Ca2+ is a major determinant of many biochemical processes in various cell types, from the beginning of new life and egg fertilization to the end of life and cell death.1 In vascular smooth muscle (VSM), physiological resting levels of intracellular free Ca2+ concentration ([Ca2+]i) in the nanomolar range are necessary to maintain basal vascular tone.2-4 VSM activation is associated with an increase in [Ca2+]i in the micromolar range,2-4 and large increases in VSM [Ca2+]i have been identified in excessive vasoconstriction disorders such as hypertension and coronary vasospasm.2,5 Activation of surface membrane receptors in VSM triggers increases in [Ca2+]i due to Ca2+ release from the intracellular stores in the sarcoplasmic reticulum, and Ca2+ entry from the extracellular space through Ca2+ channels. Ca2+ then activates specific protein kinases and phospatases that are involved in VSM contraction and relaxation.2,3,6 Ca2+ may also function as a second messenger to activate other signaling pathways such as cytosolic phospholipase A2α, phospholipase C, protein kinase C and phosphodiesterase.6-8 An increase in [Ca2+]i could also modulate plasma membrane channels and pumps such as Ca2+-activated K+ channels and the plasma membrane CaATPase (PMCA).9,10 Additionally, Ca2+ may affect sarcoplasmic reticulum channels and pumps such as the inositol 1,4,5-trisphosphate (IP3) receptor, the ryanodine-sensitive receptor and intracellular Ca2+ release channels, and the Ca2+ uptake pump (SERCA).11,12
Calmodulin, a Ca2+ Sensor and Regulatory Protein
Ca2+ performs most of its functions by interacting with specific Ca2+ binding proteins, which serve as Ca2+ sensors and regulatory proteins. Calmodulin (CaM) is a critical Ca2+ sensor and regulatory protein in VSM. CaM is a ubiquitous small acidic protein of 16.7 kDa that is involved in virtually all Ca2+-dependent intracellular events.2,3 The total intracellular concentration of CaM in the cell is significantly lower than the total concentration of its intracellular targets, making it a limiting factor in their regulation. CaM contains four EF-hand Ca2+ binding sites. The formation of Ca2+/CaM complex is necessary to activate myosin light chain (MLC) kinase, leading to MLC phosphorylation, actin-myosin interaction and VSM contraction. On the other hand, Ca2+/CaM-dependent protein phosphatase initiates MLC dephosphorylation and VSM relaxation. Also, Ca2+/CaM-dependent protein kinase II (CaMKII) is a ubiquitous mediator of Ca2+-linked signaling pathways that phosphorylates a wide range of substrates to coordinate and regulate Ca2+-mediated cellular functions.13-15 For example, CaMKII phosphorylates and inactivates MLC kinase, a process that may be essential in the regulation of VSM contraction.16
Ca2+/CaM in VSM Cell Cycle and Growth
Vascular tissue injury in response to hypoxia, hyperlipidemia, oxidative stress and nicotine smoking is associated with increased production of growth factors/promoters such as hypoxia-inducible factor, epidermal growth factor, fibroblast growth factor, platelet derived growth factor, angiotensin II, and endothelin-1.17-22 The growth factors stimulate the expression and phosphorylation of ERK1/2 and JNK, and increase the DNA binding activity of activator protein-1 (AP-1) and nuclear factor-kB (NF-kB).17,23 These nuclear events induce the phenotypic transformation of VSM into undifferentiated rapidly growing cells. VSM cell (VSMC) growth contributes to the pathogenesis of vascular hypertrophic disorders such as hypertension, atherosclerosis and vascular restenosis following angioplasty.24-26
Studies have implicated Ca2+ as a regulator of mammalian cell cycle during early G1 phase and near the G1 to S phase transition.27 However, the molecular mechanisms underlying the Ca2+-sensitivity of the G1/S phase transition in VSM cell cycle have not been clearly elucidated. Also, while some studies have suggested a role for CaM in cell cycle and have shown that Ca2+/CaM is required for cell proliferation in both unicellular and multicellular eukaryotes,27,28 the specific molecular targets of the Ca2+/CaM-dependent pathways are unclear.
