Abstract
Cell motility on extracellular matrices critically depends on matrix rigidity, which affects cell adhesion and formation of focal contacts. Receptor-like protein tyrosine phosphatase alpha (RPTPα) and the αvβ3 integrin form a rigidity-responsive complex at the leading edge. Here we show that the rigidity response through increased spreading and growth correlates with leading edge recruitment of Fyn, but not endogenous c-Src. Recruitment of Fyn requires the palmitoylation site near the N-terminus and addition of that site to c-Src enables it to support a rigidity response. In all cases, the rigidity response correlates with the recruitment of the Src family kinase to early adhesions. The stretch-activated substrate of Fyn and c-Src, p130Cas, is also required for a rigidity response and it is phosphorylated at the leading edge in a Fyn-dependent process. A possible mechanism for the fibronectin rigidity response involves force-dependent Fyn phosphorylation of p130Cas with rigidity-dependent displacement. With the greater displacement of Fyn from p130Cas on softer surfaces, there will be less phosphorylation. These studies emphasize the importance of force and nanometer-level movements in cell growth and function.
INTRODUCTION
The matrix rigidity response is important in cell motility, matrix remodeling, and development, as well as in pathological processes such as tumor formation and metastasis. The rigidity response involves interactions with extracellular matrix (ECM) mainly through integrin receptors, which regulate organization of the actin cytoskeleton (Giancotti and Ruoslahti, 1999). Our previous studies showed that the generation of force on rigid matrix contacts causes reinforcement of the integrin–cytoskeleton linkages through recruitment of focal adhesion proteins, increased cell adhesion, and spreading (Choquet et al., 1997; Giannone et al., 2004). However, fibroblasts respond to more than force and exhibit durotaxis or spread to larger areas on rigid than on soft substrates. The critical rigidity of the matrix appears to be around 3kPa, which correlates with tissue rigidities (Pelhame and Wang, 1997; Engler et al., 2004; Yeung et al., 2005). Various cell types respond to matrix rigidity in fundamentally different ways (Yeung et al., 2005). Furthermore, response to increased matrix rigidity can result in tissue disorganization and malignant transformation (Paszek et al., 2005).
One of the molecules implicated in matrix force transduction is receptor-like protein tyrosine phosphatase alpha (RPTPα; von Wichert et al., 2003). No ligands are known to bind directly to RPTPα, but this “orphan” receptor is thought to transduce force signals from αvβ3 integrin–cytoskeleton complexes to intracellular signaling pathways (Petrone and Sap, 2000; von Wichert et al., 2003). Knockout cells for RPTPα (RPTPα−/− cells) show defects in early spreading and focal contact formation (Su et al., 1999). In addition, RPTPα−/− cells fail to respond to changes in FN matrix rigidity and spread to equal areas on soft and rigid FN matrices (Jiang et al., 2006). Interestingly, knockout cells for focal adhesion kinase (FAK) spread very poorly on rigid FN substrates because of an increased contractile response on rigid substrates, whereas inhibition of Rho kinase restores normal spread area in FAK−/− cells (Chen et al., 2002).
RPTPα activates Src family kinases (SFKs), namely cellular Src (hereafter referred to as Src), Fyn, and c-Yes by dephosphorylation of a negative regulatory phosphotyrosine in the C-terminal domain of SFKs (Zheng et al., 1992; Ponniah et al., 1999; Su et al., 1999; Zheng et al., 2000; von Wichert et al., 2003). Although at later times SYF cells (Src−/−, Fyn−/−, and Yes−/−) show mild abnormalities in focal contact formation and matrix adhesion, these defects are more prominent during early phases of cell spreading (Klinghoffer et al., 1999). Reconstitution of Fyn in SYF cells activates early focal contact formation, whereas Src and c-Yes do not (von Wichert et al., 2003). Although Fyn is both myristoylated and palmitoylated in the N-terminal domain, Src is only myristoylated because it lacks the palmitoyl-acyl-transferase recognition sequence (Alland et al., 1994; Resh, 1994). Palmitoylation is known to enhance plasma membrane binding and lipid-dependent aggregation, which might facilitate recruitment to focal contacts (Wolven et al., 1997).
A major substrate of the SFKs is the docking protein p130Cas (hereafter referred to as Cas; Cary et al., 1998). Mechanical stretch of cytoskeletons stimulates the SFK-mediated phosphorylation of the interior substrate domain of Cas (Tamada et al., 2004). In addition, Cas can simultaneously bind Src and FAK, therefore facilitating Src activation of FAK (Fonseca et al., 2004). Thus, Cas might be a downstream substrate of SFKs, which is activated during the rigidity response.
In this report, we show that Fyn, unlike native Src, is involved in the rigidity response and accelerates cell spreading during the initial cell–matrix interaction. Rigidity response correlates with localization of Fyn to the leading edge and early focal contact formation on FN. Palmitoylation is required for recruitment of Fyn to early focal contacts as is RPTPα, and introduction of a palmitoylation site in Src increases its accumulation in early focal contacts as well as enabling it to restore rigidity response. Finally, the Fyn and Src substrate, Cas, is required for rigidity response and is localized to the leading edge in close proximity to Fyn in a Fyn-dependent manner.
MATERIALS AND METHODS
Constructs
Fyn-GFP and Src-GFP constructs were a gift from Professor John Woodward. Fyn(C3G)-GFP construct was generated via PCR-amplification using Fyn-GFP as template and primers including point mutations (P1: 5′ ATG GGC GGT GTG CAA TG 3′; P2: 5′ CAT TGC ACA CCG CCC AT 3′). Src(S3C)-GFP construct was generated via PCR amplification using Src-GFP as template and primers including point mutations (P1: 5′ ATG GGC TGC TCC AAG TC 3′; P2: 5′ GAC TTG GAG CAG CCC AT 3′). The correct sequence of both constructs was confirmed by sequencing. Cells were transfected with various constructs by using Fugene (Gibco BRL, Rockville, MD) according to manufacturer’s protocol.
Small Interference RNA
Silencer small interference RNA (siRNA) transfection kit and custom siRNAs sequences targeted against RPTPα and Cas (Ambion, Austin, TX) were used according to the manufacturer’s protocol. The RPTPα expression levels were tested by Western blots and immunofluorescent staining (with anti-RPTPα [Abcam, Cambridge, MA] antibody or anti-RPTPα antibody raised in D2 hybridoma cells; a gift from Professor Jan Sap), and Cas expression levels were tested by anti-p130Cas antibody (BD Transduction Laboratories, Lexington, KY). Controls with “scrambled” siRNA sequences were included.
