Abstract
RINT-1 was first identified as a Rad50-interacting protein that participates in radiation-induced G2/M checkpoint control. We have recently reported that RINT-1, together with the dynamitin-interacting protein ZW10 and others, is associated with syntaxin 18, an endoplasmic reticulum (ER)-localized SNARE involved in membrane trafficking between the ER and Golgi. To address the role of RINT-1 in membrane trafficking, we examined the effects of overexpression and knockdown of RINT-1 on Golgi morphology and protein transport from the ER. Overexpression of the N-terminal region of RINT-1, which is responsible for the interaction with ZW10, caused redistribution of ZW10. Concomitantly, ER-to-Golgi transport was blocked and the Golgi was dispersed. Knockdown of RINT-1 also disrupted membrane trafficking between the ER and Golgi. Notably, silencing of RINT-1 resulted in a reduction in the amount of ZW10 associated with syntaxin 18, concomitant with ZW10 redistribution. In contrast, no redistribution or release of RINT-1 from the syntaxin 18 complex was observed when ZW10 expression was reduced. These results taken together suggest that RINT-1 coordinates the localization and function of ZW10 by serving as a link between ZW10 and the SNARE complex comprising syntaxin 18.
INTRODUCTION
In eukaryotic cells, communication between membrane-enclosed compartments in the secretory and endocytic pathways is mediated by vesicles or membrane carriers that bud from one compartment and then tether to and fuse with another compartment (Bonifacino and Glick, 2004; Lee et al., 2004). SNAP receptors (SNAREs) play a central role in membrane fusion of transport carriers with the target compartments (Ungar and Hughson, 2003; Burri and Lithgow, 2004; Hong, 2005). SNAREs constitute a superfamily of membrane proteins that are characterized by a homologous coiled-coil domain of 60–70 amino acids (aa) in length, termed the SNARE motif (Weimbs et al., 1997). In mammalian cells, there are at least 36 SNARE members that are uniquely localized in different membrane compartments (Hong, 2005). Depending on their function and localization on two opposing membranes, SNAREs can be classified into v-SNARE (VAMP family proteins) and t-SNARE (syntaxin and SNAP-25 family proteins; Söllner et al., 1993). It has been proposed that the formation of v-SNARE/t-SNARE complex (trans-SNARE complex) perturbs the apposing membranes, thereby triggering fusion (Weber et al., 1998; McNew et al., 2000). This complex comprises a bundle of four α-helices, one supplied from VAMP, one from syntaxin, and two from SNAP-25 (Sutton et al., 1998). In cases where SNAP-25 family proteins are not involved, four SNARE molecules individually supply one helix for the formation of the trans-SNARE complex (Fukuda et al., 2000). Depending on the presence of Arg or Gln in the core binding domains of the four-helical bundle, SNAREs are alternatively categorized as R- or Q-SNAREs (Fasshauer et al., 1998).
We have previously identified an endoplasmic reticulum (ER)-associated SNARE, syntaxin 18 (Hatsuzawa et al., 2000), which is most likely the mammalian orthologue of yeast Ufe1p implicated in retrograde transport from the Golgi to the ER (Lewis and Pelham, 1996) and homotypic ER membrane fusion (Patel et al., 1998). More recently, we demonstrated that syntaxin 18 is present in a large complex comprising three SNARE proteins (p31, BNIP1, and Sec22b) and three peripheral membrane proteins (Sly1p, ZW10, and RINT-1; Hirose et al., 2004; Nakajima et al., 2004). p31 is the orthologue of yeast Use1p/Slt1p, which is an unconventional Q-SNARE because of the presence of Asp instead of Gln in the core binding domain (Belgareh-Touze et al., 2003; Burri et al., 2003; Dilcher et al., 2003). BNIP1 likely corresponds to yeast Sec20p, although their sequences as well as molecular weights are markedly different with each other (Sweet and Pelham, 1992; Nakajima et al., 2004). ZW10 and RINT-1, which were originally characterized as checkpoint proteins (Williams et al., 1992; Chan et al., 2000; Xiao et al., 2001), appear to be equivalent to Dsl1p and Tip20p, respectively (VanRheenen et al., 2001; Andag and Schmitt, 2003; Hirose et al., 2004). The equivalents of all proteins identified in the syntaxin 18 complex are present in the Ufe1p complex (Lewis et al., 1997; Andag et al., 2001; Reilly et al., 2001; Hirose et al., 2004; Nakajima et al., 2004), implying that the ER membrane fusion machinery is conserved during evolution (Kraynack et al., 2005).
In the present study we investigated the role of RINT-1 in membrane trafficking between the ER and Golgi. We showed that RINT-1 participates in ER-to-Golgi transport by regulating the localization and entry of ZW10 into the syntaxin 18 complex.
MATERIALS AND METHODS
Antibodies and Chemicals
A monoclonal antibody (mAb) against human syntaxin 18 and polyclonal antibodies against human syntaxin 18, p31, RINT-1, ZW10, and BNIP1 were produced as described (Hirose et al., 2004; Nakajima et al., 2004). A polyclonal antibody against human Sec31p was prepared in this laboratory. mAbs against human p115 and ERGIC-53 were generous gifts from Dr. M. G. Waters (Princeton University, Princeton, NJ) and Dr. H.-P. Hauri (University of Basel, Switzerland), respectively. mAbs against calnexin, p150Glued, and Rad50 were obtained from BD Transduction Laboratories (Lexington, KY). A mAb against Hsp47 and a polyclonal antibody against mannosidase II (Man II) were obtained from StressGen Biotechnologies (Victoria, British Columbia, Canada) and Chemicon (Temecula, CA), respectively. A polyclonal antibody against glutathione S-transferase (GST) was from Santa Cruz Biotechnology (Santa Cruz, CA). A mAb against α-tubulin, monoclonal and polyclonal antibodies against FLAG, nocodazole, and brefeldin A (BFA) were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture
HeLa cells (Riken Bioresource Center) were cultured in Eagle's minimum essential medium supplemented with 50 IU/ml penicillin, 50 μg/ml streptomycin, and 10% fetal calf serum. 293T cells were grown in DMEM supplemented with the same materials.
Immunofluorescence Analysis
Immunofluorescence microscopy was performed as described (Tagaya et al., 1996). Cells were fixed with methanol at −20°C for 5 min for staining of endogenous RINT-1 and ZW10, or 4% paraformaldehyde at room temperature for 20 min for Man II, Sec31p, and expressed proteins. Confocal microscopy was performed with an Olympus Fluoview 300 laser scanning microscope (Tokyo, Japan).
