Abstract
The endosomal sorting complexes required for transport, ESCRT-I, -II, and -III, are thought to mediate the biogenesis of multivesicular endosomes (MVEs) and endosomal sorting of ubiquitinated membrane proteins. Here, we have compared the importance of the ESCRT-I subunit tumor susceptibility gene 101 (Tsg101) and the ESCRT-III subunit hVps24/CHMP3 for endosomal functions and receptor signaling. Like Tsg101, endogenous hVps24 localized mainly to late endosomes. Depletion of hVps24 by siRNA showed that this ESCRT subunit, like Tsg101, is important for degradation of the epidermal growth factor (EGF) receptor (EGFR) and for transport of the receptor from early endosomes to lysosomes. Surprisingly, however, whereas depletion of Tsg101 caused sustained EGF activation of the mitogen-activated protein kinase pathway, depletion of hVps24 had no such effect. Moreover, depletion of Tsg101 but not of hVps24 caused a major fraction of internalized EGF to accumulate in nonacidified endosomes. Electron microscopy of hVps24-depleted cells showed an accumulation of EGFRs in MVEs that were significantly smaller than those in control cells, probably because of an impaired fusion with lyso-bisphosphatidic acid-positive late endosomes/lysosomes. Together, our results reveal functional differences between ESCRT-I and ESCRT-III in degradative protein trafficking and indicate that degradation of the EGFR is not required for termination of its signaling.
INTRODUCTION
Membrane-bound receptors are key transmitters of cell signaling. When activated by their respective ligands, different receptors activate various intracellular signaling pathways that control transcription, cytoskeletal functions, or membrane trafficking. Even though receptor signaling has classically been thought to occur from the plasma membrane, accumulating evidence suggests that at least some receptors can transmit signals from endomembranes such as endosomes as well. Moreover, signaling from endomembranes may be qualitatively different from signaling from the plasma membrane, thereby providing additional opportunities for spatial regulation of signal transduction (Ceresa and Schmid, 2000; Sorkin and Von Zastrow, 2002).
Owing to their involvement in carcinogenesis, epidermal growth factor (EGF) receptors (EGFRs) are among the most intensively studied receptors of mammalian cells (Rubin and Yarden, 2001), and their downstream signaling, such as the mitogen-activated protein (MAP) kinase and phosphoinositide kinase pathways, have been characterized in detail (Pawson, 2004). The finding that inhibition of endocytosis with dominant-negative dynamin constructs inhibits EGF-induced activation of the MAP kinase pathway has led to the view that this pathway is activated by ligand-bound EGFRs that reside in the limiting membrane of endosomes (Vieira et al., 1996). It has thus been proposed that EGF-activated MAP kinase activation is silenced when the activated receptors become internalized into intraluminal vesicles of endosomes and subsequently are degraded by lysosomal enzymes as the multivesicular endosomes (MVEs) fuse with lysosomes (Futter et al., 1996). Such degradation is thought to be essential for termination of EGFR signaling, and its impairment has been associated with carcinogenesis (Dikic and Giordano, 2003; Bache et al., 2004b; Polo et al., 2004). It is therefore important to characterize the mechanisms of EGFR down-regulation thoroughly.
A molecular machinery, first identified in yeast as part of the vacuolar protein sorting (vps) machinery, has proven important for the endosomal sorting of EGFRs into the degradative MVE pathway (Katzmann et al., 2002; Babst et al., 2002b; Raiborg et al., 2003). On activation and internalization, the multiubiquitinated EGFR interacts with a complex that contains the ubiquitin-binding protein hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) on the endosome membrane (Bache et al., 2003b; Sigismund et al., 2005). Hrs recruits the endosomal sorting complex required for transport, ESCRT-I (Bache et al., 2003a), which is thought to capture the ubiquitinated cargo through its ubiquitin-binding subunit tumor susceptibility gene 101 (Tsg101) (Sundquist et al., 2004). By analogy with the endosomal sorting of ubiquitinated cargo in yeast, the ubiquitinated EGFR has been further assumed to be delivered to ESCRT-II before it gets internalized into MVEs through the activity of ESCRT-III (Katzmann et al., 2002). In support of this idea, depletion of ESCRT-I subunits has been shown to inhibit ligand-induced EGFR degradation (Bishop et al., 2002; Bache et al., 2003b, 2004a), as has overexpression of an N-terminal fragment of the human ESCRT-III subunit hVps24 (Yan et al., 2005). However, the relationship between endosomal trafficking of the EGFR and its signaling properties has been less explored. What is known is that hrs mutant Drosophila embryos show enhanced EGF signaling (Lloyd et al., 2002) and that EGF-induced activation of the MAP kinase pathway is enhanced in cell lines with impaired Tsg101 function (Babst et al., 2000). The roles of ESCRT-II and ESCRT-III in receptor silencing have not been examined.
In the present work, we have analyzed the function of hVps24 in the trafficking and signaling of EGFRs. Unexpectedly, we find that hVps24 depletion affects degradation but not signaling of EGFRs. This indicates that Tsg101 and hVps24 have distinct functions in the regulation of receptor trafficking and that endosomal receptor silencing occurs independently of proteolytic degradation.
MATERIALS AND METHODS
Cell Culture and Small-Interfering RNA (siRNA) Oligonucleotides
HeLa cell cultures were maintained as recommended by American Type Culture Collection (Manassas, VA). For RNA interference (RNAi) against Tsg101, we used the same oligonucleotides as described previously (Bishop et al., 2002), and for hVps24 we used the following oligonucleotides: siRNA-1, sense sequence 5′-AGCAUGGACGAUCAGGAAGTT-3′ and antisense sequence 5′-CUUCCUGAUCGUCCAUGCUTT-3′; and siRNA-2, sense sequence 5′-GCA GAA AUG GAA AUU GAC AGT T-3′ and antisense sequence 5′-CUGUCAAUUUCCAUUUCCUGCTT-3′. A BLAST search confirmed that these sequences were unique to Vps24. Both siRNA-1 and siRNA-2 were used for the experiments described in Figure 3, whereas only siRNA-1 was used in all other experiments. As negative control we used a scrambled sequence of the same nucleotides as in siRNA-1: sense scrambled control 5′-CGGGAGGCUAAGAAAUACGTT-3′ and antisense scrambled control 5′-CGUAUUUCUUAGCCUCCCGTT-3′. Transfection of HeLa cells with siRNA was performed as described previously (Bache et al., 2003b). The cells were first transfected with siRNA using the transfection reagent Oligofectamine (Invitrogen, Carlsbad, CA) for 2 or 3 d, and then the cells were replated and left for another 2 d before experiments were carried out.