Antibodies
For this study, the following antibodies were used: a mouse monoclonal antibody (mAb) against paxillin (BD Transduction Laboratories), a mouse mAb against Cas (BD Transduction Laboratories), a mouse mAb against Src (Upstate Biotechnology, Lake Placid, NY), a mouse mAb against Fyn (Chemicon, Temecula, CA), a rabbit polyclonal antibody against RPTPα (Abcam), an affinity purified polyclonal rabbit anti-phoshoY165Cas antibody (Cell Signaling Technology, Beverly, MA), horseradish peroxidase–conjugated anti-mouse and anti-rabbit antibodies (Amersham, Piscataway, NJ), an affinity-purified rabbit polyclonal phosphoY416-Src kinase family antibody (Cell Signaling Technology), a goat anti-rabbit immunoglobulin (Ig) conjugated with Alexa 647 (Molecular Probes, Eugene, OR), a goat anti-rabbit Ig conjugated with Alexa 555 (Molecular Probes), and goat anti-mouse Ig conjugated with Alexa 568 (Molecular Probes).
Cell Culture
RPTPα+/+ and RPTPα−/− fibroblasts were a generous gift from Professor Jan Sap (Sap et al., 1990). Cells were grown in high-glucose DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 20 mM HEPES (all materials from Invitrogen, Carlsbad, CA). Cells were cultured on plasma-treated tissue culture plastic (Falcon, Lincoln Park, NJ), harvested, and then plated on FN-coated coverglasses or polyacrylamide gels. Cells were not serum-starved, but serum-free DMEM was used in the spreading assays. Anti-RPTPα, antibody-producing D2 hybridoma cells were obtained from Professor Jan Sap. Cells were grown in high-glucose DMEM supplemented with 10% newborn calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 20 mM HEPES (all materials from Invitrogen). Cells were cultured in suspension and harvested, and the cell-free supernatant was used in immunocytochemistry as described elsewhere (Su et al., 1996). Cas−/− cells were generous gift from Professor Hisamaru Hirai (Honda et al., 1998) and were cultured in high-glucose DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 20 mM HEPES (all materials from Invitrogen). MCF10A human breast epithelial cells and malignant scp lines were generous gift from Professor Joan Massague. MCF10A cells were grown in 1:1 mixture of DMEM and Ham’s F-12 media supplemented with 5% horse serum, 100 μg/ml streptomycin, 2 mM l-glutamine, and 20 mM HEPES, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone and 0.02 μg/ml EGF (all materials from Invitrogen). Scp cell lines were grown in high-glucose DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 20 mM HEPES.
Spreading Assays on Polyacrylamide Substrates
The full-length FN-coated polyacrylamide substrates were prepared as described previously (Pelham and Wang, 1997). The flexibility of the substrate was manipulated by maintaining the total acrylamide concentration at 10% while varying the bis-acrylamide component between 0.08% (rigid) and 0.03% (soft; E = 3 kPa, and E = 1 kPa, respectively; Engler et al., 2004). The uniformity of FN coating on the substrate surface was examined by coating the gels with FN conjugated to Cy5 fluorophore (Amersham Biosciences) according to manufacturer’s instructions and visualized by confocal microscopy. Experiments were performed 2 h after the cells were plated on the polyacrylamide gel at a low density. Spread area was quantified for at least 50 cells for each condition, and statistical significance of the results was confirmed by t test (p < 0.01). Data are presented as mean ± SE.
Immunocytochemistry
Fibroblast cells were plated onto FN-coated coverglass (10 μg/ml FN) or FN-coated polyacrylamide gels. After incubation for the described time, cells were fixed in 3.7% formaldehyde and permeabilized with 0.1% Triton. Fixed cells were incubated with primary antibodies (described above) for 1 h followed by washing and incubation with appropriate fluorescent secondary antibodies (also described above). Fluorescent signals from all samples were visualized by confocal microscopy.
Microscopy and Analysis
Images of immunofluorescently stained samples were acquired using a Fluoview confocal microscope (Olympus, Melville, NY). Phase contrast images of the cells plated on polyacrylamide substrates were recorded with a cooled CCD camera attached to an Olympus IX81 equipped with a 10× objective. Analysis of acquired images was performed with the image analysis program, ImageJ (by W. Rasband, NIH, Bethesda, MD; http://rsb.info.nih.gov/ImageJ).
RESULTS
Fyn Restores Rigidity Response in SYF Cells and Inhibits Growth on Soft Surfaces
Because Src and Fyn kinases can be activated by RPTPα during early phases of spreading (Su et al., 1999; von Wichert et al., 2003) and defects in rigidity response reported in RPTPα−/− cells may be related to early spreading defects, we tested the roles of Fyn and Src activity in the rigidity response. SYF cells were plated on soft and rigid polyacrylamide gels coated with full-length FN as previously described elsewhere (Pelham and Wang, 1997), and the total spread area and overall morphology were analyzed. FN-coated polyacrylamide gels provided a biochemically stable environment while rigidity was controlled by variations in the concentration of the bis-acrylamide cross-linker. Wild type fibroblasts spread to ∼2.5 times larger areas on rigid than on soft gels and retained more of their characteristic morphology on rigid than soft substrates (Pelham and Wang, 1997). It is important to note that this ratio between areas on rigid and soft substrates remains constant across a variety of cell lines, whereas the differences in absolute spread areas between cell lines can be significant. Interestingly, RPTPα−/− cells spread to equal areas on rigid or soft substrates (Jiang et al., 2006). Similar to RPTPα−/− cells, SYF cells spread to approximately equal areas on both soft and rigid substrates (Figure 1, A and B). SYF cells seemed to retain normal overall morphology on both soft and rigid substrates (although cells were more elongated on rigid and slightly rounder on soft; Figure 1B). These results were consistent with the hypothesis that the RPTPα-mediated activation of Src, Fyn, or c-Yes was involved in rigidity response.
Figure 1.
Fyn, but not Src restores rigidity response in SYF cells. (A) SYF cells spread to approximately equal area on soft and rigid FN-coated polyacrylamide gels. Reconstitution with Fyn-GFP restores rigidity response by inhibiting the spreading on soft substrates, and reconstitution with Src-GFP has no effect. Spread areas in SYF, SYF/Fyn-GFP, and SYF/Src-GFP cells were quantified, and results are presented as mean ± SE for at least 50 cells. (B) DIC images of SYF cells plated on FN-coated glass (left), rigid FN-coated polyacrylamide gels (middle), and FN-coated soft polyacrylamide gels (right). Scale bar, 20 μm. (C–F) immunofluorescence staining for the focal contact marker paxillin (red) of SYF/Fyn-GFP and SYF/Src-GFP transiently transfected cells plated on rigid (C and E) and soft FN-coated polyacrylamide gels (D and F). Fyn (green) accumulates in focal contacts that are elongated and rich in paxillin in SYF/Fyn-GFP cells plated on rigid substrates (C). On soft substrates, accumulations of Fyn and paxillin appear shorter (D). Paxillin accumulations in SYF/Src-GFP cells are shorter and punctate regardless of substrate rigidity (E and F). Scale bar, 20 μm. (G) The proliferation on rigid versus soft FN-coated gels was observed over 72 h upon plating. The number of cells was counted and normalized by the number of cells initially plated. RPTPa+/+ cells were used as control. Although control cells proliferate much faster on rigid than on soft substrates, SYF cells grew equally well regardless of substrate rigidity. Introduction of Fyn-GFP, but not Src-GFP rescued wild-type phenotype on soft substrates.
