Abstract
Myelin, the insulating layers of membrane wrapped around axons by oligodendrocytes, is essential for normal impulse conduction. It forms during late stages of fetal development but continues into early adult life. Myelination correlates with cognitive development and can be regulated by impulse activity through unknown molecular mechanisms. Astrocytes do not form myelin, but these nonneuronal cells can promote myelination in ways that are not understood. Here, we identify a link between myelination, astrocytes, and electrical impulse activity in axons that is mediated by the cytokine leukemia inhibitory factor (LIF). These findings show that LIF is released by astrocytes in response to ATP liberated from axons firing action potentials, and LIF promotes myelination by mature oligodendrocytes. This activity-dependent mechanism promoting myelination could regulate myelination according to functional activity or environmental experience and may offer new approaches to treating demyelinating diseases.
Introduction
There is growing awareness that activity-dependent interactions between glia and synapses are important for nervous system development and function (Fields and Stevens-Graham, 2002; Fields, 2004), but comparatively little is known of activity-dependent interactions between axons and myelinating glia. These glial cells are far removed from synapses where neurotransmitter could act as a neuron-glial signal, and the mechanisms regulating myelination are still unclear. Myelination by oligodendrocytes in the CNS and Schwann cells in the PNS is essential for nervous system function in vertebrates, and myelin organizes the distribution of ion channels along the axon to provide the electrical properties necessary for saltatory impulse conduction (Dupree et al., 2004). Myelination is a highly regulated developmental process, but it also takes place postnatally and continues into early adult life (Yakovlev and Lecours,1967; Giedd, 2004). As with synaptic remodeling during this period, evidence suggests that action potential firing can influence myelination (Zalc and Fields, 2000; Demerens et al., 1996). More recent evidence suggests that activity-dependent effects on myelination may regulate nervous system function according to functional and cognitive activity (Schmithorst et al., 2005), learning (Bengtsson et al., 2005), and environmental input (Markham and Greenough, 2004). For review see Fields (2005). How, at a molecular and cellular level, impulse activity promotes myelination by mature oligodendrocytes, is an important question.
Two general molecular mechanisms have been revealed for activity-dependent effects on early stages of myelination: activity-dependent regulation of cell adhesion molecule expression in neurons that are necessary for myelination (Itoh et al., 1995, Stevens et al., 1998) and the release of diffusible signaling molecules from axons firing action potentials, which activate receptors on premyelinating glia and influence their proliferation and differentiation (Fields and Stevens, 2000; Stevens et al., 2002). Molecular mechanisms for effects of impulse activity on myelination at later stages of development are unknown.
Purinergic signaling, mediated by the activity-dependent release of ATP from axons (Stevens and Fields, 2000) and the subsequent hydrolysis to adenosine (Stevens et al., 2002), is one of the principal mechanisms of activity-dependent communication between axons and myelinating glia (Fields, 2006). ATP released from axons firing action potentials inhibits myelination by Schwann cells by arresting their development at an early stage (Fields and Stevens, 2000). In the CNS, adenosine, presumably generated by the hydrolysis of extracellular ATP released from axons, inhibits proliferation of oligodendrocyte progenitor cells (OPCs), and stimulates their differentiation into a premyelinating stage, thus increasing myelination (Stevens et al., 2002). In both cases, action potentials influence myelination by regulating maturation of immature premyelinating glia. The possible activity-dependent communication and effects on oligodendrocytes that are already differentiated to a promyelinating stage have not been explored.
The object of the present series of experiments was to determine if neural impulse activity can influence myelination at later stages of oligodendrocyte development, independent of the known effects of adenosine on OPC differentiation (Stevens et al., 2002). An activity-dependent effect on myelination after OPCs have matured could have relevance to treating demyelinating disease and to use-dependent effects on myelination. The results reveal a new mechanism by which action potentials influence myelination. This involves unexpected interactions between astrocytes and myelinating glia and between purinergic and cytokine signaling.
Results and Discussion
To determine effects of action potentials on myelination after oligodendrocytes have matured well beyond the progenitor stage, we conducted experiments in special culture dishes (Campenot chambers) equipped for electrical stimulation (Figure 1A). After 3-4 weeks in culture,OPCs were added to DRG neurons and co-cultured together for 7 days to allow sufficient time for OPCs to differentiate to the oligodendrocyte stage. Axons were then stimulated with a pattern of electrical activity (0.5 s at 10 Hz, every 2 s), which is known to stimulate release of ATP from axons (Stevens and Fields, 2000). 2 weeks later, myelination was assessed and found to have increased 3-fold on axons firing action potentials compared with unstimulted controls, or axons stimulated in the presence of TTX, which blocks sodium-dependent action potentials (Figure 1B) (p < 0.001).