Cell Synchronization and Immunoprecipitation
HeLa cells were synchronized by double thymidine block followed by nocodazole treatment, as described by Chan et al. (2000). Mitotic cells were collected by gently shaking off flasks. Unsynchronized and synchronized cells were homogenized in lysis buffer and immunoprecipitated with the indicated antibodies (Hatsuzawa et al., 2000).
Plasmid Construction and Transfection
The cDNAs encoding RINT-1ΔN (aa 220-792), RINT-1ΔC (aa 1-659), RINT-1N (aa 1-264), RINT-1M (aa 200-585), and RINT-1C (aa 565-792) were amplified by PCR and inserted into pFLAG-CMV 6a (Sigma-Aldrich). The plasmids for Sar1p mutants were constructed in this laboratory (Shimoi et al., 2005). The plasmid (pEBG) encoding GST was a kind gift from Dr. B. Mayer (Harvard Medical School, Boston, MA). For ZW10 binding assays, 293T cells grown on 35-mm dishes were transfected with the plasmid for the RINT-1 constructs (1 μg each) using LipofectAMINE PLUS reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol.
Protein Transport from the ER to the Golgi
The expression plasmid for vesicular stomatitis virus-encoded glycoprotein fused to green fluorescent protein (VSVG-GFP) was kindly donated by Dr. J. Lippincott-Schwartz (National Institutes of Health, Bethesda, MD). The plasmid (1 μg) was cotransfected with the plasmid for FLAG-RINT-1 full-length or deletion constructs (1 μg) into HeLa cells grown on 35-mm dishes. The cells were incubated at 40°C for 24 h and then shifted to 32°C to allow transport. The cells were fixed and processed for immunofluorescence analysis.
RNA Interference
Duplex RNAs used for targeting were RINT-1 (268) (5′-gaacaggtacttacaatttca-3′), RINT-1 (1149) (5′-ttagccactgatattccttgt-3′), β-COP (276) (5′-gagacttttacatgagatgat-3′), and lamin A/C (5′-ctggacttccagaagaacatt-3′). The duplex RNAs were purchased from Japan BioServices (Saitama, Japan), HeLa cells were grown on six-well plates or 100-mm dishes and transfected with duplex RNAs using Oligofectamine (Invitrogen) according to the manufacturer's protocol. Their final concentration was 100 nM. At 72 h after transfection, the cells were processed for immunoblotting, immunofluorescence, and immunoprecipitation. In the case of double transfection, cells were first transfected with duplex RNAs, incubated for 48–54 h, and then transfected with the plasmid for VSVG-GFP or Sar1p mutants. Transport of VSVG-GFP or redistribution of Man II to the ER was monitored after further 18–24-h incubation.
RESULTS
RINT-1 Is Associated with ZW10 and Syntaxin 18 throughout the Cell Cycle
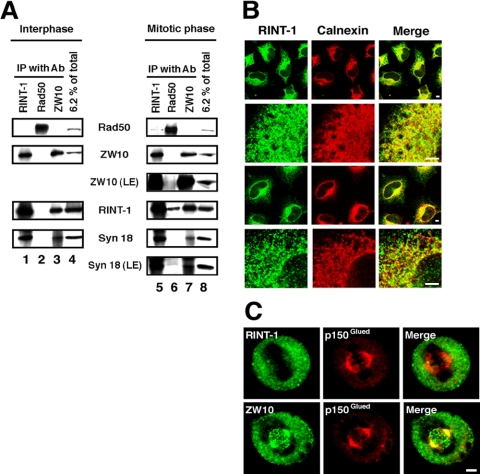
RINT-1 (Rad50-interacting protein 1) was originally discovered as a checkpoint protein that interacts with Rad50 only during late S and G2/M phases (Xiao et al., 2001). On the other hand, we have previously shown that RINT-1, together with ZW10 and other proteins, is complexed with syntaxin 18 (Hirose et al., 2004), an ER-localized SNARE implicated in membrane fusion (Hatsuzawa et al., 2000). These results suggest that there are, at least, two discrete RINT-1–containing complexes. To gain insight into the relationship between the two complexes, we performed immunoprecipitation experiments using lysates from HeLa cells that were unsynchronized (that is, predominantly interphasic) or synchronized at M phase with nocodazole (Figure 1A). In line with a previous report (Xiao et al., 2001), RINT-1 was coprecipitated with Rad50 from lysates of mitotically arrested cells (lane 6), but not from those of interphase cells (lane 2). In a reciprocal experiment, a small but significant amount of Rad50 was coprecipitated with RINT-1 from mitotic lysates (lane 5). Little, if any, ZW10 was coprecipitated with Rad50 from lysates of interphase cells (lane 2) or mitotic ones (lane 6), and vice versa (lanes 3 and 7). On the other hand, RINT-1 was coprecipitated with ZW10 regardless of whether cells were mitotically arrested or not (lanes 3 and 7). These results suggest that, unlike the case of Rad50, RINT-1 is associated with ZW10 throughout the cell cycle.
Figure 1.
RINT-1 is associated with ZW10 and syntaxin 18 throughout the cell cycle. (A) Lysates were prepared from interphasic or mitotic HeLa cells and immunoprecipitated with an antibody against RINT-1 (lanes 1 and 5), Rad50 (lanes 2 and 6), or ZW10 (lanes 3 and 7). The immunoprecipitated proteins were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies (lanes 1–3 and 5–7). Input (6.2% of total) was also analyzed (lanes 4 and 8). LE, long exposure. (B) HeLa cells were treated without (top two rows) or with 30 μg/ml digitonin (bottom two rows) for permeabilization and double-stained with antibodies against RINT-1 (first column) and calnexin (second column). Merged images are shown on the right. Bar, 5 μm. (C) Mitotic HeLa cells were fixed and double-stained with RINT-1 (top row) or ZW10 (bottom row) and p150Glued. Merged images are shown on the right. Bar, 5 μm.
ZW10, together with Rod and Zwilch, has been reported to be localized on kinetochores at prometaphase (Williams et al., 2003). As RINT-1 is associated with ZW10 throughout the cell cycle, we wondered whether or not RINT-1 is associated with kinetochores during mitosis. In interphase cells, RINT-1 gave a reticular staining pattern similar to that of an ER marker, calnexin (Figure 1B). In prometaphase cells, RINT-1 was not localized on kinetochores (Figure 1C), suggesting that it is not a component of a ZW10 complex comprising Rod and Zwilch.
