Abstract
Cyclooxygenase-2 (COX-2), a prostanoid-synthesizing enzyme that contributes to the toxicity associated with inflammation, has recently emerged as a promising therapeutic target for several illnesses, ranging from osteoarthritis to Alzheimer's disease. Although COX-2 has also been linked to ischemic stroke, its role in the mechanisms of ischemic brain injury remains controversial. We demonstrate that COX-2-deficient mice have a significant reduction in the brain injury produced by occlusion of the middle cerebral artery. The protection can be attributed to attenuation of glutamate neurotoxicity, a critical factor in the initiation of ischemic brain injury, and to abrogation of the deleterious effects of postischemic inflammation, a process contributing to the secondary progression of the damage. Thus, COX-2 is involved in pathogenic events occurring in both the early and late stages of cerebral ischemia and may be a valuable therapeutic target for treatment of human stroke.
Keywords: middle cerebral artery occlusion, prostanoids, cerebral blood flow, NS398, stroke
Stroke remains a major cause of death and disability worldwide (1–4). After many years of setbacks, effective treatments for stroke, based on thrombolysis and restoration of flow, have been developed (5, 6). However, these therapies can be safely administered only in those patients who reach medical attention during the first few hours after the onset of the stroke (7, 8). Therefore, it would be important to develop treatments that target downstream events in the ischemic cascade. Furthermore, it would be desirable to combine thrombolytic therapy with other therapeutic strategies aimed at protecting the brain from the residual ischemia (9). Immediately after induction of ischemia, activation of glutamate receptors initiates the ischemic cascade and contributes to the damage that occurs in the early stages of cerebral ischemia (refs. 10 and 11; see ref. 12 for a review). At later times after ischemia, the tissue damage continues to evolve (13), and inflammation and programmed cell death are thought to be major factors in the progression of the injury (refs. 14–17; see ref. 18 for a review).
Cyclooxygenase (COX)-1 and COX-2 are enzymes involved in the first step of the synthesis of prostanoids (see ref. 19 for a review). COX-1 is expressed constitutively in many organs and contributes to the synthesis of prostanoids involved in normal cellular functions. COX-2 is thought to be an inducible enzyme whose expression is up-regulated in pathological states, most notably those associated with inflammation (see ref. 20 for a review). There is substantial evidence that COX-2 reaction products are responsible for cytotoxicity in models of inflammation (see ref. 21 for a review). Thus, COX-2 inhibitors are potent antiinflammatory agents and have recently been introduced in clinical practice with notable success (22, 23).
In brain, COX-2 is present in selected neurons (24–26), and its expression is up-regulated in several neurological diseases, including stroke, Alzheimer's dementia, and seizures (24, 27–29). In rodents as in humans, cerebral ischemia up-regulates COX-2 in neurons, blood vessels, and inflammatory cells infiltrating the injured brain (27, 28, 30). However, experimental evidence linking COX-2 to the mechanisms of brain injury associated with these conditions is lacking. For example, although it is well established that cerebral ischemia increases COX-2 expression in the damaged brain, studies using COX inhibitors in models of focal cerebral ischemia have yielded conflicting results (28, 31, 32). Furthermore, studies using COX-2 inhibitors cannot exclude effects unrelated to COX-2, such as modification of gene expression or activation of peroxisome proliferator-activated receptors (33, 34). In view of the increasing use of COX-2 inhibitors as antiinflammatory agents in the elderly—a population with an increased risk for stroke—it would be of great interest to define the role of COX-2 in ischemic brain injury.
Mice with a null mutation of the COX-2 gene have been a useful model for investigating the role of COX-2 in systemic inflammation, thermoregulation, and cerebrovascular regulation (refs. 35 and 36; see ref. 37 for a review). In the present study, therefore, we used COX-2-deficient mice to gain further insight into the role of COX-2 in ischemic brain injury.
Methods
Animals.
COX-2-null mice were obtained from a colony established at the University of Minnesota (35, 36). Mice (SV129 × C57BL/6J) were back-crossed to C57BL/6J mice five or six times and were studied at age 2–3 months. Experiments were performed in age-matched littermates [wild-type (+/+), heterozygous (+/−), and homozygous (−/−)] to minimize confounding effects deriving from the genetic background of the mice. The genotype of all COX-2 mice was determined by PCR with the use of primers and methods described previously (35). No alterations in the anatomy of large cerebral vessels were noticed in COX-2-null mice. C57BL/6J mice were obtained from The Jackson Laboratory.
Induction of Focal Cerebral Ischemia.
Focal cerebral ischemia was produced by occlusion of the middle cerebral artery (MCA) (38). Mice were anesthetized with 2% halothane/100% oxygen. Body temperature was maintained at 37 ± 0.5°C by a thermostatically controlled infrared lamp. A 2-mm hole was drilled in the inferior portion of the temporal bone to expose the left MCA. The MCA was elevated and cauterized distal to the origin of the lenticulostriate branches. Mice in which the MCA was exposed but not occluded served as sham-operated controls. Wounds were sutured and mice were allowed to recover and were returned to their cages. Rectal temperature was controlled until mice regained full consciousness. Thereafter, rectal temperature was measured daily until the time of sacrifice. There were no major differences in rectal temperature among COX-2 +/+, +/−, and −/− mice before MCA occlusion or after ischemia. For example, 48 h after MCA occlusion rectal temperature was 36.2 ± 0.1°C in COX-2 +/+, 36.6 ± 0.1 in +/−, and 36.0 ± 0.2 in −/− mice (P > 0.05, analysis of variance).