To determine which SFK(s) was required for rigidity response, spreading of Fyn-GFP and Src-GFP transiently transfected SYF cells (transfection efficiency >70%) was analyzed on soft and rigid FN-coated substrates (Figure 1A). We confirmed that the addition of GFP to the C-terminal end of Fyn and Src did not affect their localization (Supplementary Figure 1), nor apparent enzymatic activity, as previously reported (Anders et al., 1999a, 1999b). In our hands, reconstitution with c-Yes resulted in aberrant morphology (the fraction of cells adhering to substrate was reduced, and cells were spreading very slowly, with numerous filopodial extensions). Further, these spreading defects were more pronounced in Yes transfectants plated on FN-coated gels. Therefore, we speculate that although Yes might have a regulatory role in fine-tuning of the rigidity response, its activity is insufficient to rescue the normal early spreading phenotype in absence of Fyn. Transfection with Src-GFP had no significant effect on the ratio of rigid substrate cell area to soft substrate cell area (AR = rigid area/soft area, hereafter referred to as area ratio, see Figure 1A). In contrast, SYF/Fyn-GFP cells spread to a twofold larger area on rigid versus soft substrates, which indicated that Fyn played an important role in cell response to matrix rigidity. Similar results were obtained when wild-type Src, and Fyn constructs were transfected in SYF cells (Supplementary Figure 1). It is important to note that many different normal fibroblast lines with different morphologies and different average areas all responded to rigid surfaces by spreading to larger areas. Although the spread areas of SYF/Fyn-GFP cells were significantly smaller than the spread area of wild-type fibroblasts, the rigidity response was rescued by introduction of Fyn. Because SYF cells spread to a threefold smaller area on tissue culture plastic compared with wild-type fibroblasts (unpublished data), we speculate that reconstitution with Fyn is not sufficient to rescue the cell size phenotype in SYF cells. In addition, we attempted to rescue the rigidity response and/or spread area by double-transfection with Fyn-GFP and Src-GFP. These cells spread poorly on both soft and rigid gels (325 ± 20.5 and 444 ± 33.7 μm2, respectively), and the area ratio was slightly higher than in SYF cells (AR = 1.36 vs. AR = 0.95, respectively). Therefore, we conclude that overexpression of Src and Fyn resulted in inhibited spreading, but did not have a positive effect on rigidity response. This result is consistent with our previous studies showing that double-transfection with Src and Fyn negatively affected cell spreading (von Wichert et al., 2003).
Because Fyn restored the rigidity response in SYF cells, we hypothesized that reconstitution of Fyn would also affect the formation of focal contacts as a part of the rigidity response. Therefore, we compared focal contact formation on rigid and soft substrates by immunofluorescence staining for the focal contact marker, paxillin (Turner, 2000). When plated on rigid substrates SYF/Fyn-GFP cells formed more focal contacts (76.4 ± 2.3, average for 15 representative cells). These contacts were elongated, resembling focal contacts on FN-coated glass. On soft substrates, SYF/Fyn-GFP cells formed fewer focal contacts (38.9 ± 1.3, average for 15 cells) that were shorter and punctate (Figure 1, C and D). In contrast, SYF/Src-GFP cells established a similar number of focal contacts on both soft and rigid substrates (36.2 ± 1.2, and 35.9 ± 2.1, respectively; Figure 1, E and F). These contacts were short and the intensity of paxillin staining was reduced. Thus, focal contact formation depended not only upon Fyn, but also upon rigidity of the substrate.
Further, we tested whether proliferation of SYF cells was affected by matrix rigidity. Proliferation rate of SYF cells was the same on soft and rigid FN-coated polyacrylamide substrates, unlike control wild-type fibroblasts, which grew faster on rigid than on soft matrices. However, transfection with Fyn-GFP caused inhibition of proliferation on soft surfaces. Transfection with Src-GFP had no effect on SYF cell proliferation on soft versus rigid surfaces (Figure 1G). Because RPTPα−/− cells responded to the rigidity of collagen-coated gels (Jiang et al., 2006), we tested SYF cell growth on collagen surfaces and found that growth was inhibited on the soft collagen-coated surfaces (Supplemental Figure 2). Thus, both rigidity response and proliferation on fibronectin substrates appeared to depend upon Fyn expression, whereas Src, Yes, and Fyn did not seem to regulate the cellular response to collagen rigidity.
Figure 2.
Localization of Fyn to early focal contacts is RPTPα-dependent. (A–C) immunofluorescence staining for paxillin (red) of SYF/Src-GFP (A), RPTPα+/+/Src-GFP (B), and RPTPα−/−/Src-GFP (C) fibroblast cells plated on FN-coated glass for 30 min show low concentration of Src-GFP in early focal contacts. (D) Immunostaining of SYF/Src(S3C)-GFP shows increased recruitment of Src palmitoylated mutant to focal contacts. (E and F) Immunofluorescence stainings for paxillin (red) of SYF/Fyn-GFP (E) and RPTPα+/+/Fyn-GFP (F) cells show colocalization of Fyn-GFP and paxillin in early focal contacts. (G and H) Immunofluorescence stainings for paxillin (red) of RPTPα−/−/Src-GFP (G) and SYF/Fyn(C3G)-GFP (H) cells show reduced overlap in localization of Fyn and paxillin due to the lack of RPTPα-mediated activation of Fyn and palmitoylation, respectively. Scale bar, 10 μm. Blow-up panels are 2.5× magnified sections marked by a dash line. (I) Quantitative analysis of colocalization of GFP-fusion proteins with paxillin staining. Fluorescence intensities were measured across focal contacts (identified as areas with increased red signal toward the edge of the cells) for both channels; background fluorescence was subtracted, and the ratio between intensities in green (GFP-fusion proteins) and red channel (paxillin) was calculated. Cells included in analysis were representative of the larger cell population; at least 10 cells with a minimum of 35 focal contacts were analyzed for each condition.
Fyn, But Not Src, Was Recruited to the Focal Contacts at Early Times
Previous studies showed that the αvβ3-RPTPα complex at the leading edge was critical for both early spreading and reinforcement (von Wichert et al., 2003). Because Src and Fyn were activated by RPTPα (Su et al., 1999; Zheng et al., 2000), their distribution and function in early spreading may be correlated with their role in rigidity response and RPTPα activity. Early spreading was severely impaired in SYF cells, indicating that SFK activity was required for this process (Klinghoffer et al., 1999; von Wichert et al., 2003). To further characterize the role of Fyn and/or Src in early spreading, we compared spreading and fluorescence distribution in SYF/Fyn-GFP and SYF/Src-GFP cells after 30 min on FN. Although SYF/Fyn-GFP cells did not show any abnormalities in spreading, SYF/Src-GFP cells had an abnormal spreading phenotype similar to SYF cells (Supplementary Figure 3).
Figure 3.
