Abstract
Newer insulins and easier blood glucose monitoring have greatly improved the ability to obtain excellent control of blood glucose levels with less risk of hypoglycemia. In type 1 diabetes, insulin pump therapy remains the optimal approach with the most flexibility, especially with the ultra-fast-acting analogs lispro or aspart. Otherwise, once- or twice-daily dosing with the long-acting analog glargine provides excellent basal coverage, and lispro or aspart at meals provides bolus coverage, all in the attempt to mimic physiological insulin secretion. For type 2 diabetes, although oral agents continue to be a mainstay of therapy, it is clear that many patients require insulin to attain the goal A1c of < 6.5%. Once-daily glargine is now used more commonly after 1-2 oral agents have failed, and it typically takes the place of sulfonylureas. The future will likely have better systems for continuous glucose monitoring and novel therapies to control glucose through agents that affect gut hormones.
Introduction
The treatment of diabetes was revolutionized by Banting and Best in the 1920's by the extraction of insulin from animal pancreases. Supplemental insulin administration remains the treatment for insulin deficiency, which characterizes type 1 diabetes. In type 2 diabetes, relative insulin deficiency occurs in the face of insulin resistance. In the fasting state, hepatic glucose output is matched by a continuous basal level of insulin, which maintains normal glucose levels. Many diabetes patients experience a "dawn phenomenon" in which glucose levels rise quite high in the early morning due to increased hepatic glucose output in response to rising levels of hormones, such as cortisol, that peak in the early morning. Disposal of a glucose load after a meal requires a rapid increase in insulin levels. It is often difficult to use subcutaneous insulin injections to match this rapid physiological insulin peak. The reasons include location and timing. In the normal physiological state, pancreatic insulin enters the portal vein and affects the liver first, where it is removed quickly by binding and degradation. Subcutaneously administered insulin, on the other hand, appears in the peripheral circulation first and is present for a long time, measured in hours. If one gives enough insulin to deal with the immediate postmeal glucose peak, then there is a risk of later hypoglycemia.[1-4]
Type 1 diabetic patients usually do not present the problem of insulin resistance, but often lack counterregulatory hormones and have very little or no endogenous insulin production. It is difficult to use subcutaneous insulin to duplicate the exquisitely timed insulin-release characteristics of the pancreas in response to changes in glucose levels. Amylin, which is cosecreted with insulin and affects a number of functions, including gastric emptying, is also deficient in type 1 diabetes. Thus, many type 1 diabetic patients have brittle glucose values, ranging from very high values to hypoglycemia. Improvement of glucose control has been proven to reduce the frequency of retinopathy, nephropathy, and neuropathy in type 1 and type 2 diabetes.[5,6]
The goal in managing glucose levels is to achieve normoglycemia, meaning an HbA1c less than 6.5%, a fasting glucose below 100 mg/dL, postprandial glucose below 140 mg/dL, and the avoidance of hypoglycemia. Most patients with either type 1 or type 2 diabetes present with various combinations of insulin deficiency and insulin resistance. Effective glycemic control is usually quite complex. Treatment must be individualized, with a variety of techniques to manage the features of each patient's glucose profile.
Typically, oral hypoglycemics are the initial treatment for type 2 diabetic patients. With an increasing duration of diabetes, multiple oral hypoglycemic agents in combination are usually required. The United Kingdom Prospective Diabetes Study (UKPDS) demonstrated a progressive loss of insulin-secretory capacity as diabetes progressed. As a result, in many type 2 diabetic patients, insulin injections are eventually needed to supplement levels of insulin that are relatively inadequate to overcome the insulin resistance.
The advent of self-monitoring of blood glucose (SMBG) with fingerstick devices and glucose oxidase test strips made it possible for patients to obtain immediate, precise feedback on their glucose levels. Thus, they were able to modify their insulin dosages much more precisely and with less risk of hypoglycemia. SMBG has dramatically improved the ability of patients to control their glucose levels.
The management of glucose levels in the type 1 diabetic patient is often complicated by wide glucose excursions, sometimes referred to as "brittle" diabetes. Therefore, frequent SMBG and multiple daily injections of insulin or use of an insulin pump, usually before each meal, are essential to achieve good glycemic control. It is a formidable challenge for the patient, the physician, and the rest of the diabetes team, including the nurse, dietician, Certified Diabetes Educator, and others, to carry out these programs of tight glycemic control. However, the benefits of tight control, in terms of prevention of complications, are worth the effort, the risks, and the expense. Therefore, intensive insulin regimens need to be offered to all type 1 diabetic patients and are the standard of care.