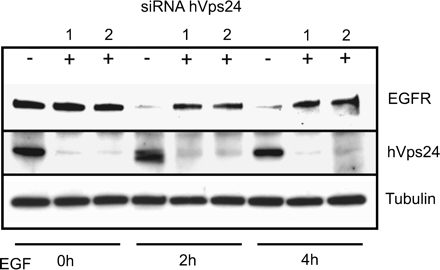
Figure 3.
Depletion of hVps24 retards EGFR down-regulation. HeLa cells treated with scrambled RNA (−) or with siRNA against hVps24 (+) were stimulated for 0, 2, and 4 h with EGF. The cells were lysed and analyzed by SDS-PAGE and sequential blotting with antibodies against EGFR and hVps24. The same membrane was then reblotted with anti-tubulin to verify equal loadings.
Antibodies and Reagents
Antiserum against hVps24 was obtained from Eurogentec (Herstal, Belgium) and raised by injecting rabbits with keyhole limpet hemocyanin coupled to two different peptides corresponding to amino acids 4–18 and 178–192 of hVps24. The peptides contained a terminal cysteine for conjugation purposes. The serum was affinity purified according to the manufacturer's procedure using a SulfoLink kit from Pierce Chemical (Rockford, IL). Human anti-early endosomal antigen (EEA)1 antiserum (Mu et al., 1995) was a gift from Ban-Hock Toh (Monash University, Melbourne, Australia). Rabbit anti-human lysosomal-associated membrane protein (LAMP)2 was a gift from Gillian Griffiths (University of Oxford, Oxford, United Kingdom). A mouse monoclonal antibody against Tsg101 was obtained from GeneTex (San Antonio, TX). Mouse monoclonal antibodies against lyso-bisphosphatidic acid (LBPA) (Kobayashi et al., 1998) were kindly provided by Jean Gruenberg (University of Geneva, Geneva, Switzerland). Mouse monoclonal antibodies against α-tubulin and CD63 were from Sigma-Aldrich (St. Louis, MO) and the Developmental Studies Hybridoma Bank of the University of Iowa (Iowa City, IA), respectively. Sheep antibodies against TGN46 were from Serotec (Oxford, United Kingdom). Sheep antibodies against EGFR were from Fitzgerald (Concord, MA). Mouse monoclonal antibodies against phospho-mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK)1/2 (Thr202/Tyr204), polyclonal rabbit antibodies against MAPK/ERK1/2 and phospho-mitogen-activated protein kinase kinase (MEK)1/2 (Ser217/221), and rabbit monoclonal antibodies against MEK1/2 were from Cell Signaling Technology (Beverly, MA). Cy2-, Cy3-, and Cy5-labeled secondary antibodies as well as streptavidin-Cy3 were from Jackson ImmunoResearch Laboratories (West Grove, PA). Biotinylated dextran with molecular mass of 10 kDa was from Invitrogen, and cycloheximide was from Sigma-Aldrich.
Down-Regulation of EGFR in Cells Depleted of hVps24
HeLa cells were transfected with siRNAs against hVps24 or a scrambled RNA duplex of the same nucleotides as control. After 48 h, the cells were replated into three 5-cm dishes with siRNA-treated cells and three 5-cm dishes with control RNA-treated cells, and left for another 48 h. The cells were starved in serum-free medium for 1 h in the presence of 10 μg/ml cycloheximide and then incubated with normal medium supplemented with 50 ng/ml EGF and 10 μg/ml cycloheximide for 0, 2, and 4 h. The cells were lysed for 15 min in lysis buffer (125 mM KAc, 25 mM HEPES, 2.5 mM MgAc, 5 mM EGTA, 0.5% NP-40, pH 7.2, and mammalian protease inhibitor cocktail [Sigma-Aldrich]), and the lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent immunoblotting using antibodies against the EGFR, hVps24, and tubulin to verify equal loadings.
Confocal Immunofluorescence Microscopy
To investigate the degradation of the EGFR using immunofluorescence microscopy, HeLa cells grown on coverslips treated or not with siRNA against hVps24 were incubated in serum-free medium for 1 h in the presence of 10 μg/ml cycloheximide. The cells were then stimulated with 50 ng/ml EGF and cycloheximide for 15 min and chased in normal medium with cycloheximide for 3 h. The cells were then permeabilized with 0.05% saponin, fixed with 3% paraformaldehyde, and stained for fluorescence microscopy as described previously (Simonsen et al., 1998). Coverslips were examined using a Zeiss LSM 510 META microscope equipped with a Neo-Fluar 100×/1.45 oil immersion objective. Image processing was done with Adobe Photoshop version 7.0 (Adobe Systems, Mountain View, CA). For studying of the fluid phase pathway in cells depleted of hVps24, HeLa and HEp2 cells treated with siRNA against hVps24 were grown on coverslips and preincubated with biotinylated dextran on ice for 1 h, before they were left to internalize the prebound dextran at 37°C for 30 min. The cells were washed and incubated at 37°C for 0 or 3 h before they were permeabilized, fixed, and stained as described above.