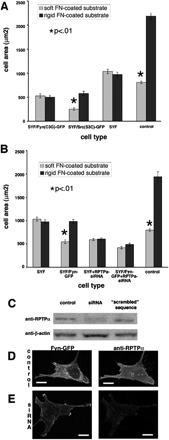
Fyn-mediated rigidity response requires both palmitoylation and RPTPα. (A) Quantitation of spread areas of SYF/Fyn(C3G)-GFP and SYF/Src(S3C)-GFP cells showing that the loss of the palmitoylation site in the N-terminus of Fyn kinase blocks its ability to restore rigidity response, whereas introduction of the palmitoylation site in the N-terminus of Src kinase enabled it to restore rigidity response in SYF cells. Results shown as mean ± SE for at least 50 cells (same as in B). (B) Quantitation of spread areas of SYF/Fyn-GFP cells treated with siRNA for RPTPα. Despite the presence of Fyn-GFP, no significant difference in spread area was observed between cells on rigid versus soft FN-coated polyacrylamide substrates, indicating that RPTPα activation of Fyn is necessary for rigidity response. (C) Western blots showing decreased levels of RPTPα in RPTPα-siRNA–treated cells compared with untreated control and control treated with “scrambled” sequence. Loading control is β-actin. (D and E) Immunofluorescence staining for RPTPα in control cells (D) and RPTPα-siRNA-treated SYF/Fyn-GFP cells (E) shows decrease in RPTPα expression levels in treated cells. Scale bar, 15 μm.
To determine if the distribution of Fyn and Src changed with time and formation of focal contacts, we followed their relative distributions as well as the emergence of focal contacts. Colocalization of Fyn-GFP with paxillin was observed in the “brushstroke-like” accumulations at the leading edge in both SYF and RPTPα+/+ fibroblasts as early as 15 min after plating (Figure 2, E and F). To quantify the accumulation of Fyn/Src-GFP, the fluorescence intensities were measured across focal contacts for both channels; background fluorescence was subtracted; and the ratio between intensities in green (GFP-fusion proteins) and red channel (paxillin) was calculated. Quantitative analysis of the overlap between green and red signal showed that the ratios between Fyn-GFP and anti-paxillin were approximately the same in SYF and RPTPα+/+ cells 30 min after plating (0.90 ± 0.02 and 0.84 ± 0.02, respectively). In contrast, in RPTPα−/− cells the overlap between Fyn-GFP and paxillin was drastically reduced (0.23 ± 0.01; Figure 2, G and I). Nevertheless, in RPTPα−/− cells that were plated on FN for 2 h or longer, colocalization of Fyn and paxillin appeared to be normal (unpublished data).
In sharp contrast, the overlap between Src-GFP and paxillin localization was significantly reduced in any of the tested cell lines for the first 30 min after plating (colocalization ratios were 0.22 ± 0.02, 0.25 ± 0.01, 0.26 ± 0.01 for SYF, RPTPα+/+, RPTPα−/−, respectively; Figure 2, A–C and I). However, no difference between Fyn and Src accumulation was found in focal contacts of the cells plated on FN for more than 2 h (unpublished data). Thus, only Fyn was recruited to the leading edge and focal complexes at early times, whereas both Src-GFP and Fyn-GFP were localized in mature focal adhesions on FN.
Palmitoylation Was Required for Recruitment of Fyn to the Leading Edge and Focal Contacts at Early Times
Previous studies had shown that the presence of cysteine on position 3 was required for palmitoylation of SFKs (Wolven et al., 1997). Unlike Fyn, which was myristoylated and double-palmitoylated (Cys3 and Cys6), endogenous Src was only myristoylated (Alland et al., 1994). Because the palmitoylation enhanced plasma membrane binding of Fyn, we tested the hypothesis that palmitoylation was also required for the recruitment of Fyn to focal contacts during early cell spreading on FN. Therefore, we mutated the critical cysteine residue to glycine (C3G) in our Fyn-GFP construct to produce Fyn(C3G)-GFP. Consistent with our hypothesis, Fyn(C3G)-GFP failed to accumulate at the leading edge or in early focal contacts of spreading RPTPα+/+ fibroblasts (Figure 2H). Further, the palmitoylation of Src (Src(S3C)-GFP) caused it to be localized to the leading edge unlike native Src-GFP (Figure 2D). Compared with wild-type Src-GFP, more colocalization of mutated Src-GFP protein with paxillin was observed at early time points during spreading on FN (Figure 2, D and I). Nevertheless, the level of Src(S3C)-GFP in early focal contacts was lower than the amount of Fyn-GFP (0.77 ± 0.02 colocalization ratio for Src(S3C)-GFP compared with 0.90±.02 for Fyn-GFP). Thus, palmitoylation on cysteine3 was required for Fyn and Src localization to the leading edge during early spreading and focal contact formation, but other factors (such as palmitoylation on cysteine6, protein–protein interactions, etc.) influenced the amount of the kinase at the leading edge.
General inhibition of palmitoylation by the pharmacological inhibitors tunicamycin and 2-bromo-palmitate had a similar effect on Fyn-GFP distribution as mutation of the palmitoylation site (unpublished data). Taken together, these results indicated that C3 palmitoylation was involved in Fyn localization at early times.
Palmitoylation Was Critical for the Matrix Rigidity Response
Because the palmitoylation affected Fyn distribution during early spreading, we tested the effect of palmitoylation on the rigidity response. Transfection of Fyn(C3G)-GFP did not restore the rigidity response to SYF cells (Figure 3A), unlike wild-type Fyn-GFP. In contrast, introduction of the palmitoylation motif to the Src-GFP construct by mutating serine to cysteine (S3C) enabled it to rescue the matrix rigidity response in SYF cells (Figure 3A). Thus, a palmitoylation signal was necessary and sufficient for Fyn or Src to restore the rigidity response to SYF cells.
RPTPα Was Required for Fyn Activation in Rigidity Response
To test whether Fyn was involved in an alternate, RPTPα-independent matrix rigidity response pathway, we used an siRNA strategy to knockdown RPTPα expression in SYF/Fyn-GFP cells. The siRNA efficiency was confirmed by Western blots (Figure 3C) and immunofluorescent staining (Figure 3, D and E). Although the expression levels of Fyn-GFP did not change because of the RPTPα-targeted siRNA treatment, the ability of SYF/Fyn-GFP cells to respond to matrix rigidity was abolished. Similar to SYF cells, RPTPα-targeted siRNA-treated SYF/Fyn-GFP cells spread to approximately equal areas on both soft and rigid substrates (Figure 3B). This result confirmed our hypothesis that Fyn cannot restore the rigidity response unless activated by RPTPα. We tested whether or not RPTPα was involved in the rigidity response in cells other than fibroblasts, by knocking down the levels of RPTPα in the epithelial cell line MCF10A. On siRNA treatment of cells, there was a loss of the rigidity response (see Supplementary Figure 4) that correlated with the loss of RPTPα protein. Thus, we concluded that RPTPα was required as an upstream regulator of Fyn activation in response to matrix rigidity and that its role in early rigidity response is present in several different cell types.
