Abstract
Rab small GTPases are involved in the transport of vesicles between different membranous organelles. RAB-3 is an exocytic Rab that plays a modulatory role in synaptic transmission. Unexpectedly, mutations in the Caenorhabditis elegans RAB-3 exchange factor homologue, aex-3, cause a more severe synaptic transmission defect as well as a defecation defect not seen in rab-3 mutants. We hypothesized that AEX-3 may regulate a second Rab that regulates these processes with RAB-3. We found that AEX-3 regulates another exocytic Rab, RAB-27. Here, we show that C. elegans RAB-27 is localized to synapse-rich regions pan-neuronally and is also expressed in intestinal cells. We identify aex-6 alleles as containing mutations in rab-27. Interestingly, aex-6 mutants exhibit the same defecation defect as aex-3 mutants. aex-6; rab-3 double mutants have behavioral and pharmacological defects similar to aex-3 mutants. In addition, we demonstrate that RBF-1 (rabphilin) is an effector of RAB-27. Therefore, our work demonstrates that AEX-3 regulates both RAB-3 and RAB-27, that both RAB-3 and RAB-27 regulate synaptic transmission, and that RAB-27 potentially acts through its effector RBF-1 to promote soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) function.
INTRODUCTION
Neurotransmitter release is accomplished by the fusion of neurotransmitter-filled synaptic vesicles at the presynaptic nerve terminal. This process occurs through a series of highly regulated steps that include synaptic vesicle transport, docking/tethering, priming, fusion, endocytosis, recycling, and neurotransmitter refilling (Sudhof, 2004). Several genes have been assigned roles in the various steps of the synaptic vesicle cycle. Rab3 (termed RAB-3 in Caenorhabditis elegans), a member of the Rab family of small GTPases, regulates synaptic transmission, possibly through the docking, priming, or fusion steps (Nonet et al., 1997; Weimer and Jorgensen, 2003; Schluter et al., 2004).
Rabs act in a variety of cell types and regulate vesicular transport between organelles. Rabs cycle on and off membranes via a GTP-dependent mechanism (Zerial and McBride, 2001). Rab activity is regulated by two proteins, which act in an antagonistic manner. The guanine nucleotide exchange factor (GEF) exchanges GTP for GDP, and the GTPase activating protein activates the intrinsic GTPase activity of a Rab (Bernards, 2003). GDP bound Rabs are held off of the membrane by a GDP dissociation inhibitor (Wu et al., 1996). The GTP/membrane-bound form of Rabs typically binds to a variety of effectors that regulate particular steps of membrane transport and cell signaling (Zerial and McBride, 2001; Spang, 2004).
Rab3 was once thought to play a central role in regulating release. However, recent work has shown that a quadruple knockout of all four isoforms of Rab3 in mice only results in a 30% reduction in evoked synaptic response (Schluter et al., 2004). This is consistent with work in C. elegans showing that mutations in the single rab-3 gene cause only mild defects in synaptic transmission (Nonet et al., 1997). Surprisingly, more dramatic phenotypes are found in Rab3 GEF knockout mice, which exhibit a marked reduction in evoked release that is not seen in the Rab3 knockout mice (Wada et al., 1997; Tanaka et al., 2001; Yamaguchi et al., 2002). Similarly, C. elegans aex-3 (Rab3 GEF homologue) mutants have more severe synaptic transmission defects than rab-3 mutants and exhibit defects in the defecation motor program not seen in rab-3 mutants (Iwasaki et al., 1997). These findings suggest that the Rab3 exchange factor homologue, AEX-3, may regulate another closely related Rab besides RAB-3.
RAB-27 is a close homologue of Rab3 and is expressed in the nervous system, making it an excellent candidate Rab to be regulated by AEX-3 (Pereira-Leal and Seabra, 2001; Barral et al., 2002). There are two isoforms of Rab27 in mammals, whereas there is only one isoform in C. elegans. Lesions in the human Rab27A gene cause Griscelli syndrome, an often fatal disease associated with albinism and immunodeficiency (Menasche et al., 2000; Sanal et al., 2002; Bahadoran et al., 2003; Menasche et al., 2003). Vertebrate Rab27 and Rab3 both bind to rabphilin, which contains a Zn2+ finger Rab-binding domain, and two C2 domains (Fukuda, 2003a; Fukuda et al., 2004; Fukuda, 2005). However, C. elegans rabphilin interacts with RAB-27 but not RAB-3 via glutathione S-transferase (GST) pull-down, in vitro coimmunoprecipitation, and yeast two-hybrid assays (Fukuda, 2003a; Fukuda et al., 2004). Previous work demonstrated that rabphilin acts independently of RAB-3 in vertebrates and invertebrates and that rabphilin potentiates soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) function in invertebrates (Schluter et al., 1999; Staunton et al., 2001). Recent work by Tsuboi et al. demonstrates that rabphilin regulates dense core vesicle docking by binding to the SNARE protein SNAP25 (Tsuboi and Fukuda, 2005). Although Rab27 is expressed in the brain, its role in neurons and the relevance of the interaction between Rab27 and rabphilin are largely unknown (Fukuda et al., 2002; Zhao et al., 2002).
We set out to address some of the outstanding questions regarding synapse-specific Rab function: Why is the Rab3 GEF homologue (aex-3) mutant phenotype more severe than the Rab3 mutant phenotype? Does AEX-3 regulate a second Rab, potentially RAB-27? Does RBF-1 (rabphilin) function as a RAB-27 effector in C. elegans? Does RAB-27 play a role in synaptic transmission? We identify rab-27 mutants in C. elegans and demonstrate that they have synaptic transmission defects. Our data reveal that AEX-3 regulates both RAB-3 and RAB-27. We demonstrate that RAB-3 and RAB-27 both regulate synaptic transmission. We also provide evidence that RBF-1 is an effector of RAB-27 in C. elegans. Together, these data suggest a model in which AEX-3 regulates RAB-3, RAB-27, and RBF-1.
MATERIALS AND METHODS
Growth and Culture of C. elegans and General Methods
Caenorhabditis elegans were grown at 22.5°C on solid medium as described by Wood (1988). Aldicarb plates were made by adding 2-methyl-2-[methylthio]proprionaldehyde 0-[methylcarbamoyl]-oxime (aldicarb) (Chem Services, West Chester, PA) to growth medium after autoclaving. Standard methods for molecular cloning and biochemistry were used unless otherwise stated (Sambrook and Russell, 2001).
Aldicarb Assays
Twenty-five young adult animals were transferred to 1 mM aldicarb plates. Worms were scored every 30 min for total number moving. Animals were considered paralyzed if they no longer moved when prodded with a platinum wire. Each assay was performed three times.
Strains Used
Isolation of aex-3(js815).
The aex-3(js815) mutant allele was isolated from a PCR screen using a knockout deletion library as described in Liu et al. (1999). Briefly, two rounds of nested PCR were used to screen an ethyl methane sulfonate (EMS) mutagenized library. Round 1 consisted of two outer primers and two inner poison primers: outer, 5′-TGTTAATGGCATTTTTCAGACG-3′ and 5′-TCAACCCAGAGGTGAACTTTTT-3′; and poison, 5′-ATGATGCGCTTGAGAAGAAGA-3′ and 5′ GGGCTCAAGTGAGTATTTTTGTG-3′. Round 2 consisted of a pair of inner or nested primers: 5′-ACAATTTGTGTCCAAGTCAACG-3′ and 5′-ATCTTTCGACAGACGCTCAACT-3′. The js815 allele has a 555-bp deletion (aa 159-264) that causes a frame shift and a premature stop. PCR using primers 5′-TGTTAATGGCATTTTTCAGACG-3′, 5′-TCAACCCAGAGGTGAACTTTTT-3′, and 5′-ATGATGCGCTTGAGAAGAAGA-3′ was used to genotype js815.
Cloning of aex-6 Mutations.