Potential Ca2+/CaM-dependent targets include the serine/threonine phosphatase calcineurin and the family of multifunctional Ca2+/CaM-dependent protein kinases (CaMKs). In mammalian cells, both types of enzymes contribute to the regulation of cell cycle progression. However, the mechanism by which Ca2+/CaM and its downstream targets, particularly calcineurin and CaMKs, regulate key cell cycle-regulatory proteins, remains enigmatic. By understanding how Ca2+/CaM regulates cell cycle progression in normal mammalian cells, we would gain insight into how hormones control cell division, and how VSMCs coordinate Ca2+ and its downstream targets during the cell transformation into the rapidly growing proliferative phenotype.28
Ca2+/CaM-Cyclin Interaction during VSM Cell Cycle
The passage of a cell through the cell cycle is controlled by proteins in the cytoplasm called cyclins. Recent studies have revealed the important role of cyclins in vascular and cardiac tissue injury, inflammation and wound repair.29 Cyclins bind to and activate specific cyclin-dependent kinases (CDKs), which in turn phosphorylate a variety of protein substrates that control the cell cycle. While the levels of CDKs in the cell remain fairly stable, the levels of certain cyclins rise and fall with the specific stages of the cell cycle. Cyclin D and CDK4 mainly control the G1 phase, cyclins A and E and CDK2 control the S-phase, while cyclins A and B and CDK1 control the mitotic phase. Growth factors stimulate the expression and tyrosine phosphorylation of ERK-1, which in turn promote the expression of cyclins and induce VSMC growth.21 On the other hand, a decrease in tyrosine phosphorylation of ERK1/2 reduces the expression of cyclins D1 and E, resulting in G1 phase cell cycle arrest and inhibition of VSMC proliferation.30
Studies have shown that depletion of Ca2+ pools in VSMC using the SERCA inhibitor thapsigargin causes inhibition of translocation of activated ERK1/2 to the nucleus, and prevents cyclin D1 expression, thus delaying the progression into the S-phase and the cell cycle.31 Also, forced gene expression of SERCA2a in a model of balloon injury of the rat carotid artery is associated with decreased cyclin D1 in VSMCs, cell cycle arrest at the G1 phase, and reduced VSMC proliferation and neointima formation.32 Although these studies suggest possible effects of intracellular Ca2+ on cyclin expression and the CDK activity, the specific molecular interactions and targets involved have not been clarified.
In this issue of Circulation Research Choi and colleagues describe a possible CaM binding site on cyclin E which could be involved in Ca2+-sensitive G1/S transition in VSMCs.33 They report that the kinase activity of cyclin E/CDK2 was responsive to physiological changes in Ca2+ concentration. Pharmacological inhibition of CaM using calmidazolium abrogated the Ca2+-sensitivity of cyclin E/CDK2, retarded VSMC proliferation and caused cell cycle arrest at G1 phase. A highly conserved 22 aa N-terminal CaM-binding motif in cyclin E genes was essential in mediating the Ca2+-sensitive kinase activity of cyclin E/CDK2. Mutant cyclin E protein, lacking this CaM-binding motif, did not respond to alterations in Ca2+ concentration. These data clearly suggest that CaM-dependent cyclin E/CDK2 activity could mediate the Ca2+-sensitivity of the G1/S transition in VSM cell cycle. These novel findings not only further elucidate the molecular mechanisms underlying VSMC growth, but may also help in the design of therapies that specifically aim at Ca2+/CaM-dependent cyclin E/CDK2 activity and can be used in the prevention/treatment of hypertrophic VSMC disorders. The study also raises interest in further understanding the role of Ca2+, CaM and cyclins in VSMC proliferation and the factors that regulate the levels of the Ca2+/CaM complex and the cyclin/CDK activity.
