Abstract
The anchors of mature glycosylphosphatidylinositol (GPI)-anchored proteins of Saccharomyces cerevisiae contain either ceramide or diacylglycerol with a C26:0 fatty acid in the sn2 position. The primary GPI lipid added to newly synthesized proteins in the ER consists of diacylglycerol with conventional C16 and C18 fatty acids. Here we show that GUP1 is essential for the synthesis of the C26:0-containing diacylglycerol anchors. Gup1p is an ER membrane protein with multiple membrane-spanning domains harboring a motif that is characteristic of membrane-bound O-acyl-transferases (MBOAT). Gup1Δ cells make normal amounts of GPI proteins but most mature GPI anchors contain lyso-phosphatidylinositol, and others possess phosphatidylinositol with conventional C16 and C18 fatty acids. The incorporation of the normal ceramides into the anchors is also disturbed. As a consequence, the ER-to-Golgi transport of the GPI protein Gas1p is slow, and mature Gas1p is lost from the plasma membrane into the medium. Gup1Δ cells have fragile cell walls and a defect in bipolar bud site selection. GUP1 function depends on the active site histidine of the MBOAT motif. GUP1 is highly conserved among fungi and protozoa and the gup1Δ phenotype is partially corrected by GUP1 homologues of Aspergillus fumigatus and Trypanosoma cruzi.
INTRODUCTION
The biosynthesis of glycosylphosphatidylinositol (GPI)-anchored proteins follows the same basic rules in all eukaryotes, and all GPI anchors harbor a conserved carbohydrate core structure linking a protein moiety to a lipid moiety. In contrast, different organisms contain widely differing kinds of lipid moieties (Kinoshita and Inoue, 2000; Ferguson et al., 2006). GPI lipid biosynthesis starts with the addition of N-acetyl-glucosamine to phosphatidylinositol (PI) by PIG-A/GPI3 (Miyata et al., 1993). In many organisms, the spectrum of lipids found on mature GPI anchors is quite different from the one displayed by the free PI. The situation in Saccharomyces cerevisiae is peculiar because two very different types of lipid moieties can be found: ceramide (Cer) and diacylglycerol. Cer is found on the majority of anchors; it mainly consists of C18:0 phytosphingosine (PHS) and a C26:0 fatty acid (Fankhauser et al., 1993). On the other hand, Gas1p, a well-characterized GPI protein of yeast, is made with a C26:0 fatty acid–containing, mild base-sensitive lipid. In both types of lipid moieties the C26:0 may be hydroxylated on C2. These lipids are introduced by remodeling reactions starting soon after the primary GPI lipid is added to the nascent proteins in the lumen of the ER. During remodeling the C16- and C18-containing diacylglycerol of the primary anchor is modified or replaced. Here we identify GUP1 as a key remodelase. GUP1 and GUP2 were initially identified in a screen for glycerol uptake–deficient mutants (Holst et al., 2000). A recent report however has shown that in gup1Δ gup2Δ mutant yeast cells the glycerol H+/symport is still detectable (Neves et al., 2004). Other reports have revealed other functions of Gup1p. GUP1 is involved in bipolar bud site selection (Ni and Snyder, 2001) and is implicated in vacuolar protein sorting (Bonangelino et al., 2002). Here we propose that GUP1 acts as an enzyme that adds C26 fatty acids to the sn2 position of lyso-PI–containing GPI anchors.
MATERIALS AND METHODS
Strains, Media, and Materials
Strains with single deletions of nonessential genes were obtained from EUROSCARF (http://web.uni-frankfurt.de/fb15/mikro/euroscarf/col_index.html), namely are1Δ, MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 are1::kanMX4; are2Δ, MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 are2::kanMX4; gup1Δ, MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 gup1::kanMX4; gup2Δ, MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 gup2::kanMX4; vps4Δ, MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 vps4::kanMX4; BY4742, MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0. Several strains were obtained from Stephen Sturley, namely SCY63, MATα ade2-1 trp1-1 ura3-1 can1-1 met14 are1::HIS3 are2::LEU2; SCY1382, MATa ade2-1 trp1-1 his3-11,15 gup1::LEU2 gup2::URA3; SCY1414, are1::HIS3 are2::TRP1 gup1::LEU2 gup2::URA3; SCY325, MATα ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1. The 5Δ strain are1::HIS3 are2::TRP1 gup1::LEU2 gup2::URA3 yor175::kanMX4 was obtained from Nicolas Jacquier. Strains made for this study were FBY8171, MATa ade2-101 ura3-52 pep4::LEU2 gup1::kanMX4; FBY8172, MATα lys2 trp1 ura3 ubc6::HIS3 ubc7::LEU2 gup1::kanMX4; FBY8173, MATα his3Δ200 ura3-52 lys2-801 trp1-1 doa4::LEU2 gup1::kanMX4; FBY8174, MATα his3Δ200 leu2Δ1 ura3-52 cim5-1 gup1::kanMX4; FBY8175, MATa his3-115 leu2-3,112 ura3 pre1-1 pre2-2 gup1::kanMX4. Strains were cultured at 24, 30, or 37°C in YPD medium or in minimal media supplemented with glucose (SD) or galactose (SG) and amino acids (aa; Sherman, 2002). Selection for integration of KanMX4-containing deletion cassettes was performed on YPD plates containing 200 μg/ml G418 (Calbiochem, San Diego, CA). Unless specified, chemicals were purchased from Sigma (St. Louis, MO). Pepstatin was obtained from Alexis (San Diego, CA), and protein A-Sepharose, octyl-Sepharose, and concanavalin A-Sepharose from Amersham Biosciences (Piscataway, NJ). Anti-Pma1p antibody was a kind gift from Barbara Gaigg.
Strain and Plasmid Construction
To delete GUP1 in different yeast strains a gup1::KanMX4 deletion cassette was made by amplifying gup1::KanMX4 genomic region of the EUROSCARF deletion strain using primers GUP1_F1 (5′-aatcatacaaaggcaaaaacaaa-3′) and GUP1_R1 (5′-taaaaatacatacatgatagcag-3′).