The N-terminal Region of RINT-1 Interacts with ZW10
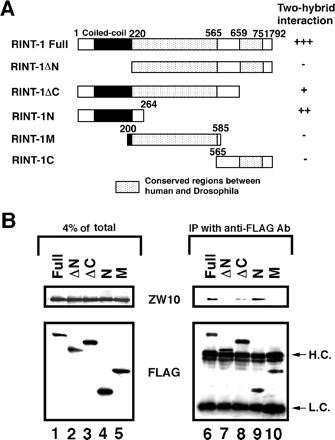
Our previous yeast two-hybrid assay demonstrated that RINT-1 binds directly to ZW10 and BNIP1 (Hirose et al., 2004; Nakajima et al., 2004). To determine which regions of RINT-1 interact with these two proteins, we constructed several truncated mutants (Figure 2A) and performed binding experiments using the yeast two-hybrid system and immunoprecipitation. Yeast two-hybrid analysis showed that deletion of the N-terminal 219 aa (RINT-1ΔN) abolishes the interaction with ZW10, and that the N-terminal 264 aa fragment (RINT-1N) is sufficient for the interaction (Figure 2A). Immunoprecipitation using FLAG-tagged RINT-1 constructs gave similar results (Figure 2B). Because the C-terminally truncated construct (RINT-1ΔC) exhibited a weaker binding to ZW10 than did the N-terminal construct (RINT-1N) in both yeast and mammalian cells, the middle region of RINT-1 might somehow suppress the interaction with ZW10. However, further study is necessary to address this possibility.
Figure 2.
The N-terminal region of RINT-1 interacts with ZW10. (A) Schematic representation of RINT-1 and its deletion constructs. The interaction of RINT-1 or its deletion constructs with ZW10 in yeast cells was evaluated by filter assays for β-galactosidase. Blue color was detected within 1 h (+++), 2 h (++) and 16 h (+). Expression of RINT-1C in yeast cells was confirmed by immunoblotting, whereas this fragment was not expressed in 293T cells (unpublished data). (B) 293T cells were transfected with the plasmids encoding the indicated FLAG-tagged constructs. At 24 h after transfection, cell lysates were prepared, and FLAG-tagged proteins were immunoprecipitated with an anti-FLAG antibody. The precipitated proteins were analyzed by immunoblotting with antibodies against ZW10 and FLAG (lanes 6–10). Lanes 1–5 show the amounts of ZW10 and FLAG-tagged proteins in 4% of the lysate. H.C. and L.C. denote immunoglobulin heavy and light chains, respectively.
Although RINT-1N, as well as full-length RINT-1, was capable of interacting with ZW10, its localization was markedly different from that of the full-length protein. RINT-1N was found to be almost exclusively localized in the cytosol, whereas full-length RINT-1 was distributed in both membranes and cytosol (Supplementary Figure S1A). Consistent with its localization, RINT-1N was not associated with the membrane components, syntaxin 18 and BNIP1 (Supplementary Figure S1B).
We could not define the BNIP1-binding site on RINT-1 because almost all constructs, to a greater or lesser extent, bind to BNIP1 (unpublished data). Perhaps, RINT-1 interacts with BNIP1 through its several distinct regions.
Overexpression of the N-terminal Region of RINT-1 Perturbs Membrane Trafficking between the ER and Golgi
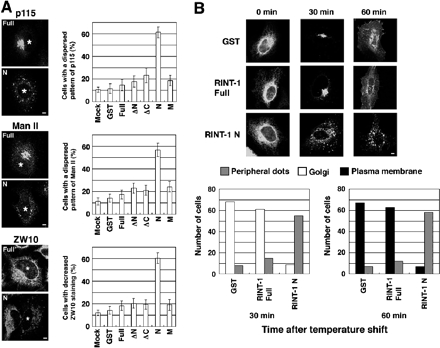
As RINT-1N interacts with ZW10 and localized in the cytosol, it may function as a dominant-negative by competing with endogenous membrane-bound RINT-1 for ZW10. We therefore examined the effect of overexpression of RINT-1N, together with full-length and other truncated constructs, on Golgi morphology (Figure 3A). In cells overexpressing RINT-1N, dispersed patterns for Golgi marker proteins, p115 and Man II, were frequently observed, whereas overexpression of full-length RINT-1 or other truncated constructs had little, if any, effect (top two and middle two panels). Notably, overexpression of RINT-1N, but not the full-length construct, resulted in a significant loss of ZW10 staining at the ER (bottom two panels), suggesting that RINT-1N induced redistribution of ZW10.
Figure 3.
Overexpression of the N-terminal region of RINT-1 perturbs membrane trafficking between the ER and Golgi. (A) HeLa cells were transfected with the plasmids encoding GST or the indicated FLAG-tagged constructs. After 24 h, the cells were fixed and double-stained. Staining for p115 (top two panels), Man II (middle two panels), and ZW10 (bottom two panels). Asterisks indicate cells overexpressing FLAG-RINT-1 full-length (Full) or FLAG-RINT-1N (N). Bar, 5 μm. The quantitative results are shown on the right. Error bars, SE of the mean for three experiments. (B) The plasmid for VSVG-GFP was cotransfected with the plasmid for GST, as a control, FLAG-RINT-1 full-length, or FLAG-RINT-1N into HeLa cells, and the protein transport assay was performed. To identity cells expressing GST or FLAG-tagged constructs, the fixed cells were stained with an anti-GST or anti-FLAG antibody followed by a Texas Red–conjugated secondary antibody. Only GFP fluorescence is shown. Bar, 5 μm. The quantitative results are shown at the bottom. “Peripheral dots” denotes the dispersed dotlike distribution pattern of VSVG-GFP. When VSVG-GFP was found to be concentrated in the perinuclear area, this localization pattern was categorized into “Golgi.” When VSVG-GFP was detected on the plasma membrane, this pattern was counted as “Plasma membrane.” In many of the cells categorized into “Plasma membrane,” VSVG-GFP was detected not only on the plasma membrane but also in the perinuclear area.