Reverse Transcription–PCR (RT-PCR).
mRNA for COX-2 was detected by RT-PCR as previously described (28). Mice were killed 24 h after ischemia (n = 4 per time point), and their brains were removed. This time point was chosen because COX-2 mRNA expression reaches a plateau 24 h after MCA occlusion in mice (39). A 4-mm-thick coronal brain slice was cut at the level of the optic chiasm, and samples including the infarcted cortex and the corresponding area of the contralateral cortex were collected and frozen in liquid nitrogen. Total RNA was extracted from the tissue according to the method of Chomczynski and Sacchi (40). RNA integrity was determined on denaturing formaldehyde gels. Aliquots of total RNA (0.25 μg) were used in the RT reaction mixed with 0.5 μg of oligo(dT) primer as directed (18-mer; New England Biolabs). First-strand cDNA synthesis was then carried out with the use of Moloney murine leukemia virus reverse transcriptase (New England Biolabs) according to the manufacturer's instructions. After heating at 95°C for 10 min, 5 μl from each RT reaction mixture was used for PCR amplification. Primers (0.2 μM each) for the sequence of interest and for porphobilinogen deaminase (PBD), a ubiquitously expressed sequence, were used in a final volume of 50 μl. COX-2 primers were as follows: forward, 5′-CCAGATGCTATCTTTGGGGAGAC-3′; reverse, 5′-GCTTGCATTGATGGTGGCTG-3′, which result in a PCR product of 249 bp. COX-1 primers were as follows: forward, 5′-GAATACCGAAAGAGGTTTGGCTTG-3′; reverse, 5′-TCATCTCCAGGGTAATCTGGCAC-3′, which yields a 374-bp product. PBD primers were as follows: forward, 5′-GCCACCACAGTCTCGGTCTGTATGCGAGC-3′; reverse, 5′-TGTCCGGTAACGGCGGCGCGGCCACAAC-3′. The “hot start” method was used with the following cycle parameters: 94°C, 15 s; 68°C, 30 s; 73°C, 20 s, for five cycles, then 94°C, 15 s; 64°C, 30 s; 73°C, 20 s, for 35 cycles; and 73°C, 15 min. Reaction products were then separated on an 8% polyacrylamide gel, stained with ethidium bromide, and photographed. Each set of PCRs included control samples run without RNA or in which the RT step was omitted to ensure that PCR products resulted from amplification from the COX-2 mRNA rather than genomic DNA. The OD of the bands was determined by a gel image analysis system (Molecular Analyst; Bio-Rad). In some studies, measurements were normalized to the OD of the PBD band used as an internal standard (28, 41).
Competitive RT-PCR was used to determine more accurately the magnitude of mRNA induction (28). A deletion construct was synthesized that consisted of the same sequence amplified from the endogenous COX-2 message but missing an internal 79-nucleotide fragment. To generate the construct, a pair of COX-2 primers was prepared: forward: 5′-CCAGATGCTATCTTTGGGGAGAC-3′; reverse: 5′-GCTTGCATTGATGGTGGCTG-3′, which result in a 249-bp PCR product. The PCR product was then digested with a restriction enzyme, Sau3AI (New England Biolabs). The sample was then religated at 14°C overnight with T4 DNA ligase (New England Biolabs), and the ligase was inactivated at 65°C for 15 min. A 1-μl aliquot of the religated sample was then amplified with the use of the COX-2 forward and reverse primers described above. Products were separated on a polyacrylamide gel, and a main product of 170 bp was excised from the gel. The construct was eluted from the crushed gel with T.E. buffer (10 mM Tris/1 mM EDTA, pH 8.0) at 55°C for 4 h and purified for use in the competition assay. The RT reaction mixtures (5 μl each) of the animals killed 24 h after ischemia (n = 4 per group) were coamplified with known amounts of deletion construct (0.25–25 fg). The PCR products were then separated on a gel, and the gel was stained with ethidium bromide and photographed. The OD of the bands was determined by image analysis. For data analysis, the log of the OD ratio (COX-2/construct) was plotted as a function of the log of the concentration of the construct and fitted by linear regression analysis (28, 42). The 0 value of the log of the ratio (COX-2/construct) (y axis) represents the point at which the COX-2 PCR product and the construct are present in equal amounts. Therefore, the amount of the construct corresponding to the 0 ratio (x axis) represents the amount of the COX-2 PCR product before PCR amplification (42, 43).
Prostaglandin E2 (PGE2) Enzyme Immunoassay.
Tissue concentration of PGE2, a COX reaction product, was measured 24 h after MCA occlusion or 3 h after N-methyl-d-aspartate (NMDA) injection. PGE2 concentration was determined in the cerebral cortex ipsilateral and contralateral to the lesion with the use of an enzyme immunoassay kit (Cayman Chemicals, Ann Arbor, MI) as previously described (28). Prostanoids were extracted with 100% methanol according to the method of Powell (see ref. 28), and the PGE2 concentration was determined spectrophotometrically according to the instructions provided with the kit.