The expression vectors harboring GUP1 or GUP1H447A were obtained as follows: the open reading frame of GUP1 was amplified by PCR using plasmid pBH2178 (kind gift from Morten Kielland-Brandt) as a template and using primers GUP1rec1sens (5′-gaattcgatatcaagcttatcgataccgatgtcgctgatcagcatcctgtctcc-3′) and GUP1rec2AS (5′-gacataactaattacatgactcgaggtcgactcagcattttaggtaaattccg-3′), underlined sequences being homologous to the target vector pGREG505 (Jansen et al., 2005). The PCR fragment was purified by a PCR purification kit (QIAGEN, Chatsworth, CA) and introduced into pGREG505 by cotransfection into yeast cells thus generating pGUP1 (Jansen et al., 2005). A point mutation to change His 447 to Ala was introduced into GUP1 by PCR amplification of two fragments of GUP1 from plasmid pBH2178 using primers GUP1rec1sens (see above) and GUP1.HIS447ALA.AS (5′-gttcgatgtcagcccatatagctacg-3′), and GUP1.HIS447ALAsens (5′-cgtagctatatgggctgacatcgaac-3′) with GUP1rec2AS (see above), underlined nucleotides representing the His to Ala mutation. The two overlapping fragments were fused by PCR using primers GUP1rec1sens and GUP1rec2AS (see above) and introduced into pGREG505 and pGREG535 by in vivo homologous recombination, yielding vectors pGUP1H447A and pHAGUP1H447A, respectively. A pGREG505 vector containing His 447 mutated to Asn (GUP1H447N) was obtained using the same strategy. Transfer of GUP1 into pGREG535 yielded pHAGUP1. To generate pHAafGUP1 and pHAtcGUP1, the GUP1 ortholog of Aspergillus fumigatus was amplified from a cDNA preparation (kindly donated by Michel Monod, Lausanne, Switzerland) using primers afGUP1-Rec1 (5′-gaattcgatatcaagcttatcgataccgatgacttcgatcctttcctggttccgg-3′ and afGUP1-Rec2 (5′-gacataactaattacatgactcgaggtcgactcaacacttcatcttgataccagcgcg-3′) and was similarly amplified from genomic DNA of Trypanosoma cruzi (prepared in the lab of Reto Brun (Basel, Switzerland) using primers tcGUP1-Rec1 (5′-gaattcgatatcaagcttatcgataccgatgagtgaggaaaaaaattgcgctaatatgc-3′) and tcGUP1-Rec2 (5′-gcgtgacataactaattacatgactcgaggtcgacttaggcaccagcggaaattccgtatc-3′). The two PCR products were then introduced into pGREG535 as above. All inserts very verified by sequencing and corresponded to the published sequences over the entire reading frame.
Preparation of Radiolabeled GPI Protein Anchor Peptides
Isolation of the lipid moieties of GPI anchors was performed as described (Guillas et al., 2000), lipids were analyzed by TLC on 20 × 20-cm silica gel 60 plates using solvent 1 (CHCl3/CH3OH/0.25% KCl, 55:45:10) or solvent 2 (CHCl3/CH3OH/0.25% KCl, 55:45:5).
Lipid Analysis
Lipids extracted from [3H]inositol–labeled cells were desalted by butanol/water partitioning and analyzed by TLC. Alkaline hydrolysis was performed using 0.1 M NaOH in CHCl3/CH3OH/H2O (10:10:3) for 1 h at 37°C. Phospholipase A2 (PLA2) treatment of lipids was done in 25 mM Tris-HCl, pH 7.5, 2 mM CaCl2, and 0.1% sodium deoxycholate for 2 h at 37°C.
Pulse-Chase Analysis
Pulse-chase analysis was performed at 30°C as described (Gaigg et al., 2005). Immunoprecipitated proteins were solubilized by boiling 5 min in sample buffer and analyzed by SDS-PAGE and visualized by fluorography or phosphorimager in order to quantify by Quantity One software (Bio-Rad Laboratories, Hercules, CA).
Raft Association Analysis
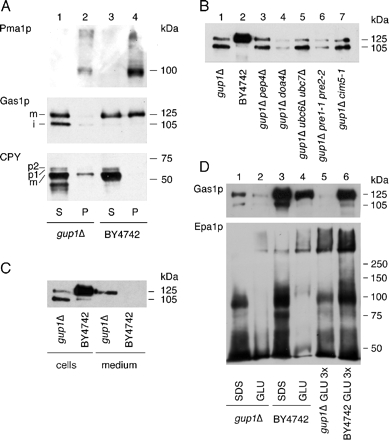
Raft association of Gas1p, CPY, and Pma1p was analyzed as described previously (Bagnat et al., 2000) with some modifications. Cells were grown at 30°C in rich medium. Twenty A600 of exponentially growing cells were collected and lysed in TEPIN buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 2.5 μg/ml antipain, 2.5 μg/ml chymostatin, 2.5 μg/ml leupeptin, and 2.5 μg/ml pepstatin) by vortexing with glass beads. The lysate was centrifuged at 3000 rpm at 4°C for 5 min to remove unbroken cells and debris. The cleared lysate was incubated with Triton X-100 added to 1% for 30 min on ice. After centrifugation at 15,000 × g for 40 min at 4°C, the supernatant was collected and precipitated with 10% TCA. The 15,000 × g microsomal pellet and the TCA precipitate were solubilized by boiling in reducing sample buffer for 5 min and analyzed by SDS-PAGE. Western blots were performed using antibodies against Gas1p, CPY, or Pma1p and revealed by chemiluminescence ECL kit.
RESULTS
The gup1Δ Mutant Is Deficient in GPI Anchor Remodeling
To find genes encoding remodelases, we analyzed the GPI anchor lipids of viable deletion strains lacking genes that show homology to known phospholipases, acyltransferases, or enzymes working on ceramides (Guillas et al., 2000; Conzelmann, 2005). To do so, deletion strains were labeled with [3H]inositol, proteins were extracted and extensively delipidated, enriched by concanavalin A-Sepharose affinity chromatography, and digested by protease to generate [3H]inositol–labeled anchor peptides. The latter were further purified by octyl-Sepharose chromatography, and their lipid moieties were liberated using nitrous acid deamination, which releases PI or inositolphosphoceramide (IPCs), depending on the GPI anchor. Finally, these lipids were desalted and analyzed by TLC. By this brute force approach, we found gup1Δ having the phenotype shown in Figure 1A. As expected, the anchors of the wild-type (wt) strain (Figure 1A, lane 9) contained a remodeled PI (pG1) that migrated faster than the PI contained in the lipid extract (lane 1) because its sn2 fatty acid is not C18:1 as in the bulk of PI, but has been exchanged for a more hydrophobic C26:0 (Sipos et al., 1997). wt anchors also contained the two ceramides: IPC/B (IPC containing PHS plus C26:0) and IPC/C (IPC containing PHS plus monohydroxylated C26:0 [C26:0-OH]; Figure 1A, lane 9). In contrast, strains deleted for GUP1 were deficient in GPI anchor remodeling (Figure 1A, lanes 4, 7, and 8). It appeared that gup1Δ strains were unable to attach pG1 or ceramides to the anchor. Their only anchor lipid seemed to be the primary, unremodeled PI. Lipid extracts of gup1Δ cells contained normal amounts of IPC/C and MIPC, although they displayed a conspicuous lack of IPC/D (IPC containing PHS and C26:0-(OH)2; Figure 1B), and this was a constant finding in several experiments. We also tested strains lacking GUP2, a homologue of GUP1 showing 53% of identities with GUP1, and strains lacking ergosterol acyltransferases ARE1 and ARE2, because all these genes also contain an MBOAT motif. As shown in Figure 1A, are1Δ, are2Δ, and gup2Δ were able to correctly make pG1, IPC/B, and IPC/C. They seemed to accumulate more unremodeled PI than wt, but unremodeled PI is occasionally also found in wt cells (unpublished data). Nevertheless, it can presently not be ruled out that Are1p and Gup2p play an auxiliary role to speed up the remodeling reaction, but they are unable to perform remodeling reactions in the absence of Gup1p. A fifth protein containing an MBOAT motif, encoded by YOR175c, has been identified. The single deletion yor175cΔ strain had normal GPI lipids, and the 5Δ strain (are1Δ are2Δ gup1Δ gup2Δ yor175cΔ) showed the same GPI anchor lipid pattern as the 4Δ strain (Supplementary Figure S1).
