Abstract
Regulation of cell migration is an important step for the development of branching tubule morphogenesis in collagen gel. Here, we showed that discoidin domain receptor (DDR) 1a/b inhibited collagen-induced tyrosine phosphorylation of signal transducers and activators of transcription (Stat) 1/3 and cell migration triggered by α2β1-integrin. Overexpression of DDR1a/b increased the interaction of DDR1 with SHP-2 and up-regulated the tyrosine phosphatase activity of SHP-2. Expression of catalytically inactive SHP-2 in DDR1-transfected cells restored the tyrosine phosphorylation of Stat3 and cell migration. We demonstrated that the Src homology-2 (SH2)-SH2 and phosphotyrosyl phosphatase (PTP) domains of SHP-2 were responsible for interaction with DDR1 and that both tyrosine phosphorylation sites 703 and 796 of DDR1 were essential for it to bind with SHP-2. Mutation of tyrosine 703 or 796 of DDR1 abolished the ability of DDR1 to inhibit the tyrosine phosphorylation of Stat1 and Stat3 and restored collagen-induced cell migration and hepatocyte growth factor-induced branching tubulogenesis in collagen gel. Together, these results demonstrate that SHP-2 is required for the DDR1-induced suppression of Stat1 and Stat3 tyrosine phosphorylation, cell migration, and branching tubulogenesis.
INTRODUCTION
Many organs, including the salivary glands, lungs, and kidneys, are formed by epithelial branching tubulogenesis during embryonic development. Numerous in vitro studies have shown that reconstituted matrices, such as collagen or basement membrane gels, promote the organization of epithelial cells into three-dimensional, tissue-like structures (Montesano et al., 1991). The attachment of epithelial cells to an extracellular matrix (ECM) is mediated through integrin and receptor tyrosine kinase (RTK). Studies using an in vitro model system with Madin-Darby canine kidney (MDCK) cells have demonstrated that α2β1-integrin regulates epithelial polarity development and tubule formation in collagen gel (Saelman et al., 1995; Chiu et al., 2002). In addition, disruption of α3β1-integrin suppressed the cell scattering, migration, and branching morphogenesis of MDCK cells (Jiang et al., 2001).
Discoidin domain receptor (DDR) is an RTK that has in its extracellular region an N-terminal domain homologous to the Dictyostelium discoideum protein discoidin I (Springer et al., 1984). There are two DDR receptors: DDR1 and DDR2 (Alves et al., 1995). DDR1 is expressed in normal epithelial and tumor cells, whereas DDR2 is expressed only in the surrounding stroma cells (Vogel, 1999). As a result of alternative splicing, there are five DDR1 isoforms identified (Alves et al., 2001). DDR1a lacks a sequence of 37 amino acids found in juxtamembrane domain of DDR1b that contains an LXNPXY sequence. DDR1c, the longest isoform found in fetal brain, is generated by insertion of six amino acids (S-F-S-L-F-S) at the beginning of the kinase domain (Alves et al., 1995). DDR1d and DDR1e are two kinase-deficient receptors differentially expressed in human colon cancer cell lines (Alves et al., 2001). Collagen type I to type VI, the ligands for DDR1, induce autophosphorylation of DDR1 with delayed kinetics (Shrivastava et al., 1997; Vogel et al., 1997). There is relatively little information concerning DDR1 downstream signal pathways and its functions of inhibiting both cell proliferation (Curat and Vogel, 2002) and apoptosis (Ongusaha et al., 2003). DDR1 can affect cell migration, but the effects are different in different cell models. In DDR1-null mice, migration of vascular smooth muscle cells toward type I collagen was reduced by 33%; in human monocytic THP-1 leukemic cells, overexpression of DDR1a resulted in increased cell migration in response to collagen, and overexpression of DDR1b decreased (Kamohara et al., 2001), all of which suggest that cell type-specific environments also modulate the function of DDR1. We found that overexpression of DDR1 in MDCK cells decreased collagen-induced cell migration (Wang et al., 2005). However, the signal mechanisms of DDR1 that regulate cell migration remain undefined.
Signal transducers and activators of transcription (Stats) are latent cytoplasmic proteins activated in response to cytokine and/or growth factor receptor stimulation (Leonard and O'Shea, 1998). In addition to cytokine receptors that transmit signals to Janus kinase (JAK) family, a variety of receptors also activate Stat proteins, for example, β1- or αv-integrin activates JAK2 and Stat5A in endothelial cells (Brizzi et al., 1999), epidermal growth factor receptor activates Stat1 (Chin et al., 1996), G protein-coupled receptors activate JAK2 and Stat1/3 (Pelletier et al., 2003), and hepatocyte growth factor receptor activates Stat3 in epithelial cells (Boccaccio et al., 1998). Several biological functions are affected in Stat3-deficient cells, including proliferation, apoptosis, and migration (Leonard and O'Shea, 1998; Sano et al., 1999). Moreover, inhibition of Stat3 activation suppresses hepatocyte growth factor-induced tubulogenesis in MDCK cells (Boccaccio et al., 1998).
RTK signaling activities are controlled by a distinct class of enzyme known as phosphotyrosyl phosphatases (PTPs). Among the nontransmembrane mammalian PTPs, only two are Src homology-2 (SH2) domain-containing, namely, SHP-1 and SHP-2 (Adachi et al., 1996). SHP-2 is a ubiquitously expressed mammalian PTP, whereas SHP-1 is primarily restricted to hematopoietic cells (Neel et al., 2003). Both PTPs have two tandem SH2 domains in their N-terminal region, a phosphatase domain in their C-terminal regions, and a tyrosine phosphorylation site in their extreme C-terminal region. SHP-2 directly binds to some autophosphorylated RTKs, such as epidermal growth factor receptor, platelet-derived growth factor receptor, or Met receptor (Neel et al., 2003). Moreover, SHP-2 binds to tyrosine-phosphorylated adaptor proteins, such as Grb2 adaptor binder (Gab) 1 and 2 (Schaeper et al., 2000), and FRS2 (Hadari et al., 1998). These interactions are essential for growth-factor- or cytokine-induced activation of the Erk pathway, for cell proliferation (Neel et al., 2003), and for HGF-induced branching tubulogenesis (Schaeper et al., 2000). In addition, SHP-2 dephosphorylates tyrosine-phosphorylated Stat1/3/5A (Ohtani et al., 2000; Wu et al., 2002; Chen et al., 2003), and down-regulates Stat3-mediated biological actions (Ohtani et al., 2000).
Our previous studies demonstrated that DDR1 (Wang et al., 2005) and integrins (Jiang et al., 2001; Chiu et al., 2002) regulate tubule formation in collagen gel. In this study, we report that SHP-2 is required for the DDR1 signaling that suppresses α2β1-integrin–mediated Stat1 and Stat3 tyrosine phosphorylation, cell migration, and HGF-induced branching morphogenesis. These results provide the first evidence of the cross-talk between DDR1 and α2β1-integrin, and they also provide a mechanistic insight into the function of DDR1.