NMDA Microinjection in Neocortex.
In halothane-anesthetized mice the dura overlying the parietal cortex was exposed, and NMDA (20 nmol in 200–300 nl of sterile 0.1 M PBS, pH 7.4) was injected with a glass micropipette (tip 40–50 μm) connected to a microinjection device. The micropipette was inserted into the parietal cortex at a site 1.5 mm caudal to bregma, 4.0 mm from the midline, and 0.8 mm below the dural surface. The micropipette was left in place for 10 min, to minimize back-flux of NMDA, and then removed. Mice were returned to their cages and allowed to survive for 3 h for PGE2 measurement and for 24 h for determination of lesion volume.
Determination of Lesion Volume.
Mice were killed 1 and 4 days after MCA occlusion or 1 day after NMDA injection. Brains were removed and frozen in cooled isopentane (−30°C). Coronal forebrain sections (thickness = 30 μm) were serially cut in a cryostat, collected at 90-μm intervals, and stained with thionin for determination of lesion volume by an image analyzer (MCID; Imaging Research, St. Catherine's, ON, Canada; ref. 38). In studies of cerebral ischemia, infarct volume in cerebral cortex was corrected for swelling to factor out the contribution of ischemic edema to the total volume of the lesion (38, 44).
Monitoring of Cerebral Blood Flow (CBF).
Techniques used for monitoring CBF after MCA occlusion have been published (38). Mice were anesthetized with halothane (maintenance 1%), and the femoral artery and trachea were cannulated. Mice were artificially ventilated with an oxygen–nitrogen mixture by a mechanical ventilator (SAR-830; CWE, Ardmore, PA). The oxygen concentration in the mixture was adjusted to maintain arterial partial O2 pressure between 120 and 140 mmHg. End-tidal CO2 was continuously monitored with a CO2 analyzer (Capstar-100; CWE) (38). CBF was monitored with two laser-Doppler flow probes (Vasamedic, Minneapolis, MN) placed through burr holes drilled in the center (3.5 mm lateral to the midline and 1 mm caudal to bregma) and the periphery (1.5 mm lateral to the midline and 1.7 mm rostral to lambda) of the ischemic territory (38). The location of the probe was selected in preliminary experiments to correspond to the region of brain that is spared from infarction in COX-2-deficient mice. After placement of the probes, the MCA was occluded and CBF was monitored for 90 min. CBF data are expressed as a percentage of the preocclusion value. Arterial pressure and blood gases did not differ between COX-2 +/+ and −/− mice (10 min after MCA occlusion: COX-2 +/+: arterial pressure: 83 ± 3 mmHg; paCO2: 34.6 ± 0.6 mmHg; arterial partial O2 pressure: 125 ± 6 mmHg; pH: 7.35 ± 0.02; COX-2 −/−: arterial pressure: 84 ± 2; paCO2: 33.1 ± 0.8; arterial partial O2 pressure: 126 ± 5; pH: 7.33 ± 0.02).
Renal Function.
Because COX-2-null mice develop renal disease (35), plasma creatinine was measured by standard colorimetric methods. Furthermore, histological analysis of the kidneys was performed in paraffin-embedded sections stained with hematoxylin and eosin. Creatinine did not differ between COX-2 +/+ (0.18 ± 0.07 mg/dl; n = 6) and +/− mice (0.18 ± 0.05; n = 6) (P > 0.05 from COX-2 +/+). In COX-2 −/− mice (n = 6), creatinine was elevated (0.43 ± 0.08; P < 0.05 from COX-2 +/+) but still in the normal range (45). COX-2 −/− mice had hypertrophy of the juxtaglomerular apparatus and macula densa but no alterations in the glomeruli or tubules.
Data Analysis.
Data in the text and figures are expressed as means ± SEM. Multiple comparisons were evaluated statistically by the analysis of variance and Tukey's test. Two-group comparisons were analyzed by the two-tailed Student's t test for independent samples. For all procedures, probability values of less than 0.05 were considered statistically significant.
Results
Postischemic COX-2 Expression Is Attenuated in COX-2-Null Mice.