Regulation of Ca2+/CaM in quiescent and growing VSMC
An important point relates to the level of Ca2+ that triggers the G1/S transition. Because of its small molecular size, Ca2+ is expected to rapidly and homogeneously distribute inside the cell. However, some studies suggest localized Ca2+ gradients and sparks in the subplasmalemmal region and perinuclear sites.4,34 Also, the regulation of Ca2+ in the nucleus, where important Ca2+-sensitive transcriptional processes reside, is a debated issue.35 Cells may also show oscillations in [Ca2+]i,4 and it is possible that the levels of the Ca2+/CaM complex may mirror those of the Ca2+ oscillations. In effect, the levels of Ca2+/CaM may play a role in the intracellular Ca2+ oscillatory behavior and in the regulation of the Ca2+ mobilization mechanisms. IP3 receptors in the sarcoplasmic reticulum are tetrameric intracellular Ca2+ channels, the opening of which is regulated by both IP3 and Ca2+. IP3 receptors are biphasically regulated by cytosolic Ca2+, which binds to two distinct sites. IP3 promotes channel opening by controlling whether Ca2+ binds to the stimulatory or inhibitory sites. Inhibition of IP3 receptors by Ca2+ may require CaM. It is likely that one lobe of CaM tethers it to the IP3 receptor, while the other lobe binds Ca2+ and then interact with a second site on the receptor to cause its inhibition.11
Also, the CaATPase (commonly called the Ca2+ pump) is a fine-tuner of intracellular Ca2+. The plasma membrane CaATPase (PMCA) plays a role in Ca2+ extrusion. PMCA is a large enzyme, with 10 transmembrane domains and a C-terminal cytosolic tail that contains regulatory sites, including a CaM-binding domain.10 The sarcoplasmic reticulum CaATPase (SERCA) plays a role in Ca2+ reuptake. Reported post-translational modifications affecting SERCA pump activity involve N-glycosylation, glutathionylation and CaMKII-dependent phosphorylation.12
CaM may also be regulated by phosphorylation. CaM is phosphorylated both in vitro and in vivo by multiple serine/threonine and tyrosine protein kinases. Casein kinase II and MLC kinase are two serine/threonine kinases that have been implicated in this process. Also, within the tyrosine kinases involved in CaM phosphorylation are receptors with tyrosine kinase activity, such as the insulin receptor and the epidermal growth factor receptor, and nonreceptor tyrosine kinases, such as the Src family kinases, Janus kinase 2, and p38Syk. CaM phosphorylation brings important consequences for the physiological cell function and the cell growth as the phosphoCaM species have differential actions as compared to nonphosphorylated CaM when acting on different CaM-dependent systems.36
Regulation of cyclin/CDK activity
The activity of CDKs is controlled not only by Ca2+/CaM and cyclins, but also by CDK inhibitors (CKIs). Growth promotion is associated with upregulation of cyclins and CDKs and downregulation of CKIs, and the reverse occurs during growth arrest. The p16(INK4a). p21(Cip1/Waf1), p27(Kip1) and p57(Kip2) are known inhibitors of CDK.23,37,38 Studies have shown that PPAR-α, a nuclear receptor that regulates lipid metabolism and inflammation, controls VSM cell cycle progression at the G1/S transition by targeting the CKI p16(INK4a).39 Also, downregulation of p27(kip1) and p57(kip2) in response to mitogenic stimulation plays a key role in the VSM cell cycle progression.38 Additionally, the mitogenic effect of hyperlipemic sera and ox-LDL in VSMC may occur via inhibition of p21(Cip1) expression, and subsequent increase in DNA synthesis and cell proliferation.22 Furthermore, antioxidants not only downregulate cyclins D and E and CDKs 2 and 4, but also upregulate p21(Cip 1) and p27(Kip 1), leading to inhibition of CDK and arrest of cell cycle progression.23 Nevertheless, it is not clear whether reduction in [Ca2+]i and Ca2+/CaM would stimulate the CKIs.
Also, the potential role of newly discovered Ca2+ sensors and binding proteins in the regulation of cyclin/CDK activity and VSM cell cycle should be investigated. A novel Ca2+- binding protein called DREAM has been shown to interact with regulatory sequences of DNA, thereby acting as a Ca2+-dependent transcriptional regulator.40 Expression of the human prodynorphin gene, which is involved in memory acquisition and pain, is regulated through its downstream regulatory element (DRE) sequence. The transcriptional repressor DRE-antagonist modulator (DREAM) specifically binds to the DRE. DREAM contains four Ca2+- binding domains of the EF-hand type. Upon stimulation by Ca2+, DREAM’s ability to bind to the DRE and its repressor function are prevented. Mutation of the EF-hands abolishes the response of DREAM to Ca2+. Also, S100B, a dimeric EF-hand Ca2+-binding protein, interacts with the cell growth suppressor p53 and controls its transcriptional activity.41
Finally, the effect of other cations on the VSM cell cycle remains to be clarified. For instance, Mg2+ is known to affect Ca2+ entry into VSM and to counteract the effects of Ca2+ on VSM contraction. However, studies have shown VSM cell cycle activation and growth regulation by Mg2+ via ERK1/2-dependent, p38 MAP kinase-independent pathways.42
Thus Ca2+ remains to be a master regulator of VSM contraction and growth. Several studies have clarified the role of CaM as a major Ca2+ sensor that regulates the activity of VSM channels, pumps and contractile proteins. New careful studies are now beginning to shed light on the intricate interaction between Ca2+, CaM, and cyclins in the regulation of VSM growth and proliferation.