Mutations in the rab-27 gene were identified by sequencing various aex-6 alleles (see Supplemental Figure S1 for identified mutations).
Other Strains Used.
Single mutants rab-3(js49) and rbf-1(js232) (Nonet et al., 1997; Staunton et al., 2001). Double mutants were made by standard crossing procedures jsIs682; aex-3(js815), aex-3(js815); jsEx740, aex-6(sa24); jsEx740, aex-6(sa24); rab-3(js49), jsIs423; aex-3(js815), aex-6(sa24); jsIs423, rab-3(js49); rbf-1(js232), and aex-6(sa24); rbf-1(js232).
Transgenic Animals.
Transgenic animals were constructed as described in Mello et al. (1991). Briefly, plasmids (see below) purified with QIAGEN (Valencia, CA) columns were coinjected with pJM23 (lin-15 marker) into lin-15(n765ts) mutant animals. Progeny were grown at 22.5°C, and transformed animals were selected based on the absence of the lin-15 phenotype. jsEx640 (NM1023-GFP::RAB-3) was integrated into chromosome III as described previously (Hope, 1999) and is referred to as jsIs682 (GFP::RAB-3). The jsIs682 (GFP::RAB-3) transgene rescues the aldicarb resistance of rab-3(js49) and is likely a functional GFP fusion (our unpublished data). jsIs423 (RBF-1::GFP) was a spontaneous integration of jsEx86 (Staunton et al., 2001). jsEx623 (NM1030-rab-27 promoter driving GFP) and jsEx740 (NM1112-GFP::RAB-27) were also used (see Supplemental Figure S1 for rab-27 clones used). The jsEx740 (GFP::RAB-27) transgene rescues the aldicarb resistance and defecation (AEX) defects found in aex-6(sa24) and is likely a functional GFP fusion (our unpublished data).
Plasmid Construction
NM1030-rab-27 Promoter Driving Enhanced GFP (eGFP).
A 3.3-kb rab-27 promoter was amplified in a PCR using oligonucleotides 5′-AAACATCACTGCAGCGATTGCACAACTCAAGGCTCTC-3′ and 5′-AATTCCATGGGATAGTCGTAGTCACCCATCTTCC-3′ digested with PstI and NcoI and inserted into PstI-NcoI NM1019.
NM1112-rab-27 Promoter Driving eGFP Fused to the rab-27 Coding Region.
A 1.8-kb rab-27 genomic region was amplified in a PCR using oligonucleotides 5′-CCCTGTACATGGGTGACTACGACTATCTCATC-3′ and 5′-AATTCGGCCGATAATCAGCAATTTGCACAATAGGAAGAAGCAGCCGATGGGTC-3′, digested with BsrGI and EagI, and inserted into BsrGI-EagI NM1030.
pMK2: A Small Genomic rab-3 Clone.
pMK2 consists of the 4.5-kb BglII rab-3 genomic fragment of cosmid F11G1 inserted in BamHI-digested pBluescript II in the orientation such that the PstI site of the vector is proximal to the rab-3 promoter in the insert.
NM1019-rab-3 Promoter Driving eGFP.
A 1.3-kb rab-3 promoter fragment was amplified from pMK2 using oligonucleotides 5′-CCCTCACTAAAGGGAACAAAAG-3′ and 5′-CGCCTTGAGGTTAACCGACCGGTGCCATCTGAAAA-3′, digested with PstI and AgeI, and inserted into AgeI/PstI NM990. This vector consists of eGFP and the 3′ untranslated region of the unc-10 gene inserted in the yeast URA3 shuttle vector pRS426 (Christianson et al., 1992). The sequence of NM990 is available at thalamus.wustl.edu/nonetlab/.
NM1023-rab-3 Promoter Driving eGFP and the rab-3 Coding Region.
The coding region of rab-3 was amplified from cDNA with oligonucleotides 5′-TCGGCTGCAGTTAGCAATTGCATTGCTGTTG and 5′-GGTTTGTACATGGCGGCTGGCGGACAACCTC, digested with BsrGI and EagI, and inserted into BsrGI/EagI-digested NM1019.
NM1122-RAB-27 Protein Expression Vector.
rab-27 was amplified from cDNA using oligonucleotide 5′-CATGGCCATGGGTGACTACGACTATCTC-3′ and 5′-TCGGGTCGACGCATAGGAAGAAGCGGCCG-3′, digested with NcoI and SalI, and inserted into NcoI-SalI pHO2d (Fasshauer et al., 1997). The final construct contained the complete rab-27 coding sequences minus the last four amino acids fused to a linker sequence [ASTSLNSG] and ending in a His6-tag.
Production of RAB-27 Antisera
NM1122 was transformed into BL21-Codon Plus-RIL cells (Stratagene, La Jolla, CA). The His6-tagged fusion protein was expressed using a 1 mM isopropyl β-d-thiogalactoside induction at room temperature for 18 h. The fusion protein was purified on Ni-NTA agarose (QIAGEN) in RB buffer (20 mM HEPES, pH 7.4, 200 mM KCl, 0.1 mM phenylmethylsulfonyl fluoride [PMSF], 0.1% β-mercaptoethanol, 5% glycerol, and 5-500 mM gradient of imidazole), and dialyzed into phosphate-buffered saline. Antiserum 209 and 217 were raised in rabbits (Covance, Denver, PA).
Immunohistochemistry
Immunohistochemistry was performed using Bouin's fixative for whole-mount staining as described previously (Nonet et al., 1997). Mouse anti-RAB-3 antibodies (m937.3) were used as described previously (Nonet et al., 1997). Rabbit anti-RAB-27 antibodies (RB209) were used at a 1:10,000 dilution. Primary antibodies were visualized with goat anti-mouse IgG Alexa 488 and goat anti-rabbit IgG Alexa 568 (Invitrogen, Carlsbad, CA) at a 1:1000 dilution.
Imaging and C. elegans Neuroanatomy
All images (with the exception of Figure 3C) were taken with the anterior end on the left, the posterior on the right, dorsal side on the top, and ventral on the bottom. These images were taken of the anterior (head) neurons that surround a large muscular organ termed the pharynx. These neurons form a synaptic-rich nerve ring, which is essentially void of neuronal cell bodies. Synaptic proteins occur as a fairly sharp ring in this region of the nervous system. The neuronal cell bodies that form synapses within the nerve ring are found both anterior and posterior to the nerve ring. There are ∼50 neurons proximal to the anterior side and 100 neurons proximal to the posterior side. In mutants that disrupt synaptic localization, the signal seems diffuse, and the outline of a number of cell bodies will become increasingly apparent. For a guide to C. elegans anatomy, we suggest www.wormatlas.org.
Figure 3.