Figure 1.

Action Potentials Stimulate Myeli-nation of DRG Axons by Mature Oligodendrocytes(A) Mouse DRG neurons were cultured 3-4 weeks in multicompartment culture dishes equipped with electrodes for stimulating action potentials in axons. Axons from DRG neurons plated into the two-side compartments grow under the barrier with the central compartment, providing a high resistance connection for electrical stimulation through platinum electrodes (Fields et al., 1992). Oligodendrocyte progenitor cells (OPCs) were added to the side compartments and cultured with DRG neurons for 7 days. Electrical stimulation in a phasic pattern (see Experimental Procedures) was applied to axons for 2-3 weeks, and the number of myelinated profiles stained with Sudan black was compared with unstimulated controls. (B) Electrical stimulation increased myelination through a mechanism requiring activation of sodium-dependent action potentials, as shown by blocking the effects of stimulation in the presence of 1 μM of the sodium channel blocker tetrodotoxin (TTX). (C) Treating cultures of mature oligodendrocytes and DRG neurons with adenosine (300μM) or the adenosine receptor agonist NECA over a wide range of concentrations failed to increase the number of Sudan-black-stained myelin profiles, but treatment with the ATP receptor agonist 2MeSATP (300 μM) mimicked the effect of electrical stimulation in increasing myelination by oligodendrocytes (n = 156 cultures, eight independent experiments). ATP is known to be released by axons firing action potentials (Stevens and Fields, 2000), suggesting that ATP may be an activity-dependent intercellular messenger regulating myelination. (D) A similar increase in myelination was seen by using immunocytochemical staining with antibodies against myelin oligodendrocyte glycoprotein (MOG) to quantify the number of myelin segments/microscope field in unstimulated (1Ca) and stimulated cultures (1Cb) (24 cultures, three independent experiments). p < 0.005 with respect to control; scale bar = 20 μm. Error bars represent SEM.
Thus, action potential firing in axons can increase myelination after oligodendrocytes have developed beyond the progenitor stage. It would be difficult to explain this result by previous studies reporting that activity-dependent production of adenosine promotes myelination by stimulating differentiation of oligodendrocytes. As expected, adenosine or the adenosine receptor agonist NECA applied over a wide range of concentrations after 7 days in coculture had no effect on myelination (Figure 1C). This is consistent with the expectation that adenosine treatment would not increase myelination if applied after the OPCs had matured.
ATP does not stimulate differentiation of OPCs, as shown in previous studies (Stevens et al., 2002), but surprisingly, treatment of cocultures at this later developmental stage with a nonhydrolysable form of ATP, 2MeSATP (300 μM), produced significantly more myelinated axons per field visualized by Sudan black staining (Figure 1C) (ANOVA, p < 0.005) or staining for the myelin oligodendrocyte glycoprotein (MOG) (Figures 1Ca, 1Cb, and 1D) (p < 0.005). Electrical stimulation combined with 2MeSATP treatment produced no further increase in myelination, suggesting that electrical activity and ATP receptor activation may increase myelination by acting through the same mechanism (Figure 2A). Because these cultures were treated with ATP receptor agonists after the oligodendrocytes had already matured to a promyelinating stage, the increased myelination must be due to effects other than promoting differentiation from the immature stage, in contrast to the actions of adenosine (Stevens et al., 2002). (This was confirmed by staining the cocultures with markers of oligodendrocyte differentiation. There was no significant difference in the proportion of cells stained for NG2, O4, and O1 compared with controls, after treating cocultures with 100 μM 2MeSATP for 2, 4, or 7 days, starting 7 days after coculturing the OPCs with DRG neurons [Figure 3] [p > 0.3 in all cases]). Cells were post mitotic during the treatment period as shown by measuring mitotic rate with a BrdU assay, and ATP treatment had no effect on cell proliferation (Figure 3). The total number of neuron (p > 0.4) and glial cells (p > 0.3) was not significantly different between controls and ATP-treated cocultures after 14 days, suggesting an effect of ATP receptor activation on myelination that is unrelated to cell survival or cell death. Figure 3 provides an independent analysis of changes in proportion of O1-, MBP-, and MOG-positive cells in cocultures (n = 280 microscope fields).