MATERIALS AND METHODS
Plasmids and Antibodies
Tyrosine phosphorylation mutant DDR1 (Y484F-DDR1, Y547F-DDR1, Y569F-DDR1, Y586F-DDR1, Y703F-DDR1, and Y796F-DDR1) was obtained by introducing the indicated point mutation to wild-type DDR1b by using complementary primers. A kit (QuikChange site-directed mutagenesis kit; Stratagene, La Jolla, CA) was used to produce the mutants. Introduction of the point mutation was confirmed by sequencing the relevant region. The pcDNA3.1 expression vectors encoding DDR1a, DDR1b, and carboxy-terminal–truncated (dominant-negative) DDR1 (DN-DDR1) construct were described previously (Wang et al., 2005). The PRcCMV vectors encoding Flag-tagged constitutive active Stat3 was kindly provided by J. F. Bromberg (Rockefeller University, New York, NY), and the Y705F-Stat3 dominant-negative mutant was provided by A. Kaptein (Centre de Recherche, ZA de Courtaboeuf, Les Ulis, France); the Flag-tagged constitutive-active Stat1 described previously (Sironi and Ouchi, 2004) was kindly provided by Toru Ouchi (New York University, New York City, NY); the pcDNA3 expression vectors encoding SHP-2 catalytically inactive (C/S) mutant were kindly provided by Hiroshi Ohnishi (Gunma University, Maebashi City, Japan); and the pGEX-3T expression vectors encoding full-length SHP-2, N-SH2 domain, C-SH2 domain, N-SH2-C-SH2, C-SH2-PTP, or PTP domain described previously in Wu et al., (2002) were kindly provided by Eugene Chin (Brown University School of Medicine, Providence, RI). Bacterially expressed glutathione S-transferase (GST) fusion proteins were purified using glutathione-agarose beads (Sigma-Aldrich, St. Louis, MO) and overnight dialysis. Rabbit polyclonal anti-Stat3, anti-phospho-Stat3 (Tyr705), and monoclonal anti-phospho-Stat3 (ser727) antibodies were from Cell Signaling Technology (Beverly, MA). Mouse monoclonal anti-SHP-1, anti-SHP-2, and rabbit polyclonal anti-DDR1, and anti-GST antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). P1E6 monoclonal antibody against α2-integrin was from Chemicon International (Temecula, CA). Mouse anti-β-actin was from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom). Rabbit polyclonal anti-α2-integrin, anti-αv-integrin, anti-β1-integrin, and anti-β3-integrin were from Biogenesis (Poole, Dorset, United Kingdom).
Cell Culture and Transfections
MDCK cell clone II 3B5 cells, NIH-3T3 cells, HeLa cells, and 293T cells were maintained in DMEM (high glucose; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum under 5% CO2 at 37°C. They were synchronized using 20–24 h of incubation in serum-free DMEM. MDCK cell clone II 3B5 cells and NIH3T3 cells grown in dishes 60 mm in diameter were transfected with 4 μg of expression plasmids using a reagent (Lipofectamine; Invitrogen) according to the manufacturer's protocol, and then selected using G418. 293T cells grown in dishes 100 mm in diameter were transiently transfected with 12 μg of expression plasmids by using the same reagent.
Preparation of Collagen Gel for Culture
Type I collagen was prepared from rat-tail tendons according to the established procedure (Jiang et al., 2001). The collagen stock solution was 1%; it contained 10 mg (dry weight) rat-tail tendon dissolved in 1 ml of 0.025 N acetic acid. For the preparation of 0.3% collagen gel, 3 volumes of 1% collagen was mixed with 5.7× DMEM (1 volume), 2.5% NaHCO3 (0.5 volume), 0.1 M HEPES (1 volume), 0.17 M CaCl2 (0.1 volume), 1 N NaOH (0.1 volume), and 4.3 volumes of 1× culture medium (DMEM + 10% fetal calf serum) under chilled conditions.
Immunoprecipitation and Western Blot
Cells were washed twice with ice-cold phosphate-buffered saline (PBS) with 1 mM sodium vanadate and lysed in modified radio immunoprecipitation assay buffer (RIPA; 150 mM NaCl, 1 mM EGTA, 50 mM Tris, pH 7.4, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) with protease inhibitor (Complete Protease Inhibitor Cocktail Tablets; Roche Diagnostics, Taiwan) and 1 mM sodium vanadate. The lysates were cleared using centrifugation at 14,000 rpm for 15 min at 4°C, analyzed using Western blot with the antibodies indicated, and followed by enhanced chemiluminescence analysis (ECL system; GE Healthcare). For the immunoprecipitation assay, 500 μg of cell lysates were mixed with anti-DDR1 or anti-SHP-2 antibodies overnight at 4°C. Protein A beads (20 μl; Sigma-Aldrich) were added and mixed at 4°C for 1 h. The beads were washed four times with RIPA buffer and subjected to SDS-PAGE followed by immunoblotting with the reciprocal antibodies.
Flow Cytometry
Cells were cultured at 37°C for 24 h and then serum-starved for 12 h before collagen treatment. Cells were then detached and incubated on ice for 30 min with PBS containing 0.1% bovine serum albumin (BSA) and primary antibodies to integrins α2, β1, αv, or β3. After being washed, the cells were incubated with FITC-conjugated goat anti-mouse IgG (Sigma-Aldrich) and analyzed on a flow cytometer (FACScan; BD Biosciences, San Jose, CA).
Cell Adhesion Assay
Cell adhesion to laminin, fibronectin, collagen, or vitronectin (each at 10 μg/ml) was performed as described previously (Manes et al., 2003). Briefly, 96-well plates were coated (16 h; 4°C) with different substrates. Cells were serum-starved in DMEM/0.1% BSA (12 h; 37°C); after trypsinization, 3 × 104 cells/well were plated (30 min; 37°C). After being washed, adhered cells were fixed and stained with 0.5% crystal violet, and optical density (OD) was measured at 595 nm. Each condition was assayed in quadruplicate.
Immunofluorescence and Confocal Study
Serum-starved cells were stimulated with collagen for 30 min, washed three times with PBS, and fixed using 4% paraformaldehyde prepared in PBS for 20 min at room temperature. Cells were then washed three times in PBS, permeabilized with 0.5% Triton X-100 in PBS for 10 min at room temperature, and then incubated with antibodies as indicated for 1 h. Cells were then washed and incubated with Alexa Fluor 488-conjugated goat anti-rabbit antibody (Invitrogen) and tetramethylrhodamine B isothiocyanate-conjugated goat anti-mouse antibody (Sigma-Aldrich) for 1 h. Immunofluorescent images were taken using confocal microscopy (TCS-SP2; Leica, Tokyo, Japan).
In Vitro Pull-Down Assay
Bacterially expressed GST fusion proteins were purified using glutathione-agarose beads (Sigma-Aldrich) and overnight dialysis. For the in vitro pull-down assay, cell lysates (in RIPA buffer without sodium vanadate) were incubated with glutathione-agarose beads and 10 μg of SHP-2 GST fusion protein for 1 h at 4°C. The incubated lysates were mildly centrifuged, and the precipitates were washed extensively with RIPA buffer, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then immunoblotted with anti-DDR1 or anti-Stat3 antibodies.
In Vitro SHP-2 Phosphatase Activity Assay
Cell lysates from serum-starved MDCK cells expressing the distinct DDR1 constructs were immunoprecipitated with anti-SHP-2 antibody (2 h; 4°C). The beads capturing total SHP-2 were suspended in 50 μl of assay buffer (20 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1 mM dithiothreitol, and 1 mM EDTA). The reaction was initiated by adding 50 μl of assay buffer containing 24 mM p-nitrophenyl phosphate (pNPP). After incubation (1 h; 37°C), the reaction was terminated with 10 μl of 5 N NaOH, and the amount of p-nitrophenol released was determined using absorbance at 405 nm. Alternatively, SHP-2 was immunoprecipitated from PC11, DA-1, and DB-9 cells by using anti-SHP-2 antibody. The immunoprecipitated SHP-2 was then incubated with interferon (INF)-α–treated (10 ng/ml for 10 min) HeLa cell lysates in HEPES reaction buffer (25 mM HEPES, pH 6.5, 50 mM NaCl, and 10 mM 2-mercaptoethanol) for 60 min at 30°C. It was then boiled for 5 min in 2× loading buffer, and the boiled samples underwent immunoblot analysis.
Cell Migration Assay
A modified Boyden chamber (NeuroProbe, Gaithersburg, MD) was used to assay cell migration ability through a chemotactic filter. To evaluate the migration potential, cells from monolayer cultures were treated with 0.2% trypsin/EDTA, washed once with serum-free medium containing 0.05% soybean trypsin inhibitor, and resuspended in serum-free medium. Cells (5 × 105) were suspended in serum-free medium and added to the upper chamber. A polycarbonate filter with 8-μm pores was used to separate the Boyden chamber into an upper and a bottom chamber. We added 30 μg/ml type I collagen, laminin, fibronectin, or vitronectin suspended in serum-free medium to the bottom chamber as the chemoattractant. After a 6-h incubation, cells on the top of the filter were scraped, and the remaining cells on the bottom side of the filter were fixed, stained, and counted under a light microscope. Ten wells were counted in a 100×-magnified field.