Figure 4.
Activation of Cas is required in Fyn-mediated FN rigidity response. (A) Cas−/− cells spread to approximately equal area on soft and rigid FN-coated polyacrylamide gels (similar to SYF cell; see Figure 1). Reconstitution with GFP-Cas restored the rigidity response by increasing spreading on rigid substrates. Results are presented as the mean spread area ± SE for at least 50 cells. (B) DIC images of Cas−/− (top panel) and Cas−/−/GFP-Cas cells (bottom panel) plated on FN-coated glass (left), rigid FN-coated polyacrylamide gels (middle), and soft FN-coated polyacrylamide gels (right). Scale bars, 20 μm. (C and D) Immunofluorescence staining for the focal contact marker paxillin (red) of Cas−/−/GFP-Cas transiently transfected cells plated on rigid (C) and soft FN-coated polyacrylamide gels (D). GFP-Cas (green) accumulated in focal contacts that were elongated and rich in paxillin in cells plated on rigid substrates (C). On soft substrates, accumulations of GFP-Cas were shorter and punctate (D). Scale bar, 10 μm. (E) Western blots showing decreased levels of Cas in Cas-siRNA–treated cells compared with untreated control and control treated with “scrambled” sequence. Loading control is β-actin. (F and G) Immunofluorescence staining for Cas (red) in control cells (G) and Cas-siRNA–treated SYF/Fyn-GFP cells (I) shows decrease in Cas expression levels in treated cells. Scale bar, 10 μm. (I) Quantitation of spread area of SYF/Fyn-GFP cells treated with siRNA for Cas. Results are shown as mean ± SE for at least 50 cells. Despite the presence of Fyn-GFP, no significant difference in spread area was observed between cells on rigid versus soft FN-coated polyacrylamide substrates, indicating that Cas phosphorylation by Fyn was necessary for the rigidity response.
Cas Was Downstream of the RPTPα-mediated Rigidity Response Mechanism
Identified as a stretch-dependent substrate for SFKs (Tamada et al., 2004), Cas was a logical candidate to have a role in rigidity response. Cas knockout cells (Cas−/− cells) showed defects in the substrate rigidity response similar to defects observed in SYF cells. Although the spread area (on both FN-coated glass and FN-coated gels) of Cas−/− cells was larger than SYF cells (which can be explained by divergent roles that SFKs and Cas play in cell growth and differentiation), the area ratio of Cas−/− cells on rigid versus soft gels was approximately one. Reconstitution of Cas−/− cells by transient transfection with GFP-Cas full-length restored a twofold area ratio for spreading on rigid versus soft polyacrylamide (Figure 4A). Interestingly, reconstitution with GFP-Cas increased spreading on rigid substrates, unlike reconstitution with Fyn-GFP in SYF background, which inhibited spreading on soft substrates. Although this difference could be explained by the existence of intersecting mechanisms of regulation, we believe that ablation of Cas (or overexpression) leads to multiple defects in cell adhesion and spreading. Because Cas−/− cells show great variation in cell area, as well as a twofold increase in absolute cell area compared with wild-type fibroblasts, it appears that other regulatory pathways are also impaired. Further, with siRNA knockdown of Cas in SYF/Fyn-GFP cells, there was not a similar increase in spread area although the cells were not able to respond to rigidity (see below). Although there are some unusual features of Cas−/− cells, the restoration of Cas is sufficient to restore the response to rigidity in those cells.
In addition, we analyzed the effect of reconstitution with GFP-Cas on cell morphology; reconstituted cells adopted a rounder shape on the soft matrices in contrast to the elongated morphology on rigid substrates. Furthermore, reconstituted cells had shorter, more actively ruffling lamellipodia than Cas−/− cells when plated on soft substrates (Figure 4B).
Immunofluorescent staining for paxillin showed that Cas−/− cells formed punctate focal contacts on both soft and rigid substrates (unpublished data). Introduction of the GFP-Cas construct resulted in shorter, punctate focal contacts on soft substrates, whereas focal contacts on rigid matrices appeared normal (Figure 4, C and D).
To examine whether other substrates of Fyn can compensate for the lack of Cas in FN rigidity response, we used siRNA technology to knockdown the expression levels of Cas in SYF/Fyn-GFP cells. SYF cells were simultaneously transfected with Fyn-GFP and siRNAs targeted against Cas. The siRNA efficiency was confirmed by Western blots (Figure 4E) and immunofluorescent staining against Cas (Figure 4, F and G). After 48 h of incubation with siRNAs, cells were replated on soft and rigid gels for 2 h, fixed, and immunofluorescently stained for Cas. In cells with very low levels of Cas (unlike in control cells with normal Cas levels), Fyn-GFP failed to rescue the rigidity response in SYF cells (Figure 4I). Thus, we decided to test further the role of Fyn-mediated phosphorylation of Cas in FN rigidity response.
Cas Phosphorylation by Fyn at the Leading Edge Was Required for the FN Rigidity Response
Previous studies showed that Cas was localized to the leading edge of spreading fibroblasts, as well as in focal contacts (Fonseca et al., 2004). Because Cas was phosphorylated by SFKs in vitro, we tested whether recruitment of Cas to the early focal contacts and its phosphorylation required SFKs in vivo. Unlike in control fibroblasts (Figure 5D), in SYF cells (plated on FN for 45 min), focal contacts (stained for paxillin) were formed but no GFP-Cas was recruited to these structures (Figure 5A). We tested whether Src and Fyn could rescue Cas recruitment to the focal contacts. In cells double-transfected with GFP-Cas and wild-type Fyn, GFP-Cas was distributed to the focal contacts. Transfection with native Src did not rescue Cas recruitment to the focal contacts (Figure 5B, C). Therefore, Fyn was required for recruitment of Cas to early focal contacts.
Figure 5.
Fyn, unlike Src rescues recruitment of Cas to early focal contacts in SYF cells. (A) Transiently transfected SYF/GFP-Cas cells were plated on FN-coated glass for 45 min, fixed, and immunostained for paxillin (red). The amount of GFP-Cas (green) in focal contacts labeled with anti-paxillin antibody (red) was much lower than in control cells (D). Reconstitution with Fyn significantly increased the amount of GFP-Cas in focal contacts (B), whereas reconstitution with Src had no effect (C). The activity of Fyn/Src was quantified by immunofluorescent staining with an anti-phosphoY416-Src family kinase antibody that recognizes the activated form of SFKs (cyan). Scale bar, 10 μm.