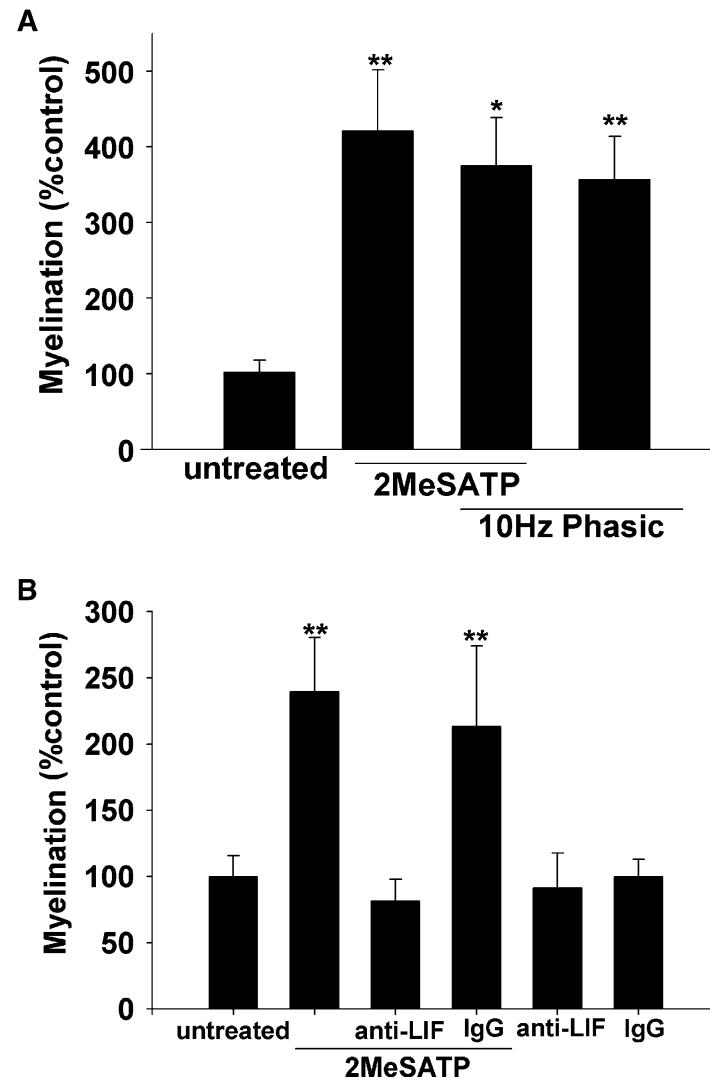
Figure 2.
Electrical Activity Stimulates Myelination through a Mechanism Involving ATP Receptor Activation and the Cytokine Leukemia Inhibitory Factor (A) Both electrical stimulation (10 Hz, phasic; 0.5 s pulse every 2 s) and ATP receptor activation with 300 μM 2MeSATP for 2 weeks increased myelination, with nonadditive effects when used in combination, suggesting action through a similar mechanism. (B) Activity-blocking antibodies against the cytokine leukemia inhibitory factor (LIF) blocked the increase in myelination produced by 2MeSATP treatment. No effects on myelination were seen after antibody treatment in the absence of 2MeSATP stimulation, suggesting the effects of LIF are activity dependent and require ATP receptor stimulation (93 experiments; three replicates). None of the treatments affected the number of oligodendrocytes (data not shown). Asterisk, p < 0.05; double asterisks, p < 0.005. Error bars represent SEM.
Figure 3.