Figure 1.
gup1Δmutant is deficient in GPI anchor remodeling. (A–C) Cells of indicated genotype were labeled at 30°C with [3H]inositol, and lipids were extracted and analyzed by TLC/fluorography using solvent 2, either directly (B) or after deacylation with NaOH (C). GPI anchors were isolated from delipidated proteins, and their lipid moiety was released with nitrous acid and analyzed by TLC/fluorography (A) along with the free lipids of wt (SCY325) cells (wt, FL) using solvent 1. 2Δ are = are1Δ are2Δ; 2Δ gup = gup1Δ gup2Δ; 4Δ = are1Δ are2Δ gup1Δ gup2Δ. pG1 is a remodeled form of PI, containing C26:0 in sn2. IPC/B contains PHS-C26:0; IPC/C contains PHS-C26:0-OH. (D) wt or gup1Δ cells were labeled at 24 or 37°C with [3H]inositol for 2 h. The delipidated labeled proteins were analyzed by SDS-PAGE/fluorography.
The gup1Δ Mutant Accumulates lyso-PI
When proteins from [3H]inositol–labeled cells were analyzed by SDS-PAGE/fluorography, it appeared that gup1Δ strains incorporated as much [3H]inositol into GPI proteins as wt cells (Figure 1D). This result is in contrast to the low amount of [3H]inositol–labeled anchor peptides one obtains from gup1Δ cells (Figure 1A). However, it became apparent that during the preparative octyl-Sepharose purification step a significant amount of labeled peptides of gup1Δ were eluted with 25% propanol, whereas the wt anchor peptides were eluted only with 50% propanol, as previously reported (Figure 2A; Guillas et al., 2000). The fractions obtained with 25% propanol were treated with HNO2, and liberated lipids were separated by TLC. This revealed that gup1Δ cells contain massive amounts of anchor lipids that migrate less and end up in the zone of lyso-PI (Figure 2B, lanes 10–13). For comparison, we treated the lipid extracts of wt cells with PLA2 to generate lyso-PI and fractioned the products on octyl-Sepharose. Lyso-PI clearly was eluted with 25% of propanol as well (Figure 2B, lanes 3–5). However, only part of the gup1Δ anchor lipids comigrating with lyso-PI were sensitive to mild base (Figure 3, lanes 1 and 2). Indeed, in several experiments we observed base-resistant anchor lipids in gup1Δ, which were much more polar than the normal IPC/B and IPC/C. One of these was only seen in gup1Δ cells (Figure 3, lanes 1 and 2, marked with asterisk), and another one could occasionally also be found in wt cells (Figure 3, lanes 5 and 6). The base-resistant lipids of gup1Δ cells were not contaminating free lipids but were truly derived from GPI anchors, because they were not present when the HNO2 treatment was omitted (Figure 3, lanes 9 and 10). Yet, from several experiments of the type shown in Figures 2 and 3, we concluded that gup1Δ cells accumulated GPI anchors containing mainly lyso-PI. This finding is in keeping with the presence of an MBOAT motif in GUP1 and with the idea that Gup1p acts as a sn2-specific acyltransferase for GPI anchors. To further support this concept, we mutated histidine 447, the predicted active site of the MBOAT motif of GUP1 (Hofmann, 2000). As shown in Figure 4A, gup1Δ cells harboring GUP1H447A still had abnormal anchor peptides because a significant part of them eluted abnormally at 25% propanol from the octyl-Sepharose column, as was the case for gup1Δ cells harboring the control vector (Figure 4A) and for gup1Δ cells (Figure 2A). To get a complete view of the situation, anchor lipids were liberated from all anchor peptides, those having been eluted with 25 as well as 50% propanol. The bulk of anchor lipids of gup1Δ cells harboring the empty vector migrated to the region of lyso-PI (Figure 4B, lanes 4 and 4′). Expression of wt HA-tagged GUP1 restored normal anchor remodeling in gup1Δ cells (Figure 4B, lane 3), whereas expression of GUP1H447A or HA-tagged GUP1H447A alleles was unable to do so (Figure 4B, lanes 5, 6, 5′, and 6′). Controls showed that significant amounts of HA-tagged GUP1 forms were present in these cells, whether or not they were mutated at His 447 (Figure 4C).
Figure 2.
GPI anchor lipids of gup1Δ cells are less hydrophobic than the ones of wt. (A) BY4742 wt and gup1Δ cells were labeled with [3H]inositol, and GPI proteins were purified, digested with pronase, and purified by chromatography on octyl-Sepharose. The octyl-Sepharose column was washed with 5%, eluted with 25% and then 50% propanol in water. Radioactivity in eluted fractions was measured by scintillation counting. (B) The fractions 1–9 of the gup1Δ derived peptides of panel A were subjected to nitrous acid treatment and the thus liberated lipid moieties from indicated fractions were analyzed by TLC in solvent 1 (lanes 9–13). In parallel, the lipid extract of [3H]inositol–labeled wt cells was treated with PLA2 and loaded onto the same octyl-Sepharose column, and eluted fractions were analyzed by TLC as above (lanes 2–7). Untreated lipid extract was run in lanes 1 and 8. Note that anchor peptides eluting with 25% propanol (lanes 10 and 11) are contaminated by hydrophobic peptides, which slightly distort the migration of lipids during TLC.
Figure 3.