Branching Morphogenesis Assay
To study branching morphogenesis, cells were cultured in collagen gel as described previously (Jiang et al., 2001). Briefly, cells were harvested from subconfluent culture by using trypsin/EDTA and resuspended at a concentration of 2 × 104 cells/ml in ice-cold collagen solution (0.3%). Aliquots of cell suspension were dispensed into six-well plates and allowed to gel at 37°C before 1 ml of culture medium was added. Culture medium with 2 ng/ml HGF (Sigma-Aldrich) was changed daily.
Image Acquisition
Images were scanned at a minimum resolution of 300 dpi. TIFF files were resized and eventually brightness adjusted (process applied to the whole image) with Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA) for figures preparation. Cultures were processed for branching morphogenesis as described above and examined on a phase contrast microscope (model CK2; Olympus, Tokyo, Japan) with 10×/0.25 and 20×/0.4 objectives (Olympus) at room temperature. Images were captured with a digital camera (model Coolpix 4500; Nikoh, Tokyo, Japan).
Statistical Analysis
All data are expressed as mean ± SE of at least three independent experiments. One-way analysis of variance was used to test for statistical difference. Statistical significance was set at p < 0.05.
RESULTS
DDR1 Inhibited Collagen-induced Cell Migration and Cell Adhesion
To determine the specificity of DDR1 in cell migration, we used MDCK cells transfected with vector only (PC11), DDR1a (DA-1), DDR1b (DB-9), or DDR1 dominant-negative (DN) mutant (DN38) (Wang et al., 2005). Migration capability was assayed using laminin, fibronectin, collagen, or vitronectin as the chemoattractant in a Boyden chamber (Figure 1A). Consistent with our previous results (Wang et al., 2005), overexpression of DDR1 inhibited collagen-induced cell migration, whereas DN-DDR1 elevated collagen-induced cell migration. There were no differences in cell migration between these cell lines when laminin, fibronectin, and vitronectin were used as the chemoattractants. Because collagen receptor α2β1-integrin is the most abundant integrin in MDCK cells (Schoenenberger et al., 1994). In addition, MDCK cells do not contain α5-integrin, which is fibronectin receptor, and harbors only minimal levels of α3-integrin, which in this cell line may be required for laminin binding. Cell adhesion to collagen is higher (∼2-fold) than that to other ECMs (Figure 1C), cell migration on collagen might be higher than that on other ECMs.
Figure 1.
DDR1 down-regulated collagen-induced cell migration. (A) Migration capability of DDR1-transfected MDCK cells on different ECM. Cell migration assays were performed in Boyden chambers using different ECM as the chemoattractant: laminin, fibronectin, type I collagen, or vitronectin. The results are mean ± SE of three individual experiments in triplicate. The expression of DDR1 in PC11, DA-1, DB-9, and DN38 cells are shown in the top panel. Western blots with DDR1, myc, and β-actin antibodies were performed. (B) NIH-3T3 cells were stably transfected with vector only (3T3-V1), DDR1a (3T3-DA), or DDR1b (3T3-DB). Migration capability of DDR1-transfected NIH-3T3 cells was assayed using type I collagen as the chemoattractant. The expression of DDR1 was analyzed using Western blot with anti-DDR1 antibody (top). (C) Adhesion of DDR1-transfected MDCK cells on different ECM. Cells were plated on different substrates: fibronectin, collagen, or vitronectin for 30 min. Cells adhesion was assessed by crystal violet staining and expressed as relative value (percentage) versus control cells (PC11) plated on fibronectin. Results are the mean ± SE of three independent experiments. *p < 0.05; compared with PC11. (D) DDR1-transfected MDCK cells were plated for 30 min on cell culture dishes coated with collagen of different concentrations. Cells adhesion was assessed by crystal violet staining and expressed as relative value (percentage) versus control cells (PC11) plated on cell culture dishes coated with 10 μg/ml collagen. (E) Expression of integrins in DDR1-transfected cells. Cell lysates were analyzed using Western blot with α2-integrin, αv-integrin, β1-integrin, and β3-integrin. The filters were probed with β-actin antibody as the control. (F) Flow cytometric analysis of surface expression of different integrins in PC11, DA-1, DB-9, and DN38 cells.
To determine whether DDR1-mediated inhibition of cell migration is a general phenomenon, we used NIH-3T3 fibroblast cells with stable transfections of empty vector (3T3-V1), DDR1a (3T3-DA), or DDR1b (3T3-DB). DDR1 expression was confirmed by Western blotting (Figure 1B). Consistent with our results in MDCK cells, collagen-induced cell migration was decreased in DDR1a- or DDR1b-expressing NIH-3T3 fibroblast cells.
To study the role of DDR1 in integrin-mediated cell adhesion, we used DDR1-transfected MDCK cells seeded on fibronectin-, collagen-, or vitronectin-coated dishes. There were no differences in cell adhesion to fibronectin, or vitronectin. However, overexpression of DDR1 reduced cell adhesion to collagen, and DN-DDR1 increased it (Figure 1, C and D). In addition, Figure 1D showed that collagen dose dependently induced cell adhesion in these four cell lines.
DDR1 Did Not Affect the Expression of α2β1-Integrin
Collagen-induced migration is triggered primarily by α2β1-integrin in MDCK cells (Wang et al., 2005). The sensitivity of cell adhesion on collagen to DDR1 expression raises the possibility that DDR1 suppressed cell adhesion by modulating the expression of α2β1-integrin. Western blotting and FACScan were used to detect the levels and surface expression of integrins, respectively. The tests showed that DDR1 neither influenced the levels of integrins (Figure 1E) nor altered their surface expression (Figure 1F).
DDR1 Regulated Cell Migration by Suppressing α2β1-Integrin-dependent Tyrosine Phosphorylation of Stat3
Our examination of whether DDR1-regulated collagen-induced cell migration by controlling the activation of Stat3 showed that tyrosine phosphorylation of Stat3 was inhibited in DA-1 and DB-9 cells but elevated in DN38 cells, independent of collagen stimulation and compared with PC11 control cells. However, there was no difference in the serine phosphorylation of Stat3 (Figure 2A). Similarly, in NIH-3T3 cells, the tyrosine phosphorylation of Stat3 was inhibited in 3T3-DA and 3T3-DB cells, but serine phosphorylation was not affected, compared with 3T3-V1 cells (Figure 2B). Using α2-integrin-specific blocking antibody (P1E6), collagen-induced Stat3 tyrosine phosphorylation, but not serine phosphorylation, was effectively blocked in PC11 cells (Figure 2C). This result confirmed that the collagen-induced tyrosine phosphorylation of Stat3 is α2β1-integrin dependent.
Figure 2.
DDR1 inhibited collagen-induced cell migration by suppressing α2β1 integrin-dependent tyrosine phosphorylation of Stat3. Serum-starved cells were treated with 100 μg/ml soluble collagen type I (Col I) for the indicated time. Cell lysates were analyzed using Western blot with antibodies as indicated. (A and B) Inhibition of collagen-induced Stat3 tyrosine-705 (Stat3-p-Tyr), instead of serine-727 (Stat3-p-Ser) phosphorylation in DDR1-transfected MDCK cells and NIH-3T3 cells, respectively. (C) Blockage of α2β1-integrin in PC11 cells inhibited collagen-induced tyrosine phosphorylation of Stat3. Serum-starved PC11 cells were preincubated with or without 30 μg/ml blocking antibody (+) against α2-integrin (anti-α2) or normal serum (−) for 60 min and then left untreated or stimulated with collagen for another 60 min. Cell lysates were analyzed using Western blot with antibodies as indicated. (D) Constitutive-active Stat3 (CAStat3) restored the migration capability of DA-1 and DB-9 cells (E). DA-1 and DB-9 cells were stably transfected with constitutive-active Stat3. Two representative clones were selected. Equal amounts of cell lysates were analyzed using Western blot with antibodies as indicated (top). Antibody to FLAG was used to indicate the Flag-tagged CAStat3. Migration assays were performed in Boyden chambers using type-I collagen as the chemoattractant (bottom). The results are presented as mean ± SE of three individual experiments in duplicate or triplicate.