Using an anti-phospho-Cas antibody (Fonseca et al., 2004), we tested the effect of matrix rigidity and SFKs on the phosphorylation of Cas at the leading edge and focal contacts. When we probed SYF/Fyn-GFP transfected cells (Figure 6A), phosphorylated Cas rapidly accumulated at the leading edge and in the focal contacts on FN-coated glass, unlike in SYF/Src-GFP cells (Figure 6D). Although the level of pCas accumulation was only slightly reduced in SYF/Fyn-GFP cells plated on rigid gels (89 ± 2% of the average signal in cells plated on glass), the accumulation of pCas on soft gels was significantly reduced (38 ± 1%; Figure 6, B, C, and E). Therefore, both Fyn and a rigid matrix were required for phosphorylation of Cas at the leading edge and in focal contacts. This provided further evidence that colocalization of Fyn and Cas at the molecular level may be part of the rigidity response process. In addition, Fyn(C3G)-GFP did not cause recruitment and phosphorylation of Cas at the leading edge, whereas Src(S3C)-GFP induced Cas phosphorylation (Supplementary Figure 5). Thus, palmitoylation of Fyn and its consequent recruitment to the leading edge are crucial for its role in the phosphorylation of Cas.
Figure 6.
Fyn-mediated phosphorylation of Cas depends on matrix rigidity. (A–C) SYF/Fyn-GFP cells were plated for 1 h on FN-coated glass (A), rigid gel (B), and soft gel (C). Left panels, distribution of Fyn-GFP; middle panels, immunostainings for pY165Cas (phosphorylated Cas); and right panels, merge. (D) Immunostaining of SYF/Src-GFP cells plated for 1 h on FN-coated glass for pY165Cas (phosphorylated Cas). Scale bar, 10 μm. (E) Quantitative analysis of immunostainings for pY165Cas (phosphorylated Cas). Fluorescence intensities were measured across focal contacts (identified as areas with increased red signal toward the edge of the cells) for red channel; background fluorescence was subtracted, and average intensity was calculated. Cells included in analysis were representative of the larger cell population; at least 10 cells with minimum of 35 focal contacts were analyzed for each condition. Results are normalized against the average pCas signal in SYF/Fyn-GFP cells plated on FN-coated glass.
DISCUSSION
Cell spreading and motility on FN-coated substrates depends strongly on the rigidity of the substrates. Previous studies have shown that force-dependent reinforcement of FN contacts is mediated through αvβ3 integrins that form a complex with RPTPα at the leading edges of active lamellipodia (von Wichert et al., 2003). Further, SFKs are activated by RPTPα upon integrin-fibronectin interaction. We report here that SYF cells are defective in the FN rigidity response. Despite structural and sequence similarities between Src, c-Yes, and Fyn, we demonstrate that only Fyn restores the rigidity response in SYF cells. Although Fyn-GFP is localized to the leading edges of active lamellipodia and to early focal contacts, Src-GFP is not. Distribution of Fyn during early spreading, and its concomitant role in the rigidity response, depend both on palmitoylation and its activation by RPTPα. The prominent substrate of SFKs, Cas, is also required for the FN rigidity response and colocalizes with Fyn during early spreading. Furthermore, Fyn-mediated recruitment and phosphorylation of Cas in early focal contacts is rigidity-dependent.
Because there is extensive evidence that RPTPα activates SFKs upon cell binding to FN-coated surfaces, it is logical to predict that the RPTPα-dependent rigidity response involves SFKs. Similar to RPTPa−/− cells, the FN rigidity response is impaired in SYF cells. Of particular interest is the specificity of Fyn in restoring the rigidity response to SYF cells. Because Fyn is both myristoylated and palmitoylated, it has a high affinity for the cell membrane. Further, the palmitoyl-acyl transferase is localized to the plasma membrane, which can facilitate Fyn activation by RPTPα-mediated dephosphorylation (Wolven et al., 1997). There is also evidence that the leading edge contains specialized lipid domains that could facilitate the localization of Fyn (Kovalenko et al., 2004; Yang et al., 2004). In contrast, Src shows lower affinity for the membrane because it is only myristoylated, and it is not recruited by RPTPα at the membrane. This hypothesis is consistent with the result that inhibition of Fyn palmitoylation prevents the rigidity response and delays recruitment of Fyn to the leading edge and focal contacts. Also, introduction of a palmitoylation site in Src restores the rigidity response in SYF cells and causes its recruitment to the leading edge and focal contacts at earlier times. Although palmitoylated forms of Fyn, Src, or c-Yes do not produce the same morphology in SYF cells, palmitoylation is necessary and sufficient for Fyn and Src to restore the matrix rigidity response as well as for their localization to the edges of active lamellipodia during early spreading.
The rigidity response is an early response of cells to FN substrates and correlates with early focal complex formation. Maturation of adhesion complexes involves changes in the integrin components (in some cases) as well as in the nature of associated cytoplasmic proteins (Zaidel-Bar et al., 2003). During later phases of focal contact formation, Src is recruited to these structures through other pathways that do not involve RPTPα (Fincham et al., 2000; Volberg et al., 2001; Arias-Salgado et al., 2003). This indicates that RPTPα-mediated signaling in focal contact formation is crucial in early phases after initial interaction between the cells and ECM. In later phases, other pathways appear to compensate for the lack of RPTPα or any downstream components such as Fyn. Although Src and Fyn exhibit a certain level of redundancy, it appears that staged activation of these proteins in integrin signaling might be of crucial importance in motility and the rigidity response. Because there are many examples of cell motility signaling pathways that involve sequential protein activation, we believe that staged responses of SFKs might enable cells to respond to the changes in microenvironment in a timely manner, which provides for complex functions such as the rigidity response. The inhibition of growth on soft surfaces by the restoration of the rigidity response further reinforces the hypothesis that the early pathway of rigidity response has critical importance for cell function.
There are many substrates for the SFKs (Thomas et al., 1995; Thomas and Brugge, 1997; Volberg et al., 2001; Playford and Schaller, 2004), but one of the logical candidates for Fyn-dependent rigidity response is Cas. Phosphorylation of Cas was recently shown to depend dramatically on the mechanical stretching of cell cytoskeletons (Tamada et al., 2004), and Cas phosphorylation in early spreading cells was localized to edges of active lamellipodia (Yi et al., 2002; Abassi et al., 2003; Tamada et al., 2004). When we tested the rigidity response in Cas−/− cells, they showed behavior similar to SYF and RPTPα−/− cells. In SYF cells, Cas was not stabilized at active lamellipodial edges or in early focal complexes. However, expression of Fyn in SYF cells restored Cas localization to active edges and focal complexes. Thus, we suggest that mechanical-dependent phosphorylation of Cas may play a critical role in the Fyn-dependent rigidity response.
Rigidity response is a complicated process that underlies many cellular functions including cell growth and transformation. Soft matrices compromise normal cell growth and oncogenic transformation enables cells to grow on soft agar. Full activation of spreading of SYF cells with Fyn-GFP but not Src-GFP on rigid matrices correlates with rigidity-dependent growth. In contrast, SYF and Cas−/− cells grow equally well on rigid and soft substrates. Thus, we suggest that on soft surfaces there is an inhibition of growth by the components of the rigidity response pathway.