gup1Δcells contain a novel base-resistant GPI lipid. The [3H]inositol–labeled GPI anchor lipids were isolated from indicated strains as in Figure 2, but omitting the purification over octyl-Sepharose except in lanes 5 and 6, which contain 50% propanol eluates. Samples were treated or not with HNO2 and/or NaOH, as indicated. The novel base resistant anchor lipid of gup1Δ is indicated by an asterisk (∗, lanes 1 and 2).
Figure 4.
The GPI remodeling activity of Gup1p requires Histidine 447. (A) The indicated strains were labeled with 40 μCi of [3H]inositol, and GPI anchor peptides were purified using octyl-Sepharose column chromatography as in Figure 2A. The columns were eluted with 25% (fractions 2–7) and 50% propanol (fractions 8–12). Ten percent of each fraction was used to determine the radioactivity. (B) Aliquots of anchor peptides (100,000 cpm/strain) from 50% propanol eluates (fractions 8–10, panel A) were treated with HNO2 (lanes 2–6) or control incubated (lane 7), and liberated lipids were run on TLC using solvent 2; the same amount of radioactivity was also taken from 25% propanol eluates (fractions 3–5 in panel A) and similarly analyzed (lanes 4′–6′). Lane 1 contains the free lipids of BY4742. The total amount of radiolabeled anchor peptides obtained in corresponding elution fractions for each strain (cpm ×10−5) is given below each lane. (C) Western blotting with anti-HA antibody of protein extracts from gup1Δ cells harboring the indicated plasmids.
We further wanted to verify that yeast Gup1p is working only on GPI-anchored proteins, but not on free GPI lipids. For this we labeled yeast microsomes with UDP-[3H]GlcNAc either in the presence or absence of C26-CoA, the presumed substrate of Gup1p. Indeed, the profile of GPI lipids made by gup1Δ microsomes was the one of wt, and the same was seen when reactions were done in the absence of divalent cations (Supplementary Figure S2).
The gup1Δ Mutants Have Fragile Cell Walls
Cell wall fragility and hypersensitivity to calcofluor white (CFW) are characteristic for mutants having defects in GPI biosynthesis. Figure 5A shows that cells deleted for GUP1 were hypersensitive to CFW. Cells harboring GUP1H447A or HA-GUP1H447A instead of wt GUP1 were similarly hypersensitive to CFW (Figure 5B), suggesting that it is the loss of an acyltransferase activity that renders gup1Δ cells hypersensitive to CFW. In contrast, the gup2Δ mutant, which is not significantly impaired in GPI anchor remodeling (Figure 1A), was not hypersensitive to CFW (Figure 5A). Overexpression of HA-GUP1H447A in wt cells had no effect on their growth rate and did not increase their CFW sensitivity (Figure 5C), suggesting that Gup1p is not part of a multisubunit enzyme complex, but that it can act on its own.
Figure 5.
The gup1Δ cells are hypersensitive to calcofluor white. (A and B) Tenfold dilutions of the indicated strains harboring the indicated plasmids were spotted on plates containing 0 or 50 μg/ml calcofluor white (CFW). The plates were incubated at 30°C for 3 d. 2Δ gup is gup1Δ gup2Δ and 4Δ signifies the are1Δ are2Δ gup1Δ gup2Δ strain, and wt is BY4742. (C) BY4742 wt cells harboring the indicated plasmids were plated either on glucose (left) or galactose in the presence of CFW (right) and incubated at 30°C for 3 d.
The ER-to-Golgi Transport of Gas1p in gup1Δ Is Delayed
The vesicular transport of the GPI proteins Gas1p and Yps1p from the ER to the Golgi is dependent on ongoing sphingolipid biosynthesis (Horvath et al., 1994; Skrzypek et al., 1997; Sutterlin et al., 1997; Watanabe et al., 2002), and Gas1p has been found to be associated with detergent resistant membrane fractions in the ER (Bagnat et al., 2000). From these findings, it was proposed that Gas1p has to partition into rafts in order to be incorporated into transport vesicles. To study the transport of Gas1p, cells were pulsed with [35S]Cys/Met for 5 min and chased for 5, 10, 30, and 60 min. Gas1p was immunoprecipitated and analyzed by fluorography (Figure 6). In the wt strain the ratio between the ER form (105 kDa) and the Golgi form (125 kDa) is already ∼1:1 after 5 min of chase, whereas in gup1Δ mutant, the ER form is predominant up to 30 min of chase, indicating a delay in Gas1p transport. Immunoprecipitation of CPY from the same lysates showed that its maturation is not delayed in gup1Δ cells (Figure 6C).
Figure 6.
The transport of Gas1p from the ER to the Golgi is delayed in gup1Δ cells. (A) Cells were pulse-labeled with [35S]Cys/Met at 30°C and chased for the indicated times. Gas1p was immunoprecipitated and analyzed by SDS-PAGE/fluorography. (B) The fraction of mature 125-kDa Gas1p was obtained from the phosphorimager analysis of panel A, as well as a second, independent experiment, and was expressed as % of the total Gas1p (mature and immature). (C) CPY was similarly immunoprecipitated in lysates of panel A and was quantitated as Gas1p.
As shown in Figure 7A, the plasma membrane ATPase Pma1p was detergent insoluble in gup1Δ, indicating that classical raft-associated proteins remain raft associated. On the other hand, Gas1p of gup1Δ was entirely detergent soluble, whereas in the wt, ∼60% of Gas1p was detergent resistant, as expected (Watanabe et al., 2002). Moreover, Western blotting showed a massive accumulation of the immature 105-kDa ER form of Gas1p in gup1Δ (Figure 7A, lane 1), again indicating a delayed ER-to-Golgi transport of Gas1p. Western blotting with anti-CPY antibodies showed that there was no accumulation of immature p1 (ER) and p2 (Golgi) forms of CPY in gup1Δ (Figure 7A). Thus, pulse-chase and Western blot experiments point to an ER-to-Golgi transport problem that specifically affects Gas1p.
Figure 7.