To determine whether DDR1 suppressed cell migration by suppressing Stat3 activation, we stably transfected constitutive active form of Stat3 (CAStat3) into DA-1 and DB-9 cells, and two representative clones of each were selected for evaluating the restoration of cell migration. Indeed, after transfection of CAStat3, migration capability was restored to ∼80% in DA-1 and 70% in DB-9 cells (Figure 2, D and E). These results suggest that DDR1 regulates cell migration by suppressing α2β1-integrin-dependent tyrosine phosphorylation of Stat3.
Phosphatase Activity of SHP-2 Was Augmented in DDR1-overexpressing MDCK Cells
To determine whether protein tyrosine phosphatases were involved in DDR1-inhibited tyrosine phosphorylation of Stat3, we assessed the tyrosine phosphorylation of Stat3 in response to the general tyrosine phosphatase inhibitor (Na3VO4). All cell lines treated with Na3VO4 had a higher level of phosphorylation of Stat3 than those not treated (Supplemental Figure S1). There were no significant differences in phosphorylation of Stat3 between PC11- and DDR1-transfected cells, suggesting that tyrosine phosphatase might be involved in DDR1-regulated Stat3 tyrosine phosphorylation.
SHP-1 dephosphorylates tyrosine-phosphorylated Stat5B (Ram and Waxman, 1997), and SHP-2 is involved in dephosphorylating Stat1/3/5A (Ohtani et al., 2000; Wu et al., 2002; Chen et al., 2003). We detected the expression of SHP-1 and SHP-2 using Western blotting. SHP-2 was expressed equally in control and DDR1-transfected MDCK cells, but SHP-1 was not expressed (Figure 3A).
Figure 3.
Phosphatase activity of Shp-2 was elevated in DDR1-overexpressed MDCK cells. (A) Expression of SHP-2, instead of SHP-1, in Jurkat cells (+), PC11 cells, and DDR1-transfected MDCK cells. (B) Serum-starved PC11, DA-1, DB-9, DN38, or catalytically inactive SHP-2 C/S mutant-expressed MDCK cell clone II 3B5 cells (Shp2 C/S) were treated with or without collagen for 30 min. PTP activity was determined in pellets using pNPP as a substrate. Values are expressed as picomoles of phosphate released per minute. Background level is indicated with a dashed line. (C) In vitro phosphatase activity assay using INF-α–treated HeLa cell lysates as the substrate. Cell lysates (1 mg) from PC11, DA-1, or DB-9 cells were subjected to immunoprecipitation with normal serum (bead) or anti-SHP-2 antibody (shp2).
To determine whether DDR1 influenced the phosphatase activity of SHP-2, we performed an in vitro phosphatase assay by using pNPP as a substrate. PTP activity in SHP-2 immunoprecipitates from catalytically inactive SHP-2 C/S mutant-expressed MDCK cells was at near-background level (Figure 3B). PTP activity was prominent in SHP-2 immunoprecipitates of cells overexpressing DDR1a or DDR1b. Phosphatase activity of SHP-2 was also assessed using INF-α–treated HeLa cell lysate as the substrate. No phosphatase activity was detected in PC11 cells, but it was elevated in DA-1 and DB-9 cells, as a decrease in Stat3 tyrosine phosphorylation showed (Figure 3C). These results indicate that expression of DDR1 increased the phosphatase activity of SHP-2.
Increased Interaction between DDR1 and SHP-2 in DDR1-transfected MDCK Cells
To determine how DDR1 increased the phosphatase activity of SHP-2, we studied the interaction between DDR1 and SHP-2 in vivo. There was detectable interaction between DDR1 and SHP-2 in PC11 cells after collagen treatment for 30 min, but no interaction was detected in DN38 cells (Figure 4A). In DA-1 and DB-9 cells, there was significant interaction between DDR1 and SHP-2 before collagen stimulation; it markedly increased within 30 min and declined 4 h after collagen treatment. However, neither stat3, PTP1B, nor PP2A showed detectable interaction with DDR1 in any cell lines (our unpublished data).
Figure 4.
SHP-2 was involved in DDR1-mediated suppression of Stat3 tyrosine phosphorylation and collagen-induced cell migration. (A) Time course of DDR1 and SHP-2 interaction in control (PC11) and DDR1-transfected MDCK cells. (B) The interaction of SHP-2 with DDR1 and Stat3 in PC11, DN38, DA-1, and DB-9 cells. Cells were treated with soluble Col I for the indicated time. Total cell lysates (1 mg) were subjected to immunoprecipitation (IP) with normal serum (IgG), anti-DDR1, or anti-SHP-2 antibodies and then immunoblotted with antibodies as indicated. (C) Restoration of the Stat3 tyrosine phosphorylation and migration capability of DA-1 cells after transfection of dominant-negative mutant SHP-2. DA-1 cells were stably transfected with catalytically inactive mutant SHP-2 (DA1-DNshp2). Two representative clones, 40 and 31, were selected. (D) DB-9 cells were stably transfected with catalytically inactive mutant SHP-2 (DB9-DNshp2). Two representative clones, 8 and 7, were selected. Serum-starved cells were stimulated with (+) or without (−) collagen for 60 min. Cell lysates were analyzed using Western blot with antibodies as indicated. Migration assays were performed in Boyden chambers by using type I collagen as the chemoattractant. The results are presented as mean ± SE of three individual experiments in duplicate or triplicate.
To further confirm the interaction between DDR1 and SHP-2, cell lysates were immunoprecipitated with anti-SHP-2 antibody and then immunoblotted with anti-DDR1 or anti-Stat3 antibodies. The result showed that SHP-2 interacted with DDR1 in PC11 cells after collagen treatment for 30 min, but no interaction was detected in DN38 cells (Figure 4B). The Stat3 levels present in SHP-2 coimmunoprecipitates were higher in PC11 cells than in DN38 cells. In addition, the interactions of SHP-2 with DDR1 and Stat3 were significantly elevated in DA-1 and DB-9 cells. The levels of DDR1 and Stat3 present in SHP-2 coimmunoprecipitates were markedly higher in DA-1 and DB-9 cells than in PC11 and DN38 cells. Our previous study (Wang et al., 2005) showed that DDR1 was initially activated 30 min after the beginning of collagen treatment in PC11 cells but that autophosphorylation of DDR1 began in DA-1 and DB-9 cells before collagen treatment. The factor that DDR1 bind to and activates tyrosine phosphatase SHP-2 is concomitant with the kinetics of DDR1 activation in MDCK cells, suggesting that tyrosine phosphorylation is critical for DDR1 to bind to and activate SHP-2.
Expression of C/S SHP-2 Restored Tyrosine Phosphorylation of Stat3 and Cell Migration in DDR1-transfected Cells
To address whether SHP-2 was involved in DDR1-mediated suppression of tyrosine phosphorylation of Stat3 and cell migration, DA-1 and DB-9 cells were stably transfected with myc-tagged mutant SHP-2 (myc-Shp2 C/S), a catalytically inactive mutant with the residual cysteine 459 substituted for serine 459 (Guan and Dixon, 1991). Two representative clones were used to assess the phosphorylation of Stat3 and cell migration. Both DA-1 and DB-9 cells with myc-Shp2 C/S showed restoration of tyrosine phosphorylation of Stat3 and cell migration (Figure 4, C and D). These results indicate that DDR1 inhibits Stat3 activation and cell migration via SHP-2 phosphatase activity.