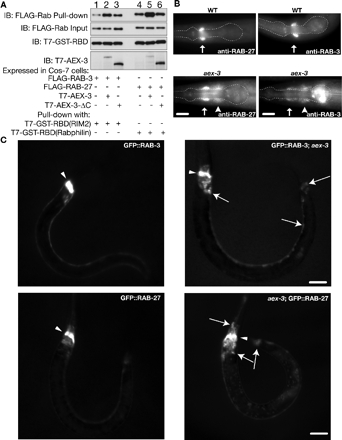
AEX-3 regulates RAB-3 and RAB-27. (A) AEX-3 increases the amounts of GTP-bound RAB-3 and RAB-27 in COS-7 cells. Lysates were prepared from COS-7 cells cotransfected with either FLAG-Rab3 alone (lane 1), FLAG-Rab3, and T7-tagged AEX-3 (T7-AEX-3; lane 2), FLAG-Rab3 and T7-tagged AEX-3 deletion mutant (T7-AEX-3-ΔC; lane 3), FLAG-Rab27 alone (lane 4), FLAG-Rab27 and T7-AEX-3 (lane 5), or FLAG-Rab27 and T7-AEX-3-ΔC (lane 6). After normalizing the amount of input FLAG-Rab between each cotransfected condition (IB: FLAG-Rab Input), the cell lysates were incubated with equal amounts of T7-/GST-tagged Rab binding domain (T7-GST-RBD) of mammalian Rim2 or rabphilin (IB: T7-GST-RBD). Because the RBD binds specifically to the GTP-bound form of each Rab, the amount of Rab pulled-down by a GST-tagged RBD is a readout for the amount of GTP-bound Rab in the lysate (IB: FLAG-Rab pull-down). The amounts of RAB-3 or RAB-27 pulled down are dramatically increased when they are coexpressed with full-length T7-AEX-3 (lanes 2 and 5), but not with coexpression of an empty vector (lanes 1 and 4) or a mutant form of AEX-3 (T7-AEX-3-ΔC, lanes 3 and 6). The expression of T7-AEX-3 or T7-AEX-3-ΔC was analyzed by immunoblotting with anti-T7 tag antibody (IB: T7-AEX-3). Ratio of band intensities of FLAG-Rab pull-down compared with FLAG-Rab input: lane 1 (0.55), lane 2 (1.86), lane 3 (0.88), lane 4 (1.52), lane 5 (3.08), and lane 6 (1.83). (B) aex-3 mutant animals mislocalize RAB-3 and RAB-27. Wild type (top) and aex-3(js815) (bottom) stained with RAB-27 (left) and RAB-3 (right) antisera. Both RAB-3 and RAB-27 are mislocalized from synapse-rich regions (arrows) in aex-3 mutants to cell bodies (arrowheads). The pharynx is outlined as an anatomical guide. Bars, 10 μm. (C) aex-3 mutant animals mislocalize GFP-tagged RAB-3 and RAB-27. Top left, visualization of GFP from a GFP-tagged RAB-3 transgenic animal (jsIs682) shows primarily nerve ring staining. Top right, GFP-tagged RAB-3 (jsIs682) is mislocalized from the nerve ring to neuronal cell bodies in aex-3(js815) mutant animals. Arrows mark representative neuronal cell bodies. Arrowheads mark the dorsal side of the nerve ring. Bottom left, visualization of GFP from a GFP-tagged RAB-27 transgenic animal (jsEx740) shows primarily nerve ring staining. Bottom right, GFP-tagged RAB-27 (jsEx740) is mislocalized from the nerve ring to neuronal cell bodies in aex-3(js815) mutant animals. Arrows mark representative neuronal cell bodies. Bars, 20 μm.
Western Analysis
Western analysis was performed as described in Weimer et al. (2003). Briefly, mixed staged animals were grown to near starvation and diluted in 5× the volume of packed worms with a sucrose:HEPES solution (0.36 M sucrose, 12 mM HEPES, and protease inhibitors). Worms were sonicated four times for 10 s on ice. Lysates were spun down at 16,000 × g for 15 min. Each lysate contained about 3 mg/ml protein, and 15 μg was loaded onto a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to nitrocellulose (Osmonics, Minnetonka, MN). Primary anti-RAB-27 (rabbit 209) was used at a 1:5000 dilution. Secondary goat anti-rabbit IgG H & L horseradish peroxidase (HRP) (Zymed Laboratories, South San Francisco, CA) was used at a 1:10,000 dilution. Blots were detected using an ECL kit (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
Measurement of GTP-bound Rab
Assay was carried out essentially as described in Coppola et al. (2002). COS-7 cells were transfected with 0.5 μg of plasmids encoding FLAG-tagged C. elegans RAB-3 (FLAG-Rab3) or RAB-27 (FLAG-Rab27). The aforementioned cells were cotransfected with 3.5 μg of plasmid encoding either an empty expression vector (pEF-BOS), T7-tagged AEX-3 (T7-AEX-3), or a C-terminal deletion mutant (Δaa1083-1409) of AEX-3 (T7-AEX-3-ΔC) (Oishi et al., 1998; Coppola et al., 2002). The cotransfected cells were incubated for 2 d at 37°C, harvested, and homogenized in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% glycerol, 1 mM PMSF, 100 μM pepstatin A, and 100 μM leupeptin). The homogenates were treated with 1% Triton X-100 for 1 h and centrifuged at 17,400 × g for 10 min. Expression of T7-AEX-3 or T7-AEX-3-ΔC was determined by Western analysis with anti-T7 tag antibodies (Figure 3A, IB: T7-AEX-3). The relative abundance of FLAG-Rab3 or FLAG-Rab27 in each lysate was determined by immunoblotting with HRP-conjugated anti-FLAG tag antibody (1/5000 dilution) (Figure 3A, IB: FLAG-Rab Input). The amount of FLAG-Rab3 or FLAG-Rab27 input in each experimental condition was equalized before pulling-down, so that approximately the same amount of FLAG-Rab was used in each experiment (Figure 3A, IB: FLAG-Rab Input). The equalized lysate (FLAG-Rab3 or FLAG-Rab27) was incubated for 1 h at 4°C with a T7-/GST-tagged Rab binding domain (RBD) of mammalian Rim2 or rabphilin (T7-GST-Rim2-RBD or T7-GST-Rph-RBD, respectively; (Fukuda, 2004; Fukuda et al., 2004), which had been coupled to glutathione-Sepharose 4B (GE Healthcare). After washing the beads with lysis buffer three times, the amount of FLAG-Rab that was pulled-down was analyzed by 12.5% SDS-PAGE followed by immunoblotting with the HRP-conjugated anti-FLAG tag antibody (1/5000 dilution) (Figure 3A, IB: FLAG-Rab pull-down). HRP-conjugated anti-T7 tag antibody (1/5000 dilution) was used for detection of T7-GST-Rim2-RBD and T7-GST-Rph-RBD (Figure 3A, IB: T7-GST-RBD).
Electrophysiology
The technique for recording evoked postsynaptic currents (ePSCs) at the C. elegans neuromuscular junction has been described previously (Wang et al., 2001), which was based on a technique originally developed by others (Richmond et al., 1999). Postsynaptic currents were amplified with a Multiclamp 700A amplifier (Molecular Devices, Sunnyvale, CA) and acquired with the Clampex software (Molecular Devices). Data were sampled at a rate of 10 kHz after filtering at 2 kHz. The recording pipette solution contained 120 mM KCl, 20 mM KOH, 5 Mm Tris, 0.25 mM CaCl2, 4 mM MgCl2, 36 Mm sucrose, 5 mM EGTA, and 4 mM Na2ATP (pH adjusted to 7.2 with HCl). The external solution included 140 mM NaCl, 5 mM KCl, 5 mM CaCl2, 5 mM MgCl2, 11 mM dextrose, and 5 mM HEPES (pH adjusted to 7.2 with NaOH).
The frequency and mean amplitude of miniature postsynaptic currents (mPSCs) of each experiment were determined using MiniAnalysis (Synaptosoft, Decatur, GA) with the amplitude detection threshold set at 10 pA. The analysis was assisted by visual inspection to include undetected smaller events (≥5 pA) and to exclude falsely detected events resulting from baseline fluctuations. Amplitudes of ePSCs were measured with Clampfit (Molecular Devices). The averaged amplitude of the two largest ePSCs from each experiment was used for statistical analysis. Statistical comparisons were made by one-way analysis of variance (ANOVA) followed by Tukey's post hoc tests. A p < 0.05 is considered statistically significant.