Phosphorylation of ERK1/2 and MEK1/2 in Cells Depleted of Tsg101 and hVps24
HeLa cells treated with siRNA against Tsg101, hVps24, or scrambled RNA oligonucleotides were incubated for 72 h and replated in one six-well plate each. After 48 h, the cells were starved in serum-free medium supplemented with 10 μg/ml cycloheximide for 4 h. All wells, except controls, were stimulated with 5 ng/ml EGF for 5 min and chased in normal medium with cycloheximide for the indicated times. All the cells were washed in ice-cold phosphate-buffered saline and placed on ice. Then, 100 μl of 2× sample buffer for SDS-PAGE was added to each well, and the viscous lysate was transferred to QIAshredder columns (QIAGEN, Hilden, Germany) and centrifuged at 14,000 rpm for 5 min. The now liquefied lysates were boiled and analyzed by SDS-PAGE and subsequent immunoblotting using antibodies against phospho- and total-MEK1/2, and phospho- and total-MAPK/ERK1/2. The intensities of the different bands obtained by immunoblotting were quantified using the software provided by the ChemiGenius imaging system (Syngene, Cambridge, United Kingdom) and plotted as percentage of the respective phosphorylation intensities after 5 min of EGF stimulation without further chase. Intensities were adjusted for different loadings.
Vesicular pH Measurement of EGF-containing Endocytic Organelles
The luminal pH of endocytic vesicles containing EGF-fluorescein isothiocyanate (FITC) was measured by single-cell fluorescence ratio image analysis similarly to that described previously (Sharma et al., 2004). After 2.5-h serum starvation, HeLa cells were incubated in the presence of 100 nM EGF-FITC (Invitrogen) for 1 h, followed by a 30-min chase in serum-free medium at 37°C. Then, cells were transferred into NAKH medium (140 mM NaCl, 5 mm KCl, 20 mM HEPES, 10 mM glucose, 0.1 mM CaCl2, and 1 mM MgCl2, pH 7.3) and imaged on Axiovert 100 microscope (Carl Zeiss MicroImaging, Thornwood, NY) equipped with a cooled charge-coupled device camera (Princeton Instruments, Princeton, NJ) and a 63× numerical aperture 1.4 Planachromat objective. During image acquisition, cells were kept in a thermostated chamber at 30°C. Images were acquired at 490 ± 5 and 440 ± 10 nm excitation wavelength, using a 535 ± 25-nm emission filter. In situ calibration curves were obtained on HeLa cells internalized transferrin-FITC (Invitrogen) by clamping the vesicular pH between 4.5 and 7.0 in buffered K+-rich medium (135 mM KCl, 10 mM NaCl, 20 mM HEPES or 20 mM MES, 1 mM MgCl2, and 0.1 mM CaCl2) with10 μM nigericin and 10 μM monensin (Sigma-Aldrich). The fluorescence ratios as a function of extracellular pH provided the standard curve for pH determination of EGF-FITC–containing vesicles. Image analysis was performed with the Metafluor software (Molecular Devices, Sunnyvale, CA). The background subtracted fluorescence ratios of ∼100–300 vesicles were determined, and their distributions were fitted to mono- or multipeak Gaussian curves using Origin 7.0 software (OriginLab, Northampton, MA). The average pH of each type of vesicle population was calculated as the arithmetic mean of the data and was identical to the Gaussian mean. In Tsg101 siRNA treatment, the data were best fitted with a two-component Gaussian distribution, and the mean ratio was obtained by weighted averaging.
Electron Microscopy (EM)
To follow EGFR endocytosis on the EM level, we followed a pre-embedding approach. Cells were transfected with the RNAi Vps24/scrambled construct (for transfection details, see above) and incubated for 72 h. The cells were then labeled with EGFR antibody (catalog no. 555996; BD Biosciences PharMingen, San Diego, CA) at 4°C for 20 min and washed three times, followed by a 20-min incubation with 10-nm protein A-gold (PAG). After washing, the cells were treated with 50 ng/ml EGF for 20 min at 37°C and chased in EGF-free medium for 3 h. Cells were fixed in 2% glutaraldehyde in 0.1 M phosphate buffer at room temperature for 1 h, scraped, and pelleted at 10,000 rpm. Samples were postfixed with 2% OsO4 and 1.5% KFeCN in H2O, stained with 4% uranyl acetate, and prepared for conventional plastic embedding. Sections (40–50 nm; Leica microtome) were contrasted with lead citrate and observed in a Philips CM10 electron microscope. The mean diameter of EGFR-containing endosomes was estimated by measuring 70–80 EGFR/PAG-positive endosomes per group (control/RNAi-treated cells) from three separate experiments.
RESULTS
Endogenous hVps24 Is Found on Late Endosomes and Colocalizes with Internalized EGFRs
Immunofluorescence and immuno-EM has shown that Hrs resides on the limiting membrane of early endosomes and MVEs (Raiborg et al., 2002; Sachse et al., 2002). In contrast, it has been difficult to determine the precise subcellular localizations of endogenous ESCRT proteins because of the paucity of ESCRT antibodies suited for immunocytochemistry. That these proteins tend to aggregate when overexpressed in cells also makes it difficult to follow their localizations by epitope-tagging approaches. Nevertheless, it has been possible to determine that endogenous Tsg101 localizes mainly to late endosomes (Bache et al., 2003a). Endogenous hVps24 has previously been reported to localize to the trans-Golgi network (TGN) (Yan et al., 2005), which is difficult to reconcile with a role in protein sorting from endosomes to lysosomes. We therefore attempted to revisit the localization of this ESCRT-III subunit. For this purpose, an antiserum was raised against a peptide sequence specific for hVps24 and used to stain fixed HEp2 cells. Interestingly, the anti-hVp24 antibody showed a punctate cytoplasmic staining (Figure 1A) that was more reminiscent of endosomes than of the TGN. Indeed, we were unable to detect any significant colocalization with the TGN marker TGN46 (Figure 1, J–L), and we only detected little colocalization with EEA1 (Figure 1, A–C). In contrast, hVp24 was found to colocalize partially with the late endosomal markers CD63 (Figure 1, D–F) and LAMP1 (Figure 1, G–I), with CD63 showing the highest extent of colocalization. A similar colocalization with late endosomal markers was found using HeLa cells, although these cells expressed lower levels of hVps24 than HEp2 cells (our unpublished data). We conclude from this that hVps24 resides mainly on a subset of late endosomes.