SHP-2 N-Terminal SH2N-SH2C Domain and C-Terminal Catalytic Domain (PTP) Interacted with DDR1
To characterize the domains of SHP-2 that interact with DDR1, we used various GST recombinant fusion proteins of SHP-2, either full length or with different functional domains (Wu et al., 2002). GST-SH2-SH2, GST-PTP, GST-SH2C-PTP, and GST-SHP-2 pulled down DDR1 (Figure 5A). Our results revealed that neither the SHP-2 N-terminal N-SH2 nor C-SH2 domain (residues 1–220) alone was able to precipitate DDR1 (Figure 5A). A weak signal was visible after extended exposure time, however, indicating that DDR1 and these domains had weakly interacted. We confirmed interaction between these GST fusion proteins and DDR1 using 3T3-DB whole-cell lysates. GST-SH2-SH2, GST-PTP, and GST-SHP-2 pulled down DDR1 (Figure 5B). These data indicate that the SHP-2 N-terminal SH2N-SH2C domain and PTP domain mediate interaction with DDR1. In Figure 5, A and B, the different molecular mass of DDR1 perhaps represents different phosphorylated form of DDR1 that is dephosphorylated by the SHP-2 constructs containing the PTP activity.
Figure 5.
Direct interaction between C-terminal catalytic domain (PTP) of SHP-2 with DDR1 tyrosine phosphorylation sites 703 and 796. (A) Schematic representation of GST fusion protein containing full-length SHP-2 or various deletion mutants as indicated. Open boxes correspond to SHP-2 protein and numbers in brackets define the N- and C-terminal residues of the proteins used. Thin lines correspond to the GST fusion portion. Cell lysates (1 mg) of DB-9 cells were incubated with GST fusion proteins. Cellular proteins bound to GSTs were then Western blotted with anti-DDR1 antibody. The middle panel shows GST fusion proteins stained with Coomassie brilliant blue. The bottom panel shows that equal amounts of lysates were used in each of the reactions as indicated using anti-β-actin antibody. (B) Interaction of SH2-SH2 domain, PTP domain, and full-length SHP-2 with DDR1 in 3T3-DB cells. (C) Interactions of N-SH2 and PTP domain of SHP-2 with Stat3 and Stat1. (D) Schematic representation of DDR1 functional domains and a number of tyrosine-residue mutant constructs, with the tyrosine residues substituted for phenylalanine in the cytoplasmic region of DDR1. (E) Direct interaction between SHP-2 and DDR1 tyrosine phosphorylated sites 703 and 796. MDCK cell clone II 3B5 cells were stably transfected with tyrosine-residue mutant DDR1 as indicated. Cells were stimulated with type I collagen for 30 min. Total cell lysates were immunoprecipitated with normal serum (IgG) or anti-DDR1 antibody. For quantification, precipitated DDR1 was Western blotted with anti-DDR1 antibody. An equal amount of precipitated DDR1 was applied to each lane for Western blot with anti-SHP-2 antibody (top) and reprobed with anti-DDR1 antibody (bottom). (F) 293T cells were transiently transfected with DDR1a (DA), DDR1B (DB), or tyrosine-residue mutant DDR1. Cell lysates were analyzed as in E. (G) PTP activity assay of Y547F, Y703F, or Y796F DDR1 mutant-transfected MDCK cells by using pNPP as a substrate. (H) NIH-3T3 cells were stably transfected with DDR1b (3T3-DB) or DDR1 tyrosine-residue mutants Y703F (3T3-Y703F) or Y796F (3T3-Y796F). Serum-starved cells were stimulated with collagen for 30 min. Cell lysates (1 mg) were incubated with GST only or GST-SH2-SH2 (2SH2), GST-PTP (PTP), or GST-SHP-2 (shp2). Cellular proteins bound to GSTs were then Western blotted with anti-DDR1 antibody. Western blotting with anti-β-actin antibody was used as the internal control. “Irrelevant” is proteins that were pulled down by the GST-SH2-SH2 domain, which can be recognized by goat anti-rabbit secondary antibody alone.
SHP-2 is associated with Stat3 in vivo (Figure 4B). To assess which domains of SHP-2 interacted with Stat3, we used SHP-2 GST fusion proteins incubated with DB-9 cell lysates, and analyzed their interaction using anti-Stat3 antibody. GST-SH2N, GST-SH2-SH2, and GST-PTP pulled down both Stat3 and Stat1 (Figure 5C). This indicates that both the SHP-2 N-terminal SH2 (aa 1–107) and PTP domains mediate interaction with Stat1 and 3, consistent with a previous report for Stat1 (Wu et al., 2002).
Both Tyrosine Residues 703 and 796 of DDR1 Were Required for Interaction with SHP-2 to Augment Its PTP Activity
Treatment of the DB-9 and 3T3-DB lysates with λ-phosphatase eliminated DDR1/SHP-2 associations (Supplemental Figure S2), suggesting that tyrosine phosphorylation of DDR1 is critical for interaction with SHP-2. To determine which tyrosine residues of DDR1 were required for formation of the complex, we generated a number of tyrosine-residue mutants in the cytoplasmic domain of DDR1 (Figure 5D), transfected them into MDCK or human embryonic kidney 293T cells and assessed their ability to bind SHP-2. As shown in Figure 5, E and F, all the mutant forms of DDR1 interacted with SHP-2, with the notable exception of the Y703F and Y796F forms.
To examine whether DDR1 tyrosine 703 and 796 are critical for binding and thus increasing the PTP activity of SHP-2, we assessed PTP activity in cells harboring DDR1 tyrosine 703 and 796 mutants. As shown in Figure 5G, DDR1 mutants harboring Y703F or Y796F amino acid substitutions were unable to support activation of SHP-2, indicating that these residues in DDR1 must interact with SHP-2 to augment its PTP activity.
To characterize that SHP-2 N-terminal SH2N-SH2C domain or PTP domain mediated the interaction with 703 and 796 tyrosine residues of DDR1, we used SHP-2 GST fusion proteins incubated with cell lysates of NIH-3T3 cells harboring DDR1 Y703F or Y796F plasmids (3T3-Y703F or 3T3-Y796F) and analyzed their interaction using anti-DDR1 antibody (Figure 5H). Consistent with the in vivo results, neither 703 nor 796 tyrosine-mutated DDR1 interacted with GST-PTP or GST-SHP-2. However, these two mutants still can interact with GST-SH2-SH2. These results indicate that both 703 and 796 tyrosine residues of DDR1 interact with SHP-2 PTP domain.
Both 703 and 796 Tyrosine Phosphorylation Sites of DDR1 Were Important for Suppressing Tyrosine Phosphorylation of Stat3 and Cell Migration
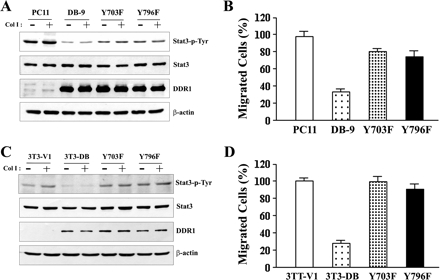
We tested whether the SHP-2–targeted DDR1 in 703 and 796 tyrosine residues caused DDR1-mediated suppression of Stat3 tyrosine phosphorylation and cell migration. MDCK cells overexpressing the mutants DDR1 Y703F or Y796F showed higher levels of tyrosine phosphorylation of Stat3 than those of DB-9 cells but less than those of PC11 cells (Figure 6A). Cell migration capability was restored to ∼80% in MDCK cells overexpressing mutants DDR1 Y703F or Y796F (Figure 6B). 3T3-Y703F and 3T3-Y796F cells, too, showed higher tyrosine phosphorylation of Stat3 (Figure 6C) and greater cell migration (Figure 6D) than 3T3-DB cells. Both 3T3-Y703F and 3T3-Y796F cells exhibited tyrosine phosphorylation of Stat3 and migration capability similar to 3T3-V1 cells. These results suggested that both tyrosine sites 703 and 796 of DDR1 were required for the DDR1-mediated suppression of tyrosine phosphorylation of Stat3 and cell migration.
Figure 6.
Tyrosine phosphorylation sites 703 and 796 of DDR1 were important for regulating Stat3 activation and collagen-induced cell migration. Expression of DDR1 tyrosine mutants Y703F and Y796F in MDCK cells abolished DDR1-mediated suppression of Stat3 tyrosine phosphorylation (A) and collagen-induced cell migration (B). Expression of DDR1 tyrosine mutants Y703F and Y796F in NIH-3T3 cells abolished DDR1-mediated suppression of Stat3 tyrosine phosphorylation (C) and collagen-induced cell migration (D). Serum-starved cells were stimulated with (+) or without (−) Col I for 60 min, and the cell lysates were immunoblotted with antibodies as indicated. Migration assays were done in Boyden chambers by using Col I as the chemoattractant. The results are presented as mean ± SE of three individual experiments in duplicate or triplicate.