RESULTS
RAB-27 Is a Synaptic Protein
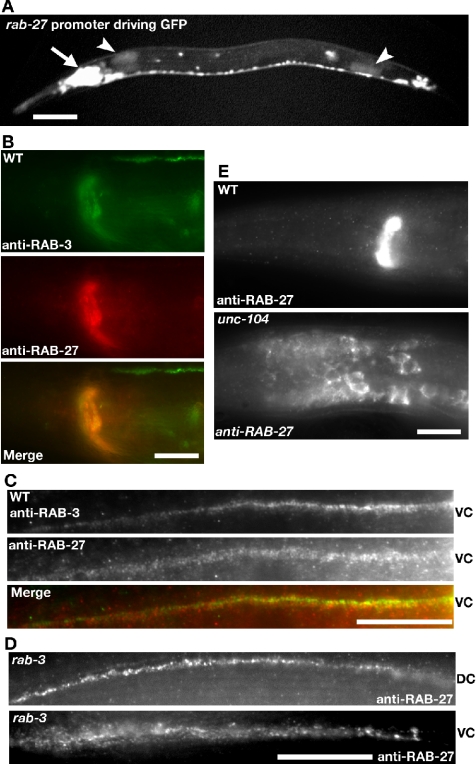
The closest C. elegans homologue of the vertebrate Rab27 family members is the gene Y87G2A.4, which we have named rab-27 in accordance with standard C. elegans nomenclature (Horvitz et al., 1979; Pereira-Leal and Seabra, 2001). To determine which cells express RAB-27, we constructed transgenic animals that express GFP under the rab-27 promoter (see Supplemental Figure S1 and Materials and Methods for rab-27 constructs and protein sequence alignment). This rab-27 reporter is detected pan-neuronally and in the intestine (Figure 1A). C. elegans rab-27 expression is consistent with that of mammalian Rab27B, which is also expressed in both brain and intestine as well as other secretory cells (Barral et al., 2002; Zhao et al., 2002). To determine the subcellular localization of RAB-27, we raised antibodies against the full-length recombinant RAB-27 protein. Immunostaining of wild-type and rab-3(js49) mutant animals shows that the RAB-27 protein is enriched in synapse-rich regions of the nervous system, such as the nerve ring, dorsal cord, and ventral cord (Figure 1, B–D). RAB-27 largely colocalizes with synaptic vesicle-associated RAB-3, although this colocalization is not complete (Figure 1, B and C). RAB-27 immunoreactivity in rab-27 mutants is absent suggesting that the RAB-27 antibody is specific (see below). RAB-27 immunostaining is normal in rab-3 mutants, suggesting that RAB-27 localization is independent of RAB-3 function (Figure 1D). Although the GFP reporter shows RAB-27 expression in the intestine, no immunoreactivity was detected in the intestine, possibly due to diffuse localization, low expression, or the absence of endogenous expression in the intestine.
Figure 1.
Synaptic Localization of RAB-27. (A) rab-27 is expressed in the nervous system. Animals carrying a transgene expressing GFP under the rab-27 promoter. Expression is seen in the neuronal ganglion (arrow) and intestinal cells (arrow head). Bar, 20 μm. (B) Colocalization of RAB-3 and RAB-27 in synapse-rich regions. Wild-type animals costained with RAB-3 (green) and RAB-27 (red) antisera. Immunoreactivity is most apparent at synapse-rich regions of the nerve ring. Bar, 10 μm. (C) Colocalization of RAB-3 and RAB-27 at the synapse. Wild-type animals costained with RAB-3 (green) and RAB-27 (red) antisera. RAB-3 and RAB-27 seem to colocalize within the synapse-rich ventral cord (VC), although their colocalization is not complete. Bar, 10 μm. (D) RAB-27 synaptic localization is independent of RAB-3. rab-3(js49) mutant animals were stained for RAB-27. Immunoreactivity remained abundant in the synapse-rich regions of the dorsal cord (DC) and VC. Bar, 10 μm. (E) RAB-27 localization is UNC-104 dependent. Wild-type and unc-104(e1265) mutant animals stained with RAB-27 antisera. RAB-27 staining is found in cell bodies anterior and posterior to the synaptic-rich nerve ring in unc-104 mutant animals. Bar, 10 μm.
In C. elegans, synaptic vesicle- and dense core vesicle-associated proteins share a unique dependence on the kinesin UNC-104 for anterograde transport (Hall and Hedgecock, 1991; Jacob and Kaplan, 2003; Zahn et al., 2004). Consistent with RAB-27 being present on either synaptic and/or dense-core vesicles, we found that RAB-27 is retained in cell bodies in unc-104(e1265) mutants (Figure 1E). RAB-3 is similarly mislocalized in unc-104(e1265) mutants (Nonet et al., 1997). Thus, C. elegans RAB-27 displays characteristics of a synaptic vesicle and/or dense-core vesicle-associated protein, although we do not exclude the possibility that RAB-27 is associated with another membrane organelle.
aex-6 Encodes RAB-27
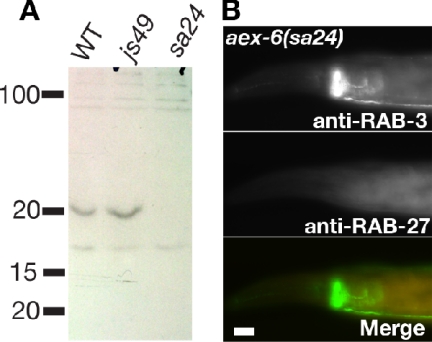
To address the role of RAB-27 in the nervous system, we set out to identify mutations in the rab-27 gene. We reasoned that if RAB-27 is indeed regulated by AEX-3 (see below), then rab-27 mutants might have similar phenotypes as aex-3 mutants, and they might have been isolated in previous genetic screens. aex-6 emerged as a candidate to encode RAB-27 through the aforementioned reasoning. aex-6 was originally isolated as a mutant defective in the anterior body wall muscle contraction (aBoc) and expulsion steps of the defecation motor program in C. elegans (Thomas, 1990). In addition, aex-6 mutants are egg-laying defective, lethargic, and form dauers at high temperatures (Thomas, 1990; Ailion and Thomas, 2003). aex-3 mutants share many of these phenotypes. We performed a series of analyses to determine whether aex-6 encodes RAB-27. Several lines of evidence support this prediction. First, aex-6 mutants map to an interval on chromosome I that encompasses the rab-27 locus (Thomas, 1990). Second, sequencing of three aex-6 alleles reveal point mutations in the rab-27 coding region, including two alleles containing early stop codons (Supplemental Figure S1). Third, RAB-27 immunoreactivity is absent in aex-6 alleles both by immunohistochemistry and by Western blot analyses (Figure 2, A and B). Fourth, a transgene containing GFP-tagged RAB-27 rescues both the aldicarb and AEX phenotypes (our unpublished observation; see Supplemental Figure S1 for transgene constructs). Thus, aex-6 encodes RAB-27. We will continue to refer to mutations in the rab-27 gene as aex-6.
Figure 2.
aex-6(sa24) is a null allele. (A) Western blot confirming loss of RAB-27 in aex-6(sa24). Approximately 15 μg of total worm lysate from wild-type, rab-3(js49), and aex-6(sa24) were separated by SDS-PAGE and blotted to nitrocellulose. Band of approximately 20 kDa disappears in aex-6(sa24) mutants. n = 3. (B) aex-6(sa24) mutant animals lack RAB-27. aex-6(sa24) mutant animals were costained for RAB-3 and RAB-27. Although RAB-3 was properly localized to the synapse-rich nerve-ring, RAB-27 was not detected. Bars, 10 μm.
AEX-3 Regulates Both RAB-3 and RAB-27
The similarity between the rab-27 and RAB3 GEF aex-3 mutant phenotypes suggested that both RAB-3 and RAB-27 might be regulated by this guanine nucleotide exchange factor. Association of the Rab protein with the vesicle membrane often requires the activity of the GEF protein (Pfeffer, 2005). In particular, RAB-3 association with synaptic vesicles requires AEX-3 (Iwasaki et al., 1997). Thus, we tested whether RAB-27 is also mislocalized in aex-3 mutants. We found that, like RAB-3, RAB-27 is more abundant in cell bodies in aex-3(js815) mutants than in wild-type animals (Figure 3B). A transgene carrying an N-terminal GFP fusion to RAB-27 (GFP::RAB-27) was similarly mislocalized in aex-3(js815) (Figure 3C). The mislocalization of RAB-27 was less drastic than that of a GFP::RAB-3 transgene (Figure 3, B and C). It is worth noting that each GFP fusion rescued the corresponding Rab loss-of-function mutation (see Materials and Methods). These results are consistent with AEX-3 regulating both RAB-3 and RAB-27.