Figure 1.
hVps24 is localized to late endosomes. Hep2 cells were permeabilized before fixation. The cells were stained with antibodies against hVps24 (red) and EEA1 (green; A–C), CD63 (green; D–F), LAMP2 (green; G–I), or TGN46 (green; J–L). Colocalization between hVps24 and the endosome/TGN markers is indicated in yellow.
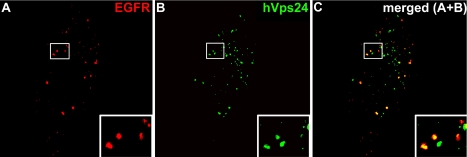
To address whether internalized EGFRs traffic through hVps24-containing endosomes, HEp2 cells were stimulated with EGF for 5 min and chased in EGF-free medium for 15 min to allow trafficking through endosomes. They were then stained with antibodies against hVps24 and EGFR. This analysis showed that most of the EGFR-containing endosomes were positive for hVps24 (Figure 2, indicated with yellow in the merged image). The extensive colocalization of internalized EGFRs with hVps24 is consistent with the possibility that ESCRT-III mediates endosomal trafficking of these receptors.
Figure 2.
Localization of hVps24 and EGFR in EGF-stimulated cells. HEp2 cells were starved overnight before stimulation with 50 ng/ml EGF (5 min) followed by 15-min chase in 10 μg/ml cycloheximide-containing medium. The cells were permeabilized before fixation and stained for EGFR (red) and hVps24 (green). Colocalization between EGFR and hVps24 is indicated in yellow in the merged image.
hVps24 Is Essential for Ligand-induced Degradative EGFR Trafficking
The idea that hVps24 is involved in degradative protein sorting is supported by the finding that overexpression of the N-terminal part of hVps24 inhibits EGFR degradation (Yan et al., 2005). However, because ESCRT-III proteins are thought to engage in heteromultimeric complexes (Babst et al., 2002a), an indirect effect of this overexpression on EGFR degradation could not be excluded. We therefore investigated whether depletion of hVps24 affects EGFR degradation. Two siRNA duplexes specific for distinct sequences within hVps24 (siRNA-1 and siRNA-2, respectively) were found to yield strong knockdowns of hVps24 in comparison with a scrambled sequence RNA duplex, as determined by Western blotting (Figure 3, middle). Moreover, although the EGFR was largely degraded after 2 and 4 h of EGF stimulation in cells treated with the control RNA duplex, siRNA against hVps24 strongly inhibited EGF-induced receptor degradation (Figure 3, top). This indicates that hVps24, like Hrs and ESCRT-I, is required for ligand-induced degradation of the EGFR.
To study in more detail the consequences of hVps24 depletion on EGFR trafficking, we studied the cells by confocal immunofluorescence microscopy. In cells treated with control RNA, EGF stimulation for 30 min resulted in a redistribution of the EGFR into EEA1-positive early endosomes (our unpublished data). After a 3-h chase, the receptor was hardly detectable, in accordance with the biochemical data in Figure 3 (Figure 4A). In contrast, in hVps24-depleted cells, the EGFR was detected in EEA1-positive endosomes both after a 30-min EGF stimulation (our unpublished data) and after a 3-h chase (Figure 4B). In a minority (∼20%) of the siRNA-treated cells, enlarged EGFR-positive endosomes could be observed (our unpublished data). There was essentially no colocalization between the EGFR and the late endosomal/lysosomal marker LBPA in the hVps24-depleted cells. Together, our results indicate that hVps24 is important for trafficking of the EGFR from EEA1-positive early endosomes to LBPA-positive late endosomes/lysosomes.
Figure 4.
EGFR is retained in early endosomes in the absence of hVps24. HeLa cells treated with scrambled RNA (A) or with siRNA against hVps24 (B) were given a 30-min pulse of EGF and chased for 3 h. The cells were permeabilized before fixation and stained with antibodies against the EGFR (green), EEA1 (red), and LBPA (blue). Colocalization between EGFR and EEA1 is shown in yellow.
hVps24 Is Not Essential for Fluid Phase Transport between Early Endosomes and Lysosomes
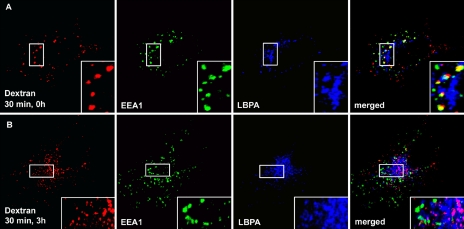
Previous studies have shown that EGFR trafficking but not fluid phase transport from early to late endosomes requires phosphatidylinositol 3-phosphate (PI3P), a lipid that is crucial for Hrs recruitment and thereby for ESCRT-I function (Petiot et al., 2003). We therefore asked whether hVps24 is required for fluid phase transport from early endosomes to late endosomes/lysosomes. To address this, hVps24 was knocked down in HeLa cells by siRNA, and the cells were incubated with fluorescently labeled dextran for 30 min to allow endocytic uptake. Thereafter, the cells were chased for 3 h in the absence of dextran to allow trafficking of the endocytosed dextran to late endosomes. In cells treated with scrambled RNA duplex, dextran was seen to colocalize with EEA1 after 30 min and with LBPA after a 3-h chase, as expected (our unpublished data). Importantly, in hVps24-depleted cells, the internalized dextran also colocalized with EEA1 after 30 min (Figure 5A) and was also transported to LBPA-positive late endosomes after a 3-h chase (Figure 5B). This indicates that hVps24, similarly to PI3P, mediates EGFR trafficking but not fluid phase transport from early to late endosomes.
Figure 5.
