), verapamil (10 μM), verapamil and PSC833 (2 μM), NSC73306 (up to 50 μM) or NSC73306 and Prazosin. Values represent relative increase of ATPase activity as compared to the basal activity measured in the absence of added compounds (set to 1). (B) Calcein assay was performed in the presence of the indicated compounds, as described in the Methods section. KB-3-1 cells are negative for P-gp and, therefore, are brightly fluorescent; verapamil and NSC73306 have no effect on Calcein accumulation. KB-V1 cells are dimly fluorescent secondary to P-gp-mediated Calcein AM efflux; Unlike verapamil, NSC73306 at concentrations from 10 to 50 μM does not affect the P-gp-mediated efflux of Calcein AM.
Discussion
In this study, we demonstrate the use of a small molecule thiosemicarbazone (NSC73306) to exploit P-gp-mediated MDR in human cancer cells. This drug family has shown therapeutic antiretroviral, antimalarial, antibacterial, antihypertensive, and anticancer activity, among others (35). For example, 2-formylpyridine thiosemicarbazone was reported to inhibit the growth of P388 lymphocytic leukemia and L1210 lymphoid leukemia cells both in vitro and in vivo, albeit by an unknown mechanism. Often, the biological effects of thiosemicarbazones are attributed to metal chelation or inhibition of ribonucleotide reductase (36, 37). No relationship between these mechanisms and P-gp-mediated MDR has yet been identified.
To characterize the selectivity of NSC73306 for P-gp-expressing cells, four KB epidermoid carcinoma cell lines expressing increasing levels of P-gp were evaluated. Comparison of those nearly isogenic lines led to three major conclusions: i) Although NSC73306 was most effective in cells expressing the greatest amounts of P-gp, with associated high-level drug resistance, substantial sensitivity was observed even at lower levels of P-gp expression. Preferential toxicity of NSC73306 was observed even in the KB-8-5 human epidermoid cell line that expresses P-gp at modest levels typical of human tumors in vivo (20, 21), ii) no growth inhibition was observed in P-gp-negative cells when used at concentrations effective in P-gp-positive cells, and iii) sensitivity to NSC73306 was proportional to both the expression and function of P-gp. These findings substantiated and complemented our previous results using a tetracycline-regulated expression system, which relied upon binary ‘all-or none’ expression models (19).
The results obtained using multiple inhibitors (PSC833 and XR9576) and RNAi experiments strongly supported the third conclusion. Interestingly, the extent of cytotoxic modulation mediated by RNAi directed against MDR1 was inferior to that of PSC833, a chemical-based P-gp inhibitor. This small, but statistically significant effect is most likely explained by the incomplete inhibition of MDR1 by RNAi, as residual P-gp expression (i.e., P-gp has a long half-life on the plasma membrane) is likely to be sufficient to maintain intermediate resistance to doxorubicin and sensitivity to NSC73306.
The effect of NSC73306 on the HCT-15 human colon cancer cell line was particularly encouraging, since as many as 73% of colon cancers constitutively express high levels of P-gp (38-40). Furthermore, P-gp-dependent sensitivity of HCT-15 colon cancer cells to NSC73306 suggests that hypersensitivity occurs not just in chemotherapy-selected cell lines, but also in cancers that intrinsically express P-gp. Our results provide preliminary evidence for the applicability of NSC73306 to other tumor types, such as leukemia, sarcoma, renal and adrenocortical cancers that also intrinsically express P-gp (11, 39). For several tumor types, the precise contribution of P-gp to drug resistance has been difficult to quantify because these tumors are often resistant to MDR1 substrates and non-substrates alike. Further experiments will therefore be required to determine if drug sensitivity also extends to these diverse tumor types.
Unexpectedly, expression of P-gp in MDR cell lines decreased after only a three-week exposure to NSC73306. This loss of P-gp expression further supports the causal link between the toxicity of NSC73306 and P-gp function. It is unknown whether reduction of P-gp levels was secondary to adaptation of P-gp-positive cells or was the result of the death of P-gp-positive cells with resultant selection for P-gp-negative cells. If ascribed to adaptation of P-gp-positive cells, then NSC73306 may, in fact, have the added benefit of P-gp inhibition, albeit due to protein down-regulation rather than interference with efflux function. Immunofluorescence studies using a P-gp-GFP fusion protein and anti-P-gp fluorescent antibodies should aid in resolving this dilemma and are now in progress.