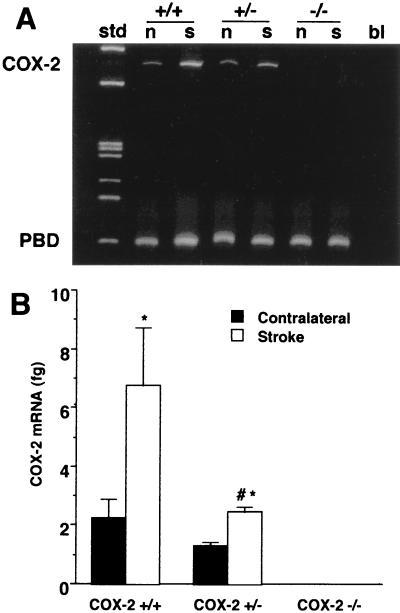
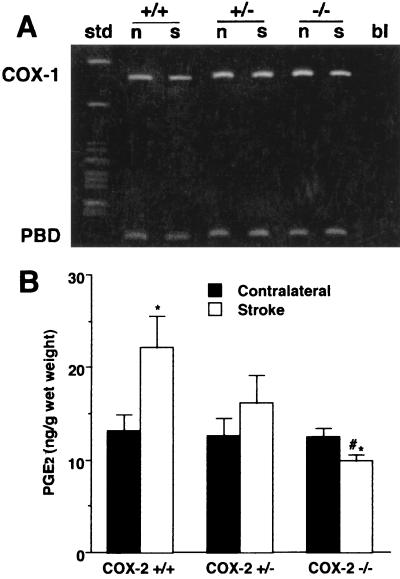
First, we sought to establish whether the increase in COX-2 expression that occurs after cerebral ischemia is reduced in COX-2-null mice. In COX-2 +/+ mice, COX-2 mRNA was increased in the ischemic cortex 24 h after MCA occlusion by 2- to 3-fold (Fig. 1A). The up-regulation was reduced in COX-2 +/− (P < 0.05, analysis of variance) (Fig. 1B). COX-2 mRNA was not detected in either the ischemic or the nonischemic cortex in COX-2 −/− mice. In contrast, expression of COX-1 mRNA was comparable in COX-2 +/+, +/−, and −/− mice (Fig. 2A). To determine whether the reduction in COX-2 mRNA expression is associated with a reduction in COX-2 enzymatic activity, the concentration of the COX reaction product PGE2 was measured in the ischemic and nonischemic cortex 24 h after MCA occlusion. PGE2 concentration was significantly elevated in the ischemic cortex of COX-2 +/+ mice (Fig. 2B). The elevation was attenuated in COX-2 +/− mice and absent in COX-2 −/− mice (Fig. 2B).
Figure 1.
COX-2 mRNA expression in the brain of COX-2-null mice 24 h after MCA occlusion. (A) Representative gel illustrating COX-2 mRNA expression in the ischemic cortex (s) and contralateral cortex (n) assessed by RT-PCR. The ubiquitous sequence PBD was also studied and used as an internal control. std, standards; bl, sample without the reverse transcriptase step. (B) COX-2 mRNA expression assessed by competitive PCR. COX-2 expression is reduced in COX-2 +/− mice and is absent in COX-2 −/− mice (n = 4 per group; *, P < 0.05 from contralateral; #, P < 0.05 from +/+ stroke).
Figure 2.
COX-1 mRNA expression and PGE2 concentration in the brain of COX-2-null mice 24 h after MCA occlusion. (A) Representative gel illustrating COX-1 mRNA expression assessed by RT-PCR 24 h after MCA occlusion. Similar results were obtained in four separate groups of COX-2 +/+, +/−, and −/− mice. COX-1/PBD OD ratios in the ischemic cortex of COX-2 +/+, +/−, and −/− mice were 1.7 ± 0.4, 1.6 ± 0.2, and 1.8 ± 0.5, respectively (n = 4 per group; P > 0.05). Abbreviations are as in Fig. 1. (B) Effect of MCA occlusion on PGE2 concentration in the ischemic cortex (Stroke) and contralateral cortex. The PGE2 elevation in the ischemic cortex in COX-2 +/+ (n = 8) was attenuated in COX-2 +/− (n = 7) and abolished in COX-2 −/− mice (n = 7) (*, P < 0.05 from contralateral; #, P < 0.05 from +/+ stroke).
Ischemic Brain Injury Is Reduced in COX-2-Null Mice.
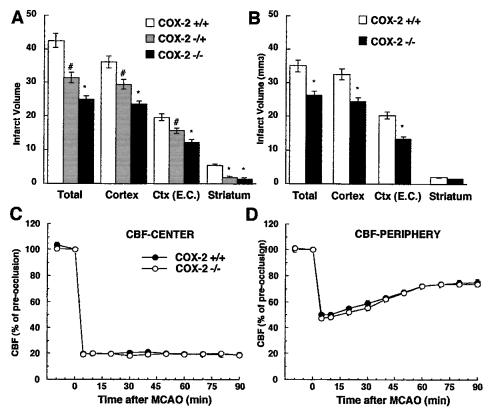
We then studied the brain injury produced by MCA occlusion in COX-2-null mice. Mice were killed 96 h after ischemia, and the infarct volume (mm3) was measured in brain sections stained with thionin (38). The 96-h time point was selected on the basis of the fact that, at this time, the damage resulting from postischemic inflammation is fully expressed (38). Infarct volume was significantly smaller in COX-2-null mice than in wild-type littermates (Fig. 3A). The reduction was greater in COX-2 −/− (−38 ± 4%) than in +/− mice (−20 ± 3%) (Fig. 3A). These data suggest that COX-2 contributes to ischemic brain injury.
Figure 3.
(A) Infarct size in COX-2 +/+ (n = 6), +/− (n = 7), and −/− (n = 8) null mice 96 h after MCA occlusion (MCAO). Ctx (E.C.), cerebral cortical infarct corrected for swelling. (*, P < 0.05 from COX-2 +/+ and +/− mice; #, P < 0.05 from COX-2 −/− and +/+ mice). (B) Infarct size in COX-2-null mice 24 h after MCAO (n = 6 per group; *, P < 0.05 from COX-2 +/+ mice). (C and D) CBF reduction in the center (C) and periphery (D) of the ischemic region in COX-2 +/+ and −/− mice after MCAO (n = 6 per group).