Fluid phase transport from early to late endosomes is unaffected in hVps24-depleted cells. HeLa cells treated with siRNA against hVps24 were left to internalize prebound dextran for 30 min at 37°C. The cells were washed and chased for 0 h (A) and 3 h (B) before they were permeabilized, fixed, and stained for immunofluorescence microscopy. Dextran is shown in red, EEA1 in green, and LBPA in blue. Colocalization between dextran and EEA1 is shown in yellow, and colocalization between dextran and LBPA is in purple.
hVps24, Unlike Tsg101, Is Not Required for Silencing of the EGFR
Cell lines with impaired Tsg101 function show sustained activation of the MAP kinase pathway downstream of the activated EGFR, suggesting that ESCRT-I is essential for silencing of EGFR signaling from the endosome membrane (Babst et al., 2000). Because ESCRT-III is thought to function downstream of ESCRT-I in degradative receptor trafficking (Babst et al., 2002a), we therefore asked whether depletion of hVps24 causes a similar sustainment of EGF signaling. HeLa cells were treated with control RNA or with siRNAs against Tsg101 or hVps24 and then serum starved, stimulated with EGF for 5 min, and chased for up to 120 min. Activation of the MAP kinase pathway was then followed by analyzing cell lysates by immunoblotting with antibodies against phosphorylated MEK1/2 and ERK1/2. Although there was little phosphorylation of the two kinases in starved cells, both were strongly phosphorylated after 5 min of EGF stimulation (Figure 6, A and B). In cells treated with control RNA, phosphorylation of both MEK1/2 and ERK1/2 was strongly reduced already after a 30-min chase (Figure 6, A and B). When Tsg101 was depleted, there was a sustained activation of both MEK1/2 and ERK1/2 (Figure 6, A–C), in agreement with previous results (Babst et al., 2000). Surprisingly, however, depletion of hVps24 did not cause sustained phosphorylation of MEK1/2 and ERK1/2 (Figure 6, A–C). On the contrary, phosphorylation of ERK1/2 was slightly less sustained in hVps24-depleted cells than in control cells (Figure 6, A and C). This indicates that, although hVps24 is required for degradation of the EGFR, hVps24 is not required for silencing of receptor signaling.
Figure 6.
Different signaling downstream of the EGFR in cells depleted of Tsg101 or hVps24. (A) HeLa cells treated with scrambled RNA, siRNA against Tsg101, or siRNA against hVps24 were plated out in one six-well dish each and starved for 3 h in the presence of cycloheximide. All wells except one in each dish were given a 5-min pulse of EGF and then chased in normal medium supplied with cycloheximide for the indicated times. The cells were then lysed and analyzed by SDS-PAGE and antibodies against phospho-MEK, total MEK, phospho-ERK, and total ERK as described in Materials and Methods. The intensities of the phospho-MEK (B) and phospho-ERK (C) bands were quantified by using the software provided by the ChemiGenius imaging system and plotted as percentage of the respective phosphorylation intensities after 5 min of EGF stimulation and 0 min of chase. Intensities are adjusted for different loadings. Note that the values in C are obtained from a separate set of experiments with shorter chase times, an example of which is provided in Supplemental Figure S1. Control cells, square symbols; Tsg101 siRNA-treated cells, triangle symbols; and hVps24 siRNA-treated cells, round symbols. The error bars represent SEM of four experiments.
Depletion of Tsg101 and hVps24 Differentially Affects the pH of EGF-containing Endosomes
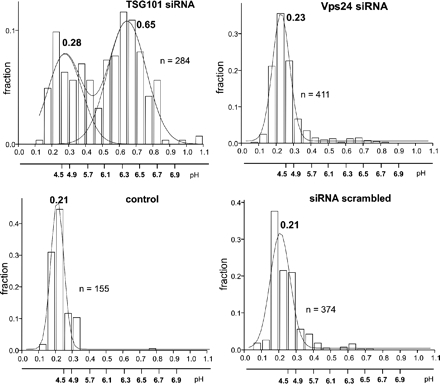
How can the differential requirements for Tsg101 and hVps24 in EGFR silencing be explained? Because dissociation of ligand at low endosomal pH is thought to contribute to receptor silencing (Skarpen et al., 1998), we considered the possibility that depletion of Tsg101 and hVps24 may differentially affect receptor accumulation in acidified endosomes. To address this, we used FITC-labeled EGF as a pH probe for EGFR-containing compartments. The validity of this probe was confirmed by control experiments showing that internalized EGF and EGFR colocalized strongly under the experimental conditions used (Supplemental Figure S2). HeLa cells were depleted for Tsg101 or hVps24, incubated with FITC-labeled EGF for 60 min, chased for another 30 min in serum-free medium, and intraendosomal pH was measured by fluorescence ratio imaging. This analysis showed that FITC-EGF reached a compartment with a pH of ∼4.5 in cells treated with control RNA, indicative of transport to late endosomes or lysosomes (Figure 7). By comparison, FITC-labeled transferrin reached a compartment with a pH value of 6.4, presumably corresponding to recycling endosomes (our unpublished data). Interestingly, FITC-EGF–containing endosomes in hVps24-depleted cells showed the same low pH as in control cells, whereas FITC-EGF showed a bimodal distribution in Tsg101-depleted cells (Figure 7). Some of the FITC-EGF was found in a compartment with pH of ∼4.5, whereas the strongest signal was found in a compartment with pH of ∼6.4, suggestive of a delay in trafficking between two different types of endosomes. This indicates that Tsg101, but not hVps24, is required for normal accumulation of EGF in acidic endosomes. This raises the possibility that intraluminal pH could be a crucial factor in the silencing of EGFRs.
Figure 7.
Effect of hVps24 and TSG101 ablation on the vesicular pH of EGF-containing endocytic vesicles. Ratiometric fluorescence video image analysis of internalized EGF-FITC (and transferrin-FITC; our unpublished data) containing vesicles was performed in live HeLa cells transfected with the indicated siRNA as described in Materials and Methods. The mean pH of vesicle population was calculated by the Origin software, measured in five independent experiments (±SEM). In each experiment, the luminal pH of 100–300 endocytic vesicles was determined.