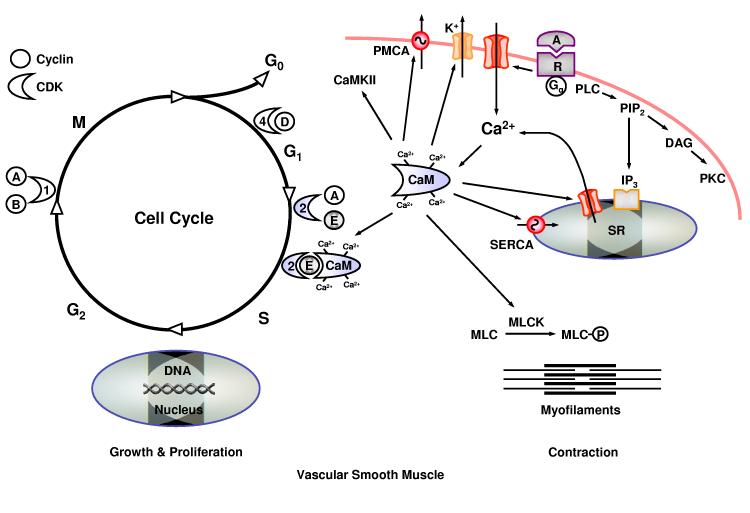
Agonist (A)-receptor (R) interaction increases the hydrolysis of phosphatidylinositol 4,5- bisphosphate (PIP2) and IP3 production in VSM. IP3 stimulates Ca2+ release from the sarcoplasmic reticulum (SR). Also, extracellular Ca2+ enters VSMC through Ca2+ channels. Ca2+ binds calmodulin (CaM), which could activate myosin light chain (MLC) kinase and initiate contraction, or regulate the activity of K+ channels, Ca2+ release channels, Ca2+ pumps, and CaM kinase II. During vascular injury, VSMC transforms into undifferentiated phenotype and enters a cell cycle which consists of G1, growth and preparation of the chromosomes for replication; S, synthesis of DNA; G2, preparation for mitosis; and M, mitosis. Ca2+/CaM increases the activity of cyclin E/CDK2 and stimulates G1/S transition, and thereby promotes VSM growth and proliferation.
ACKNOWLEDGEMENTS
The authors acknowledge the support of grants from the National Heart, Lung & Blood Institute (HL65998, HL70659).