To determine whether COX-2 is also involved in pathogenic processes that occur in the initial stages of cerebral ischemia, we studied infarct volume in mice killed 24 h after ischemia. At this time the damage resulting from postischemic inflammation does not contribute to tissue injury (38). For example, in mice lacking inducible nitric oxide synthase, an enzyme that plays a critical role in inflammatory responses, infarct volume is not reduced 24 h after ischemia, but only at 96 h (38). We found that in COX-2-null mice infarct volume is also reduced 24 h after ischemia (−34 ± 3%; Fig. 3B). These data suggest that COX-2 is also involved in pathogenic events occurring in the early stages of cerebral ischemia.
Effect of MCA Occlusion on CBF in COX-2-Null Mice.
The intensity of the ischemic insult has a profound impact on the ensuing brain damage (see ref. 46 for a review). We therefore studied the effect of MCA occlusion on neocortical CBF in COX-2 +/+ and −/− mice. CBF was monitored both in the center of the ischemic territory and in the peripheral region that is spared from infarction in COX-2 −/− mice. As illustrated in Fig. 3 C and D, the reduction in CBF in the center or periphery of the ischemic territory did not differ between COX-2 +/+ and −/− mice (P > 0.05). These data demonstrate that the reduction in CBF in areas that are spared from infarction in COX-2 −/− mice is comparable to that observed in COX-2 +/+ mice. Therefore, differences in the intensity of the ischemic insult cannot account for the neuroprotection observed in COX-2-null mice.
NMDA-Mediated Injury in Vivo Is Attenuated in COX-2-Null Mice.
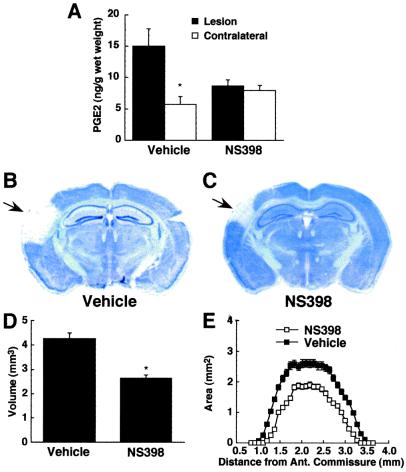
Glutamate receptors play a critical role in the initiation of ischemic brain injury (12). Therefore, we sought to determine whether the reduction in ischemic damage in COX-2-null mice could be attributed to reduced susceptibility to glutamate receptor-mediated damage. The glutamate receptor agonist NMDA was microinjected directly into the cerebral cortex, and injury volume was determined in thionin-stained brain sections 24 h later. At this time after NMDA microinjection, there is no histological evidence of inflammation in the area of the lesion (data not shown). In normal mice, NMDA microinjection increased the local concentration of PGE2 and produced a well-defined neocortical lesion (Fig. 4 A and B). The PGE2 elevation was attenuated by treatment with the COX-2 inhibitor NS398 (20 mg/kg i.p.; 1 h before NMDA), demonstrating that COX-2 was responsible for the increase in this prostaglandin (Fig. 4A). NS398 administration (20 mg/kg i.p.; 1 h before and 1 h after NMDA) also reduced the volume of the lesion (Fig. 4 C and D). In addition, the NMDA-induced damage was markedly attenuated in COX-2 −/− mice (Fig. 5). Administration of NS398 reduced injury volume in COX-2 +/+ but not in COX-2 −/− mice (Fig. 5C), attesting to the specificity of the effect of NS398 on COX-2. Thus the brain damage produced by the activation of glutamate receptors is attenuated by COX-2 inhibition and in COX-2-null mice.
Figure 4.
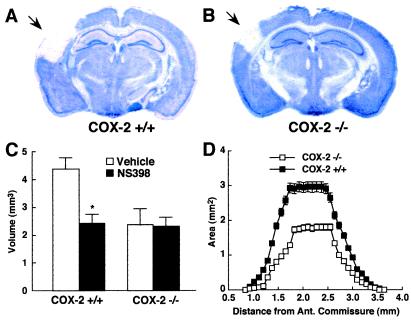
Effect of NS398 on the lesion produced by direct microinjection of NMDA into the cerebral cortex of C57BL/6J mice. (A) PGE2 concentration in the injured cortex, 3 h after NMDA injection, in mice receiving vehicle (n = 5) or NS398 (n = 5; *, P < 0.05 from vehicle). (B and C) Thionin-stained brain sections showing the lesion produced by NMDA (arrow) in mice receiving vehicle or NS398. (D) Effect of NS398 (n = 6) or vehicle (n = 7) on the volume of the lesion produced by NMDA (*, P < 0.05 from vehicle). (E) Effect of NS398 on the lesion area at different rostrocaudal levels from the anterior commissure.
Figure 5.