In giving insulin therapy to both type 1 and type 2 diabetic patients, the issues are overall dose amounts and the timing of insulin delivery. Fortunately, there have been a number of developments that improve our capability to deliver insulin with better timing characteristics. With regard to short-acting insulin, lispro insulin (Humalog) and insulin aspart (Novolog) are synthetic products with an exchange of amino acids in the polypeptide chains that make up insulin. As a result of these changes, lispro and aspart insulin are more quickly absorbed from subcutaneous tissue and disappear more quickly.[7-11] Thus, it is possible to give a dose much closer (often 10-15 minutes) to mealtime than with regular insulin, and there is less risk of hypoglycemia at a later time.[12-14] It is also easier to give larger doses than with regular insulin, creating higher peaks to deal with postmeal glucose levels with less risk of hypoglycemia.
Lispro/aspart insulin has largely replaced regular insulin in the management of mealtime glucose levels in type 1 diabetes. Lispro/aspart insulin is effectively used in insulin infusion pumps[15,16] and in pregnant diabetic women.[17] However, there are certainly patients who require a longer insulin peak post meals. In these patients, regular insulin may still have a place and even longer acting insulins may be needed.
On the other end of the insulin-action spectrum, it is necessary to have a long-acting injected insulin that provides a basal insulin level. This basal level must remain low between meals to avoid hypoglycemia. NPH and Lente insulin, and even Ultralente insulin, have definite peak-and-trough levels that may be unpredictable and contribute to unexpected hyperglycemia and hypoglycemia. The advent of glargine insulin, another insulin analog, affords us an insulin with very consistent, long-acting flat insulin levels. Glargine insulin carries several amino acid modifications, in particular, 2 arginine substitutions, which result in precipitation at physiological pH. As a result of this precipitation, a depot of insulin is formed resulting in a slow release of insulin into the circulation. Glargine insulin is given as a once-daily injection and maintains a relatively constant insulin level over a 24-hour period. Thus, there are fewer peaks of intermediate-acting insulin at unplanned times as with NPH, Lente, or Ultralente (Figure 1).
Figure 1.
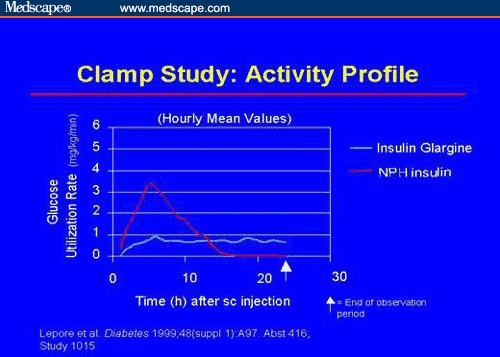
Glucose clamp comparison of glargine insulin vs NPH: NPH insulin has a peak activity, whereas glargine insulin has a very flat profile.
The use of glargine may afford smoother control in the type 1 diabetic patient.[18,19] It is also very useful as a supplemental insulin dose in type 2 diabetic patients in whom oral hypoglycemic combinations no longer can meet targets.[20,21] Occasionally, in type 1 diabetic patients, glargine seems to wear off in less than 24 hours and has to be given twice daily for optimal control.
Continuous Subcutaneous Infusion of Insulin
The administration of insulin by continuous subcutaneous infusion of insulin (CSII) has great advantages in terms of permitting the programmed timing of insulin levels. At present, insulin pumps are available, which permit preprogrammed delivery of basal insulin profiles as well as quick premeal infusion of bolus insulin doses. Insulin pumps typically deliver insulin through an indwelling subcutaneous catheter, which must be changed every 48-72 hours. Modern insulin pumps have proven safe and effective in a wide variety of type 1 and type 2 diabetic patients, representing all ages and education levels, and are very well accepted by patients. Implanted pumps have also been developed.[22-24] The implanted pumps deliver insulin directly into the peritoneal cavity and then to the portal venous system, thus allowing a first pass in the liver before the peripheral circulation, similar to that of normal physiological pancreatic insulin. However, the catheters have tended to clog over time, and the need to change the internal catheter surgically on an annual or more frequent schedule has constituted an impediment to dissemination of the implanted pumps.[25,26]
The great revolution in insulin pump use will be the advent of automated glucose feedback control. The algorithms to administer insulin in response to glucose levels are successful in maintaining target levels.[27,28] The problem has been the lack of continuous glucose monitoring feedback. The ability to sample minute amounts of skin fluid for glucose promises to remedy this problem. An external glucose oxidase electrode has been developed and used successfully by Medtronic MiniMed, named the Continuous Glucose Monitoring System (CGMS). The present-day device is similar in appearance to an insulin pump and does require a similar indwelling subcutaneous catheter.[29,30] The device is downloaded at the end of 3 days of use and provides a retrospective tracing of approximately 288 blood glucose values for each of the 3 days. Analysis of this can help both the patient and the physician to better manage meals, exercise, and high and low blood glucose levels, and to improve glucose control with less hypoglycemia.[31-33] The device has not yet been approved for use by patients to control their insulin pump delivery, but we expect this evolution in the next few years.