Depletion of hVps24 Affects the Morphology of EGFR-containing Endosomes
Taking into account that hVps24-depleted endosomes show normal acidification, why is their degradation of EGFRs inhibited? The most straightforward explanation is that their fusion with lysosomes and thus their acquisition of lysosomal hydrolases might be inhibited. To address this possibility, we studied the endosomal morphology of endosomes in hVps24-depleted cells by EM. HeLa cells treated with control RNA or with siRNA against hVps24 were incubated on ice with antibodies against the EGFR, followed by colloidal gold-labeled secondary antibodies. After stimulation with EGF for 30 min and chase for 3 h at 37°C, the cells were fixed, embedded in plastic, and sectioned for transmission EM. Internalization of bound gold particles was not observed in cells treated with an irrelevant antibody (our unpublished data). In control cells, EGFR-containing endosomes were readily detected by the presence of colloidal gold and had a typical MVB appearance with numerous intraluminal vesicles. EGFR-containing endosomes could also be detected in hVps24-depleted cells, but these endosomes had a different morphology than those of the control cells. The endosomes seemed to contain fewer intraluminal vesicles than the control endosomes, and, most notably, the EGFR-positive endosomes in hVps24-depleted cells were significantly smaller than those in the control cells (Figure 8, A–D). A quantitative analysis confirmed this (Figure 8E). We noted that the colloidal gold particles mostly localized to intraendosomal membranes even in hVps24-depleted cells, suggesting that EGFR sorting from the limiting membrane does not require hVps24 (Figure 8B). It was also our impression that the morphology of the intraluminal vesicles of hVps24-depleted endosomes was less uniform than that seen in control endosomes. Together, these findings indicate that the formation/maturation of MVEs was affected by hVps24 depletion, whereas EGFR sorting was unaffected.
Figure 8.
Depletion of hVps24 causes accumulation of small MVEs. EM of EGFR-labeled endosomes/MVEs. (A) EGFR labeling in control cell within a typical endosome with multivesicular appearance. Note that the MVE contains numerous intraluminal vesicles and that most of the labeling is found on these vesicles. (C) Overview of a group of endosomes illustrating the homogenous morphology of EGFR-positive endosomes. (B) In RNAi-treated cells, the EGFR-positive vesicles are significantly smaller than control MVEs and contain seemingly fewer intraluminal vesicles. EGFR labeling is found on these vesicles, which do not seem so homogeneous in size compared with control cells. (D) Overview of a group of endosomes and lysosomes in an RNAi-treated cell. Arrowheads in C and D indicate EGFR labeling. Bars, 200 nm. (E) Estimation of the mean vesicle diameter of EGFR-positive endosomes in control and RNAi-treated cells (control cells, n = 76; RNAi-treated cells, n = 68; 3 separate experiments). The difference in diameter between the two groups is ∼30%. Significance level by Student's t test (p ≤ 0.005).
DISCUSSION
The termination of receptor signaling in endosomes is thought to be of great importance for the duration of cell signaling events, and its impairment has been implicated in carcinogenesis (Dikic and Giordano, 2003). Here, we have shown that two ESCRT subunits play differential roles in silencing of the EGFR. Although the ESCRT-I subunit Tsg101 is required for efficient silencing of EGF-induced activation of the MAP kinase pathway, this was not the case for the ESCRT-III subunit hVps24. Because both Tsg101 and hVps24 were found important for ligand-induced degradation of the EGFR, this reveals that silencing of MEK/ERK signaling from endosomes can occur independently of EGFR degradation.
Based on previous studies with yeast Vps24 (Babst et al., 2002a) and with the N-terminal part of hVps24 (Yan et al., 2005), the requirement of hVps24 for EGFR degradation was expected. However, it was unexpected that MEK/ERK activity was silenced in the virtual absence of hVps24. It seems implausible that this can be explained by inefficient siRNA-mediated knockdown, because the extent of hVps24 depletion was high and had a strong effect on EGFR degradation. In principle, the receptor could be silenced in four different ways: 1) by proteolytic degradation, 2) by dissociation of ligand at low endosomal pH, 3) by engagement of the cytosolic tail of the receptor by ESCRT complexes that prevent access of signaling mediators, or 4) by sequestration of the receptor in intraluminal vesicles of the endosome. Our results indicate that the EGFR is silenced through one of the three latter mechanisms, or through a combination of these mechanisms.
How can it be explained that knockdown of Tsg101 and hVps24 similarly inhibits degradation of the EGFR but differentially affects its silencing? There may be several explanations for this paradox. First, although a recent study showed that Tsg101 depletion causes the formation of multicisternal endosomes without intraluminal vesicles (Doyotte et al., 2005), we found here that hVps24 depletion results in the accumulation of EGFRs in small endosomes that do contain intraluminal vesicles. This raises the possibility that EGFRs can signal from the limiting membranes of multicisternal endosomes in Tsg101-depleted cells, but not from intraluminal endosomal vesicles in hVps24-depleted cells. Second, depletion of Tsg101 caused EGF to accumulate in endosomes with a close to neutral pH, whereas EGF-containing endosomes in hVps24-depleted cells were acidic. Because EGF only dissociates from its receptor at low pH (Skarpen et al., 1998), this could mean that receptors in the limiting membranes of endosomes from Tsg101-depleted cells are ligand bound and therefore active, whereas endosomal EGFRs in hVps24-depleted cells are signaling incompetent because of low pH-induced dissociation of EGF. Third, it is known that depletion of Tsg101 causes depletion of the entire ESCRT-I (Bache et al., 2004a), whereas Vps24 is not required for the intactness of ESCRT-I, ESCRT-II, and the Vps20/Vps32 subcomplex of ESCRT-III (Babst et al., 2002a). This raises the possibility that EGFRs can signal from the limiting membrane of endosomes in Tsg101-depleted cells, whereas receptors in hVps24-depleted cells may be engaged in ESCRT complexes that render them inaccessible to the signaling machinery. Fourth, even though the detected MEK and ERK phosphorylation was dependent on EGF stimulation, we cannot rule out the possibility that the aberrant MEK/ERK activity observed in Tsg101-depleted cells may involve mechanisms that are independent of EGFR activity. Regardless of the mechanism, our results have uncovered a difference in the requirements of the ESCRT-I subunit Tsg101 and the ESCRT-III subunit hVps24 in the silencing of EGF-activated MAP kinase activation. The relationships between ESCRT-I and ESCRT-III may therefore be less linear than previously thought.