Lesion produced by microinjection of NMDA into the cerebral cortex of COX-2 +/+ and −/− mice. (A and B) Thionin-stained brain sections showing the lesion produced by NMDA injection (arrow) in COX-2 +/+ (A) and −/− (B) mice. (C) Effect of NS398 on the volume of the lesion produced by NMDA microinjection in COX-2 +/+ and −/− mice (n = 6 per group; *, P < 0.05 from vehicle). (D) Lesion area at different rostrocaudal levels relative to the anterior commissure in COX-2 +/+ and −/− mice (n = 6 per group).
Discussion
We used COX-2-null mice to investigate the role of COX-2 in the mechanisms of cerebral ischemic injury. We found that, after MCA occlusion, COX-2-null mice do not express COX-2 mRNA and have a reduction in the elevation in PGE2 produced by cerebral ischemia. Furthermore, the volume of brain damage produced by MCA occlusion is markedly reduced in COX-2-null mice. The reduction in injury volume, like the elevation in COX-2 mRNA and PGE2, is more marked in homozygous than in heterozygous null mice. These findings provide strong evidence that COX-2 reaction products are involved in ischemic brain damage. Furthermore, the observation that, in COX-2-null mice, infarct volume is also reduced 24 h after MCA occlusion, supports the hypothesis that COX-2 is also involved in pathogenic events that take place in the early stages of cerebral ischemia.
The neuroprotection observed in COX-2-null mice cannot be attributed to differences in the intensity of the ischemic insult, because the reduction in CBF produced by MCA occlusion is comparable in COX-2 +/+ and −/− mice. However, COX-2-null mice were found to be more resistant to the damage produced by the glutamate receptor antagonist NMDA. The reduction in excitotoxicity cannot be a consequence of alterations in the NMDA receptors in COX-2-null mice, because acute administration of the COX-2 inhibitor NS398 produced comparable attenuation in NMDA-induced injury. Conversely, the neuroprotection exerted by NS398 is unlikely to be due to effects of the drug unrelated to COX-2 inhibition, because NS398 did not confer protection in COX-2-null mice. These observations, collectively, provide evidence that COX-2 reaction products contribute to NMDA-mediated cytotoxicity. This conclusion is also supported by the observations that NS398 protects neuronal cultures from NMDA (47) and that neuronal cultures from transgenic mice overexpressing COX-2 are more susceptible to glutamate excitotoxicity (48). Considering the critical role that NMDA receptors play in ischemic brain injury (see ref. 12 for a review), the data suggest that attenuation of glutamate receptor-dependent ischemic damage contributes to the reduction in ischemic injury observed in COX-2-null mice.
The mechanisms by which COX-2 contributes to neurotoxicity remain to be defined. Superoxide radicals, a COX-2 reaction product (49, 50), are well known to participate in ischemic brain injury and could mediate tissue damage either directly or by reacting with nitric oxide to form the strong oxidant peroxynitrite (see ref. 51 for a review). Furthermore, COX-2 reaction products could activate poly(ADP-ribose) polymerase, an enzyme involved in DNA repair that contributes to neurotoxicity (52, 53). In addition, PGE2, a major reaction product of COX-2 (54), could mediate toxicity by facilitating glutamate release from astrocytes (55). However, the role of PGE2 in neurotoxicity remains controversial because this prostaglandin has also been reported to counter the cytotoxic effects of glutamate (56).
However, attenuation of glutamate neurotoxicity is not the sole mechanism of the protection from ischemic injury observed in COX-2-null mice. Cerebral ischemia is associated with an inflammatory reaction that contributes to tissue damage (57). COX-2 is a critical factor in the cytotoxicity associated with inflammation (21). It is therefore likely that COX-2 plays a role also in the mechanisms by which inflammation contributes to ischemic damage. This hypothesis is supported by the observation that the COX-2 inhibitor NS398 reduces ischemic injury even when administered 6 h after MCA occlusion (28). At this time after focal ischemia, activation of NMDA receptors does not contribute to the damage, as evidenced by the fact that NMDA receptor antagonists are no longer protective (58). Therefore, the evidence suggests that COX-2 also plays a role in the late stages of ischemic brain injury.
The findings of the present study suggest that COX-2 is a promising pharmacological target for the treatment of ischemic stroke. COX-2 inhibition offers several advantages over other prospective neuroprotective strategies. First, by targeting both “early” and “late” components of ischemic injury, COX-2 inhibitors can act as a bimodal neuroprotective strategy, with a likelihood of success greater than that of unimodal therapies. Second, potent and selective COX-2 inhibitors have already proved to be relatively safe and well tolerated (22). Therefore, examining their efficacy in stroke patients would be easier than testing potential neuroprotective agents with an unknown safety profile. Third, a large number of patients already take COX-2 inhibitors for treatment of osteoarthritis or pain (22). Epidemiological studies could provide important clues to the usefulness of COX-2 inhibitors in stroke prevention and improvement of outcome. On the basis of these considerations, COX-2 inhibition seems an attractive therapeutic strategy for stroke and other diseases associated with glutamate excitotoxicity.