At present, little is known about the mechanism of action. NSC73306 could interact directly with P-gp, or with a downstream, P-gp-dependent event. Functional assays, measuring P-gp-mediated ATP consumption and drug efflux, were performed to evaluate these possibilities. P-gp-mediated transport is coupled to ATP hydrolysis that is often stimulated by transported substrates (34, 41). The profile of the drug-stimulated ATPase reflects the nature of interaction: compounds may be substrates, inhibitors, or may have no effect on the transporter. In the presence of transported substrates, the ATPase activity of P-gp usually increases. Noncompetitive inhibitors, or compounds transported at a lower rate, inhibit the ATPase activity of the stimulated transporter. NSC73306 neither stimulated, nor inhibited the ATPase, suggesting that it does not interact with P-gp. However, there are substrates and inhibitors that have little effect on the P-gp-mediated ATPase, and the assay conditions using insect cell membranes may not be relevant if active metabolites are formed in cancer cells. Consequently, the ability of NSC73306 to inhibit P-gp-mediated transport was also evaluated in an intact cell system. When extrusion by P-gp is blocked by an agent that interferes with P-gp function (e.g. verapamil), fluorescent Calcein rapidly accumulates within the cell (Fig. 5B) (42). KB-V1 cells remained dim in the presence of NSC73306, suggesting that it does not compete with Calcein-AM transport. Thus, NSC73306 doesn't seem to be a substrate or inhibitor of P-gp (at least for Calcein-AM transport), indicating that the increased sensitivity of KB-V1 cells cannot be explained by the direct effect of NSC73306 on P-gp. Therefore, we hypothesize that the target of NSC73306 is a downstream event. This event is both P-gp-dependent, as the potentiation of NSC73306 toxicity requires functional P-gp, and P-gp-specific, as expression of ABCC1 (MRP1) or ABCG2 failed to sensitize cells to NSC73306 (data not shown).
Collateral sensitivity of cells expressing high levels of P-gp to detergents and verapamil is well documented in the literature (43-45). Although never fully explained, their effects have been attributed either to alterations in plasma membrane properties or activation of ATPase to a level that produces cell stress. This latter mechanism of cytotoxicity has already been reported for poly(ethylene oxide)-poly(propylene oxide) block co-polymers (Pluronic or ‘poloxamer’) which effectively rob the cell of its energy supply (46). These poloxamer type molecules, however, have no direct cytotoxicity when used alone, but only when combined with a P-gp substrate capable of stimulating transporter-mediated drug efflux. Similarly, P-gp expressing cells have been shown to be hypersensitive to 2-deoxyglucose, presumably because reserves of ATP are depleted by rapid consumption that occurs during active transporter function (47). Since NSC73306 does not promote P-gp-mediated ATP hydrolysis, its enhanced toxicity in drug-resistant cells is not likely to be mediated by ATP-depletion caused by P-gp (39, 48).
The precise mechanism of action of NSC73306 remains an active area of investigation. Elucidation of this mechanism should not only facilitate nascent methods of circumventing drug resistance in cancer, but will likely further our understanding of ABC transporter function and regulation. Future preclinical studies evaluating the pharmacokinetic properties and therapeutic index of this novel antineoplastic will, hopefully, lead to additional targeted therapies for improved care of patients with cancer. As with other P-gp-targeted therapeutics under development, translation to the clinical domain must proceed with caution, with particular attention to potential toxicities in normal human tissues (e.g., blood brain barrier, placenta, liver, colon, and kidney) that intrinsically express P-gp.
In summary, we have shown that NSC73306 exhibits selective toxicity in P-gp expressing cancer cells. This occurs by two separate, but closely related, mechanisms. First, the toxicity of NSC73306 is directly proportional to the levels of functional P-gp. Second, P-gp expression decreases in cells treated with NSC73306, which renders cells sensitive to the very P-gp substrates against which they had previously shown resistance. Taken together, these two mechanisms offer the promise of improving cancer treatment, especially in those tumors that are most resistant to MDR1 substrates.