DDR1 Down-Regulated Tyrosine Phosphorylation of Stat1 via Regulation of SHP-2 Activity
Because active Stat3 did not completely restore DDR1-overexpression–induced down-regulation of cell migration, the data prompted us to examine whether other Stat members, such as Stat1, were also regulated by DDR1. SHP-2 controls Stat1 activation by dephosphorylation of specific tyrosine resides (Wu et al., 2002). Indeed, Stat1 was hyperphosphorylated on the tyrosine phosphorylation site 701 (Y701) in DN38 cells and dephosphorylated in DA-1 and DB-9 cells (Figure 7A). Collagen-induced tyrosine phosphorylation of Stat1 was triggered through α2β1-integrin, because α2β1-integrin neutralizing antibody completely blocked its activation (Figure 7B). In addition, SHP-2 interacted with Stat1 in DA-1 and DB-9 cells (Figure 7C). We tested whether DDR1 suppressed Stat1 phosphorylation through SHP-2. MDCK cells overexpressing mutants of DDR1 Y703F or Y796F showed restored Stat1 tyrosine phosphorylation (Figure 7D).
Figure 7.
Tyrosine phosphorylation sites 703 and 796 of DDR1 are required for inhibition of Stat1 activation. (A) Collagen-induced Stat1 tyrosine-701 (Stat1-p-Tyr) phosphorylation was suppressed in DA-1 and DB-9 cells but increased in DN38 cells. Serum-starved cells were treated with 100 μg/ml soluble Col I for the indicated time. (B) Blockage of α2β1-integrin in PC11 cells inhibited collagen-induced Stat1 tyrosine phosphorylation. Serum-starved PC11 cells were pre-incubated without or with 30 μg/ml blocking antibody (+) against α2-integrin (anti-α2) or normal serum (−) for 60 min and then left untreated or stimulated with collagen for another 60 min. Cell lysates were analyzed using Western blot with antibodies as indicated. (C) SHP-2 interacted with Stat1 in DA-1 and DB-9 cells. Cell lysates (1 mg) were subjected to immunoprecipitation (IP) with normal serum (IgG) or anti-SHP-2 antibodies and then analyzed using Western blot with anti-Stat1 and anti-SHP-2 antibodies. (D) Expression of DDR1 tyrosine mutants Y703F and Y796F in MDCK cells abolished DDR1-mediated suppression of Stat1 tyrosine phosphorylation. Serum-starved cells were stimulated with (+) or without (−) collagen for 60 min, and the cell lysates were analyzed using Western blot. (E) Constitutive-active Stat1 restored collagen-induced migration capability in DA-1 and DB-9 cells. DA-1 and DB-9 cells were stably transfected with the constitutive-active form of Stat1 (CAStat1). Equal amounts of cells lysates were analyzed using Western blot with antibodies as indicated. Antibody to FLAG was used to indicate Flag-tagged CAStat1. Migration assays were performed in Boyden chambers by using Col I as the chemoattractant.
To determine whether Stat1 is also involved in DDR1-induced suppression of cell migration, we stably transfected constitutive-active form of Stat1 (CAStat1) into DA-1 and DB-9 cells and evaluated the restoration of cell migration. After transfection of CAStat1, migration capability was restored to ∼85% in DA-1 and 70% in DB-9 cells (Figure 7E). Together, these results indicate that DDR1 down-regulates Stat1 activation by activating SHP-2, which may also be involved in the DDR1-induced decrease in cell migration.
Mutation of Tyrosine 703 or 796 of DDR1 Abrogated the Inhibitory Effects of DDR1 in HGF-induced Branching Tubulogenesis
Cell migration capability is one important influence on the sprouting of cell processing and subsequent branching tubule morphogenesis (Montesano et al., 1991). We demonstrated that DDR1 inhibits HGF-induced branching tubulogenesis of MDCK cells in collagen gel by suppressing cell migration (Wang et al., 2005). To assess the role of DDR1 tyrosine residues and SHP-2 in branching tubulogenesis, MDCK cells overexpressing various tyrosine mutations of DDR1 were cultured in collagen gel with 2 ng/ml HGF. Expression of DDR1 in these cells was detected using Western blotting (Figure 8A). After 2 d in collagen gel with HGF, PC11 cells showed the sprouting of membrane processes. This process was inhibited in DB-9 cells as well as in MDCK cells expressing mutant DDR1 Y484F, Y569F, and Y586F (Figure 8B). After 6 d, PC11 cells produced normal and branching tubules in the presence of HGF, whereas DB-9 cells and MDCK cells overexpressing mutant DDR1 Y484F, Y569F, or Y586F produced shorter tubes with less branching (Figure 8C). In contrast, MDCK cells overexpressing mutant DDR1 Y703F or Y796F exhibited normal branching tubulogenesis similar to that of PC11 cells. These results suggest that mutation of tyrosine 703 or 796 of DDR1 reverses the inhibitory effects of DDR1 in HGF-induced branching tubulogenesis. Together, all these results indicate that SHP-2 is significantly involved in DDR1-regulated cell functions.
Figure 8.
HGF-induced morphogenesis of DDR1 transfectants cultured in collagen gel. (A) Expression of DDR1 in DDR1-transfected MDCK cells. MDCK cell clone II 3B5 cells were stably transfected with DDR1 tyrosine-residue mutants. Cell lysates were analyzed using Western blot with anti-DDR1 antibody. The filters were probed with β-actin antibody as a control. (B) Mutation of tyrosine 703 or 796 of DDR1 restored the inhibitory effects of DDR1 in HGF-induced cell processing in collagen gel. Representative phase-contrast pictures were taken from the parental or DDR1-transfected MDCK cells cultured in Col I gel with 2 ng/ml HGF for 2 d. Each clone was tested in three tubulogenesis assays with consistent results. Bar, 50 μm. (C) Representative phase-contrast pictures taken from the parental or DDR1-transfected MDCK 3B5 cells cultured in type I collagen gels with 2 ng/ml HGF for 6 d. Bar, 100 μm.
DISCUSSION
Many biological processes are jointly regulated by receptor tyrosine kinases and integrins. Their cooperation, including reciprocal transactivation, modulation of their cellular compartments, and signaling cross-talk, often involves regulation of the activities of kinase or phosphatase (Adachi et al., 1996; Neel et al., 2003). In the present study, we not only identified an interacting partner of DDR1 but we also unraveled the functional mechanism of DDR1. We report here a novel collagen signaling pathway showing the cross-talk between DDR1 and α2β1-integrin, through an original association of DDR1 and SHP-2 to negatively regulate α2β1-integrin–dependent signaling (Figure 9). We found that DDR1 binds SHP-2 both in vitro and in vivo and that this interaction is mediated by a docking sequence in the cytoplasmic domain of DDR1. We showed that the SH2-SH2 and PTP domains of SHP-2 were responsible for interaction with DDR1. Mutation of tyrosine residues 703 and 796 in DDR1 abolished the interaction of DDR1 with SHP-2 and PTP activity, which consequently restored Stat1 and Stat3 activation, collagen-induced cell migration, and HGF-induced branching tubulogenesis.
Figure 9.
Schematic depiction of collagen signal pathways in regulation of cell migration through DDR1/SHP-2 interactions in suppression of α2β1-integrin–mediated Stat3 activation.
Several tyrosine residues in the cytoplasmic region of DDR1 and DDR2 are flanked by consensus sequences that suggest roles as autophosphorylation and substrate attachment sites (Alves et al., 1995). For example, the sequences flanking Tyr-844 (YELM) in DDR1 in the carboxy-terminal region of the kinase core domain contain the YXXM binding motif for association of the p85 subunit of phosphatidylinositol 3′-kinase (PI3-kinase) (Fantl et al., 1992; Alves et al., 1995). Moreover, Tyr-506 (YSGD) in the juxtamembrane region of DDR1 might serve as a receptor binding site for SHC, and the sequence flanking Tyr-547 (YMEP) in DDR1b might serve as the binding site for GTPase-activating protein (Alves et al., 1995). Here, we showed that the sequences flanking Tyr-703 (YMEN) and Tyr-796 (YYRV) in DDR1b might serve as binding sites for SHP-2.