From the physical viewpoint, rigidity is defined as the amount of displacement per unit force on the surface. There are two major theoretically acceptable mechanisms for rigidity response: kinetic mechanisms where cells “measure” rigidity by the rate of increase in force as the cell pulls on the matrix or positional mechanisms where cells sense the distance that integrins move for a given force. We favor the latter mechanism because components of rigidity response are localized to the leading edges of active lamellipodia. These studies have now defined a leading edge pathway from rigid FN-integrin binding to the RPTPα-mediated activation and recruitment of Fyn to the leading edge. We postulate that Fyn then phosphorylates adjacent Cas in a force and position-dependent manner, and phospho-Cas catalyzes downstream signaling events. In addition, the fact that Cas is not involved in early spreading responses to the rigidity of collagen indicates that at least two parallel rigidity response mechanisms are present in fibroblasts.
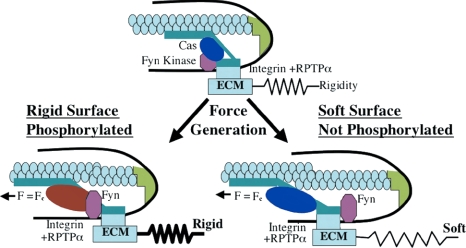
On the basis of the known physical and biochemical aspects of the components involved in the rigidity response, we propose a possible model for the FN matrix rigidity response (Figure 7). In the first step, FN binding to αvβ3 integrin activates RPTPα through a force-dependent conformational change. Activated RPTPα dephosphorylates Fyn, which is abundantly present in the submembrane compartment due to its palmitoylation, and Fyn is immobilized at the leading edge. In parallel, FN-bound integrins are linked to the actin cytoskeleton and are pulled rearward. If Cas is associated with the integrin–FN complex (possibly part of the integrin–cytoskeleton linkage), then a soft matrix could allow the complex to move relative to Fyn. On activation of Fyn and/or modification of Cas by force, we postulate that Fyn could phosphorylate the tyrosines in the substrate domain, depending on substrate rigidity. When the FN matrix is rigid, a short movement would create sufficient force to activate phosphorylation while Cas is adjacent to Fyn. When the FN matrix is soft, the complex would move a greater distance before creating sufficient force for activation, thereby sterically preventing Fyn phosphorylation. Thus, in this model the greater movement of Cas away from Fyn on soft surfaces would decrease Cas phosphorylation and all downstream signaling associated with a rigid matrix.
Figure 7.
Model of rigidity response though RPTPα-mediated Fyn activation. Schematic drawing depicts how RPTPα-mediated activation of Fyn upon cell adhesion to FN-coated substrate regulates rigidity response. Cells attach to rigid or soft FN-coated substrate through αvβ3 integrins, which causes formation of the integrin–cytoskeleton complex at the leading edge. This complex is connected to acto-myosin gel via actin-binding proteins in the complex. Inactive Fyn (pink) is bound to membrane due to palmitoylation. Force is exerted on the leading edge complex in response to matrix rigidity. At low forces, RPTPα is activated by a conformation change in the αvβ3 integrin, and it subsequently activates Fyn by dephosphorylating it. Simultaneously, rearward movement of actin begins driven by myosin motors. The leading edge complex moves along with the actin network, but at a slower rate because of the slip bond between integrins and cytoskeleton. On rigid substrates force is developed rapidly; therefore, it reaches the critical force (Fc) required for Fyn or Cas activation while all components are in close proximity. On soft substrates, the displacement needed to reach the activation force threshold is greater; therefore, inactive Cas (blue) moves away from Fyn before force-dependent activation can cause phosphorylation. On rigid substrates, phosphorylation of Cas (red) is followed by accumulation of focal contact proteins and reinforcement of the integrin–cytoskeleton bond. This stabilizes leading edge and accelerates cell spreading. On soft substrates, unphosphorylated Cas fails to play its role in reinforcement, which results in impaired spreading.
Many other studies suggest that Cas is an important component in various aspects of integrin signaling, which is consistent with our hypothesis that Cas is a crucial component in the rigidity response during early spreading. It has been shown that phosphorylated Cas may serve as a critical scaffolding protein for the activation of a number of small G proteins, including Rap1 and Rac (Cho and Klemke, 2000; Gotoh et al., 2000; Cho and Klemke, 2002; Tamada et al., 2004). In addition, Cas binds to Vav, which may have a critical role in activation of leading edge actin polymerization complexes (Arthur et al., 2004).
Both RPTPα (Ardini et al., 2000) and Cas (Brinkman et al., 2000; van der Flier et al., 2000) have been previously implicated in breast cancer, and SFKs are known proto-oncogenes. The pathway we propose appears to be general because knocking down expression of RPTPα in wild-type breast epithelial cells also leads to impaired rigidity response on FN. Based on our unpublished experiments, a similar pathway plays an important role in the interaction between neurons and fibronectin (Kostic and Sheetz, unpublished results). Thus, components described above are required for rigidity responses in various cell types. In addition, the engineering principle of recruiting a kinase and a force-dependent substrate to a localized region for probing the mechanical properties of the environment provides a general mechanism for the rigidity response.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Drs. J. Sap, J. Woodward, H. Hirai, and J. Massague for generous gifts of plasmids and cell lines. We also thank A. Meshel, H. G. Doeberreiner, and B. Dubin-Thaler for comments on the manuscript. This work was supported by the National Institutes of Health (to M.P.S.).