Acknowledgments
We thank the staff of NCI DTP for generation of the pharmaceutical database used in this study, Dr. Thomas Spande (NIDDK) for performing APCI mass spectrometry, George Leiman for editorial assistance, Dr. Anna Maria Calcagno for critically reviewing this manuscript, and we thank Qiagen Inc. for the design and synthesis of siRNAs. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Pluen A, Boucher Y, Ramanujan S, et al. Role of tumor-host interactions in interstitial diffusion of macromolecules: Cranial vs. subcutaneous tumors. PNAS. 2001;98:4628–33. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46:149–68. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 3.Durand RE, Olive PL. Resistance of tumor cells to chemo- and radiotherapy modulated by the three-dimensional architecture of solid tumors and spheroids. Methods Cell Biol. 2001;64:211–33. doi: 10.1016/s0091-679x(01)64015-9. [DOI] [PubMed] [Google Scholar]
- 4.Green SK, Frankel A, Kerbel RS. Adhesion-dependent multicellular drug resistance. Anticancer Drug Des. 1999;14:153–68. [PubMed] [Google Scholar]
- 5.Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50:4478–84. [PubMed] [Google Scholar]
- 6.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Research. 2001;11:1156–66. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 7.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1976;455:152–62. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 8.Ueda K, Cardarelli C, Gottesman MM, et al. Expression of a full-length cDNA for the human “MDR1” gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987;84:3004–8. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gros P, Croop J, Housman D. Mammalian multidrug resistance gene: Complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986;47:371–80. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- 10.Sauna ZE, Ambudkar SV. Characterization of the catalytic cycle of ATP hydrolysis by human P-glycoprotein. The two ATP hydrolysis events in a single catalytic cycle are kinetically similar but affect different functional outcomes. J Biol Chem. 2001;276:11653–61. doi: 10.1074/jbc.M011294200. [DOI] [PubMed] [Google Scholar]
- 11.Fojo AT, Ueda K, Slamon DJ, et al. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987;84:265–9. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marie JP, Zittoun R, Sikic BI. Multidrug resistance (mdr1) gene expression in adult acute leukemias: correlations with treatment outcome and in vitro drug sensitivity. Blood. 1991;78:586–92. [PubMed] [Google Scholar]
- 13.Yuen AR, Sikic BI. Multidrug resistance in lymphomas. J Clin Oncol. 1994;12:2453–9. doi: 10.1200/JCO.1994.12.11.2453. [DOI] [PubMed] [Google Scholar]
- 14.Baldini N, Scotlandi K, Barbanti-Brodano G, et al. Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N Engl J Med. 1995;333:1380–5. doi: 10.1056/NEJM199511233332103. [DOI] [PubMed] [Google Scholar]
- 15.Hsia TC, Lin CC, Wang JJ, et al. Relationship between chemotherapy response of small cell lung cancer and P-glycoprotein or multidrug resistance-related protein expression. Lung. 2002;180:173–9. doi: 10.1007/s004080000091. [DOI] [PubMed] [Google Scholar]
- 16.Burger H, Foekens JA, Look MP, et al. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin Cancer Res. 2003;9:827–36. [PubMed] [Google Scholar]
- 17.Nicolantonio FD, Knight LA, Glaysher S, et al. Ex vivo reversal of chemoresistance by tariquidar (XR9576) Anticancer Drugs. 2004;15:861–9. doi: 10.1097/00001813-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Robert J, Jarry C. Multidrug resistance reversal agents. J Med Chem. 2003;46:4805–17. doi: 10.1021/jm030183a. [DOI] [PubMed] [Google Scholar]
- 19.Szakacs G, Annereau JP, Lababidi S, et al. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell. 2004;6:129–37. doi: 10.1016/j.ccr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama S, Fojo A, Hanover JA, et al. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985;11:117–26. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- 21.Shen DW, Cardarelli C, Hwang J, et al. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986;261:7762–70. [PubMed] [Google Scholar]
- 22.Cohen JS, Lyon RC, Chen C, et al. Differences in phosphate metabolite levels in drug-sensitive and -resistant human breast cancer cell lines determined by 31P magnetic resonance spectroscopy. Cancer Res. 1986;46:4087–90. [PubMed] [Google Scholar]
- 23.Scudiero DA, Monks A, Sausville EA. Cell line designation change: multidrug-resistant cell line in the NCI anticancer screen. J Natl Cancer Inst. 1998;90:862. doi: 10.1093/jnci/90.11.862. [DOI] [PubMed] [Google Scholar]
- 24.Roschke AV, Tonon G, Gehlhaus KS, et al. Karyotypic complexity of the NCI-60 drug-screening panel. Cancer Res. 2003;63:8634–47. [PubMed] [Google Scholar]
- 25.Ambudkar SV, Lelong IH, Zhang J, et al. Purification and reconstitution of human P-glycoprotein. Methods Enzymol. 1998;292:492–504. doi: 10.1016/s0076-6879(98)92038-9. [DOI] [PubMed] [Google Scholar]