The SHP-2 N-SH2 and C-SH2 domains are thought to be important for binding with phosphotyrosyl (pTyr)-containing proteins and for C-terminal PTP regulatory actions (Neel et al., 2003). Most proteins that bind a SHP-2 SH2 domain contain at least one immunoreceptor tyrosine-based inhibitory motif (ITIM): [I/V/L]xY(p)xx[I/V/L] (Ravetch and Lanier, 2000). Indeed, an ITIM motif was found in the carboxy-terminal region of DDR1 sequence flanking Tyr-740 ([I] SYPM [L]). However, our in vitro protein–protein interaction studies revealed that neither the N-SH2 nor C-SH2 domain alone interacted with DDR1, but that the SH2-SH2 domain interacted with DDR1 instead. In addition, the SH2-SH2 domain interacted with DDR1 tyrosine-residue mutants that, with tyrosine residues 484, 547, 569, 586, 703, or 796, substituted for phenylalanine in the cytoplasmic region of DDR1 (our unpublished data). In light of these findings, we conclude that the SHP-2 SH2-SH2 domain may provide a complete and stronger docking site than N-SH2 or C-SH2 alone to bind with the ITIM motif of DDR1. Ample evidence indicates that SHP-2 positively regulates cell migration, which is mediated, at least in part, by the GTP binding protein RhoA, although there is still controversy as to whether SHP-2 regulates RhoA activity positively or negatively (Inagaki et al., 2000; Kodama et al., 2000; Schoenwaelder et al., 2000). Here, we show that the DDR1/SHP-2 complex mediates the suppression of collagen-induced Stats activation and cell migration. Despite the implication of SHP-2 and RhoA activity in cell migration, we found that DDR1 did not affect the RhoA-GTP levels in MDCK cells (our unpublished data), suggesting that RhoA is not involved in DDR1–SHP-2 pathway to modulate cell migration.
The present study also showed that α2β1-integrin–mediated signaling modulates collagen-induced cell migration by regulating tyrosine phosphorylation of Stat1 and Stat3. However, the signaling mechanism of α2β1-integrin–dependent activation of Stat1 and Stat3 is still unclear. One possibility is that integrin contributes by regulating the focal adhesion kinase (FAK) or JAK/Stat pathway. Integrin-mediated endothelial cell adhesion induces JAK2 and Stat5 activation (Brizzi et al., 1999). In addition, it has been proposed that FAK is downstream of JAK2, which associates with JAK2 after growth hormone stimulation (Zhu et al., 1998). FAK with a C-terminal deletion abolishes the interaction of FAK with Stat1, integrin-mediated Stat1 activation, and cell migration (Xie et al., 2001). Our finding that DDR1 does not alter phosphorylation of FAK and that overexpression of FAK or FRNK in MDCK cells does not influence collagen-induced phosphorylation of Stat3 suggests that FAK is not involved in α2β1-integrin–mediated Stat3 activation (our unpublished data). It is possible that DDR1 may block activation of Stats by inhibiting JAK activity in cytoplasm through SHP-2 (You et al., 1999). However, our findings that SHP-2 physically interacts with Stat1 and Stat3 in vivo and in vitro as well as that SHP-2 isolated from DDR1-transfected MDCK cells can dephosphorylate the INF-α-stimulated tyrosine phosphorylation of Stat3 in HeLa cell lysates, suggest that DDR1 may inhibit activation of Stat1 and Stat3 by directly binding SHP-2 with Stat1 and Stat3.
Recent evidence (Sano et al., 1999; Xie et al., 2001) indicates that Stats are involved in cell motility. Our data support this and demonstrate that Stats are the major targets of DDR1 in signaling modulation of collagen-induced cell migration. The mechanism by which Stats modulate cell migration is related to their transcriptional-activation function (Leonard and O'Shea, 1998). The mechanism by which Stat3 modulates cell motility in ovarian cancer cells has been described recently (Silver et al., 2004). Tyrosine-phosphorylated Stat3 is localized in nuclei and focal adhesions, and it interacts with FAK and paxillin. It has been proposed that membrane-associated Stat3 may serve as a sensor or as an adapter protein of adhesion when modulating cell migration (Silver et al., 2004). The fact that FAK and paxillin interacted with Stat3 in MDCK cells after treatment with collagen (our unpublished data) suggests that membrane-associated Stat3 in MDCK cells may also be involved in modulating cell migration.
Epithelial branching morphogenesis is a fundamental developmental process that gives rise to many epithelial organs, including the prostate, kidneys, lungs, and mammary glands. Our previous studies (Wang et al., 2005) indicate that the functions of DDR1 include the inhibition of cell proliferation and suppression of cell migration, which result in dampening HGF-induced branching tubulogenesis in collagen gel. In the present study, we demonstrated that mutated Tyr-703 and Tyr-796 residues of DDR1, which abolish the interaction between DDR1 and SHP-2, restore Stat1 and Stat3 activation, cell migration, sprouting, and branching tubulogenesis in collagen gel. Furthermore, our finding that cells harboring mutated Tyr-703 or Tyr-796 DDR1 produce normal branching tubules, but that the total number of tubes (restored to ∼60%) is smaller than in PC11 cells, indicated that cell proliferation in these mutants was partially restored. We therefore propose that DDR1–SHP-2 signaling may contribute primarily to regulating cell migration and secondarily to extending the suppression of cell proliferation.