Intracellular transport and sorting of GPI proteins in gup1Δ cells. (A) Cells were grown in YPD, harvested, and lysed using glass beads. The lysate was cleared of unbroken cells by centrifugation and extracted with 1% Triton X-100 for 30 min on ice. The samples were then centrifuged for 40 min at 15,000 × g to yield a detergent-resistant pellet (P) and a soluble (S) fraction. Proteins were then precipitated with TCA and analyzed by Western blotting using antibodies against the indicated proteins. (B) Cells were grown at 30°C to exponential phase, except for gup1Δ pre1-1 pre2-2 and gup1Δ cim5-1 thermosensitive strains that were grown at 24°C and incubated 2 h at 37°C. Proteins were extracted (Kushnirov, 2000) and analyzed by Western blotting using anti-Gas1p antibody. (C) Cells were grown at 30°C to exponential phase and harvested at a density (A600) of 1.6 and 1.98 for gup1Δ and BY4742, respectively. Proteins were extracted from the cells (Kushnirov, 2000), and secreted proteins were precipitated from the medium using TCA. Proteins were analyzed by Western blotting using anti-Gas1p antibody. (D) Cells harboring pBC322 (obtained from Brendan Cormack), a plasmid containing HA-tagged EPA1, were grown to exponential phase. Cells were broken with glass beads, the lysate was spun at 15,000 × g, and the pellet was first extracted by boiling in SDS (Frieman and Cormack, 2003). After extensive washing of SDS-insoluble cell walls, covalently attached cell wall proteins were liberated by β1,3-glucanase treatment as described (Frieman and Cormack, 2003). Cellular proteins extracted by boiling in SDS or cell wall proteins extracted with glucanase (GLU) were analyzed by Western blotting using Gas1p (top panel) or anti-HA antibody (bottom panel). Material from equivalent numbers of cells was loaded in lanes 1–4, whereas lanes 5 and 6 contained three times the amount of the material loaded in lanes 2 and 4, respectively (GLU 3×).
In several Western blot experiments there was relatively little mature Gas1p and the sum of all forms of Gas1p of gup1Δ seemed to be less than normal. Therefore we tried to see if part of the Gas1p of gup1Δ underwent either vacuolar or proteasomal degradation. As shown in Figure 7B, the genetic inactivation of these degradation pathways did not increase the amounts of mature and immature Gas1p of gup1Δ mutants. On the other hand, we found a significant amount of mature Gas1p in the medium (Figure 7C). The fact that only mature Gas1p was present in the medium indicated that this material did not originate from dying cells. Thus, it would appear that in gup1Δ cells, Gas1p, having either an unremodeled PI or a lyso-PI as its lipid moiety, is transported inefficiently out of the ER, is directed in the trans-Golgi toward the plasma membrane, not the vacuole, and, upon arrival at the plasma membrane, is lost, at least partially, into the medium. The residual mature Gas1p nevertheless seems to be functionally important for gup1Δ, because the gup1Δ gas1Δ double mutant is very sick (Schuldiner et al., 2005).
Because a part of Gas1p is covalently associated with the cell wall (De Sampaio et al., 1999; Yin et al., 2005), we wondered if the secretion of Gas1p reflects a defect in the cell wall integration of GPI proteins. This integration is probably operated by Dfg5p and/or Dcw1p, the enzymes proposed to transfer GPI proteins from GlcN-PI onto β1,6-glucans, thus incorporating them into the cell wall (Lu et al., 1995; Kollar et al., 1997; Kitagaki et al., 2002). To investigate this issue, we introduced into gup1Δ cells EPA1, an HA-tagged GPI protein that is displayed at the cell surface (Frieman and Cormack, 2003). As shown in Figure 7D, the amount of EPA1 that could be liberated only by β-glucosidase treatment was comparable in wt and gup1Δ cells (lanes 2 vs. 4 and 5 vs. 6). On the other hand, the fraction of glucan-linked Gas1p was much higher in wt than in gup1Δ cells (lanes 5 vs. 6). Thus, gup1Δ cells still incorporate EPA1, but not Gas1p into the cell wall. It is conceivable that in gup1Δ cells, Gas1p mainly contains a lyso-PI, whereas EPA1 contains some atypical base-resistant lipid anchor and that this would be decisive. Further experiments are required to analyze the anchor lipids of these proteins and to see if the incorporation of GPI proteins into the cell wall is dependent on their anchor lipid moiety.
GUP1 Homologues from T. cruzi and A. fumigatus Partially Complement the Defect of gup1Δ Cells
The Blast link for GUP1 at NCBI lists homologues in all phyla except for archaea, but curiously, the closest homologues of GUP1 are all of fungal origin (scores between 1921 and 887), the next closest homologues are from D. discoideum or protozoa (T. cruzi, T. brucei, L. major; scores from 765 to 366), and the next closest homologues are all metazoan (scores from 254 to 181). This latter class also contains mammalian homologues. The relatively strong conservation of GUP1 among fungi and protozoa is also seen in the alignment shown in Figure 8A. Of the 79 residues conserved among fungal GUP1 homologues, 66 were located in the MBOAT region. On the other hand, conventional alignment programs failed to correctly align the MBOAT motif and active site histidine of metazoan homologues (Danio, Tetraodon, Rattus, Canis, Xenopus, Mus, and Anopheles) with yeast GUP1. Expression of GUP1 homologues of T. cruzi and A. fumigatus significantly increased the CFW resistance of gup1Δ cells (Figure 8B). As judged by Western blotting, they failed to reduce the abnormally high amounts of the immature Gas1p form, but an increase of the mature form of Gas1p was noted in cells expressing afGUP1 (Figure 8C) so that the ratio of immature/total Gas1p was significantly lower in afGUP1-complemented gup1Δ than in the empty vector control (Figure 8D). Expression of tcGUP1 and afGUP1 also significantly reduced the secretion of Gas1p (Supplementary Figure S3). The analysis of the anchor lipids showed that the expression of GUP1 homologues of T. cruzi and A. fumigatus in gup1Δ cells reduced the fraction of lipids in the region of lyso-PI but did not restore synthesis of pG1 (unpublished data).
Figure 8.
GUP1 homologues of T. cruzi and A. fumigatus partially complement gup1Δ cells. (A) The amino acid sequence of S. cerevisiae Gup1p is shown with its putative transmembrane domains (black lines, obtained with the TMHMM Server, v. 2.0 at http://www.cbs.dtu.dk/services/TMHMM/), the MBOAT motif (gray line, aa 271-510), and the predicted Histidine 447 active site (*). The closest homologues, namely 17 fungal and 13 protozoan sequences were aligned, each phylogentic group separately, with scGup1p using Clustalv W, and identities and similarities within each alignment were indicated with stars and points below the Gup1p sequence. (B) Calcofluor white resistance of BY4742 wt and gup1Δ cells was assayed as in Figure 5B. (C) Proteins of wt or gup1Δ mutants harboring different plasmids were extracted and analyzed in Western blots for the presence of Gas1p and Wbp1p. (D) Quantification of immature Gas1p (as a percentage of total Gas1p) in four independent experiments, one being the one shown in C. The Student's t test was utilized to compare the values obtained for gup1Δ cells harboring the empty vector with the ones obtained for gup1Δ cells harboring other plasmids: ∗p = 0.40; ∗∗p = 0.0034; ∗∗∗p = 0.188.