Contributor Information
Vera V. Koledova, Division of Vascular Surgery, Brigham and Women’s Hospital, Boston, Massachusetts 02115
Raouf A. Khalil, Harvard Medical School, Boston, Massachusetts 02115
REFERENCES
- 1.Santella L, Ercolano E, Nusco GA. The cell cycle: a new entry in the field of Ca2+ signaling. Cell Mol Life Sci. 2005;62(21):2405–13. doi: 10.1007/s00018-005-5083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil RA, van Breemen C. Mechanisms of calcium mobilization and homeostasis in vascular smooth muscle and their relevance to hypertension. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management. Raven Press; New York: 1995. pp. 523–40. [Google Scholar]
- 3.Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev. 1996;76(4):967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- 4.Lee CH, Poburko D, Kuo KH, Seow CY, van Breemen C. Ca2+ oscillations, gradients, and homeostasis in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282(5):H1571–83. doi: 10.1152/ajpheart.01035.2001. [DOI] [PubMed] [Google Scholar]
- 5.Cain AE, Khalil RA. Pathophysiology of essential hypertension: role of the pump, the vessel, and the kidney. Semin Nephrol. 2002;22(1):3–16. [PubMed] [Google Scholar]
- 6.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70(11):1537–47. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirabayashi T, Murayama T, Shimizu T. Regulatory mechanism and physiological role of cytosolic phospholipase A2. Biol Pharm Bull. 2004;27(8):1168–73. doi: 10.1248/bpb.27.1168. [DOI] [PubMed] [Google Scholar]
- 8.Goraya TA, Cooper DM. Ca2+-calmodulin-dependent phosphodiesterase (PDE1): current perspectives. Cell Signal. 2005;17(7):789–97. doi: 10.1016/j.cellsig.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 10.Guerini D, Coletto L, Carafoli E. Exporting calcium from cells. Cell Calcium. 2005;38(34):281–9. doi: 10.1016/j.ceca.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Taylor CW, Laude AJ. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+ Cell Calcium. 2002;32(56):321–34. doi: 10.1016/s0143416002001859. [DOI] [PubMed] [Google Scholar]
- 12.Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium. 2005;38(34):291–302. doi: 10.1016/j.ceca.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Zhou ZH, Ando S, Furutsuka D, Ikebe M. Characterization of Ca2+/calmodulin-dependent protein kinase II from smooth muscle. Biochem J. 1995;310(Pt 2):517–25. doi: 10.1042/bj3100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim I, Je HD, Gallant C, Zhan Q, Riper DV, Badwey JA, Singer HA, Morgan KG. Ca2+- calmodulin-dependent protein kinase II-dependent activation of contractility in ferret aorta. J Physiol. 2000;526(Pt 2):367–74. doi: 10.1111/j.1469-7793.2000.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfleiderer PJ, Lu KK, Crow MT, Keller RS, Singer HA. Modulation of vascular smooth muscle cell migration by calcium/calmodulin-dependent protein kinase II-delta 2. Am J Physiol Cell Physiol. 2004;286(6):C1238–45. doi: 10.1152/ajpcell.00536.2003. [DOI] [PubMed] [Google Scholar]
- 16.Pfitzer G. Invited review: regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001;91(1):497–503. doi: 10.1152/jappl.2001.91.1.497. [DOI] [PubMed] [Google Scholar]
- 17.Zahradka P, Werner JP, Buhay S, Litchie B, Helwer G, Thomas S. NF-kappaB activation is essential for angiotensin II-dependent proliferation and migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2002;34(12):1609–21. doi: 10.1006/jmcc.2002.2111. [DOI] [PubMed] [Google Scholar]
- 18.Kintscher U, Bruemmer D, Blaschke F, Unger T, Law RE. p38 MAP kinase negatively regulates angiotensin II-mediated effects on cell cycle molecules in human coronary smooth muscle cells. Biochem Biophys Res Commun. 2003;305(3):552–6. doi: 10.1016/s0006-291x(03)00802-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YM, Wang KQ, Zhou GM, Zuo J, Ge JB. Endothelin-1 promoted proliferation of vascular smooth muscle cell through pathway of extracellular signal-regulated kinase and cyclin D1. Acta Pharmacol Sin. 2003;24(6):563–8. [PubMed] [Google Scholar]
- 20.Pestana IA, Vazquez-Padron RI, Aitouche A, Pham SM. Nicotinic and PDGF-receptor function are essential for nicotine-stimulated mitogenesis in human vascular smooth muscle cells. J Cell Biochem. 2005;96(5):986–95. doi: 10.1002/jcb.20564. [DOI] [PubMed] [Google Scholar]