Next, we investigated the effect of full-length RINT-1 and RINT-1N on protein transport from the ER to the Golgi. We used a well-known morphological transport assay that utilizes GFP fused to a temperature-sensitive VSVG mutant that is unable to exit the ER at the nonpermissive temperature (40°C; Presley et al., 1997). At 30 min of shifting to the permissive temperature (32°C), VSVG-GFP had exited the ER and accumulated at the perinuclear, Golgi region in cells expressing full-length RINT-1, as observed in nontransfected cells (unpublished data) and GST-expressing cells (Figure 3B). In cells expressing RINT-1N, on the other hand, VSVG-GFP had exited the ER but remained in dotlike structures at the cell periphery. Double staining revealed that the distribution of VSVG-GFP fairly overlaps with that of ERGIC-53, a marker for the ER-Golgi intermediate compartment, and β-COP, a COPI component, but less with that of Sec31p, an ER exit site marker (Supplementary Figure S2), suggesting that VSVG-GFP spots observed in RINT-1N–expressing cells represent the ERGIC, Collectively, these results indicate that overexpression of RINT-1N disturbs membrane trafficking between the ER and Golgi.
Depletion of RINT-1 Affects Membrane Trafficking between the ER and Golgi
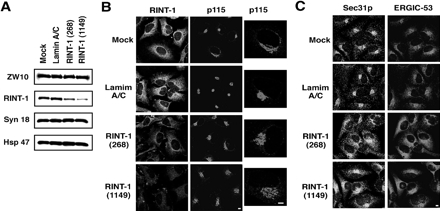
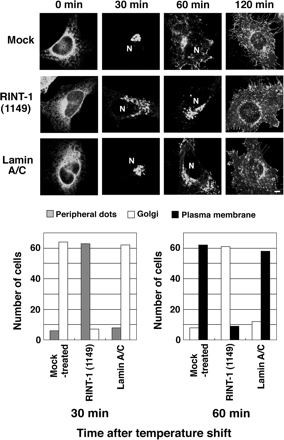
To assess the requirement of RINT-1 for membrane trafficking, RINT-1 expression was knocked down by RNA interference (RNAi). Two short interfering RNAs (siRNAs) named RINT-1 (268) and RINT-1 (1149) markedly blocked RINT-1 expression, although the latter effect was more prominent (Figure 4A). Concomitant with the reduction in RINT-1 expression, the intensity of RINT-1 staining was decreased, verifying the specificity of the anti-RINT-1 antibody (Figure 4B, bottom two rows). In addition, the distribution of p115 was changed from a compact pattern to a moderately dispersed one, reminiscent of the pattern of p115 staining in cells depleted of ZW10 (Hirose et al., 2004) or syntaxin 18 (unpublished data). The degree of the dispersion of p115 appeared to correlate with the degree of the suppression of RINT-1 expression. Such a change was not observed in mock-treated cells (Figure 4B, top row) or cells transfected with lamin A/C siRNA (second row). In cells where RINT-1 expression was suppressed, another Golgi protein, GM130, also showed a dispersed pattern (unpublished data). The localization of Sec31p and ERGIC-53 was also changed (Figure 4C). We noticed that the overall intensity of ERGIC-53 staining in RINT-1-knockdown cells was markedly higher than that in control cells. Although the reason for this is unclear at this moment, images obtained by scanning at lower PMT voltage showed dispersion of Sec31p and ERGIC-53 in RINT-1–depleted cells (Supplementary Figure S3). A morphological transport assay revealed that the transport of VSVG-GFP from the ER was substantially delayed in cells transfected with RINT-1 (1149) compared with mock-treated cells or cells transfected with the lamin A/C siRNA (Figure 5).
Figure 4.
Silencing of RINT-1 causes dispersion of proteins located in compartments of the early secretory pathway. (A) HeLa cells were transfected without (mock) or with lamin A/C siRNA, RINT-1 (268), or RINT-1 (1149). At 72 h after transfection, the cells were solubilized in phosphate-buffered saline with 0.5% SDS. Equal amounts of total proteins were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. (B) Immunofluorescence microscopic analysis of mock-treated cells, and cells transfected with lamin A/C siRNA, RINT-1 (268), or RINT-1 (1149). At 72 h after transfection, the cells were fixed and double-stained with antibodies against RINT-1 and p115. Expanded images for p115 are shown on the right. Bar, 5 μm. (C) Cells were treated as described in B and double-stained with antibodies against Sec31p and ERGIC-53. Bar, 5 μm.
Figure 5.
Delay in VSVG-GFP transport from the ER in cells with reduced expression of RINT-1. HeLa cells were successively transfected with siRNA and the plasmid for VSVG-GFP. Transport of VSVG-GFP from the ER was monitored as described in Materials and Methods. Typical images at 0, 30, 60, and 120 min after the temperature shift to 32°C are shown. N indicates the position of the nucleus. Bar, 5 μm. The quantitative results are shown at the bottom.
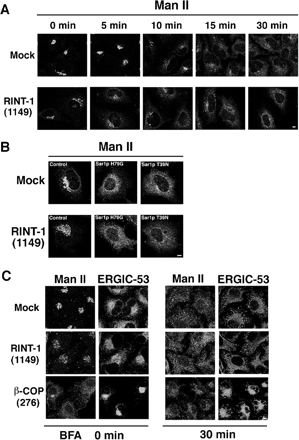
Depletion of RINT-1 Does Not Substantially Affect BFA- or Sar1p Mutant-induced Redistribution of Man II to the ER
To explore the possibility that RINT-1 is involved in retrograde transport from the Golgi to the ER, we first investigated the effect of knockdown of RINT-1 on BFA-induced redistribution of Man II. BFA is a well-known reagent that induces the retrograde transport of Golgi enzymes to the ER (Klausner et al., 1992). On BFA treatment, Man II was redistributed to the ER in RINT-1–depleted cells, with kinetics similar to that observed in mock-treated cells, although the localization patterns of Man II in RINT-1–depleted cells and mock-treated cells were substantially different before BFA treatment (Figure 6A). We next examined whether depletion of RINT-1 affects Sar1p mutant–induced redistribution of Man II to the ER. Both the GTP-restricted form, H79G, and the GDP-restricted form, T39N, have been shown to inhibit membrane trafficking from the ER, thereby causing Golgi enzymes to be redistributed to the ER (Kuge et al., 1994; Shima et al., 1998; Ward et al., 2001; Jiang and Storrie, 2005). As shown in Figure 6B, upon expression of Sar1pH79G or T39N, Man II was redistributed to the ER in RINT-1–depleted cells, as observed in control cells.
Figure 6.