Another device attempting automated, minimally invasive glucose monitoring is the GlucoWatch. Again, the principle is that of subcutaneous fluid sampling, in this case by reverse iontophoresis. The device sits on the wrist and samples many times during a 12-hour period and provides values via an electric current, which brings up fluid for measurement. Use of the GlucoWatch has been limited by discomfort and local skin irritation, shut down by heat or humidity, and related to technical problems.[34-37]
Clearly, the use of wireless communication between a continuous glucose sampling device and an insulin pump is the next step. It will make possible truly automatic glucose regulation, a closed-loop system or an "electromechanical pancreas." We expect that external insulin pumps coupled with effective glucose feedback will be available within the next 5 years. This will make the concept of the "artificial pancreas" a reality. These devices will be more expensive as compared with present-day treatment approaches, both in the initial hardware costs and in ongoing supplies, but will be an excellent value if they really provide optimal glycemic control.
The coming of inhaled insulin could offer a novel approach to the management of diabetes. Insulin is a relatively small peptide that can be absorbed by the mucosal surface. The lungs provide an enormous surface area for absorption. Only a fraction of inhaled insulin is actually absorbed. It was always believed that variable absorption would lead to erratic glucose levels. The development of dry microparticulate powders has improved homogeneous, reproducible absorption. The rapid absorption and disappearance of inhaled insulin provide excellent timing, similar to that of lispro and aspart insulin. As a result, inhaled insulin at meals does permit good control of glucose levels without excessive hypoglycemia (Figure 2).[38,39]
Figure 2.
Inhaler for insulin powder.
Inhaled insulin is short-acting with timing characteristics similar to lispro insulin. Thus, its use must be coupled with a long-acting insulin to maintain appropriate basal levels. Glargine insulin used with inhaled insulin is a most promising combination for type 1 diabetic patients. It is expected that most such patients could be managed with 1 glargine insulin shot daily and multiple inhalations of short-acting insulin.
To be sure, glucose control with inhaled insulin taken before meals is likely to be less optimal than that achievable with glucose feedback-controlled continuous insulin delivery. Furthermore, there are significant questions as to possible long-term effects on the lungs. A large amount of immunogenic protein is delivered to the lung surface with inhaled insulin. If inhaled insulin is proven safe in long-term studies, we expect that its use will be driven by patient demand. Present-day inhaled insulin delivery systems are large and cumbersome. The systems will, no doubt, develop improved usage characteristics. Yet, patients, even today, are highly enthusiastic about the concept of needleless insulin administration. This enthusiasm is clearly a matter of personal perception. Obviously, an insulin pump requires a needlestick only once in a 24-72-hour period. Yet, there does appear to be a remarkable desire to obtain the inhaled devices.
We expect that the innovations in insulin management and glucose feedback will make it possible to control glucose levels much closer to the goal of normal HbA1c in type 1 diabetes. We also expect that the use of supplemental insulin with oral agents, which reduce resistance to insulin, will achieve this goal in type 2 diabetes In the 21st century, we expect to see the benefits of normalization of glucose levels on the serious microvascular and macrovascular complications of diabetes.
Footnotes
The author received a grant from the Association of Diabetes Investigators to support the preparation of this manuscript. This grant was partially supported by unrestricted educational grants from Aventis, GlaxoSmithKline, Novartis, Takeda, and Sanofi-Synthelabo.
This article is supported by an independent educational grant from Pfizer, Inc.