Acknowledgments
We thank Dr. Dale Cooper for help with the measurement of blood urea nitrogen and creatinine, Dr. Carlos Manivel for pathological examination of mouse kidneys, Ms. Tracy Aber for assistance in experiments involving RT-PCR, and Mr. Tim Murphy and Ms. Andrea Hyde for editorial assistance. This work was supported by National Institutes of Health Grant NS35806.
Abbreviations
- NMDA
N-methyl-d-aspartate
- COX
cyclooxygenase
- MCA
middle cerebral artery
- RT-PCR
reverse transcription–PCR
- PBD
porphobilinogen deaminase
- CBF
cerebral blood flow
- PGE2
prostaglandin E2
References
- 1.Jorgensen H S, Nakayama H, Pedersen P M, Kammersgaard L, Raaschou H O, Olsen T S. Clin Geriatr Med. 1999;15:785–799. [PubMed] [Google Scholar]
- 2.Johansson B, Norrving B, Lindgren A. Stroke (Dallas) 2000;31:481–486. doi: 10.1161/01.str.31.2.481. [DOI] [PubMed] [Google Scholar]
- 3.Kita Y, Okayama A, Ueshima H, Wada M, Nozaki A, Choudhury S R, Bonita R, Inamoto Y, Kasamatsu T. Int J Epidemiol. 1999;28:1059–1065. doi: 10.1093/ije/28.6.1059. [DOI] [PubMed] [Google Scholar]
- 4.Warlow, C. P. (1998) Lancet352, Suppl. 3, SIII1–4. [DOI] [PubMed]
- 5.Lyden P D, Grotta J C, Levine S R, Marler J R, Frankel M R, Brott T G. Neurology. 1997;49:14–20. doi: 10.1212/wnl.49.1.14. [DOI] [PubMed] [Google Scholar]
- 6.Kwiatkowski T G, Libman R B, Frankel M, Tilley B C, Morgenstern L B, Lu M, Broderick J P, Lewandowski C A, Marler J R, Levine S R, Brott T. N Engl J Med. 1999;340:1781–1787. doi: 10.1056/NEJM199906103402302. [DOI] [PubMed] [Google Scholar]
- 7.Hamann G F, del Zoppo G J, von Kummer R. Thromb Haemost. 1999;82,Suppl. 1:92–94. [PubMed] [Google Scholar]
- 8.Lyden P D. Prog Cardiovasc Dis. 1999;42:175–183. doi: 10.1016/s0033-0620(99)70001-0. [DOI] [PubMed] [Google Scholar]
- 9.Steiner T, Hacke W. Eur Neurol. 1998;40:1–8. doi: 10.1159/000007947. [DOI] [PubMed] [Google Scholar]
- 10.Benveniste H, Drejer J, Schousboe A, Diemer N H. J Neurochem. 1984;43:1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 11.Butcher S P, Bullock R, Graham D I, McCulloch J. Stroke (Dallas) 1990;21:1727–1733. doi: 10.1161/01.str.21.12.1727. [DOI] [PubMed] [Google Scholar]
- 12.Lee J M, Zipfel G J, Choi D W. Nature (London) 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 13.Dereski M O, Chopp M, Knight R A, Rodolosi L C, Garcia J H. Acta Neuropathol. 1993;85:327–333. doi: 10.1007/BF00227730. [DOI] [PubMed] [Google Scholar]
- 14.Du C, Hu R, Csernansky C A, Hsu C Y, Choi D W. J Cereb Blood Flow Metab. 1996;16:195–201. doi: 10.1097/00004647-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gomez-Isla T, Hyman B T, Moskowitz M A. J Cereb Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Chopp M, Li Y, Jiang N, Zhang R L, Prostak J. J Cereb Blood Flow Metab. 1996;16:578–584. doi: 10.1097/00004647-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Connolly E S, Jr, Winfree C J, Springer T A, Naka Y, Liao H, Yan S D, Stern D M, Solomon R A, Gutierrez-Ramos J C, Pinsky D J. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dirnagl U, Iadecola C, Moskowitz M A. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 19.Vane J R, Bakhle Y S, Botting R M. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 20.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Isakson P. Adv Exp Med Biol. 1997;400A:167–170. doi: 10.1007/978-1-4615-5325-0_24. [DOI] [PubMed] [Google Scholar]
- 21.Seibert K, Masferrer J, Zhang Y, Gregory S, Olson G, Hauser S, Leahy K, Perkins W, Isakson P. Agents Actions Suppl. 1995;46:41–50. doi: 10.1007/978-3-0348-7276-8_5. [DOI] [PubMed] [Google Scholar]
- 22.Hawkey C J. Lancet. 1999;353:307–314. doi: 10.1016/s0140-6736(98)12154-2. [DOI] [PubMed] [Google Scholar]
- 23.Willoughby D A, Moore A R, Colville-Nash P R. Lancet. 2000;355:646–648. doi: 10.1016/S0140-6736(99)12031-2. [DOI] [PubMed] [Google Scholar]