Developmental Profile of Oligodendrocytes in Cocultures and Effects of P2-Receptor Activation The state of differentiation of oligodendrocytes in cocultures was determined by staining with antibodies against markers of OPC differentiation after 7, 9, 11, and 14 days in coculture with DRG neurons in the presence and absence of 100 μM 2MeSATP, and cell proliferation was measured by BrdU incorporation (magenta). Hoechst nuclear stain was used to identify all cells in the culture (blue) and to calculate the percentage of cells identified with each marker. Mean percentage and SEM of cells stained positively with each marker are indicated in the lower right corner of each figure (n = 40 microscope fields in each). Immunocytochemical staining for a marker of early oligodendrocytes O4 is shown in green; a marker for intermediate stage oligodendrocytes, myelin basic protein (MBP), is shown in red; and the myelin oligodendrocyte glycoprotein (MOG) expressed by mature oligodendrocytes is shown in yellow. The results show that OPCs have progressed into a postmitotic phase and begun to express the O4 antigen in large numbers by 7 days in culture, the time when treatment with 2MeSATP was begun. ATP receptor activation with 2MeSATP had no effects on maturation or proliferation of oligodendrocytes when applied after 7 days in coculture, but it did increase the number of cells expressing the mature oligodendrocyte marker MOG after 11 and 14 days (4-7 days 2MeSATP treatment; p < 0.001). The results show that ATP receptor activation increases myelination by increasing development of oligodendrocytes at the mature MOG positive stage, rather than acting on cells at the progenitor stage.
There is a large family of ATP receptors (P2 receptors) that could allow glia to detect ATP release from axons. (See Burnstock [2004] for review of purinergic receptors.) These receptors activate a wide range of intracellular signaling cascades that could interact with other axonal signals regulating myelination. Experiments with more selective P2 receptor agonists showed that the P2Y receptor agonist UTP (300 μM) was ineffective in increasing myelination (90.1% ± 13.9% relative to control), but the P2X receptor agonist α-β methyl ATP (300 μM) increased myelination 169.4% ± 37.0%. Considerable additional research will be required to fully identify the specific P2 receptor subtypes responsible for this increase in myelination, but these initial findings do not provide support for involvement of the P2X3, P2Y2, or P2Y4 receptor subtypes. Further experiments suggest that ATP released from axons firing action potentials stimulates myelination by mature oligodendrocytes through an unexpected mechanism involving cytokine signaling.
Interaction between ATP and LIF Receptor Signaling in Myelination
A search of the literature for factors that might affect myelination after OPCs have differentiated to a promyelinating phenotype suggested possible involvement of cytokines. Recently published studies demonstrate a pronounced increase in myelination in cultures of dissociated cerebral cortex, which is proportional to the concentration of exogenous ciliary neurotrophic factor (CNTF) or leukemia inhibitory factor (LIF) applied (Stankoff et al., 2002). The positive effect of these cytokines on myelination results from effects on late-stage oligodendrocytes; specifically those cells expressing MOG, a cell-surface glycoprotein of mature oligodendrocytes (Scolding et al., 1989), which is not expressed in oligodendrocytes before 7 days in coculture. Consistent with this, we observed that the proportion of oligodendrocytes staining positively for MOG was increased after 7 days 2MeSATP treatment begun after 1 week in co-cultur e (33% ± 0.888% versus 64% ± 3.41%; p < 0.001), but no difference in number of MOG-positive cells was seen after 2 or 4 days 2MeSATP treatment begun 7 days after coculture. (See also data from a replicate experiment in Figure 3, D11 and D14.) Similar effects were seen after treating oligodendrocytes in monoculture with 2MeSATP for seven days (44.8% ± 2.24% versus 22.6% ± 1.19% MOG-positive oligodendrocytes in treated versus control cultures; p < 0.001), indicating that the LIF response does not require indirect effects involving neurons. (It is important to note, in the context of results to be discussed below, that monocultures of oligodendrocytes contain approximately 3% GFAP-positive astrocytes, increasing to 8% by 7 days in culture.)
To test the hypothesis that ATP might promote myelination through an LIF-dependent mechanism, we added antibodies against LIF to cocultures treated with the ATP receptor agonist 2MeSATP (Figure 2B). The increase in myelin induced by 2MeSATP was blocked by the presence of antibody blocking LIF activity, but myelination was not affected by antibody against LIF alone. This indicates that the effects of endogenous LIF on myelination require activation of P2 receptors. The result was specific to LIF antibody, as normal goat IgG had no effect on the increased myelination induced by 2MeSATP, and normal goat IgG had no effect on myelination in the absence of 2MeSATP (Figure 2B).