Retrograde transport is not significantly impaired in cells with reduced expression of RINT-1. (A) HeLa cells were transfected without (mock) or with RINT-1 (1149). At 72 h after transfection, the cells were incubated with 10 μM BFA for the indicated times, fixed, and stained with an antibody against Man II. Bar, 5 μm. (B) At 54 h after transfection, the cells were further transfected with the plasmids for Sar1p mutants and incubated for 18 h. Redistribution of Man II to the ER occurred in almost all RINT-1–depleted and control cells irrespective of the expression levels of Sar1p mutants (unpublished data). Bar, 5 μm. (C) RINT-1 or β-COP expression was knocked down by RNAi. The expression level of β-COP was decreased by 86% (unpublished data). The cells were incubated with 10 μM BFA for 30 min, fixed, and double-stained with antibodies against Man II and ERGIC-53. Bar, 5 μm.
Given that RINT-1 depletion had no marked effect on BFA- or Sar1p mutant–induced redistribution of Man II to the ER, we sought to compare the phenotype of cells defective in retrograde transport with that of RINT-1–depleted cells. To this end, β-COP, a component of COP I vesicles involved in retrograde trafficking (Bonifacino and Glick, 2004; Lee et al., 2004), was knocked down by RNAi. On β-COP depletion, Man II became dispersed, whereas ERGIC-53 remained concentrated at the perinuclear region, with loss of its peripheral distribution (Figure 6C, BFA 0 min). Addition of BFA did not induce redistribution of ERGIC-53 from the perinuclear region to the ER (Figure 6C, BFA 30 min). These results suggest that retrograde transport of the ERGIC is blocked as a consequence of β-COP depletion. Obviously, the phenotype of β-COP–depleted cells is different from that of RINT-1–depleted cells (Figures 4 and 6C).
Loss of RINT-1 Affects the Distribution and Binding of ZW10 to Syntaxin 18
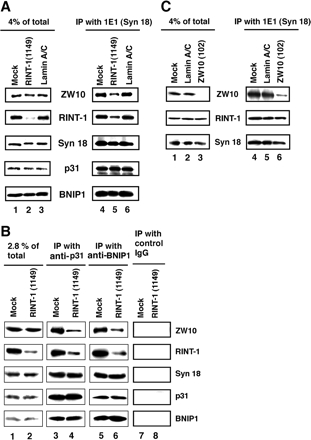
To gain insight into the mechanism underlying the ER-to-Golgi transport defect in RINT-1–depleted cells, we examined the effect of RINT-1 depletion on syntaxin 18 complex assembly. For this purpose, RINT-1 was knocked down, and the syntaxin 18 complex was precipitated with a monoclonal anti-syntaxin 18 antibody (mAb 1E1). As shown in Figure 7A, ZW10, a partner of RINT-1, was not efficiently coprecipitated with syntaxin 18 from lysates of cells transfected with RINT-1 (1149; lane 5) compared with mock-treated cells (lane 4) or lamin A/C siRNA-transfected cells (lane 6). The fact that the relative amount of RINT-1 coprecipitated with syntaxin 18 (lane 5) was larger than that in cell lysates (lane 2) may imply that RINT-1 complexed with ZW10 and other proteins may be more stable than free RINT-1. In contrast to ZW10, the efficiencies of BNIP1 and p31 coprecipitation were indistinguishable between RINT-1–depleted and control cells (lanes 4–6). Similar results were obtained when immunoprecipitation was performed using antibodies against p31 and BNIP1 (Figure 7B, lanes 3–6).
Figure 7.
Silencing of RINT-1 causes a decrease in the amount of ZW10 associated with syntaxin 18. (A) HeLa cells were transfected without (mock) or with RINT-1 (1149) or lamin A/C siRNA. At 72 h after transfection, lysates were prepared and immunoprecipitated with an anti-syntaxin 18 antibody. The precipitated proteins were detected by immunoblotting with the indicated antibodies (lanes 4–6). Input (4% of total) was also analyzed (lanes 1–3). (B) Cell lysates were prepared as described in A, and immunoprecipitated with an anti-p31 (lanes 3 and 4), anti-BNIP1 (lanes 5 and 6), or control IgG (lanes 7 and 8). Input (2.8% of total) was also analyzed (lanes 1 and 2). (C) HeLa cells were transfected without (mock) or with lamin A/C siRNA or ZW10 (102). At 72 h after transfection, lysates were prepared and immunoprecipitated with an anti-syntaxin 18 antibody. The precipitated proteins were analyzed by immunoblotting with the indicated antibodies (lanes 4–6). Input (4% of total) was also analyzed (lanes 1–3).
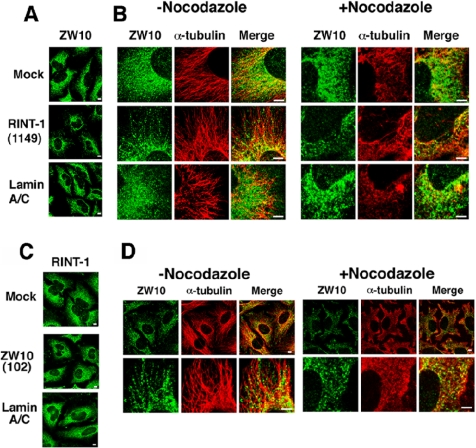
ZW10 in interphase cells has been reported to be distributed in entire ER membranes (Hirose et al., 2004). Given that overexpression of RINT-1N causes the redistribution of ZW10 (Figure 3A, bottom row), we were interested in the distribution of ZW10 in RINT-1–depleted cells. As shown in Figure 8, A and B, the distribution of ZW10 was significantly changed concomitant with the suppression of RINT-1 expression. ZW10 staining in RINT-1–depleted cells was not observed in the entire ER but rather in dispersed, short filament/dotlike structures. Of note, ZW10 expression was not suppressed in RINT-1–depleted cells (Figure 7). Some of ZW10-positive structures were observed along microtubules. Indeed, nocodazole treatment abolished linear arraylike staining for ZW10 (Figure 8B).
Figure 8.