In the light of current models for MVE biogenesis, the differential effects of Tsg101 and hVps24 depletion on the pH on EGF-containing endosomes was unexpected and suggest that Tsg101 but not hVps24 is required for normal transport of EGF from early endosomes to a compartment with low pH. Alternatively, Tsg101 but not hVps24 might be needed for the acquisition of proton pumps in endosomal membranes. However, because of the bimodal distribution of FITC-EGF in acidic and less acidic endosomes and the highly acidic pH (<5, measured by FITC-dextran; our unpublished data) of lysosomes in Tsg101-depleted cells (Figure 7), we favor the interpretation that Tsg101 is required for EGF trafficking, not endosomal acidification as such. But if the EGFR accumulates in properly acidified endosomes in hVps24-depleted cells, why is degradation of the EGFR inhibited? One possibility is that hVps24 might be required for activation of cathepsin B, which is known to mediate degradation of the EGFR (Authier et al., 1995). However, we did not detect any change in cathepsin B activity in hVps24-depleted cells (our unpublished data). Our immunofluorescence and electron microscopic analyses indicated that normal MVE biogenesis and progression into late endosomes/lysosomes were attenuated in hVps24-depleted cells. We therefore propose that hVps24 is important for the fusion of MVEs with lysosomes, which contain hydrolytic enzymes needed for receptor degradation. This implies a novel function for hVps24 distinct from its suggested role in MVE biogenesis and sorting (Babst et al., 2002a).
Previous studies have revealed that fluid phase transport from early endosomes to lysosomes is regulated differentially than receptor trafficking (Petiot et al., 2003; Gruenberg and Stenmark, 2004). The finding that EGFR trafficking but not fluid phase transport from early endosomes to lysosomes requires hVps24 is consistent with this concept. In this context, it is also worth noting that EGFRs have recently been found to accumulate in a subset of MVEs that are distinct from LBPA-containing MVEs (White et al., 2006). This indicates the existence of multiple parallel pathways between early endosomes and lysosomes.
A previous study concluded that endogenous hVps24 is localized to the TGN (Yan et al., 2005), whereas here we found hVps24 to localize to late endosomes, especially those positive for CD63. The differential results are unlikely to have arisen from use of different cell lines, because both studies used HeLa cells as one of two cell lines examined. However, because late endosomes are often found in the juxtanuclear area, where the TGN is also located, TGN and late endosomal markers may sometimes yield partially overlapping staining patterns at the light-microscopic level. A localization of hVps24 to late endosomes is consistent with a previously found localization of Tsg101 to these organelles, and with a function for hVps24 in endosomal trafficking. Even though hVps24 is enriched on late endosomes, this does not mean that hVps24 is initially recruited to these organelles. On overexpression of Hrs, Tsg101 can be seen to accumulate on early endosomes, suggesting that ESCRT-I is initially recruited to early endosomes (Bache et al., 2003a). Similarly, overexpressed hVps24 can be found on early endosomes (Yan et al., 2005), and we did observe a few EEA1-positive structures that labeled for endogenous hVps24 (Figure 1, A–D). We therefore hypothesize that hVps24 is recruited to early endosomes but remains endosome associated through endosomal maturation or trafficking. Because early endosomes are more short lived than late endosomes, the bulk of hVps24 is therefore detected on late endosomes at steady state.
In the present work, we have revealed some unexpected differences in the functions of the ESCRT-I subunit Tsg101 and the ESCRT-III subunit hVps24. Both subunits are required for ligand-induced degradation of EGFRs, but only Tsg101 is required for termination of EGF signaling from endosomes. This indicates that endosomal silencing of receptor signaling is more complex than previously thought and warrants further studies on the function of ESCRTs in receptor trafficking and signaling. It is interesting to note that although no ESCRT-III subunits have so far been implicated in cancer, this has been the case for two ESCRT-I subunits, Tsg101 and hVps37A/HCRP1 (Li and Cohen, 1996; Xu et al., 2003). It is thus possible that ESCRT-I plays a particularly important role in signal termination on endosomes. Further studies on the connections between the endosomal sorting machinery and carcinogenesis will elucidate this role.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gillian Griffiths and Jean Gruenberg for reagents. C. R. and T. S. are postdoctoral fellows of the Norwegian Cancer Society. S. S., L. M., and A. B. are supported by the FUGE program. This work was also supported by the Research Council of Norway, the Novo-Nordisk Foundation, and by the Canadian Institute of Health Research.