This association between ATP receptor activation and LIF in promoting myelination suggests that action potential firing could promote myelination by the activity-dependent release of LIF in cocultures. To test this hypothesis, we measured the concentration of LIF in these cultures to determine if LIF was released when axons were stimulated to fire action potentials. Neurons and oligodendrocytes were cultured together in multi-compartment cell culture dishes equipped with platinum electrodes to stimulate the axons as they traverse the barrier between central and side compartments (Figure 1A) (Fields et al., 1992). Axons were stimulated with a phasic stimulus (0.5 s at 10 Hz every 2 s), and the culture medium was collected and assayed for LIF content with an ELISA (R&D Systems Quantikine murine). Action potential firing significantly increased the concentration of LIF in cocultures measured after 24 hr stimulation (Figure 4A). The increase in LIF concentration in the culture medium declined with prolonged electrical stimulation, but the elevated levels of LIF persisted for at least one week of electrical stimulation (Figure 4A). (Tests with purified CNTF confirmed that the ELISA assay does not crossreact with the cytokine CNTF.)
Figure 4.

Action Potential Firing Causes LIF Release from Cocultures of DRG Neurons and Oligodendrocytes, and LIF Treatment Affects Myelination in a Concentration-Dependent Manner(A) 10 Hz phasic stimulation of DRG axons (open triangles) increased the concentration of LIF in medium sampled from cocultures compared with unstimulated cultures (filled circles). The stimulus-induced release of LIF decreased with time in culture, but remained elevated for approximately 1 week (asterisk, p < 0.03 at day 7). Antibody against LIF did not interfere with the LIF release (data not shown). Error bars represent SEM. (B) LIF was applied to 7 day old cocultures over a wide range of concentrations, and myelination was assessed by Sudan black and MOG staining. Low concentrations of exogenous LIF (<5 ng/ml) promoted myelination (p < 0.003, linear regression). Error bars represent SEM. (C) LIF concentrations above 5 ng/ml inhibited myelination (p < 0.0001, linear regression). Error bars represent SEM. (D) Representative images of cocultures stained with MOG are shown; the optimal concentration of LIF was near 0.1 ng/ml. This suggests that endogenously released LIF could influence the timing and extent of myelination. An average of 11 myelinated segments/microscope field were seen in response to 300 μM 2MeSATP. There were no significant differences in the numbers of oligodendrocytes or DRGs in these experiments at the time of myelin quantification. Bar = 10 μm. Error bars represent SEM.
There are some discrepancies reported in the literature on the effects of LIF on myelination. Using similar cell culture models, Stankoff and colleagues (2002) showed a significant concentration-dependent increase in myelination after LIF treatment, but in another study, treatment of cocultures with LIF was reported to inhibit myelin formation (Park et al., 2001). This controversy was investigated and resolved by applying a wide range of LIF concentrations to cocultures of DRG neurons and oligodendrocytes after 7 days, and assessing the amount of myelin formed 14-21 days later. The results revealed that myelination was increased significantly by low concentrations of supplemental LIF (<5 ng/ml) (Figures 4B and 4D) but that high concentrations (>5 ng/ml) were inhibitory (Figures 4C and 4D). Such nonlinear concentration-response functions are not unusual for cytokines in many biological processes. Note that the concentration of LIF measured in cocultures treated with 2MeSATP for 24 hr was 0.014 ng/ml, which is in the optimal range for a positive effect of LIF on myelination.
If the increase in myelination produced by electrical stimulation was dependent on endogenous LIF released in response to ATP, which is known to be liberated by electrically active axons (Stevens and Fields, 2000), the increase in myelination should be inhibited by electrical stimulation in the presence of antibodies against LIF. The results confirmed this hypothesis (Figure 5A).
Figure 5.
Action Potentials Increase Myelination via an LIF-Dependent Mechanism with Astrocytes Providing the Major Source of LIF in Response to 2MeSATP Treatment(A) LIF neutralizing antibody blocked both the 2MeSATP and action potential-mediated increase in myelination (ANOVA, p = 0.43, n = 68), suggesting that action potentials and 2MeSATP increase myelination via the same LIF-dependent mechanism. Error bars represent SEM. (B) The release of LIF after 24 hr electrical stimulation of DRG axons in coculture with oligodendrocytes was blocked by stimulation in the presence of 30 U/ml apyrase, an enzyme that rapidly degrades extracellular ATP (p < 0.0001, ANOVA among all four groups; p < 0.05 elect. Stim. versus elect. Stim. + apyrase). This is consistent with the hypothesis that ATP released from axons firing action potentials in turn induces release of LIF by a mechanism involving activation of P2 purinergic receptors. Treatment with apyrase alone had no effect on LIF release. Error bars represent SEM. (C) Astrocytes release LIF in response to 2MeSATP treatment in a concentration-dependent manner, with a maximal response between 100 and 300 μM. Error bars represent SEM. (D) To identify the cellular source of LIF in co-cultures, astrocytes, DRG neurons, and oligodendrocytes were cultured separately and treated with 100 μM 2MeSATP for 1-3 days. In contrast to the large amount of LIF released by 2MeSATP treatment of astrocytes, monocultures of DRG neurons or oligodendrocytes released little LIF. The concentration of LIF in treated versus untreated cultures of astrocytes was significantly different (0.247 6 0.055 versus 7.21 6 0.049 ng/ml LIF in control versus 2MeSATP; p < 0.0001). The small amount of LIF released in oligodendrocyte cultures could derive from contaminating GFAP-positive cells, which increase in oligodendrocyte cultures from 3% to 8% by 10 days in culture. Double asterisks, p < 0.001. Error bars represent SEM.