Silencing of RINT-1 causes redistribution of ZW10. (A and B) HeLa cells were transfected without (mock) or with RINT-1 (1149) or lamin A/C siRNA. At 72 h after transfection, the cells were treated with or without 10 μg/ml nocodazole for 90 min. The cells were fixed and double-stained with antibodies against ZW10 and α-tubulin. Bar, 5 μm. (C and D) HeLa cells transfected without (mock) or with ZW10 (102) or lamin A/C siRNA were incubated for 72 h and stained for RINT-1. As observed previously (Hirose et al., 2004), p115 was modestly dispersed in ZW10 (102)-transfected cells (unpublished data), confirming the depletion of ZW10. Alternatively, the cells were incubated with or without 10 μg/ml nocodazole for 90 min, fixed, and double-stained with antibodies against ZW10 and α-tubulin. Expanded images are shown (bottom row). Bar, 5 μm.
Loss of ZW10 does not Affect the Distribution and Binding of RINT-1 to Syntaxin 18
We investigated whether depletion of ZW10 affects the association of RINT-1 with syntaxin 18. Cells were transfected with siRNA named ZW10 (102) (Hirose et al., 2004), lysed, and immunoprecipitated using anti-syntaxin 18 antibody (mAb 1E1). As shown in Figure 7C, RINT-1 was efficiently coprecipitated with syntaxin 18 from lysates of ZW10-depleted cells (lane 6), as well as mock-treated cells (lane 4) and lamin A/C siRNA-transfected cells (lane 5).
We next examined if depletion of ZW10 affects the localization of RINT-1. As shown in Figure 8C, the distribution of RINT-1 was not changed when the expression level of ZW10 was lowered. Interestingly, as seen in RINT-1–depleted cells, residual ZW10 was distributed along microtubules, and this localization was nocodazole-sensitive (Figure 8D).
DISCUSSION
We have previously shown that two cell cycle-related proteins, ZW10 and RINT-1, are tightly associated with syntaxin 18 (Hirose et al., 2004). ZW10 was first characterized as a kinetochore-associated protein involved in spindle checkpoint (Williams et al., 1992; Chan et al., 2000). RINT-1, on the other hand, was shown to interact with Rad50 and participate in G2/M checkpoint control (Xiao et al., 2001). Our previous results provided evidence that ZW10 during interphase plays a role in membrane trafficking between the ER and Golgi (Hirose et al., 2004). In the present study, we demonstrated that RINT-1 is also implicated in this transport process in interphase cells.
Two-hybrid and immunoprecipitation analyses revealed that the N-terminal region of RINT-1 is responsible for the interaction with ZW10. Overexpression of this region inhibited the ER-to-Golgi transport of VSVG-GFP and caused Golgi disassembly, accompanied by a change in the distribution of ZW10. In contrast, overexpression of full-length RINT-1 neither inhibited protein transport nor induced Golgi disassembly. These results suggest that the N-terminal region of RINT-1 acts as a dominant-negative and raise the possibility that RINT-1 coordinates the localization and function of ZW10. This possibility was supported by the finding that depletion of RINT-1 by RNAi causes the release of ZW10 from the syntaxin 18 complex and affects the distribution of ZW10. In RINT-1–depleted cells, some ZW10 was localized in short filament/dotlike structures along microtubules, whereas it appeared to be distributed in entire ER membranes in normal cells. Perhaps, ZW10 is distributed in both microtubules and ER membranes in normal cells, and its release from the ER, as a consequence of RINT-1 depletion, might accentuate microtubule localization. This explanation may be consistent with the observation that, when ZW10 expression was suppressed by RNAi, residual ZW10 exhibited a pattern similar to that observed in RINT-1–depleted cells.
The present results suggest that RINT-1 plays a role in anterograde transport from the ER to the Golgi. Given a link between ZW10 and dynamitin, a subunit of dynactin that functions as an adaptor for the minus end–directed motor dynein (Starr et al., 1998), it is tempting to speculate that the ZW10/RINT-1 complex acts as an anchor for dynein–dynactin on the ER to facilitate membrane transport to the Golgi. Because a substantial fraction of RINT-1 is associated with the ER membrane (Hirose et al., 2004), RINT-1 may be associated, through its distinct regions, not only with the syntaxin 18 complex but also with other ER proteins that mediate the export of certain secretory and membrane proteins. This possibility is now under investigation.
Tip20p and Dsl1p, the yeast homologues of RINT-1 and ZW10, respectively, form a complex implicated in retrograde transport from the Golgi to the ER (Cosson et al., 1997; Lewis et al., 1997; Reilly et al., 2001; Kraynack et al., 2005). Given that Tip20p directly binds an ER SNARE, Sec20p (Sweet and Pelham, 1993), and that Dsl1p interacts with subunits of coatomer (Andag et al., 2001; Reilly et al., 2001; Andag and Schmitt, 2003), which coats vesicles for retrograde transport (Bonifacino and Glick, 2004; Lee et al., 2004), it has been postulated that the Dsl1p/Tip20p complex functions as a tether in analogy to known tethering complexes (Reilly et al., 2001; Andag and Schmitt, 2003; Lupashin and Sztul, 2005). However, a recent study showed that Tip20p also participates in anterograde transport by prohibiting back-fusion of COPII vesicles that mediate export from the ER (Kamena and Spang, 2004). The finding that the protein responsible for retrograde transport also plays a role in anterograde transport is surprising but understandable, because bidirectional transport is intimately coupled. Hammond and Glick (2000) have proposed that transitional ER sites, where COPII vesicles are formed (Bannykh et al., 1996; Rossanese et al., 1999), are created by retrograde membrane trafficking from the Golgi. Although our results suggest the involvement of RINT-1 in anterograde transport, they could not exclude the possibility that RINT-1 has some role in a retrograde transport process. Our analysis using BFA and Sar1p mutants may not be sensitive enough to assess the role of RINT-1 in retrograde trafficking.
Quite recently, Kraynack et al. (2005) reported the results of a detailed analysis of interactions between subunits of the complex comprising Dsl1p and Tip20p. Our present finding that RINT-1 regulates the entry of ZW10 to the syntaxin 18 complex is in accordance with their result that mutations within Tip20p have the most drastic effect on the integrity of the whole complex. However, one marked difference between Tip20p and RINT-1 is that Tip20p is required for the efficient assembly of the Q-SNARE complex consisting of Ufe1p, Sec20p, and Use1p/Slt1p (Kraynack et al., 2005), whereas RINT-1 does not contribute to the formation of the equivalent complex. On the basis of their observation, Kraynack et al. (2005) have proposed a model in which the Dsl1p complex stabilizes the Q-SNARE helices and makes them ready for the binding of R-SNARE on retrograde membrane carriers. The different major roles of Tip20p and RINT-1, i.e., Tip20p for retrograde transport and RINT-1 for anterograde transport, may be related to their different modes of interactions with the subunits of the yeast and mammalian complexes.