At least 127 nonessential genes belonging to 11 different functional classes are required for the maintenance of the typical bipolar bud site selection of diploid cells (Ni and Snyder, 2001). Among them is GUP1. We found that gup1Δ/gup1Δ cells indeed mostly budded randomly and that GUP1 homologues of T. cruzi and A. fumigatus were able to partially restore bipolar bud site selection (Figure 9). Thus, GUP1 of T. cruzi and A. fumigatus seem to be true orthologues of yeast GUP1, but they only partially can complement the phenotype of gup1Δ cells.
Figure 9.
GUP1 homologues of T. cruzi and A. fumigatus partially complement the bud site selection defect of gup1Δ/gup1Δ cells. The indicated diploid strains were grown exponentially for 24 h, stained with CFW, and viewed under the microscope. Two hundred cells present on randomly taken pictures were analyzed for each strain. The percentage of cells showing the normal bipolar budding pattern is indicated on the right side.
DISCUSSION
The lipids found on GPI anchors of different organisms are very often not representative of the lipids present on the PI, on which the GPI structure is initially built. Lipid moieties may be changed either before or after the addition of the GPI to the nascent proteins. The best studied case for the former scenario is the GPI remodeling of the blood form of T. brucei, where both of the primary fatty acids are exchanged for myristate through sequential enzymatic steps leading from lipid A′ via the lyso form θ to A″ and furthermore to the final A form, which is attached to proteins (Masterson et al., 1990). The remodeling occurring in S. cerevisiae is different in that the lipids are exchanged only once the GPI has been added to the GPI protein. Yet, the Gup1p-mediated remodeling reaction leading from PI to pG1 may formally be similar to the first reaction occurring in T. brucei, i.e., the replacement of a fatty acid in sn2 (Masterson et al., 1990).
Our present model for anchor lipid remodeling is that an unknown lipase first removes the acyl in sn2, that Gup1p adds the C26:0 to generate pG1, and that the thus remodeled anchor then can be further remodeled through an exchange of diacylglycerol for ceramide or phosphatidic acid for Cer-1-phosphate. This implies that the remodeling reaction operated by Gup1p greatly facilitates the introduction of PHS-C26:0 and PHS-C26:0-OH ceramides into the anchor, in that Gup1p produces the substrate for the still hypothetical remodelases that introduce PHS-C26:0 and PHS-C26:0-OH ceramides. (Gup1p-mediated remodeling may however not be an absolute requirement for the introduction of PHS-C26:0, because in 1 of 8 experiments we still could detect significant amounts of IPC/B-type anchor lipids in gup1Δ cells.)
The only findings that are not predicted by this model are the fact that gup1Δ cells contain reduced amounts of IPC/D and that they still contain a minor base-resistant anchor lipid that is not found in wt. Although these two phenomena may be secondary to the absence of a normal remodeling process, we cannot at present exclude other models to explain the importance of GUP1 for lipid remodeling. One such model would say that Gup1p may be involved in the ordinary glycerophospholipid biosynthesis and act as a 1-acyl-glycerol-3-phosphate acyltransferase able to add not only C16 and C18 but also C26:0 into sn2. Indeed such an sn2-specific acyltransferase may exist in yeast, because the only other gene known to perform the same reaction, SLC1, is not essential. If we thus assume that Gup1p acts as sn2 acyltransferase to produce phosphatidic acid, the normal remodeling of GPI anchors from PI to pG1 would have to be a phospholipase C (PLC) or D (PLD)-mediated exchange of the primary GPI anchor lipid for a C26:0-containing lipid, and the observed appearance of lyso-PI on the GPI anchors would then have to be explained as a subsidiary hydrolytic reaction that only takes place when the cells cannot make the appropriate C26:0-containing lipid. Although this model is valuable, it also would not explain the reduced amounts of IPC/D in gup1Δ and several other findings render it less likely: 1) an exhaustive screen including all yeast strains deleted in genes having homology to PLC or PLD has not revealed any mutant having a problem with anchor remodeling (unpublished results). 2) The gup1Δ slc1Δ double mutant is perfectly viable (unpublished data). 3) Overexpression of SLC1-1, a gain-of-function allele of SLC1 able to transfer C26:0 to sn2 (Nagiec et al., 1993) does not relieve the calcofluor white hypersensitivity of gup1Δ (Supplementary Figure S4A), even in the presence of low amounts of myriocin, which, by blocking the sphingolipid biosynthesis, deviates C26-CoA toward other acyltransferases. Also, overexpression of SLC1-1 does not correct the remodeling defect of gup1Δ cells (Supplementary Figure S4B). 4) Overexpression of GUP1 cannot rescue the thermosensitivity of lcb1-100 cells (Supplementary Figure S5).
A further question raised by our findings concerns the nature of the base-resistant lipid attached to GPI anchors in gup1Δ cells (Figure 3, lipid marked by asterisk). This lipid may consist of an unusual ceramide, a long-chain base, or a lyso-alkyl-glycerol.
The gup1Δ null mutation shows a synthetic sick interaction with CHS3, CHS5, and CHS6, genes required for chitin synthesis in a synthetic genetic array (SGA) analysis, whereas no such synthetic effect of chsΔ alleles with gup2Δ, are1Δ, are2Δ, or yor175wΔ were noted (Lesage et al., 2005). The specific role of Gup1p in GPI anchor remodeling explains these synthetic sick interactions between the cshΔ and gup1Δ deletion mutations.
GPI anchoring seems to be important for the maintenance of the typical bipolar budding pattern of diploid cells. This may not have been appreciated earlier, because most genes involved in GPI anchor biosynthesis or anchor processing are essential. However, GPI7, BST1, and GUP1 are nonessential, GPI-related genes, and all of them are important for bud site selection. The same is true for GAS1 and CCW12 encoding GPI proteins (Ni and Snyder, 2001). Moreover, bipolar bud site selection is deficient in four genes involved in lipid biosynthesis, namely ELO2, ELO3, ERG3, and ERG4, the former two of which also affect GPI anchoring, because they affect biosynthesis of C26 (Ni and Snyder, 2001). Other GPI proteins may not have been identified in the screen of Ni and Snyder because they are redundant.
A previous large-scale screen for CPY secretion showed that GUP1 is involved in trafficking of CPY, Pep4p, and Pho8p to the vacuole and that gup1Δ cells display vacuolar protein sorting class C mutant vacuolar morphologies (Bonangelino et al., 2002). Abnormal secretion of CPY by gup1Δ was confirmed (Supplementary Figure S6), and our data suggest that the defect in vacuolar sorting does not cause abnormal accumulation of the Golgi p2 from of CPY (Figure 7A).