References
- 1.Stephenson JM, Schernthaner G. Dawn phenomenon and Somogyi effect in IDDM. Diabetes Care. 1989;12:245-251. [DOI] [PubMed] [Google Scholar]
- 2.Perriello G, De Feo P, Torlone E, et al. The dawn phenomenon in type 1 (insulin-dependent) diabetes mellitus: magnitude, frequency, variability, and dependency on glucose counterregulation and insulin sensitivity. Diabetologia. 1991;34:21-28. [DOI] [PubMed] [Google Scholar]
- 3.Arslanian S, Ohki Y, Becker DJ, Drash AL. The dawn phenomenon: comparison between normal and insulin-dependent diabetic adolescents. Pediatr Res. 1992;31:203-206. [DOI] [PubMed] [Google Scholar]
- 4.Bolli GB, Perriello G, Fanelli CG, De Feo P. Nocturnal blood glucose control in type I diabetes mellitus. Diabetes Care. 1993;16(suppl3):71-89. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes in the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853. [PubMed] [Google Scholar]
- 7.Koivisto VA. The human insulin analogue insulin lispro. Ann Med. 1998;30:260-266. [DOI] [PubMed] [Google Scholar]
- 8.Colombel A, Murat A, Krempf M, Kuchly-Anton B, Charbonnel B. Improvement of blood glucose control in Type 1 diabetic patients treated with lispro and multiple NPH injections. Diabet Med. 1999;16:319-324. [DOI] [PubMed] [Google Scholar]
- 9.Lalli C, Ciofetta M, Del Sindaco P, et al. Long-term intensive treatment of type 1 diabetes with the short-acting insulin analog lispro in variable combination with NPH insulin at mealtime. Diabetes Care. 1999;22:468-477. [DOI] [PubMed] [Google Scholar]
- 10.Mudalier SR, Lindberg FA, Joyce M, et al. Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care. 1999;22:1501-1506. [DOI] [PubMed] [Google Scholar]
- 11.Raskin P, Guthrie RA, Leiter L, Riis A, Jovanovic L. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care. 2000;23:583-588. [DOI] [PubMed] [Google Scholar]
- 12.Heller SR, Amiel SA, Mansell P. Effect of the fast-acting insulin analog lispro on the risk of nocturnal hypoglycemia during intensified insulin therapy. U.K. Lispro Study Group. Diabetes Care. 1999;22:1607-1611. [DOI] [PubMed] [Google Scholar]
- 13.Holleman F, Schmitt H, Rottiers R, Rees A, Symanowski S, Anderson JH. Reduced frequency of severe hypoglycemia and coma in well-controlled IDDM patients treated with insulin lispro. The Benelux-UK Insulin Lispro Study Group. Diabetes Care. 1997;20:1827-1832. [DOI] [PubMed] [Google Scholar]
- 14.Chase HP, Lockspeiser T, Peery B, et al. The impact of the diabetes control and complications trial and humalog insulin on glycohemoglobin levels and severe hypoglycemia in type 1 diabetes. Diabetes Care. 2001;24:430-434. [DOI] [PubMed] [Google Scholar]
- 15.Melki V, Renard E, Lassmann-Vague V, et al. Improvement of HbA1c and blood glucose stability in IDDM patients treated with lispro insulin analog in external pumps. Diabetes Care. 1998;21:977-982. [DOI] [PubMed] [Google Scholar]
- 16.Hanaire-Broutin H, Melki V, Bessieres-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. The Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care. 2000;23:1232-1235. [DOI] [PubMed] [Google Scholar]
- 17.Jovanovic L, Ilic S, Pettitt DJ, et al. Metabolic and immunologic effects of insulin lispro in gestational diabetes. Diabetes Care. 1999;22:1422-1427. [DOI] [PubMed] [Google Scholar]
- 18.Raskin P, Klaff L, Bergenstal R, Halle JP, Donley D, Mecca T. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care. 2000;23:1666-1671. [DOI] [PubMed] [Google Scholar]
- 19.Rosenstock J, Park G, Zimmerman J. Basal insulin glargine (HOE 901) versus NPH insulin in patients with type 1 diabetes on multiple daily insulin regimens. U.S. Insulin Glargine (HOE 901) Type 1 Diabetes Investigator Group. Diabetes Care. 2000;23:1137-1142. [DOI] [PubMed] [Google Scholar]
- 20.Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136. [DOI] [PubMed] [Google Scholar]