In support of the original finding by Xiao et al. (2001), RINT-1 was found to be associated with Rad50 during mitosis. This was not a consequence of the cell cycle–dependent switch of the interacting partner. RINT-1 was found to be associated with the syntaxin 18 complex throughout the cell cycle. Recent studies showed the requirement of GRASP-65 and clathrin for mitosis (Sutterlin et al., 2002, 2005; Preisinger et al., 2005, Royle et al., 2005, Yoshimura et al., 2005). At present the reason why proteins involved in membrane trafficking also function in mitosis remains an enigma. Future studies may clarify apparently different, but common traits between membrane trafficking and mitosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. M. G. Waters, H.-P. Hauri, J. Lippincott-Schwartz, and B. Mayer for gifts of reagents. This work was supported in part by Grants-in-Aid for Scientific Research (16370089, 16044242, 16048229, and 16657309) from the Ministry of Education, Science, Sports, and Culture of Japan. K.A. is a recipient of a Japan Society for the Promotion of Science research fellowship.
Abbreviations used:
- aa
amino acid
- BFA
brefeldin A
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- mAb
monoclonal antibody
- Man II
mannosidase II
- RNAi
RNA interference
- siRNA
short interfering RNA
- SNARE
SNAP receptor
- VSVG
vesicular stomatitis virus-encoded glycoprotein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-10-0973) on March 29, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Andag U., Neumann T., Schmitt H. D. The coatomer-interacting protein Dsl1p is required for Golgi-to-endoplasmic reticulum retrieval in yeast. J. Biol. Chem. 2001;276:39150–39160. doi: 10.1074/jbc.M105833200. [DOI] [PubMed] [Google Scholar]
- Andag U., Schmitt H. D. Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J. Biol. Chem. 2003;278:51722–51734. doi: 10.1074/jbc.M308740200. [DOI] [PubMed] [Google Scholar]
- Bannykh S.. I., Rowe T., Balch W. E. The organization of endoplasmic reticulum export complexes. J. Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgareh-Touze N., Corral-Debrinski M., Launhardt H., Galan J. M., Munder T., Le Panse S., Haguenauer-Tsapis R. Yeast functional analysis: identification of two essential genes involved in ER to Golgi trafficking. Traffic. 2003;4:607–617. doi: 10.1034/j.1600-0854.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. The mechanism of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Burri L., Varlamov O., Doege C. A., Hofmann K., Beilharz T., Rothman J. E., Söllner T. H., Lithgow T. A SNARE required for retrograde transport to the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2003;100:9873–9877. doi: 10.1073/pnas.1734000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri L., Lithgow T. A complete set of SNAREs in yeast. Traffic. 2004;5:45–52. doi: 10.1046/j.1600-0854.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- Chan G.K.T., Jablonski S. A., Starr D. A., Goldberg M. L., Yen T. J. Human Zw10 and ROD are mitotic checkpoint proteins that bind to kinetochores. Nat. Cell Biol. 2000;2:944–947. doi: 10.1038/35046598. [DOI] [PubMed] [Google Scholar]
- Cosson P., Schroder-Kohne S., Sweet D. S., Demolliere C., Hennecke S., Frigerio G., Letouneur F. The Sec20/Tip20p complex is involved in ER retrieval of dilysine-tagged proteins. Eur. J. Cell Biol. 1997;73:93–97. [PubMed] [Google Scholar]
- Dilcher M., Veith B., Chidambaram S., Hartmann E., Schmitt H. D., Fischer von Mollard G. Use1p is a yeast SNARE protein required for retrograde traffic to the ER. EMBO J. 2003;22:3664–3674. doi: 10.1093/emboj/cdg339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D., Sutton R. B., Brunger A. T., Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R., McNew J. A., Weber T., Parlati F., Engel T., Nickel W., Rothman J. E., Söllner T. H. Functional architecture of an intracellular membrane t-SNARE. Nature. 2000;407:198–202. doi: 10.1038/35025084. [DOI] [PubMed] [Google Scholar]
- Hammond A. T., Glick B. S. Dynamics of transitional endoplasmic reticulum sites in vertebrate cells. Mol. Biol. Cell. 2000;11:3013–3030. doi: 10.1091/mbc.11.9.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsuzawa K., Hirose H., Tani K., Yamamoto A., Scheller R. H., Tagaya M. Syntaxin 18, a SNAP receptor that functions in the endoplasmic reticulum, intermediate compartment, and cis-Golgi vesicle trafficking. J. Biol. Chem. 2000;275:13713–13720. doi: 10.1074/jbc.275.18.13713. [DOI] [PubMed] [Google Scholar]
- Hirose H., Arasaki K., Dohmae N., Takio K., Hatsuzawa K., Nagahama M., Tani K., Yamamoto A., Tohyama M., Tagaya M. Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. EMBO J. 2004;23:1267–1278. doi: 10.1038/sj.emboj.7600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W. SNAREs and traffic. Biochim. Biophys. Acta. 2005;1744:120–144. doi: 10.1016/j.bbamcr.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Jiang S., Storrie B. Cisternal Rab proteins regulate Golgi apparatus redistribution in response to hypotonic stress. Mol. Biol. Cell. 2005;16:2586–2596. doi: 10.1091/mbc.E04-10-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamena F., Spang A. Tip20p prohibits back-fusion of COPII vesicles with the endoplasmic reticulum. Science. 2004;304:286–289. doi: 10.1126/science.1095049. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 1992;16:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynack B. A., Chan A., Rosenthal E., Essid M., Umansky B., Waters M. G., Schmitt H. D. Dsl1p, Tip20p, and the novel Dsl3 (Sec39) protein are required for the stability of the Q/t-SNARE complex at the endoplasmic reticulum in yeast. Mol. Biol. Cell. 2005;16:3963–3977. doi: 10.1091/mbc.E05-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge O., Dascher C., Orci L., Rowe T., Amherdt M., Plutner H., Ravazzola M., Tanigawa G., Rothman J. E., Balch W. E. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J. Cell Biol. 1994;125:51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.C.S., Miller E. A., Goldberg J., Orci L., Schekman R. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Lewis M., Pelham H.R.B. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;85:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Lewis M. J., Rayner J. C., Pelham H. R. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J. 1997;16:3017–3024. doi: 10.1093/emboj/16.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupashin V., Sztul E. Golgi tethering factors. Biochim. Biophys. Acta. 2005;1744:325–339. doi: 10.1016/j.bbamcr.2005.03.013. [DOI] [PubMed] [Google Scholar]