Abbreviations used:
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ESCRT
endosomal sorting complex required for transport
- Hrs
hepatocyte growth factor-regulated tyrosine kinase substrate
- MVE
multivesicular endosome
- PI3P
phosphatidylinositol 3-phosphate
- siRNA
small-interfering RNA
- Tsg101
tumor susceptibility gene 101
- vps
vacuolar protein sorting
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-10-0915) on March 22, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Authier F., Mort J. S., Bell A. W., Posner B. I., Bergeron J. J. Proteolysis of glucagon within hepatic endosomes by membrane-associated cathepsins B and D. J. Biol. Chem. 1995;270:15798–15807. doi: 10.1074/jbc.270.26.15798. [DOI] [PubMed] [Google Scholar]
- Babst M., Katzmann D. J., Estepa-Sabal E. J., Meerloo T., Emr S. D. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 2002a;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Babst M., Katzmann D. J., Snyder W. B., Wendland B., Emr S. D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 2002b;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Babst M., Odorizzi G., Estepa E. J., Emr S. D. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- Bache K. G., Brech A., Mehlum A., Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J. Cell Biol. 2003a;162:435–442. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache K. G., Raiborg C., Mehlum A., Stenmark H. STAM and Hrs are subunits of a multivalent Ubiquitin-binding complex on early endosomes. J. Biol. Chem. 2003b;278:12513–12521. doi: 10.1074/jbc.M210843200. [DOI] [PubMed] [Google Scholar]
- Bache K. G., Slagsvold T., Cabezas A., Rosendal K. R., Raiborg C., Stenmark H. The growth-regulatory protein HCRP1/hVps37A is a subunit of mammalian ESCRT-I and mediates receptor down-regulation. Mol. Biol. Cell. 2004a;15:4337–4346. doi: 10.1091/mbc.E04-03-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache K. G., Slagsvold T., Stenmark H. Defective downregulation of receptor tyrosine kinases in cancer. EMBO J. 2004b;23:2707–2712. doi: 10.1038/sj.emboj.7600292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N., Horman A., Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J. Cell Biol. 2002;157:91–101. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresa B. P., Schmid S. L. Regulation of signal transduction by endocytosis. Curr. Opin. Cell Biol. 2000;12:204–210. doi: 10.1016/s0955-0674(99)00077-0. [DOI] [PubMed] [Google Scholar]
- Dikic I., Giordano S. Negative receptor signaling. Curr. Opin. Cell Biol. 2003;15:128–135. doi: 10.1016/s0955-0674(03)00004-8. [DOI] [PubMed] [Google Scholar]
- Doyotte A., Russell M. R., Hopkins C. R., Woodman P. G. Depletion of TSG101 forms a mammalian “class E” compartment: a multicisternal early endosome with multiple sorting defects. J. Cell Sci. 2005;118:3003–3017. doi: 10.1242/jcs.02421. [DOI] [PubMed] [Google Scholar]
- Futter C. E., Pearse A., Hewlett L. J., Hopkins C. R. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J. Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Stenmark H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell. Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- Katzmann D. J., Odorizzi G., Emr S. D. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell. Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Stang E., Fang K. S., de Moerloose P., Parton R. G., Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- Li L., Cohen S. N. Tsg 101, a novel tumor susceptibility gene isolated by controlled homozygous functional knockout of allelic loci in mammalian cells. Cell. 1996;85:319–329. doi: 10.1016/s0092-8674(00)81111-3. [DOI] [PubMed] [Google Scholar]
- Lloyd T. E., Atkinson R., Wu M. N., Zhou Y., Pennetta G., Bellen H. J. Hrs regulates endosome invagination and receptor tyrosine kinase signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- Mu F. T., Callaghan J. M., Steele-Mortimer O., Stenmark H., Parton R. G., Campbell P. L., McCluskey J., Yeo J. P., Tock E. P., Toh B. H. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J. Biol. Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Pawson T. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell. 2004;116:191–203. doi: 10.1016/s0092-8674(03)01077-8. [DOI] [PubMed] [Google Scholar]
- Petiot A., Faure J., Stenmark H., Gruenberg J. PI3P signaling regulates receptor sorting but not transport in the endosomal pathway. J. Cell Biol. 2003;162:971–979. doi: 10.1083/jcb.200303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo S., Pece S., Di Fiore P. P. Endocytosis and cancer. Curr. Opin. Cell Biol. 2004;16:1–6. doi: 10.1016/j.ceb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Raiborg C., Bache K. G., Gillooly D. J., Madshus I. H., Stang E., Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- Raiborg C., Rusten T. E., Stenmark H. Protein sorting into multivesicular endosomes. Curr. Opin. Cell Biol. 2003;15:446–455. doi: 10.1016/s0955-0674(03)00080-2. [DOI] [PubMed] [Google Scholar]
- Rubin I., Yarden Y. The basic biology of HER2. Ann. Oncol. 2001;12(Suppl 1):S3–S8. doi: 10.1093/annonc/12.suppl_1.s3. [DOI] [PubMed] [Google Scholar]
- Sachse M., Urbe S., Oorschot V., Strous G. J., Klumperman J. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., et al. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J. Cell Biol. 2004;164:923–933. doi: 10.1083/jcb.200312018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Bremnes B., Rønning E., Aasland R., Stenmark H. Syntaxin-16, a putative Golgi t-SNARE. Eur. J. Cell Biol. 1998;75:223–231. doi: 10.1016/S0171-9335(98)80116-7. [DOI] [PubMed] [Google Scholar]
- Skarpen E., et al. Endocytosed epidermal growth factor (EGF) receptors contribute to the EGF-mediated growth arrest in A431 cells by inducing a sustained increase in p21/CIP. Exp. Cell Res. 1998;243:161–172. doi: 10.1006/excr.1998.4127. [DOI] [PubMed] [Google Scholar]
- Sorkin A., Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat. Rev. Mol. Cell. Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- Sundquist W. I., Schubert H. L., Kelly B. N., Hill G. C., Holton J. M., Hill C. P. Ubiquitin recognition by the human TSG101 protein. Mol. Cell. 2004;13:783–789. doi: 10.1016/s1097-2765(04)00129-7. [DOI] [PubMed] [Google Scholar]
- Vieira A. V., Lamaze C., Schmid S. L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- White I. J., Bailey L. M., Aghakhani M. R., Moss S. E., Futter C. E. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Liang L., Wang H., Li T., Zhao M. HCRP1, a novel gene that is downregulated in hepatocellular carcinoma, encodes a growth-inhibitory protein. Biochem. Biophys. Res. Commun. 2003;311:1057–1066. doi: 10.1016/j.bbrc.2003.10.109. [DOI] [PubMed] [Google Scholar]
- Yan Q., Hunt P. R., Frelin L., Vida T. A., Pevsner J., Bean A. J. mVps24p functions in EGF receptor sorting/trafficking from the early endosome. Exp. Cell Res. 2005;304:265–273. doi: 10.1016/j.yexcr.2004.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.