The mechanism responsible for the increase in LIF in cocultures induced by action potential firing was investigated and found to depend on activity-dependent ATP release and ATP receptor activation. Measurements showed that LIF was released from cocultures in response to direct application of 2MeSATP (100 μM), in the absence of electrical stimulation (0.674 6 0.171 versus 13.6 6 1.38 pg/ml LIF; control versus 2MeSATP for 24 hr; p < 0.003). The hypothesis that electrical activity triggers LIF release by an ATP-receptor-dependent mechanism was tested and supported by finding that the release of LIF from electrically stimulated cocultures was blocked in the presence of the apyrase (30 units/ml), an enzyme that rapidly degrades extracellular ATP (Figure 5B). The results suggest that action potentials stimulate release of ATP from axons, which in turn stimulates LIF release promoting myelination of late-stage (MOG-positive) oligodendrocytes. This raises the important question of how LIF is released in an activity-dependent manner.
Astrocytes Link ATP and LIF Signaling with Impulse Activity in Axons
The most probable source of LIF in the CNS is from astrocytes and microglia. No evidence for microglia could be found in these cultures by using immunocytochemistry for OX42 or tomato lectin staining. Cells positive for GFAP (a cytoskeletal protein in astrocytes) comprise approximately 3%-13% of the total cells in cocultures of DRG neurons and OPCs. The number of GFAP-positive cells increased with time in coculture (4.55% ± 0.468%; 7.5% ± 0.704%; 12.6% ± 0.645%; after 5, 7, and 14 days in vitro, respectively). 2MeSATP treatment did not affect the number of GFAP-positive cells (7.5% ± 0.7% versus 9.1% ± 1.1% p > 0.2; 12.6% ± 0.64% versus 12.32% ± 0.68% p > 0.7; control versus 2MeSATP for 5 and 7 days treatment, respectively). Similarly, electrical stimulation did not affect the number of GFAP-positive cells in DRG cultures (7.8% ± 1.79% versus 4.8% ± 1.68%, electrical stimulation versus control; p < 0.02).
Immunocytochemistry (Figure 6A), Western blot (Figure 6B), and RT-PCR (Figure 6C) provide strong evidence for LIF synthesis in astrocytes. Further experiments showed that electrical activity in axons increased the level of mRNA for LIF in astrocytes (Figure 6C). This was determined by coculturing astrocytes on DRG axons in the central compartment of the Campenot chamber, which excludes DRG cell bodies (Figure 6D). Astrocytes isolated from rat were used together with species-specific PCR primers, to ensure that the LIF mRNA in these measurements derived from astrocytes, rather than a possible contaminant from mouse neurons in the side compartment. 24 hr after axons were stimulated electrically, the level of LIF mRNA in astrocytes cocultured on DRG axons increased (Figure 6C). This increase in LIF mRNA was inhibited by electrical stimulation in the presence of apyrase, an enzyme that rapidly degrades extracellular ATP. This inhibition is consistent with the hypothesis that the increase in LIF mRNA in astrocytes growing on electrically active DRG axons is dependent upon release of ATP from axons. Examination of optic nerve from 10 day old mice confirms the presence of LIF in glia in vivo during the period of myelination (Figure 6E). Thus, LIF expression in astrocytes is not an artifact of cell culture (Figure 6A). Specificity of the antibody used for immunocytochemistry was confirmed by the absence of staining in optic nerve from LIF-/-mice (Stewart et al., 1992) (Figure 6F).
Figure 6.