- McNew J. A., Parlati F., Fukuda R., Johnston R. J., Paz K., Paumet F., Söllner T. H., Rothman J. E. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Hirose H., Taniguchi M., Kurashina H., Arasaki K., Nagahama M., Tani K., Yamamoto A., Tagaya M. Involvement of BNIP1 in apoptosis and endoplasmic reticulum membrane fusion. EMBO J. 2004;23:3216–3226. doi: 10.1038/sj.emboj.7600333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. K., Indig F. E., Olivieri N., Levine N. D., Latterich M. Organelle membrane fusion: a novel function for the syntaxin homolog Ufe1p in ER membrane fusion. Cell. 1998;92:611–620. doi: 10.1016/s0092-8674(00)81129-0. [DOI] [PubMed] [Google Scholar]
- Preisinger C., Korner R., Wind M., Lehmann W. D., Kopajtich R., Barr F. A. Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signalling. EMBO J. 2005;24:753–765. doi: 10.1038/sj.emboj.7600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley J. F., Cole N. B., Schroer T. A., Hirschberg K., Zaal K. J., Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Reilly B. A., Kraynack B. A., VanRheenen S. M., Waters M. G. Golgi-to-endoplasmic reticulum (ER) retrograde traffic in yeast requires Dsl1p, a component of the ER target site that interacts with a COPI coat subunit. Mol. Biol. Cell. 2001;12:3783–3796. doi: 10.1091/mbc.12.12.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossanese O. W., Soderholm J., Bevis B. J., Sears I. B., O'Connor J., Williamson E. K., Glick B. S. Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J. Cell Biol. 1999;145:69–81. doi: 10.1083/jcb.145.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle S. J., Bright N. A., Lagnado L. Clathrin is required for the function of the mitotic spindle. Nature. 2005;434:1152–1157. doi: 10.1038/nature03502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima D. T., Cabrera-Poch N., Pepperkok R., Warren G. An ordered inheritance strategy for the Golgi apparatus: visualization of mitotic disassembly reveals a role for the mitotic spindle. J. Cell Biol. 1998;141:955–966. doi: 10.1083/jcb.141.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoi W., Ezawa I., Nakamoto K., Uesaki S., Gabreski G., Aridor M., Yamamoto A., Nagahama M., Tagaya M., Tani K. p125 is localized in endoplasmic reticulum exit sites and involved in their organization. J. Biol. Chem. 2005;280:10141–10148. doi: 10.1074/jbc.M409673200. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Starr D. A., Williams B. C., Hays T. S., Goldberg M. L. ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterlin C., Hsu P., Mallabiabarrena A., Malhotra V. Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell. 2002;109:359–369. doi: 10.1016/s0092-8674(02)00720-1. [DOI] [PubMed] [Google Scholar]
- Sutterlin C., Polishchuk R., Pecot M., Malhotra V. The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol. Biol. Cell. 2005;16:3211–3222. doi: 10.1091/mbc.E04-12-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R. B., Fasshauer D., Jahn R., Brunger A. T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Sweet D. J., Pelham H. R. The Saccharomyces cerevisiae SEC20 gene encodes a membrane glycoprotein which is sorted by the HDEL retrieval system. EMBO J. 1992;11:423–432. doi: 10.1002/j.1460-2075.1992.tb05071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet D. J., Pelham H. R. The TIP1 gene of Saccharomyces cerevisiae encodes an 80 kDa cytoplasmic protein that interacts with the cytoplasmic domain of Sec20p. EMBO J. 1993;12:2831–2840. doi: 10.1002/j.1460-2075.1993.tb05944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaya M., Furuno A., Mizushima S. SNAP prevents Mg2+-ATP-induced release of N-ethylmaleimide-sensitive factor from the Golgi apparatus in digitonin-permeabilized PC12 cells. J. Biol. Chem. 1996;271:466–470. doi: 10.1074/jbc.271.1.466. [DOI] [PubMed] [Google Scholar]
- Ungar D., Hughson F. M. SNARE protein structure and function. Annu. Rev. Cell Dev. Biol. 2003;19:493–517. doi: 10.1146/annurev.cellbio.19.110701.155609. [DOI] [PubMed] [Google Scholar]
- VanRheenen S. M., Reilly B. A., Chamberlain S. J., Waters M. G. Dsl1p, an essential protein required for membrane traffic at the endoplasmic reticulum/Golgi interface in yeast. Traffic. 2001;2:212–231. doi: 10.1034/j.1600-0854.2001.020307.x. [DOI] [PubMed] [Google Scholar]
- Ward T. H., Polishchuk R. S., Caplan S., Hirschberg K., Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 2001;155:557–570. doi: 10.1083/jcb.200107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Söllner T. H., Rothman J. E. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Weimbs T., Low S. H., Chapin S. J., Mostov K. E., Bucher P., Hofmann K. A conserved domain is present in different families of vesicular fusion proteins: a new superfamily. Proc. Natl. Acad. Sci. USA. 1997;94:3046–3051. doi: 10.1073/pnas.94.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. C., Karr T. L., Montgomery J. M., Goldberg M. L. The Drosophila l(1) zw10 gene product, required for accurate mitotic chromosome segregation, is redistributed at anaphase onset. J. Cell Biol. 1992;118:759–773. doi: 10.1083/jcb.118.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. C., Li Z.-X., Liu S., Williams E. V., Leung G., Yen T. J., Goldberg M. L. Zwilch, a new component of the ZW10/ROD complex required for kinetochore functions. Mol. Biol. Cell. 2003;14:1379–1391. doi: 10.1091/mbc.E02-09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Liu C.-C., Chen P.-L., Lee W.-H. RINT-1, a novel rad50-interacting protein, participates in radiation induced G2/M checkpoint control. J. Biol. Chem. 2001;276:6105–6111. doi: 10.1074/jbc.M008893200. [DOI] [PubMed] [Google Scholar]
- Yoshimura S., Yoshioka K., Barr F. A., Lowe M., Nakayama K., Ohkuma S., Nakamura N. Convergence of cell cycle regulation and growth factor signals on GRASP65. J. Biol. Chem. 2005;280:23048–23056. doi: 10.1074/jbc.M502442200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.