To obtain biochemical evidence that AEX-3 regulates both RAB-3 and RAB-27, we assayed levels of GTP-bound Rabs in the presence or absence of AEX-3 (Coppola et al., 2002). GTP-bound RAB-3 specifically binds to Rim2, and GTP-bound RAB-27 specifically binds to rabphilin (Supplemental Figure S2, A and B; Fukuda, 2003a). GDP-bound forms of these Rab proteins do not bind these targets. We used GST-tagged Rab-binding domains of Rim2 and rabphilin in pull-down assays from COS-7 cells. Coexpression of AEX-3 with RAB-3 increased binding of RAB-3 to Rim (Figure 3A). Similarly, coexpression of AEX-3 with RAB-27 increased binding of RAB-27 to rabphilin (Figure 3A). It is the nucleotide exchange function of AEX-3 that is likely responsible for this activity, because an AEX-3 mutant (T7-AEX-3-ΔC) that lacks GEF activity does not stimulate binding (Figure 3A; Coppola et al., 2002). By contrast, AEX-3 did not stimulate RAB-5 binding to its effector Rabenosyn-5 (Supplemental Figure S2C; Nielsen et al., 2000). These results strongly suggest that AEX-3 regulates the activity of both RAB-3 and RAB-27.
RAB-3 and RAB-27 Control Synaptic Transmission
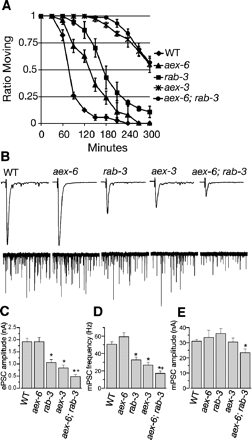
Because both RAB-3 and RAB-27 are expressed in the nervous system and both are regulated by AEX-3, we hypothesized that these Rabs regulate synaptic release. To test this hypothesis, we used a well-established pharmacological assay to analyze the changes in cholinergic signaling (Miller et al., 1996). In this assay, animals are exposed to the cholinesterase inhibitor aldicarb, and a time course of paralysis is used to quantify defects in synaptic function. As previously demonstrated, rab-3 mutants show a moderate resistance to aldicarb (Nonet et al., 1997), whereas aex-3 mutants exhibit stronger resistance compared with wild-type animals (Figure 4A). aex-6(sa24) mutants are slightly more aldicarb resistant than wild-type animals, indicating only a slight disruption of cholinergic signaling. Consistent with our hypothesis that RAB-3 and RAB-27 act to regulate synaptic transmission, and that AEX-3 regulates both proteins, aex-6; rab-3 double mutants exhibit stronger resistance to aldicarb than either single mutant and are comparable in resistance to aex-3 mutants.
Figure 4.
Synaptic transmission defects in aex-3 and aex-6. (A) Aldicarb resistance of aex-3 and aex-6. Wild-type (diamond), aex-6(sa24) (triangle), rab-3(js49) (square), aex-3(js815) (star), and aex-6(sa24); rab-3(js49) (circle) were exposed to 1 mM aldicarb and scored for percentage of the animals still moving every 30 min. Error bars represent SEM. (B) Less evoked release in aex-6; rab-3 double mutant, and aex-3 mutant animals than in rab-3 or aex-6 mutant animals. Representative traces of evoked release (top) and miniature postsynaptic currents, mPSC (bottom) of wild-type, aex-6(sa24), rab-3(js49), aex-3(js815), and aex-6(sa24); rab-3(js49) double mutant animals. (C) The mean evoked release, or ePSC, was determined for each mutant. Although there is no significant change in evoked release between wild-type (WT) and aex-6(sa24) mutant animals, there is a significant reduction between WT and rab-3(js49), aex-3(js815), and aex-6(sa24); rab-3(js49) mutant animals (∗p < 0.05). Consistent with RAB-3 and RAB-27 both regulating synaptic transmission, there is a significant decrease in evoked release between the aex-6(sa24); rab-3(js49) double mutants and either single mutant (+p < 0.05). WT (n = 40), aex-6(sa24) (n = 16), rab-3(js49) (n = 28), aex-3(js815) (n = 30), and aex-6(sa24); rab-3(js49) (n = 20). (D) The decrease in the frequency of miniature postsynaptic currents, or mPSCs, mirrored the decrease in evoked release (C). rab-3(js49), aex-3(js815), and aex-6(sa24); rab-3(js49) mutant animals all exhibit a significant decrease in the frequency of mPSCs from wild-type (∗p < 0.05). There is a significant decrease in the frequency of mPSCs between aex-6(sa24);rab-3(js49) double mutants and either single mutant (+p < 0.05). WT (n = 32), aex-6(sa24) (n = 9), rab-3(js49) (n = 16), aex-3(js815) (n = 16), and aex-6(sa24); rab-3(js49) (n = 10). (E) There is no significant change in the amplitude of mPSCs between any of the mutants and wild type, with the exception of the aex-6(sa24); rab-3(js49) double mutant animals (∗p < 0.05). n values are the same as in D.
To confirm our findings from the aldicarb assay, we analyzed miniature and evoked postsynaptic currents at the C. elegans neuromuscular junction. Consistent with the aldicarb data, rab-3(js49) mutants exhibit a reduction of evoked release, or ePSCs, to ∼55% of wild-type (Figure 4, B and C). Remarkably, the evoked release defects in aex-3(js815) and aex-6(sa24); rab-3(js49) (∼43% and ∼25% of wild type, respectively) mirror the results of the aldicarb assays (Figure 4, B and C). We also find that rab-3(js49) mutants exhibit a robust ∼35% decrease in miniature postsynaptic current (mPSC) frequency compared with wild-type animals (Figure 4, B and D). Interestingly, aex-3(js815) and aex-6(sa24); rab-3(js49) double mutants exhibit an even greater decrease in mPSC frequency (∼53 and ∼34% of wild-type, respectively). In contrast, mPSC amplitude was not affected significantly in any of the mutants we tested, except for a modest <14% reduction in the aex-6(sa24); rab-3(js49) double mutant, suggesting there is little disruption of postsynaptic function (Figure 4, B and E). These data strongly suggest that presynaptic RAB-3 and RAB-27 both regulate synaptic transmission.