- 26.Morgan DM. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol Biol. 1998;79:179–83. doi: 10.1385/0-89603-448-8:179. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S, Currier SJ, Bruggemann EP, et al. Use of recombinant P-glycoprotein fragments to produce antibodies to the multidrug transporter. Biochem Biophys Res Commun. 1990;166:180–6. doi: 10.1016/0006-291x(90)91928-l. [DOI] [PubMed] [Google Scholar]
- 28.Germann UA, Chambers TC, Ambudkar SV, et al. Characterization of phosphorylation-defective mutants of human P-glycoprotein expressed in mammalian cells. J Biol Chem. 1996;271:1708–16. doi: 10.1074/jbc.271.3.1708. [DOI] [PubMed] [Google Scholar]
- 29.Ramachandra M, Ambudkar SV, Chen D, et al. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry. 1998;37:5010–9. doi: 10.1021/bi973045u. [DOI] [PubMed] [Google Scholar]
- 30.Szakacs G, Ozvegy C, Bakos E, et al. Role of glycine-534 and glycine-1179 of human multidrug resistance protein (MDR1) in drug-mediated control of ATP hydrolysis. Biochem J. 2001;356:71–5. doi: 10.1042/0264-6021:3560071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boesch D, Gaveriaux C, Jachez B, et al. In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res. 1991;51:4226–33. [PubMed] [Google Scholar]
- 32.Martin C, Berridge G, Mistry P, et al. The molecular interaction of the high affinity reversal agent XR9576 with P-glycoprotein. Br J Pharmacol. 1999;128:403–11. doi: 10.1038/sj.bjp.0702807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63:1515–9. [PubMed] [Google Scholar]
- 34.Sarkadi B, Price EM, Boucher RC, et al. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992;267:4854–8. [PubMed] [Google Scholar]
- 35.Pandeya SN, Dimmock JR. Recent evaluations of thiosemicarbazones and semicarbazones and related compounds for antineoplastic and anticonvulsant activities. Pharmazie. 1993;48:659–66. [PubMed] [Google Scholar]
- 36.Atassi G, Dumont P, Harteel JC. Potentiation of the antitumour activity of 2-formylpyridine thiosemicarbazone by metal chelation: 2-formylpyridine thiosemicarbazone zinc sulphate (NSC 294721) Eur J Cancer. 1979;15:451–9. doi: 10.1016/0014-2964(79)90080-x. [DOI] [PubMed] [Google Scholar]
- 37.Rappa G, Lorico A, Liu MC, et al. Overexpression of the multidrug resistance genes mdr1, mdr3, and mrp in L1210 leukemia cells resistant to inhibitors of ribonucleotide reductase. Biochem Pharmacol. 1997;54:649–55. doi: 10.1016/s0006-2952(97)00210-4. [DOI] [PubMed] [Google Scholar]
- 38.Iwahashi T, Okochi E, Ono K, et al. Establishment of multidrug resistant human colorectal carcinoma HCT-15 cell lines and their properties. Anticancer Res. 1991;11:1309–12. [PubMed] [Google Scholar]
- 39.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Sakaeda T, Ohmoto N, et al. Real-time quantitative polymerase chain reaction for MDR1, MRP1, MRP2, and CYP3A-mRNA levels in Caco-2 cell lines, human duodenal enterocytes, normal colorectal tissues, and colorectal adenocarcinomas. Drug Metab Dispos. 2002;30:4–6. doi: 10.1124/dmd.30.1.4. [DOI] [PubMed] [Google Scholar]
- 41.Ambudkar SV, Dey S, Hrycyna CA, et al. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–98. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 42.Homolya L, Hollo M, Muller M, et al. A new method for a quantitative assessment of P-glycoprotein-related multidrug resistance in tumour cells. Br J Cancer. 1996;73:849–55. doi: 10.1038/bjc.1996.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orlowski S, Selosse MA, Boudon C, et al. Effects of detergents on P-glycoprotein atpase activity: differences in perturbations of basal and verapamil-dependent activities. Cancer Biochem Biophys. 1998;16:85–110. [PubMed] [Google Scholar]
- 44.Schuldes H, Dolderer JH, Zimmer G, et al. Reversal of multidrug resistance and increase in plasma membrane fluidity in CHO cells with R-verapamil and bile salts. European Journal of Cancer. 2001;37:660. doi: 10.1016/s0959-8049(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 45.Carlsen SA, Till JE, Ling V. Modulation of membrane drug permeability in Chinese hamster ovary cells. Biochim Biophys Acta. 1976;455:900–12. doi: 10.1016/0005-2736(76)90059-6. [DOI] [PubMed] [Google Scholar]
- 46.Batrakova EV, Li S, Elmquist WF, et al. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br J Cancer. 2001;85:1987–97. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bentley J, Bell SE, Quinn DM, et al. 2-deoxy-D-glucose toxicity and transport in human multidrug-resistant KB carcinoma cell lines. Oncol Res. 1996;8:77–84. [PubMed] [Google Scholar]
- 48.Bell SE, Quinn DM, Kellett GL, et al. 2-Deoxy-D-glucose preferentially kills multidrug-resistant human KB carcinoma cell lines by apoptosis. Br J Cancer. 1998;78:1464–70. doi: 10.1038/bjc.1998.708. [DOI] [PMC free article] [PubMed] [Google Scholar]