- 21.Rosenstock J, Schwartz SL, Clark CM Jr, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636. [DOI] [PubMed] [Google Scholar]
- 22.Lassmann-Vague V, Guerci B, Hanaire-Broutin H, et al. Use of implantable insulin pumps: the EVADIAC position. Recommendations of ALFEDIAM (French Language Association for the Study of Diabetes and Metabolic Diseases). Evaluation dans le Diabete du Traitement par Implants Actifs. Diabetes Metab. 1997;23:234-250. [PubMed] [Google Scholar]
- 23.Duckworth WC, Saudek CD, Giobbie-Hurder A, et al. The Veterans Affairs Implantable Insulin Pump Study: effect on cardiovascular risk factors. Diabetes Care. 1998;21:1596-1602. [DOI] [PubMed] [Google Scholar]
- 24.Jaremko J, Rorstad O. Advances toward the implantable artificial pancreas for treatment of diabetes. Diabetes Care. 1998;21:444-450. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JS, Duckworth WC, Saudek CD, Giobbie-Hurder. Surgical experience with implantable insulin pumps. Department of Veterans Affairs Implantable Insulin Pump Study Group. Am J Surg. 1998;176:622-626. [DOI] [PubMed] [Google Scholar]
- 26.Belicar P, Lassmann-Vague V. Local adverse events associated with long-term treatment by implantable insulin pumps. The French EVADIAC Study Group experience. Evaluation dans le Diabete du Traitement par Implants Actifs. Diabetes Care. 1998;21:325-326. [DOI] [PubMed] [Google Scholar]
- 27.Mokan M, Gerich JE. A simple insulin infusion algorithm for establishing and maintaining overnight near-normoglycemaia in type I and type II diabetes. J Clin Endocrinol Metab. 1992;74:943-945. [DOI] [PubMed] [Google Scholar]
- 28.Parker RS, Doyle FJ III, Peppas NA. A model-based algorithm for blood glucose control in type I diabetic patients. IEEE Trans Biomed Eng. 1999;46:148-157. [DOI] [PubMed] [Google Scholar]
- 29.Mastrototaro J. The MiniMed Continuous Glucose Monitoring System (CGMS). J Pediatr Endocrinol Metab. 1999;12(suppl3):751-758. [PubMed] [Google Scholar]
- 30.Chase HP, Kim LM, Owen SL, et al. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics. 2001;107:222-226. [DOI] [PubMed] [Google Scholar]
- 31.Bode BW, Gross TM, Thornton KR, Mastrototaro JJ. Continuous glucose monitoring used to adjust diabetes therapy improves glycosylated hemoglobin: a pilot study. Diabetes Res Clin Pract. 1999;46:183-190. [DOI] [PubMed] [Google Scholar]
- 32.Bode BW, Lane W, Levetan R, et al. Therapy adjustments based on CGMS data lower HbA1c with less hypoglycemia than blood glucose meter data alone. Diabetes. 2003;52(suppl1):386. [Google Scholar]
- 33.Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics. 2003;111:933-938. [DOI] [PubMed] [Google Scholar]
- 34.Tamada JA, Garg S, Jovanovic L, Pitzer KR, Fermi S, Potts RO. Noninvasive glucose monitoring: comprehensive clinical results. Cygnus Research Team. JAMA. 1999;282:1839-1844. [DOI] [PubMed] [Google Scholar]
- 35.Garg SK, Potts RO, Ackerman NR, Fermi SJ, Tamada JA, Chase HP. Correlation of fingerstick blood glucose measurements with GlucoWatch biographer glucose results in young subjects with type 1 diabetes. Diabetes Care. 1999;22:1708-1714. [DOI] [PubMed] [Google Scholar]
- 36.Tierney MJ, Tamada JA, Potts RO, et al. The GlucoWatch biographer: a frequent automatic and noninvasive glucose monitor. Ann Med. 2000;32:632-641. [DOI] [PubMed] [Google Scholar]
- 37.Pitzer KR, Desai S, Dunn T, et al. Detection of hypoglycemia with the GlucoWatch biographer. Diabetes Care. 2001;24:881-885. [DOI] [PubMed] [Google Scholar]
- 38.Cefalu WT, Skyler JS, Kourides IA, et al. Inhaled human insulin treatment in patients with type 2 diabetes mellitus. Ann Intern Med. 2001;134:203-207. [DOI] [PubMed] [Google Scholar]
- 39.Skyler JS, Cefalu WT, Kourides IA, et al. Efficacy of inhaled human insulin in type 1 diabetes mellitus: a randomised proof-of-concept study. Lancet. 2001;357:331-335. [DOI] [PubMed] [Google Scholar]