RAB-27 Regulates RBF-1
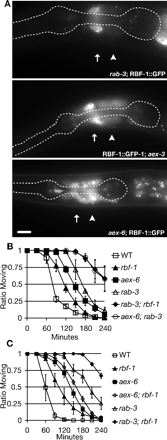
Because rab-3 and aex-6 mutant animals have distinct (e.g., defecation defect in aex-6 mutants) and overlapping (e.g., synaptic transmission defect) phenotypes and both Rabs are regulated by the same Rab3 GEF homologue (AEX-3), we hypothesized that RAB-3 and RAB-27 have distinct effectors. In vertebrates, rabphilin was originally identified as a Rab3 effector (Shirataki et al., 1993; Li et al., 1994; Ostermeier and Brunger, 1999); however, this does not seem to be the case in C. elegans (Staunton et al., 2001). Similarly, studies in mice have shown that rabphilin is not required for Rab3 function (Schluter et al., 1999). Later work demonstrated that rabphilin interacts with both vertebrate and invertebrate Rab27 (Fukuda, 2003a; Fukuda et al., 2004). To determine whether RBF-1 is an effector of RAB-27 in vivo, we looked at RBF-1 localization in aex-3 and aex-6 mutant animals. We found that GFP-tagged RBF-1 (RBF-1::GFP) is mislocalized from synapse-rich regions in aex-3(js815) and aex-6(sa24) mutant animals, but RBF-1::GFP is normally localized in rab-3(js49) animals (Figure 5A; Staunton et al., 2001). We also noted that RBF-1::GFP fluorescence is weaker in aex-6 mutant animals than in wild type. The intensity of RBF-1::GFP decreases rapidly as the aex-6 mutant animals develop beyond the young adult stage (our unpublished data). These results suggest that RBF-1 is an effector of RAB-27. To provide functional evidence that RBF-1 is an effector of RAB-27, we tested whether rbf-1 mutants enhance the aldicarb resistance found in rab-3 mutants. We found that rab-3; rbf-1 mutant animals exhibit an aldicarb resistance similar to aex-6; rab-3 mutant animals (Figure 5B). In contrast, aex-6; rbf-1 mutant animals do not exhibit an enhancement in aldicarb-resistance with respect to aex-6 single mutants (Figure 5C). These results strongly suggest that the synaptic transmission defect of aex-6 mutants is due to the loss of RBF-1 function. Our data show that RAB-3 and RAB-27 function together to regulate synaptic transmission; however, RAB-27 function diverges from RAB-3 through its effector protein RBF-1.
Figure 5.
RBF-1 is an effector of RAB-27. (A) RBF-1::GFP localization is dependent on AEX-3 and AEX-6. Visualization of GFP from an integrated RBF-1::GFP transgenic animal (jsIs423) shows primarily nerve ring staining. RBF-1::GFP is mislocalized to neuronal cell bodies in aex-3(js815) mutant animals, and the mislocalization is more severe in aex-6(sa24) mutant animals. The pharynx is outlined as an anatomical guide. Synaptic-rich regions are marked with and arrow, and neuronal cell bodies are indicated by an arrowhead. Bars, 10 μm. (B) rbf-1 enhances rab-3. Wild type (open box), rbf-1(js232) (closed triangle), aex-6(sa24) (closed box), rab-3(js49) (open triangle), rab-3(js49); rbf-1(js232) (closed diamond), and aex-6(sa24); rab-3(js49) (open circle) were exposed to 1 mM aldicarb and scored for percentage of the animals still moving every 30 min. Error bars represent SEM. (C) rbf-1 does not enhance rab-27. Wild type (open box), rbf-1(js232) (closed triangle), aex-6(sa24) (closed box), aex-6(sa24); rbf-1(js232) (open diamond); rab-3(js49) (open triangle), and rab-3(js49); rbf-1(js232) (closed diamond) were exposed to 1 mM aldicarb and scored for percentage of the animals still moving every 30 min. Error bars represent SEM.
DISCUSSION
Here, we identify AEX-3 as a regulator of RAB-27 and characterize the role of RAB-27 in the nervous system. In peripheral tissues, Rab27 has been studied due to its role in Griscelli syndrome. The immunodeficiency of Griscelli syndrome patients is due to a defect in lytic granule exocytosis from cytotoxic T-cells and may be mediated by effectors such as Munc13-4 and granuphilin (Feldmann et al., 2003; Goishi et al., 2004; Shirakawa et al., 2004; Torii et al., 2004; Yamamoto et al., 2004; Neeft et al., 2005). In addition, Rab27 plays a role in exocytosis of dense core granules from both endocrine and exocrine cells (Chen et al., 2003; Fukuda, 2003b; Izumi et al., 2003; Chen et al., 2004; Goishi et al., 2004; Imai et al., 2004; Matsumoto et al., 2004). Until now, the role of Rab27 in the nervous system was unknown. Previous work on the role of Rabs in synaptic transmission focused primarily on Rab3 (Sudhof, 2004). In this study, we identified mutations in the rab-27 gene and showed that these mutants exhibit mild synaptic and behavioral defects. We also showed that the exchange factor homologue AEX-3 regulates both RAB-3 and RAB-27 activity. This is noteworthy because AEX-3 is the first exchange factor homologue found to regulate Rab27 in invertebrates or mammals. Also, few exchange factors have been shown to regulate multiple Rabs (Jones et al., 2000). Our findings also suggest that other mechanisms, in addition to exchange factors, specifically control different Rab functions. RAB-3 and RAB-27 seem to be coregulated at the synapse, but functionally diverged through their distinct effectors.
Many exocytic proteins are conserved from yeast to vertebrates. Several of these proteins seem to play critical, if not essential, roles in exocytosis. These include the SNARE proteins and certain SNARE regulators (Bennett and Scheller, 1993; Sollner, 2003; Weimer and Jorgensen, 2003; Sudhof, 2004). The yeast exocytic Rab Sec4 is required for exocytosis (Salminen and Novick, 1987). Surprisingly, knockout of Rab3, an exocytic Rab, in mice and rab-3 mutant C. elegans exhibit only a mild decrease in synaptic transmission (Nonet et al., 1997; Schluter et al., 2004). One outstanding question is whether exocytic Rabs are required for exocytosis in metazoans. Our results demonstrate a role for two distinct Rab proteins (RAB-3 and RAB-27) in synaptic transmission. Although these results do not determine whether exocytic Rabs are required for synaptic transmission (or exocytosis in metazoans), they do demonstrate the ability of distinct Rabs, such as RAB-3 and RAB-27, to play similar or redundant roles in the same tissue. Our findings imply that there may even be a third or fourth Rab, similar to Rab3 or Rab27, regulating synaptic transmission.
We document that RAB-27 is found primarily at the synapse-rich regions of the C. elegans nervous system, notably the nerve ring and both ventral and dorsal nerve cords. RAB-27 localization only partially overlaps with that of RAB-3, suggesting that RAB-27 is likely on a distinct set of exocytic vesicles from RAB-3. One possibility is that RAB-27 is found mostly on dense-core vesicles, and RAB-3 is mostly on synaptic vesicles. Alternatively, RAB-3 and RAB-27 maybe on different subpopulations of synaptic-, dense-core, or even a novel set of exocytic vesicles. Our data are consistent with RAB-3 and RAB-27 both being localized to exocytic vesicles of the nervous system.
We show that RBF-1 is an effector of RAB-27 in C. elegans. RBF-1 is related to the mammalian protein granuphilin, which is thought to form a complex with Rab27 and the SNARE protein syntaxin (Torii et al., 2004). Recently, the C2B domain of rabphilin was shown to interact with SNAP25 and may regulate docking (Tsuboi and Fukuda, 2005). In addition, data from our laboratory demonstrates that RBF-1 potentiates SNARE function through the SNAP25 homologue RIC-4 (Staunton et al., 2001). Together, these findings suggest a mechanism in which the vesicle protein RAB-27 links/docks vesicles to release sites via an interaction with rabphilin, which then binds to a SNARE protein. Our model is consistent with work demonstrating that mutations in C. elegans rab-3, a close homologue of rab-27, have fewer synaptic vesicles at synapses and that Rab3a knockout mice lack activity-dependent recruitment of synaptic vesicles to the active zone (Nonet et al., 1997; Leenders et al., 2001). This model is also strengthened by our data showing a decrease in mPSC frequency in rab-3 mutants and a further reduction in mPSC frequency in aex-3 and aex-6; rab-3 double mutants, which could be indicative of a decrease in the readily releasable pool. It is worth noting that other studies of Rab3A knockout mice have not observed alterations in either the readily releasable pool or in the numbers of docked or total synaptic vesicles at synapses (Schluter et al., 2004) and have argued that Rab3a acts at the fusion step rather than in earlier docking steps. Whether these differences represent synapse-type specific or organism specific differences will require a more detailed understanding of the mechanisms of Rab modulation of synaptic release.