- 24.Yamagata K, Andreasson K I, Kaufmann W E, Barnes C A, Worley P F. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann W E, Worley P F, Pegg J, Bremer M, Isakson P. Proc Natl Acad Sci USA. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breder C D, Dewitt D, Kraig R P. J Comp Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miettinen S, Fusco F R, Yrjanheikki J, Keinanen R, Hirvonen T, Roivainen R, Narhi M, Hokfelt T, Koistinaho J. Proc Natl Acad Sci USA. 1997;94:6500–6505. doi: 10.1073/pnas.94.12.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nogawa S, Zhang F, Ross M E, Iadecola C. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasinetti G M. J Neurosci Res. 1998;54:1–6. doi: 10.1002/(SICI)1097-4547(19981001)54:1<1::AID-JNR1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 30.Iadecola C, Forster C, Nogawa S, Clark H B, Ross M E. Acta Neuropathol. 1999;98:9–14. doi: 10.1007/s004010051045. [DOI] [PubMed] [Google Scholar]
- 31.Hara K, Kong D L, Sharp F R, Weinstein P R. Neurosci Lett. 1998;256:53–56. doi: 10.1016/s0304-3940(98)00755-1. [DOI] [PubMed] [Google Scholar]
- 32.Cole D J, Patel P M, Reynolds L, Drummond J C, Marcantonio S. J Pharmacol Exp Ther. 1993;266:1713–1717. [PubMed] [Google Scholar]
- 33.Lehmann J M, Lenhard J M, Oliver B B, Ringold G M, Kliewer S A. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 34.Grilli M, Pizzi M, Memo M, Spano P. Science. 1996;274:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- 35.Morham S G, Langenbach R, Loftin C D, Tiano H F, Vouloumanos N, Jennette J C, Mahler J F, Kluckman K D, Ledford A, Lee C A, et al. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 36.Niwa K, Araki E, Morham S G, Ross M E, Iadecola C. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langenbach R, Loftin C D, Lee C, Tiano H. Ann NY Acad Sci. 1999;889:52–61. doi: 10.1111/j.1749-6632.1999.tb08723.x. [DOI] [PubMed] [Google Scholar]
- 38.Iadecola C, Zhang F, Casey R, Nagayama M, Ross M E. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nogawa S, Forster C, Zhang F, Nagayama M, Ross M E, Iadecola C. Proc Natl Acad Sci USA. 1998;95:10966–10971. doi: 10.1073/pnas.95.18.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 41.Iadecola C, Zhang F, Casey R, Clark H B, Ross M E. Stroke (Dallas) 1996;27:1373–1380. doi: 10.1161/01.str.27.8.1373. [DOI] [PubMed] [Google Scholar]
- 42.Galea E, Feinstein D L. PCR Methods Appl. 1992;2:66–69. doi: 10.1101/gr.2.1.66. [DOI] [PubMed] [Google Scholar]
- 43.Diviacco S, Norio P, Sentilin L, Menzo S, Clementi M, Biamonti G, Riva S, Falaschi A, Giacca M. Gene. 1992;122:313–320. doi: 10.1016/0378-1119(92)90220-j. [DOI] [PubMed] [Google Scholar]
- 44.Lin T-N, He Y Y, Wu G, Khan M, Hsu C Y. Stroke (Dallas) 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- 45.Loeb W F, Quimby F W. The Clinical Chemistry of Laboratory Animals. New York: Pergamon; 1989. [Google Scholar]
- 46.Hossmann K-A. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 47.Hewett S J, Uliasz T F, Vidwans A S, Hewett J A. J Pharmacol Exp Ther. 2000;293:417–425. [PubMed] [Google Scholar]
- 48.Kelley K A, Ho L, Winger D, Freire-Moar J, Borelli C B, Aisen P S, Pasinetti G M. Am J Pathol. 1999;155:995–1004. doi: 10.1016/S0002-9440(10)65199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kontos H A, Wei E P, Povlishock J T, Dietrich W D, Magiera C J, Ellis E F. Science. 1980;209:1242–1245. doi: 10.1126/science.7403881. [DOI] [PubMed] [Google Scholar]
- 50.Chan P H, Fishman R A. J Neurochem. 1980;35:1004–1007. doi: 10.1111/j.1471-4159.1980.tb07100.x. [DOI] [PubMed] [Google Scholar]
- 51.Chan P H. Stroke (Dallas) 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 52.Eliasson M J, Sampei K, Mandir A S, Hurn P D, Traystman R J, Bao J, Pieper A, Wang Z Q, Dawson T M, Snyder S H, Dawson V L. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 53.Endres M, Scott G, Namura S, Salzman A L, Huang P L, Moskowitz M A, Szabo C. Neurosci Lett. 1998;248:41–44. doi: 10.1016/s0304-3940(98)00224-9. [DOI] [PubMed] [Google Scholar]
- 54.Brock T G, McNish R W, Peters-Golden M. J Biol Chem. 1999;274:11660–11666. doi: 10.1074/jbc.274.17.11660. [DOI] [PubMed] [Google Scholar]
- 55.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini B L, Pozzan T, Volterra A. Nature (London) 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 56.Cazevieille C, Muller A, Meynier F, Dutrait N, Bonne C. Neurochem Int. 1994;24:395–398. doi: 10.1016/0197-0186(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 57.Barone F C, Feuerstein G Z. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Hossmann K-A. Brain Pathol. 1994;4:23–36. doi: 10.1111/j.1750-3639.1994.tb00808.x. [DOI] [PubMed] [Google Scholar]