Abbreviations used:
- ECM
extracellular matrix
- FN
fibronectin
- RPTPα
receptor-like protein tyrosine phosphatase alpha
- SFK
Src family kinase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-12-1161) on April 5, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Abassi Y. A., Rehn M., Ekman N., Alitalo K., Vuori K. p130Cas Couples the tyrosine kinase Bmx/Etk with regulation of the actin cytoskeleton and cell migration. J. Biol. Chem. 2003;278:35636–35643. doi: 10.1074/jbc.M306438200. [DOI] [PubMed] [Google Scholar]
- Alland L., Peseckis S. M., Atherton R. E., Berthiaume L., Resh M. D. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J. Biol. Chem. 1994;269:16701–16705. [PubMed] [Google Scholar]
- Anders D. L., Blevins T., Sutton G., Chandler L. J., Woodward J. J. Effects of c-Src tyrosine kinase on ethanol sensitivity of recombinant NMDA receptors expressed in HEK 293 cells. Alcohol Clin. Exp. Res. 1999a;23:357–362. [PubMed] [Google Scholar]
- Anders D. L., Blevins T., Sutton G., Swope S., Chandler L. J., Woodward J. J. Fyn tyrosine kinase reduces the ethanol inhibition of recombinant NR1/NR2A but not NR1/NR2B NMDA receptors expressed in HEK 293 cells. J. Neurochem. 1999b;72:1389–1393. doi: 10.1046/j.1471-4159.1999.721389.x. [DOI] [PubMed] [Google Scholar]
- Ardini E., Agresti R., Tagliabue E., Greco M., Aiello P., Yang L. T., Menard S., Sap J. Expression of protein tyrosine phosphatase alpha (RPTPalpha) in human breast cancer correlates with low tumor grade, and inhibits tumor cell growth in vitro and in vivo. Oncogene. 2000;19:4979–4987. doi: 10.1038/sj.onc.1203869. [DOI] [PubMed] [Google Scholar]
- Arias-Salgado E. G., Lizano S., Sarkar S., Brugge J. S., Ginsberg M. H., Shattil S. J. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl. Acad. Sci. USA. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur W. T., Quilliam L. A., Cooper J. A. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J. Cell Biol. 2004;167:111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman A., van der Flier S., Kok E. M., Dorssers L. C. BCAR1, a human homologue of the adapter protein p130Cas, and antiestrogen resistance in breast cancer cells. J. Natl. Cancer Inst. 2000;92:112–120. doi: 10.1093/jnci/92.2.112. [DOI] [PubMed] [Google Scholar]
- Cary L. A., Han D. C., Polte T. R., Hanks S. K., Guan J. L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. H., Tzen J. T., Bresnick A. R., Chen H. C. Roles of Rho-associated kinase and myosin light chain kinase in morphological and migratory defects of focal adhesion kinase-null cells. J. Biol. Chem. 2002;277:33857–33863. doi: 10.1074/jbc.M204429200. [DOI] [PubMed] [Google Scholar]
- Cho S. Y., Klemke R. L. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J. Cell Biol. 2000;149:223–236. doi: 10.1083/jcb.149.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. Y., Klemke R. L. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J. Cell Biol. 2002;156:725–736. doi: 10.1083/jcb.200111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D., Felsenfeld D. P., Sheetz M. P. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Engler A., Bacakova L., Newman C., Hategan A., Griffin M., Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham V. J., Brunton V. G., Frame M. C. The SH3 domain directs acto-myosin-dependent targeting of v-Src to focal adhesions via phosphatidylinositol 3-kinase. Mol. Cell. Biol. 2000;20:6518–6536. doi: 10.1128/mcb.20.17.6518-6536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca P. M., Shin N. Y., Brabek J., Ryzhova L., Wu J., Hanks S. K. Regulation and localization of CAS substrate domain tyrosine phosphorylation. Cell Signal. 2004;16:621–629. doi: 10.1016/j.cellsig.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Giannone G., Dubin-Thaler B. J., Dobereiner H. G., Kieffer N., Bresnick A. R., Sheetz M. P. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- Gotoh T., Cai D., Tian X., Feig L. A., Lerner A. p130Cas regulates the activity of AND-34, a novel Ral, Rap1, and R-Ras guanine nucleotide exchange factor. J. Biol. Chem. 2000;275:30118–30123. doi: 10.1074/jbc.M003074200. [DOI] [PubMed] [Google Scholar]
- Honda H., et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat. Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- Jiang G., Huang A. H., Cai Y., Tanase M., Sheetz M. P. Rigidity sensing at the leading edge through αvβ3 Integrins and RPTPα. Biophys. J. 2006;90:1804–1809. doi: 10.1529/biophysj.105.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinghoffer R. A., Sachsenmaier C., Cooper J. A., Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko O. V., Yang X., Kolesnikova T. V., Hemler M. E. Evidence for specific tetraspanin homodimers: inhibition of palmitoylation makes cysteine residues available for cross-linking. Biochem. J. 2004;377:407–417. doi: 10.1042/BJ20031037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek M. J., et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pelham R. J., Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone A., Sap J. Emerging issues in receptor protein tyrosine phosphatase function: lifting fog or simply shifting? J. Cell Sci. 2000;113(Pt 13):2345–2354. doi: 10.1242/jcs.113.13.2345. [DOI] [PubMed] [Google Scholar]
- Playford M. P., Schaller M. D. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- Ponniah S., Wang D. Z., Lim K. L., Pallen C. J. Targeted disruption of the tyrosine phosphatase PTPalpha leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol. 1999;9:535–538. doi: 10.1016/s0960-9822(99)80238-3. [DOI] [PubMed] [Google Scholar]
- Resh M. D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Sap J., D’Eustachio P., Givol D., Schlessinger J. Cloning and expression of a widely expressed receptor tyrosine phosphatase. Proc. Natl. Acad. Sci. USA. 1990;87:6112–6116. doi: 10.1073/pnas.87.16.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Muranjan M., Sap J. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 1999;9:505–511. doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- Su J., Yang L. T., Sap J. Association between receptor protein-tyrosine phosphatase RPTPalpha and the Grb2 adaptor. Dual Src homology (SH) 2/SH3 domain requirement and functional consequences. J. Biol. Chem. 1996;271:28086–28096. doi: 10.1074/jbc.271.45.28086. [DOI] [PubMed] [Google Scholar]
- Tamada M., Sheetz M. P., Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev. Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Brugge J. S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Soriano P., Imamoto A. Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature. 1995;376:267–271. doi: 10.1038/376267a0. [DOI] [PubMed] [Google Scholar]
- Turner C. E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2000;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- van der Flier S., Brinkman A., Look M. P., Kok E. M., Meijer-van Gelder M. E., Klijn J. G., Dorssers L. C., Foekens J. A. Bcar1/p130Cas protein and primary breast cancer: prognosis and response to tamoxifen treatment. J. Natl. Cancer Inst. 2000;92:120–127. doi: 10.1093/jnci/92.2.120. [DOI] [PubMed] [Google Scholar]
- Volberg T., Romer L., Zamir E., Geiger B. pp60(c-src) and related tyrosine kinases: a role in the assembly and reorganization of matrix adhesions. J. Cell Sci. 2001;114:2279–2289. doi: 10.1242/jcs.114.12.2279. [DOI] [PubMed] [Google Scholar]
- von Wichert G., Jiang G., Kostic A., De Vos K., Sap J., Sheetz M. P. RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. J. Cell Biol. 2003;161:143–153. doi: 10.1083/jcb.200211061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolven A., Okamura H., Rosenblatt Y., Resh M. D. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Mol. Biol. Cell. 1997;8:1159–1173. doi: 10.1091/mbc.8.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Kovalenko O. V., Tang W., Claas C., Stipp C. S., Hemler M. E. Palmitoylation supports assembly and function of integrin-tetraspanin complexes. J. Cell Biol. 2004;167:1231–1240. doi: 10.1083/jcb.200404100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T., Georges P. C., Flanagan L. A., Marg B., Ortiz M., Funaki M., Zahir N., Ming W., Weaver V., Janmey P. A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskel. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- Yi J., Kloeker S., Jensen C. C., Bockholt S., Honda H., Hirai H., Beckerle M. C. Members of the Zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. J. Biol. Chem. 2002;277:9580–9589. doi: 10.1074/jbc.M106922200. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R., Ballestrem C., Kam Z., Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- Zheng X. M., Resnick R. J., Shalloway D. A phosphotyrosine displacement mechanism for activation of Src by PTPalpha. EMBO. J. 2000;19:964–978. doi: 10.1093/emboj/19.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X. M., Wang Y., Pallen C. J. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature. 1992;359:336–339. doi: 10.1038/359336a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.