Our results illustrating that Rab27, a gene linked to Griscelli syndrome, regulates synaptic transmission are consistent with recent findings from patients with Chediak–Higashi syndrome, a disorder related to Griscelli syndrome (Tardieu et al., 2005). The authors found that after successful bone-marrow transplantation to treat the immunological defects, patients later develop severe neurological disorders in the absence of any structural damage that could have resulted from the disease. These neurological disorders could be the result of impaired neuronal dense-core or synaptic vesicle release. We find that RAB-27 clearly plays a role in synaptic transmission at least partially through its effector RBF-1. We propose that the vertebrate homologue of AEX-3 may play a role in the molecular mechanisms of melanosome movement, secretory vesicle release, and Griscelli syndrome via RAB-27.
Supplementary Material
ACKNOWLEDGMENTS
We thank Eiko Kanno for technical assistance and Scott Deken for comments regarding the manuscript. Some strains used in this work were obtained from the Caenorhabditis Genetics Center, which is supported by the National Institutes of Health National Center for Research Resources. This work was supported by grants from the U.S. Public Health Service (to M.L.N. and Z.-W.W.) and grants from the Ministry of Education, Culture, Sports and Technology of Japan; the Kato Memorial Bioscience Foundation; and The Sumitomo Foundation. M.F.T.M. was supported by a training grant from the U.S. Public Health Service.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-12-1170) on March 29, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Ailion M., Thomas J. H. Isolation and characterization of high-temperature-induced dauer formation mutants in Caenorhabditis elegans. Genetics. 2003;165:127–144. doi: 10.1093/genetics/165.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadoran P., et al. Characterization of the molecular defects in Rab27a, caused by RAB27A missense mutations found in patients with Griscelli syndrome. J. Biol. Chem. 2003;278:11386–11392. doi: 10.1074/jbc.M211996200. [DOI] [PubMed] [Google Scholar]
- Barral D. C., Ramalho J. S., Anders R., Hume A. N., Knapton H. J., Tolmachova T., Collinson L. M., Goulding D., Authi K. S., Seabra M. C. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J. Clin. Investig. 2002;110:247–257. doi: 10.1172/JCI15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. The molecular machinery for secretion is conserved from yeast to neurons. Proc. Natl. Acad. Sci. USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim. Biophys. Acta. 2003;1603:47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- Chen X., Li C., Izumi T., Ernst S. A., Andrews P. C., Williams J. A. Rab27b localizes to zymogen granules and regulates pancreatic acinar exocytosis. Biochem. Biophys. Res. Commun. 2004;323:1157–1162. doi: 10.1016/j.bbrc.2004.08.212. [DOI] [PubMed] [Google Scholar]
- Chen Y., Guo X., Deng F. M., Liang F. X., Sun W., Ren M., Izumi T., Sabatini D. D., Sun T. T., Kreibich G. Rab27b is associated with fusiform vesicles and may be involved in targeting uroplakins to urothelial apical membranes. Proc. Natl. Acad. Sci. USA. 2003;100:14012–14017. doi: 10.1073/pnas.2436350100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Coppola T., Perret-Menoud V., Gattesco S., Magnin S., Pombo I., Blank U., Regazzi R. The death domain of Rab3 guanine nucleotide exchange protein in GDP/GTP exchange activity in living cells. Biochem. J. 2002;362:273–279. doi: 10.1042/0264-6021:3620273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D., Otto H., Eliason W. K., Jahn R., Brunger A. T. Structural changes are associated with soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor complex formation. J. Biol. Chem. 1997;272:28036–28041. doi: 10.1074/jbc.272.44.28036. [DOI] [PubMed] [Google Scholar]
- Feldmann J., et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2. Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J. Biol. Chem. 2003a;278:15373–15380. doi: 10.1074/jbc.M212341200. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Slp4-a/granuphilin-a inhibits dense-core vesicle exocytosis through interaction with the GDP-bound form of Rab27A in PC12 cells. J. Biol. Chem. 2003b;278:15390–15396. doi: 10.1074/jbc.M213090200. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Alternative splicing in the first alpha-helical region of the Rab-binding domain of Rim regulates Rab3A binding activity: is Rim a Rab3 effector protein during evolution? Genes Cells. 2004;9:831–842. doi: 10.1111/j.1365-2443.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Versatile role of Rab27 in membrane trafficking: focus on the Rab27 effector families. J. Biochem. 2005;137:9–16. doi: 10.1093/jb/mvi002. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kanno E., Saegusa C., Ogata Y., Kuroda T. S. Slp4-a/granuphilin-a regulates dense-core vesicle exocytosis in PC12 cells. J. Biol. Chem. 2002;277:39673–39678. doi: 10.1074/jbc.M205349200. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kanno E., Yamamoto A. Rabphilin and Noc2 are recruited to dense-core vesicles through specific interaction with Rab27A in PC12 cells. J. Biol. Chem. 2004;279:13065–13075. doi: 10.1074/jbc.M306812200. [DOI] [PubMed] [Google Scholar]
- Goishi K., Mizuno K., Nakanishi H., Sasaki T. Involvement of Rab27 in antigen-induced histamine release from rat basophilic leukemia 2H3 cells. Biochem. Biophys. Res. Commun. 2004;324:294–301. doi: 10.1016/j.bbrc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Hall D. H., Hedgecock E. M. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Hope I. A. C. elegans: A Practical Approach. Oxford, NY: Oxford University Press; 1999. [Google Scholar]
- Horvitz H. R., Brenner S., Hodgkin J., Herman R. K. A uniform genetic nomenclature for the nematode Caenorhabditis elegans. Mol. Gen. Genet. 1979;175:129–133. doi: 10.1007/BF00425528. [DOI] [PubMed] [Google Scholar]
- Imai A., Yoshie S., Nashida T., Shimomura H., Fukuda M. The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J. Cell Sci. 2004;117:1945–1953. doi: 10.1242/jcs.01048. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Staunton J., Saifee O., Nonet M., Thomas J. H. aex-3 encodes a novel regulator of presynaptic activity in C. elegans. Neuron. 1997;18:613–622. doi: 10.1016/s0896-6273(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Izumi T., Gomi H., Kasai K., Mizutani S., Torii S. The roles of Rab27 and its effectors in the regulated secretory pathways. Cell Struct. Funct. 2003;28:465–474. doi: 10.1247/csf.28.465. [DOI] [PubMed] [Google Scholar]
- Jacob T. C., Kaplan J. M. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J. Neurosci. 2003;23:2122–2130. doi: 10.1523/JNEUROSCI.23-06-02122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Newman C., Liu F., Segev N. The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Mol. Biol. Cell. 2000;11:4403–4411. doi: 10.1091/mbc.11.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders A. G., Lopes da Silva F. H., Ghijsen W. E., Verhage M. Rab3a is involved in transport of synaptic vesicles to the active zone in mouse brain nerve terminals. Mol. Biol. Cell. 2001;12:3095–3102. doi: 10.1091/mbc.12.10.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Takei K., Geppert M., Daniell L., Stenius K., Chapman E. R., Jahn R., De Camilli P., Sudhof T. C. Synaptic targeting of rabphilin-3A, a synaptic vesicle Ca2+/phospholipid-binding protein, depends on rab3A/3C. Neuron. 1994;13:885–898. doi: 10.1016/0896-6273(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Liu L. X., et al. High-throughput isolation of Caenorhabditis elegans deletion mutants. Genome Res. 1999;9:859–867. doi: 10.1101/gr.9.9.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., et al. Noc2 is essential in normal regulation of exocytosis in endocrine and exocrine cells. Proc. Natl. Acad. Sci. USA. 2004;101:8313–8318. doi: 10.1073/pnas.0306709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche G., Feldmann J., Houdusse A., Desaymard C., Fischer A., Goud B., de Saint Basile G. Biochemical and functional characterization of Rab27a mutations occurring in Griscelli syndrome patients. Blood. 2003;101:2736–2742. doi: 10.1182/blood-2002-09-2789. [DOI] [PubMed] [Google Scholar]