It seems quite possible that the anchor remodeling of S. cerevisiae is paradigmatic for many other fungi and protozoans. Although the MBOAT motif occurs in a large variety of acyl-CoA–dependent acyltransferases dedicated to lipid biosynthesis in bacteria and eukaryotes, many fungi and protozoan organisms contain GUP1 homologues having a high degree of homology to yeast GUP1 and containing small homology regions also outside the MBOAT motif (Figure 8A). Indeed, GUP1 homologues of T. cruzi and A. fumigatus could partially rescue the CFW hypersensitivity, the loss of Gas1p from the cell surface, and the bud site selection defect of gup1Δ cells (Figures 8 and 9 and Supplementary Figure S3). HA-afGUP1 was more efficient than HA-tcGUP1 in this respect (Figure 8), although HA-tcGUP1 could be expressed to higher levels than HA-afGUP1 (unpublished data). T. cruzi contains both alkylacylglycerol- as well as ceramide-based GPI anchors, whereas A. fumigatus only contains the latter type (Fontaine et al., 2003; Ferguson et al., 2006). The alkylacylglycerol anchors of T. cruzi contain C16 and C18 fatty acids in sn2 (Ferguson et al., 2006), and we thus can speculate that tcGUP1 expression in gup1Δ cells may not allow adding C26:0 acids in sn2 as does yeast GUP1, but that it only may alter the proportions between lyso-PI and PI anchors. Recent studies show that, similar to yeast, the first steps of GPI biosynthesis in T. cruzi do not use ceramide as the lipid support, suggesting that ceramide is added by remodeling of protein-bound anchors also in T. cruzi (Bertello et al., 2004). Our study raises the possibility that in both, T. cruzi and A. fumigatus the addition of the normal set of ceramides would be dependent on a foregoing remodeling by GUP1. GUP1 homologues are also found in other organisms that contain ceramide anchors such as certain plants, Paramecium, and Dictyostelium (Ferguson et al., 2006). Thus, a remodeling step catalyzed by a GUP1 homologue may be occurring in many organisms, even if their sole anchor lipid is a ceramide.
Interestingly, a recent report shows that mammalian GPI anchors seem to be remodeled and undergo a deacylation–reacylation cycle in the Golgi, mediated by PGAP2, an enzyme that is completely unrelated to GUP1 (Tashima et al., 2006). This suggests that GPI lipid remodeling may be a common event during the transport of GPI proteins to the surface.
Supplementary Material
ACKNOWLEDGMENTS
We thank Stephen Sturley, Morten Kielland-Brandt, Marja Makarow, Michel Monod, Reto Brun, Roger Schneiter, and their lab members for strains, antibodies, cDNA libraries, DNA, and plasmids. We also acknowledge the excellent technical help provided by Christine Vionnet, Mélanie Bapst, and Joel Robert. This work was supported by a grant from the Swiss National Science Foundation (31-67188.01).
Abbreviations used:
- Cer
ceramide
- CFW
calcofluor white
- DHS
dihydrosphingosine
- GPI
glycosylphosphatidylinositol
- IPC
inositolphosphoceramide
- MIPC
mannosylated IPC
- PHS
phytosphingosine
- PI
phosphatidylinositol
- TLC
thin-layer chromatography
- wt
wild type.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-02-0104) on April 5, 2006.
REFERENCES
- Bagnat M., Keranen S., Shevchenko A., Shevchenko A., Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertello L. E., Alves M. J., Colli W., de Lederkremer R. M. Inositolphosphoceramide is not a substrate for the first steps in the biosynthesis of glycoinositolphospholipids in Trypanosoma cruzi. Mol. Biochem. Parasitol. 2004;133:71–80. doi: 10.1016/j.molbiopara.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Bonangelino C. J., Chavez E. M., Bonifacino J. S. Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol. Biol. Cell. 2002;13:2486–2501. doi: 10.1091/mbc.02-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A. Biosynthesis, remodeling and targeting of GPI proteins in the yeast Saccharomyces cerevisiae. In: Daum G., editor. Cell Biology and Dynamics of Yeast Lipids. Trivandrum, India: Research Signpost; 2005. pp. 133–159. [Google Scholar]
- De Sampaio G., Bourdineaud J. P., Lauquin G. J. A constitutive role for GPI anchors in Saccharomyces cerevisiae: cell wall targeting. Mol. Microbiol. 1999;34:247–256. doi: 10.1046/j.1365-2958.1999.01585.x. [DOI] [PubMed] [Google Scholar]
- Fankhauser C., Homans S. W., Thomas-Oates J. E., McConville M. J., Desponds C., Conzelmann A., Ferguson M. A. Structures of glycosylphosphatidylinositol membrane anchors from Saccharomyces cerevisiae. J. Biol. Chem. 1993;268:26365–26374. [PubMed] [Google Scholar]
- Ferguson M.A.J., Kinoshita T., Hart G. W. Glycophospholipid anchors. In: Varki A., Bertozzi C., Cummings R., Etzler M., Esko J., Freeze H., Hart G., Stanley P., editors. Essential Glycobiology. 2nd ed. 2006. (in press) [Google Scholar]
- Fontaine T., Magnin T., Melhert A., Lamont D., Latge J. P., Ferguson M. A. Structures of the glycosylphosphatidylinositol membrane anchors from Aspergillus fumigatus membrane proteins. Glycobiology. 2003;13:169–177. doi: 10.1093/glycob/cwg004. [DOI] [PubMed] [Google Scholar]
- Frieman M. B., Cormack B. P. The omega-site sequence of glycosylphosphatidylinositol-anchored proteins in Saccharomyces cerevisiae can determine distribution between the membrane and the cell wall. Mol. Microbiol. 2003;50:883–896. doi: 10.1046/j.1365-2958.2003.03722.x. [DOI] [PubMed] [Google Scholar]
- Gaigg B., Timischl B., Corbino L., Schneiter R. Synthesis of sphingolipids with very long chain fatty acids but not ergosterol is required for routing of newly synthesized plasma membrane ATPase to the cell surface of yeast. J. Biol. Chem. 2005;280:22515–22522. doi: 10.1074/jbc.M413472200. [DOI] [PubMed] [Google Scholar]
- Guillas I., Pfefferli M., Conzelmann A. Analysis of ceramides present in glycosylphosphatidylinositol anchored proteins of Saccharomyces cerevisiae. Methods Enzymol. 2000;312:506–515. doi: 10.1016/s0076-6879(00)12935-0. [DOI] [PubMed] [Google Scholar]
- Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem. Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- Holst B., Lunde C., Lages F., Oliveira R., Lucas C., Kielland-Brandt M. C. GUP1 and its close homologue GUP2, encoding multimembrane-spanning proteins involved in active glycerol uptake in Saccharomyces cerevisiae. Mol. Microbiol. 2000;37:108–124. doi: 10.1046/j.1365-2958.2000.01968.x. [DOI] [PubMed] [Google Scholar]
- Horvath A., Sutterlin C., Manning-Krieg U., Movva N. R., Riezman H. Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J. 1994;13:3687–3695. doi: 10.1002/j.1460-2075.1994.tb06678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof I., Flury I., Vionnet C., Roubaty C., Egger D., Conzelmann A. Glycosylphosphatidylinositol (GPI) proteins of Saccharomyces cerevisiae contain ethanolamine phosphate groups on the alpha1,4-linked mannose of the GPI anchor. J. Biol. Chem. 2004;279:19614–19627. doi: 10.1074/jbc.M401873200. [DOI] [PubMed] [Google Scholar]
- Jansen G., Wu C., Schade B., Thomas D. Y., Whiteway M. Drag&Drop cloning in yeast. Gene. 2005;344:43–51. doi: 10.1016/j.gene.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Inoue N. Dissecting and manipulating the pathway for glycosylphosphatidylinositol-anchor biosynthesis. Curr. Opin. Chem. Biol. 2000;4:632–638. doi: 10.1016/s1367-5931(00)00151-4. [DOI] [PubMed] [Google Scholar]
- Kitagaki H., Wu H., Shimoi H., Ito K. Two homologous genes, DCW1 (YKL046c) and DFG5, are essential for cell growth and encode glycosylphosphatidylinositol (GPI)-anchored membrane proteins required for cell wall biogenesis in Saccharomyces cerevisiae. Mol. Microbiol. 2002;46:1011–1022. doi: 10.1046/j.1365-2958.2002.03244.x. [DOI] [PubMed] [Google Scholar]
- Kollar R., Reinhold B. B., Petrakova E., Yeh H. J., Ashwell G., Drgonova J., Kapteyn J. C., Klis F. M., Cabib E. Architecture of the yeast cell wall. Beta(1→6)-glucan interconnects mannoprotein, beta(1→3)-glucan, and chitin. J. Biol. Chem. 1997;272:17762–17775. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- Kushnirov V. V. Rapid and reliable protein extraction from yeast. Yeast. 2000;16:857–860. doi: 10.1002/1097-0061(20000630)16:9<857::AID-YEA561>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lesage G., Shapiro J., Specht C. A., Sdicu A. M., Menard P., Hussein S., Tong A. H., Boone C., Bussey H. An interactional network of genes involved in chitin synthesis in Saccharomyces cerevisiae. BMC Genet. 2005;6:8. doi: 10.1186/1471-2156-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. F., Montijn R. C., Brown J. L., Klis F., Kurjan J., Bussey H., Lipke P. N. Glycosyl phosphatidylinositol-dependent cross-linking of alpha-agglutinin and beta 1,6-glucan in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 1995;128:333–340. doi: 10.1083/jcb.128.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson W. J., Raper J., Doering T. L., Hart G. W., Englund P. T. Fatty acid remodeling: a novel reaction sequence in the biosynthesis of trypanosome glycosyl phosphatidylinositol membrane anchors. Cell. 1990;62:73–80. doi: 10.1016/0092-8674(90)90241-6. [DOI] [PubMed] [Google Scholar]
- Miyata T., Takeda J., Iida Y., Yamada N., Inoue N., Takahashi M., Maeda K., Kitani T., Kinoshita T. The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science. 1993;259:1318–1320. doi: 10.1126/science.7680492. [DOI] [PubMed] [Google Scholar]
- Morita Y. S., Acosta-Serrano A., Buxbaum L. U., Englund P. T. Glycosyl phosphatidylinositol myristoylation in African trypanosomes. New intermediates in the pathway for fatty acid remodeling. J. Biol. Chem. 2000;275:14147–14154. doi: 10.1074/jbc.275.19.14147. [DOI] [PubMed] [Google Scholar]
- Nagiec M. M., Wells G. B., Lester R. L., Dickson R. C. A suppressor gene that enables Saccharomyces cerevisiae to grow without making sphingolipids encodes a protein that resembles an Escherichia coli fatty acyltransferase. J. Biol. Chem. 1993;268:22156–22163. [PubMed] [Google Scholar]
- Neves L., Lages F., Lucas C. New insights on glycerol transport in Saccharomyces cerevisiae. FEBS Lett. 2004;565:160–162. doi: 10.1016/j.febslet.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Ni L., Snyder M. A genomic study of the bipolar bud site selection pattern in Saccharomyces cerevisiae. Mol. Biol. Cell. 2001;12:2147–2170. doi: 10.1091/mbc.12.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner M., et al. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell. 2005;123:507–519. doi: 10.1016/j.cell.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- Sipos G., Reggiori F., Vionnet C., Conzelmann A. Alternative lipid remodelling pathways for glycosylphosphatidylinositol membrane anchors in Saccharomyces cerevisiae. EMBO J. 1997;16:3494–3505. doi: 10.1093/emboj/16.12.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypek M., Lester R. L., Dickson R. C. Suppressor gene analysis reveals an essential role for sphingolipids in transport of glycosylphosphatidylinositol-anchored proteins in Saccharomyces cerevisiae. J. Bacteriol. 1997;179:1513–1520. doi: 10.1128/jb.179.5.1513-1520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterlin C., Doering T. L., Schimmoller F., Schroder S., Riezman H. Specific requirements for the ER to Golgi transport of GPI-anchored proteins in yeast. J. Cell Sci. 1997;110:2703–2714. doi: 10.1242/jcs.110.21.2703. [DOI] [PubMed] [Google Scholar]
- Tashima Y., Taguchi R., Murata C., Ashida H., Kinoshita T., Maeda Y. PGAP2 is essential for correct processing and stable expression of GPI-anchored proteins. Mol. Biol. Cell. 2006;17:1410–1420. doi: 10.1091/mbc.E05-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Funato K., Venkataraman K., Futerman A. H., Riezman H. Sphingolipids are required for the stable membrane association of glycosylphosphatidylinositol-anchored proteins in yeast. J. Biol. Chem. 2002;277:49538–49544. doi: 10.1074/jbc.M206209200. [DOI] [PubMed] [Google Scholar]
- Yin Q. Y., de Groot P. W., Dekker H. L., de Jong L., Klis F. M., de Koster C. G. Comprehensive proteomic analysis of Saccharomyces cerevisiae cell walls: identification of proteins covalently attached via glycosylphosphatidylinositol remnants or mild alkali-sensitive linkages. J. Biol. Chem. 2005;280:20894–20901. doi: 10.1074/jbc.M500334200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.