- 21.Schultz K, Fanburg BL, Beasley D. Hypoxia and hypoxia-inducible factor-1{alpha} promote growth factor-induced proliferation of human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.01077.2005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Taylor AM, Li F, Thimmalapura P, Gerrity RG, Sarembock IJ, Forrest S, Rutherford S, McNamara CA. Hyperlipemia and oxidation of LDL induce vascular smooth muscle cell growth: an effect mediated by the HLH factor Id3. J Vasc Res. 2006;43(2):123–30. doi: 10.1159/000090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin SJ, Shyue SK, Shih MC, Chu TH, Chen YH, Ku HH, Chen JW, Tam KB, Chen YL. Superoxide dismutase and catalase inhibit oxidized low-density lipoprotein-induced human aortic smooth muscle cell proliferation: Role of cell-cycle regulation, mitogen-activated protein kinases, and transcription factors. Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.02.044. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3 Pt 2):581–7. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 25.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev. 2001;81(3):999–1030. doi: 10.1152/physrev.2001.81.3.999. [DOI] [PubMed] [Google Scholar]
- 26.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 27.Kahl CR, Means AR. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr Rev. 2003;24:719–36. doi: 10.1210/er.2003-0008. [DOI] [PubMed] [Google Scholar]
- 28.Kortvely E, Gulya K. Calmodulin, and various ways to regulate its activity. Life Sci. 2004;74(9):1065–70. doi: 10.1016/j.lfs.2003.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Boehm M, Nabel EG. The cell cycle and cardiovascular diseases. Prog Cell Cycle Res. 2003;5:19–30. [PubMed] [Google Scholar]
- 30.Yang M, Huang HL, Zhu BY, Tuo QH, Liao DF. Onychin inhibits proliferation of vascular smooth muscle cells by regulating cell cycle. Acta Pharmacol Sin. 2005;26(2):205–11. doi: 10.1111/j.1745-7254.2005.00526.x. [DOI] [PubMed] [Google Scholar]
- 31.Shukla N, Rowe D, Hinton J, Angelini GD, Jeremy JY. Calcium and the replication of human vascular smooth muscle cells: studies on the activation and translocation of extracellular signal regulated kinase (ERK) and cyclin D1 expression. Eur J Pharmacol. 2005;509(1):21–30. doi: 10.1016/j.ejphar.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 32.Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, Hajjar RJ, Lompre AM. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97(5):488–95. doi: 10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- 33.Choi J, Chiang A, Taulier N, Gros R, Pirani A, Husain M. A calmodulin binding site on cyclin E mediates Ca2+-sensitive G1/S transitions in vascular smooth muscle cells. Circ Res. 2006 doi: 10.1161/01.RES.0000223059.19250.91. [DOI] [PubMed] [Google Scholar]
- 34.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34(3):211–29. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 35.Nagel DJ, Aizawa T, Jeon KI, Liu W, Mohan A, Wei H, Miano JM, Florio VA, Gao P, Korshunov VA, Berk BC, Yan C. Role of nuclear Ca2+/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res. 2006;98(6):777–84. doi: 10.1161/01.RES.0000215576.27615.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benaim G, Villalobo A. Phosphorylation of calmodulin. Functional implications. Eur J Biochem. 2002;269(15):3619–31. doi: 10.1046/j.1432-1033.2002.03038.x. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto K, Kato S, Arima N, Fujii T, Morimatsu M, Imaizumi T. Cyclin-dependent kinase inhibitor, p21Waf1, regulates vascular smooth muscle cell hypertrophy. Hypertens Res. 2004;27(4):283–91. doi: 10.1291/hypres.27.283. [DOI] [PubMed] [Google Scholar]
- 38.Nakano N, Urasawa K, Takagi Y, Saito T, Kaneta S, Ishikawa S, Higashi H, Tsutsui H, Hatakeyama M, Kitabatake A. Downregulation of cyclin-dependent kinase inhibitor; p57(kip2), is involved in the cell cycle progression of vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;338(3):1661–7. doi: 10.1016/j.bbrc.2005.10.093. [DOI] [PubMed] [Google Scholar]
- 39.Gizard F, Amant C, Barbier O, Bellosta S, Robillard R, Percevault F, Sevestre H, Krimpenfort P, Corsini A, Rochette J, Glineur C, Fruchart JC, Torpier G, Staels B. PPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J Clin Invest. 2005;115(11):3228–38. doi: 10.1172/JCI22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398(6722):80–4. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- 41.Ikura M, Osawa M, Ames JB. The role of calcium-binding proteins in the control of transcription: structure to function. Bioessays. 2002;24(7):625–36. doi: 10.1002/bies.10105. [DOI] [PubMed] [Google Scholar]
- 42.Touyz RM, Yao G. Modulation of vascular smooth muscle cell growth by magnesium-role of mitogen-activated protein kinases. J Cell Physiol. 2003;197(3):326–35. doi: 10.1002/jcp.10393. [DOI] [PubMed] [Google Scholar]