- Menasche G., et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Alfonso A., Nguyen M., Crowell J. A., Johnson C. D., Rand J. B. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. USA. 1996;93:12593–12598. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeft M., et al. Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol. Biol. Cell. 2005;16:731–741. doi: 10.1091/mbc.E04-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E., Christoforidis S., Uttenweiler-Joseph S., Miaczynska M., Dewitte F., Wilm M., Hoflack B., Zerial M. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J. Cell Biol. 2000;151:601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M. L., Staunton J. E., Kilgard M. P., Fergestad T., Hartwieg E., Horvitz H. R., Jorgensen E. M., Meyer B. J. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J. Neurosci. 1997;17:8061–8073. doi: 10.1523/JNEUROSCI.17-21-08061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi H., Sasaki T., Nagano F., Ikeda W., Ohya T., Wada M., Ide N., Nakanishi H., Takai Y. Localization of the Rab3 small G protein regulators in nerve terminals and their involvement in Ca2+-dependent exocytosis. J. Biol. Chem. 1998;273:34580–34585. doi: 10.1074/jbc.273.51.34580. [DOI] [PubMed] [Google Scholar]
- Ostermeier C., Brunger A. T. Structural basis of Rab effector specificity: crystal structure of the small G protein Rab3A complexed with the effector domain of rabphilin-3A. Cell. 1999;96:363–374. doi: 10.1016/s0092-8674(00)80549-8. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal J. B., Seabra M. C. Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. A model for Rab GTPase localization. Biochem. Soc. Trans. 2005;33:627–630. doi: 10.1042/BST0330627. [DOI] [PubMed] [Google Scholar]
- Richmond J. E., Davis W. S., Jorgensen E. M. UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat. Neurosci. 1999;2:959–964. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987;49:527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Russell D. W. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Sanal O., Ersoy F., Tezcan I., Metin A., Yel L., Menasche G., Gurgey A., Berkel I., de Saint Basile G. Griscelli disease: genotype-phenotype correlation in an array of clinical heterogeneity. J. Clin. Immunol. 2002;22:237–243. doi: 10.1023/a:1016045026204. [DOI] [PubMed] [Google Scholar]
- Schluter O. M., Schmitz F., Jahn R., Rosenmund C., Sudhof T. C. A complete genetic analysis of neuronal Rab3 function. J. Neurosci. 2004;24:6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter O. M., Schnell E., Verhage M., Tzonopoulos T., Nicoll R. A., Janz R., Malenka R. C., Geppert M., Sudhof T. C. Rabphilin knock-out mice reveal that rabphilin is not required for rab3 function in regulating neurotransmitter release. J. Neurosci. 1999;19:5834–5846. doi: 10.1523/JNEUROSCI.19-14-05834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa R., Higashi T., Tabuchi A., Yoshioka A., Nishioka H., Fukuda M., Kita T., Horiuchi H. Munc13-4 is a GTP-Rab27-binding protein regulating dense core granule secretion in platelets. J. Biol. Chem. 2004;279:10730–10737. doi: 10.1074/jbc.M309426200. [DOI] [PubMed] [Google Scholar]
- Shirataki H., Kaibuchi K., Sakoda T., Kishida S., Yamaguchi T., Wada K., Miyazaki M., Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol. Cell. Biol. 1993;13:2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner T. H. Regulated exocytosis and SNARE function (Review) Mol. Membr. Biol. 2003;20:209–220. doi: 10.1080/0968768031000104953. [DOI] [PubMed] [Google Scholar]
- Spang A. Vesicle transport: a close collaboration of Rabs and effectors. Curr. Biol. 2004;14:R33–R34. doi: 10.1016/j.cub.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Staunton J., Ganetzky B., Nonet M. L. Rabphilin potentiates soluble N-ethylmaleimide sensitive factor attachment protein receptor function independently of rab3. J. Neurosci. 2001;21:9255–9264. doi: 10.1523/JNEUROSCI.21-23-09255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof T. C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Tanaka M., et al. Role of Rab3 GDP/GTP exchange protein in synaptic vesicle trafficking at the mouse neuromuscular junction. Mol. Biol. Cell. 2001;12:1421–1430. doi: 10.1091/mbc.12.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M., Lacroix C., Neven B., Bordigoni P., de Saint Basile G., Blanche S., Fischer A. Progressive neurological dysfunctions twenty years after allogeneic bone-marrow transplantation for Chediak-Higashi syndrome. Blood. 2005;106:40–42. doi: 10.1182/blood-2005-01-0319. [DOI] [PubMed] [Google Scholar]
- Thomas J. H. Genetic analysis of defecation in Caenorhabditis elegans. Genetics. 1990;124:855–872. doi: 10.1093/genetics/124.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S., Takeuchi T., Nagamatsu S., Izumi T. Rab27 effector granuphilin promotes the plasma membrane targeting of insulin granules via interaction with syntaxin 1a. J. Biol. Chem. 2004;279:22532–22538. doi: 10.1074/jbc.M400600200. [DOI] [PubMed] [Google Scholar]
- Tsuboi T., Fukuda M. The C2B domain of rabphilin directly interacts with SNAP-25 and regulates the docking step of dense core vesicle exocytosis in PC12 cells. J. Biol. Chem. 2005;280:39253–39259. doi: 10.1074/jbc.M507173200. [DOI] [PubMed] [Google Scholar]
- Wada M., Nakanishi H., Satoh A., Hirano H., Obaishi H., Matsuura Y., Takai Y. Isolation and characterization of a GDP/GTP exchange protein specific for the Rab3 subfamily small G proteins. J. Biol. Chem. 1997;272:3875–3878. doi: 10.1074/jbc.272.7.3875. [DOI] [PubMed] [Google Scholar]
- Wang Z. W., Saifee O., Nonet M. L., Salkoff L. SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron. 2001;32:867–881. doi: 10.1016/s0896-6273(01)00522-0. [DOI] [PubMed] [Google Scholar]
- Weimer R. M., Jorgensen E. M. Controversies in synaptic vesicle exocytosis. J. Cell Sci. 2003;116:3661–3666. doi: 10.1242/jcs.00687. [DOI] [PubMed] [Google Scholar]
- Weimer R. M., Richmond J. E., Davis W. S., Hadwiger G., Nonet M. L., Jorgensen E. M. Defects in synaptic vesicle docking in unc-18 mutants. Nat. Neurosci. 2003;6:1023–1030. doi: 10.1038/nn1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. The Nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Wu S. K., Zeng K., Wilson I. A., Balch W. E. Structural insights into the function of the Rab GDI superfamily. Trends Biochem. Sci. 1996;21:472–476. doi: 10.1016/s0968-0004(96)10062-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Tanaka M., Mizoguchi A., Hirata Y., Ishizaki H., Kaneko K., Miyoshi J., Takai Y. A GDP/GTP exchange protein for the Rab3 small G protein family up-regulates a postdocking step of synaptic exocytosis in central synapses. Proc. Natl. Acad. Sci. USA. 2002;99:14536–14541. doi: 10.1073/pnas.212511399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., et al. Identification of novel MUNC13-4 mutations in familial haemophagocytic lymphohistiocytosis and functional analysis of MUNC13-4-deficient cytotoxic T lymphocytes. J. Med. Genet. 2004;41:763–767. doi: 10.1136/jmg.2004.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn T. R., Angleson J. K., MacMorris M. A., Domke E., Hutton J. F., Schwartz C., Hutton J. C. Dense core vesicle dynamics in Caenorhabditis elegans neurons and the role of kinesin UNC-104. Traffic. 2004;5:544–559. doi: 10.1111/j.1600-0854.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell. Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhao S., Torii S., Yokota-Hashimoto H., Takeuchi T., Izumi T. Involvement of Rab27b in the regulated secretion of pituitary hormones. Endocrinology. 2002;143:1817–1824. doi: 10.1210/endo.143.5.8823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.