In summary, our data support the hypothesis that DDR1-SHP2-Stats signaling modulates collagen-induced cell migration as well as epithelial-branching morphogenesis in well characterized MDCK models. Moreover, our data demonstrate that Stat1 and Stat3 are the targets of cell signaling by DDR1 and α2β1-integrin that negatively and positively, respectively, regulate cell migration. A relative increase in SHP-2 activity is largely responsible for the effects of DDR1 activation in regulating Stat1 and Stat3 phosphorylation and in inhibiting branching morphogenesis. Further investigation is required to assess the various roles of DDR1 and integrins in orchestrating diverse environmental cues that in concert control epithelial organogenesis in vitro and in vivo.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to J. F. Bromberg, A. Kaptein, Hiroshi Ohnishi, Toru Ouchi, and Eugene Chin for kindly providing us plasmids and to Tsu-Ling Chen for technical assistance. This work was supported by National Science Council Grant NSC 92-2320-B006-084 and the Ministry of Education Program for Promoting Academic Excellence in Universities Grant 91-B-FA09-1-4 to Ming-Jer Tang. We also thank Center of Bioscience and Biotechnology for generous support on the accessibility of core facility.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-11-1068) on April 12, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Adachi M., et al. Mammalian SH2-containing protein tyrosine phosphatases. Cell. 1996;85:15. doi: 10.1016/s0092-8674(00)81077-6. [DOI] [PubMed] [Google Scholar]
- Alves F., Saupe S., Ledwon M., Schaub F., Hiddemann W., Vogel W. F. Identification of two novel, kinase-deficient variants of discoidin domain receptor 1, differential expression in human colon cancer cell lines. FASEB J. 2001;15:1321–1323. doi: 10.1096/fj.00-0626fje. [DOI] [PubMed] [Google Scholar]
- Alves F., Vogel W., Mossie K., Millauer B., Hofler H., Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–618. [PubMed] [Google Scholar]
- Boccaccio C., Ando M., Tamagnone L., Bardelli A., Michieli P., Battistini C., Comoglio P. M. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391:285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- Brizzi M. F., Defilippi P., Rosso A., Venturino M., Garbarino G., Miyajima A., Silengo L., Tarone G., Pegoraro L. Integrin-mediated adhesion of endothelial cells induces JAK2 and STAT5A activation: role in the control of c-fos gene expression. Mol. Biol. Cell. 1999;10:3463–3471. doi: 10.1091/mbc.10.10.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wen R., Yang S., Schuman J., Zhang E. E., Yi T., Feng G. S., Wang D. Identification of Shp-2 as a Stat5A phosphatase. J. Biol. Chem. 2003;278:16520–16527. doi: 10.1074/jbc.M210572200. [DOI] [PubMed] [Google Scholar]
- Chin Y. E., Kitagawa M., Su W. C., You Z. H., Iwamoto Y., Fu X. Y. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996;272:719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- Chiu S. J., Jiang S. T., Wang Y. K., Tang M. J. Hepatocyte growth factor upregulates alpha2beta1 integrin in Madin-Darby canine kidney cells: implications in tubulogenesis. J. Biomed. Sci. 2002;9:261–272. doi: 10.1007/BF02256073. [DOI] [PubMed] [Google Scholar]
- Curat C. A., Vogel W. F. Discoidin domain receptor 1 controls growth and adhesion of mesangial cells. J. Am. Soc. Nephrol. 2002;13:2648–2656. doi: 10.1097/01.asn.0000032419.13208.0c. [DOI] [PubMed] [Google Scholar]
- Fantl W. J., Escobedo J. A., Martin G. A., Turck C. W., del Rosario M., McCormick F., Williams L. T. Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell. 1992;69:413–423. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J. Biol. Chem. 1991;266:17026–17030. [PubMed] [Google Scholar]
- Hadari Y. R., Kouhara H., Lax I., Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K., Yamao T., Noguchi T., Matozaki T., Fukunaga K., Takada T., Hosooka T., Akira S., Kasuga M. SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility. EMBO J. 2000;19:6721–6731. doi: 10.1093/emboj/19.24.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S. T., Chiu S. J., Chen H. C., Chuang W. J., Tang M. J. Role of alpha(3)beta(1) integrin in tubulogenesis of Madin-Darby canine kidney cells. Kidney Int. 2001;59:1770–1778. doi: 10.1046/j.1523-1755.2001.0590051770.x. [DOI] [PubMed] [Google Scholar]
- Kamohara H., Yamashiro S., Galligan C., Yoshimura T. Discoidin domain receptor 1 isoform-a (DDR1alpha) promotes migration of leukocytes in three-dimensional collagen lattices. FASEB J. 2001;15:2724–2726. doi: 10.1096/fj.01-0359fje. [DOI] [PubMed] [Google Scholar]
- Kodama A., Matozaki T., Fukuhara A., Kikyo M., Ichihashi M., Takai Y. Involvement of an SHP-2-Rho small G protein pathway in hepatocyte growth factor/scatter factor-induced cell scattering. Mol. Biol. Cell. 2000;11:2565–2575. doi: 10.1091/mbc.11.8.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., O'Shea J. J. Jaks and STATs: biological implications. Annu. Rev. Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- Manes T., Zheng D. Q., Tognin S., Woodard A. S., Marchisio P. C., Languino L. R. Alpha(v)beta3 integrin expression up-regulates cdc2, which modulates cell migration. J. Cell Biol. 2003;161:817–826. doi: 10.1083/jcb.200212172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Matsumoto K., Nakamura T., Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Gu H., Pao L. The ‘Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Ohtani T., et al. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity. 2000;12:95–105. doi: 10.1016/s1074-7613(00)80162-4. [DOI] [PubMed] [Google Scholar]
- Ongusaha P. P., Kim J. I., Fang L., Wong T. W., Yancopoulos G. D., Aaronson S. A., Lee S. W. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. EMBO J. 2003;22:1289–1301. doi: 10.1093/emboj/cdg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier S., Duhamel F., Coulombe P., Popoff M. R., Meloche S. Rho family GTPases are required for activation of Jak/STAT signaling by G protein-coupled receptors. Mol. Cell. Biol. 2003;23:1316–1333. doi: 10.1128/MCB.23.4.1316-1333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram P. A., Waxman D. J. Interaction of growth hormone-activated STATs with SH2-containing phosphotyrosine phosphatase SHP-1 and nuclear JAK2 tyrosine kinase. J. Biol. Chem. 1997;272:17694–17702. doi: 10.1074/jbc.272.28.17694. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Lanier L. L. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- Saelman E. U., Keely P. J., Santoro S. A. Loss of MDCK cell alpha 2 beta 1 integrin expression results in reduced cyst formation, failure of hepatocyte growth factor/scatter factor-induced branching morphogenesis, and increased apoptosis. J. Cell Sci. 1995;108:3531–3540. doi: 10.1242/jcs.108.11.3531. [DOI] [PubMed] [Google Scholar]
- Sano S., Itami S., Takeda K., Tarutani M., Yamaguchi Y., Miura H., Yoshikawa K., Akira S., Takeda J. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18:4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeper U., Gehring N. H., Fuchs K. P., Sachs M., Kempkes B., Birchmeier W. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 2000;149:1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger C. A., Zuk A., Zinkl G. M., Kendall D., Matlin K. S. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J. Cell Sci. 1994;107:527–541. doi: 10.1242/jcs.107.2.527. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S. M., Petch L. A., Williamson D., Shen R., Feng G. S., Burridge K. The protein tyrosine phosphatase Shp-2 regulates RhoA activity. Curr. Biol. 2000;10:1523–1526. doi: 10.1016/s0960-9822(00)00831-9. [DOI] [PubMed] [Google Scholar]
- Shrivastava A., et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol. Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- Silver D. L., Naora H., Liu J., Cheng W., Montell D. J. Activated signal transducer and activator of transcription (STAT) 3, localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res. 2004;64:3550–3558. doi: 10.1158/0008-5472.CAN-03-3959. [DOI] [PubMed] [Google Scholar]
- Sironi J. J., Ouchi T. STAT1-induced apoptosis is mediated by caspases 2, 3, and 7. J. Biol. Chem. 2004;279:4066–4074. doi: 10.1074/jbc.M307774200. [DOI] [PubMed] [Google Scholar]
- Springer W. R., Cooper D. N., Barondes S. H. Discoidin I is implicated in cell-substratum attachment and ordered cell migration of Dictyostelium discoideum and resembles fibronectin. Cell. 1984;39:557–564. doi: 10.1016/0092-8674(84)90462-8. [DOI] [PubMed] [Google Scholar]
- Vogel W. Discoidin domain receptors: structural relations and functional implications. FASEB J. 1999;(13 suppl):S77–S82. doi: 10.1096/fasebj.13.9001.s77. [DOI] [PubMed] [Google Scholar]
- Vogel W., Gish G. D., Alves F., Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- Wang C. Z., Hsu Y. M., Tang M. J. Function of discoidin domain receptor I in HGF-induced branching tubulogenesis of MDCK cells in collagen gel. J. Cell Physiol. 2005;203:295–304. doi: 10.1002/jcp.20227. [DOI] [PubMed] [Google Scholar]
- Wu T. R., Hong Y. K., Wang X. D., Ling M. Y., Dragoi A. M., Chung A. S., Campbell A. G., Han Z. Y., Feng G. S., Chin Y. E. SHP-2 is a dual-specificity phosphatase involved in Stat1 dephosphorylation at both tyrosine and serine residues in nuclei. J. Biol. Chem. 2002;277:47572–47580. doi: 10.1074/jbc.M207536200. [DOI] [PubMed] [Google Scholar]
- Xie B., Zhao J., Kitagawa M., Durbin J., Madri J. A., Guan J. L., Fu X. Y. Focal adhesion kinase activates Stat1 in integrin-mediated cell migration and adhesion. J. Biol. Chem. 2001;276:19512–19523. doi: 10.1074/jbc.M009063200. [DOI] [PubMed] [Google Scholar]
- You M., Yu D. H., Feng G. S. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol. Cell. Biol. 1999;19:2416–2424. doi: 10.1128/mcb.19.3.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Goh E. L., Lobie P. E. Growth hormone stimulates the tyrosine phosphorylation and association of p125 focal adhesion kinase (FAK) with JAK2. Fak is not required for stat-mediated transcription. J. Biol. Chem. 1998;273:10682–10689. doi: 10.1074/jbc.273.17.10682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.