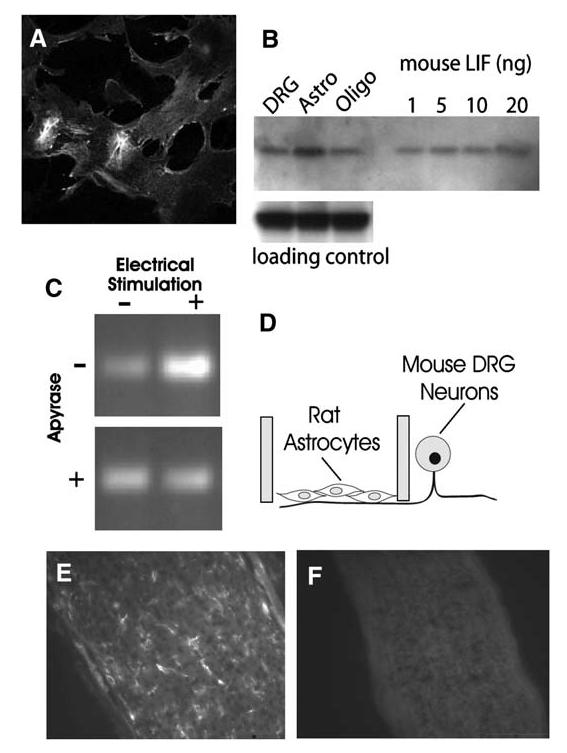
LIF Is Synthesized by Astrocytes and Regulated by Action Potential Firing(A) Astrocytes from E19 rat cortex stained strongly with anti-LIF antibody. DRG neurons and oligodendrocytes showed only weak staining with anti-LIF antibody, and astrocytes from LIF-/- mice were not stained (data not shown). (B) Western blot analysis confirmed the immunostaining results and indicated astrocytes as the predominant source of LIF in cocultures. (No microglia were detectable by immunocytochemistry.) Equal concentrations of protein (35 mg) from purified cultures of DRG neurons, astrocytes, and oligodendrocytes were compared. Antibody against ankyrin G was used as a control for equal loading in each lane. Purified mouse LIF confirmed the specificity of the LIF antibody and indicated that the amount of LIF in the astrocyte sample exceeds 20 ng. (C) The level of LIF mRNA in astrocytes grown on DRG axons was increased after 24 hr electrical stimulation of axons (top). This increase was prevented by stimulation in the presence of apyrase (30 U/ml), which degrades extracellular ATP (bottom). This indicates that the activity-dependent regulation of LIF mRNA levels in astrocytes occurs through a mechanism involving P2 purinergic receptor activation by ATP released from axons firing action potentials. (D) The experiments on effects of action potential firing on LIF mRNA in astrocytes were performed in multicompartment cell-culture chambers that separate the DRG cell bodies from axons. Astrocytes were derived from rats and plated onto mouse axons in the central compartment. Species-specific primers for LIF were used to ensure that the measurements did not derive from LIF mRNA possibly synthesized by neurons. As a control, PCR for 28s ribosomal RNA showed no change after any of the treatments in (C) (data not shown). (E) LIF is present in glia in vivo during the period of myelination in mouse optic nerve, as indicated by immunocytochemical staining of optic nerve from 10 day old mice. (F) Specificity of the antibody is shown by the lack of staining in LIF-/-mice.
Activation of P2 receptors on astrocytes has been shown previously to increase the expression of LIF mRNA in astrocytes (Yamakuni et al., 2002), but the present findings are the first to show that ATP induces release of LIF from astrocytes (Figure 5C) and that action potentials can initiate this release (Figure 5B). Treatment with 2MeSATP caused a large increase in LIF concentration in the culture medium of astrocytes in monoculture tested after 3 hr to 2 days of 100 μM 2MeSATP treatment (Figure 5Da) (p < 0.0001). The amount of LIF in conditioned medium showed a concentration-dependent relationship to the concentration of 2MeSATP applied (Figure 5C). The concentration of LIF in conditioned medium reached a maximum at 100-300 μM 2MeSATP, the concentration range used in the myelination experiments. In contrast, treatment of purified cultures of oligodendrocytes (Figure 5Dc) or DRG neurons (Figure 5Db) with 2MeSATP produced only very small increases in LIF. We cannot rule out the possibility that the small amount of LIF measured in purified cultures of DRG or oligodendrocytes may originate from the small number of GFAP-positive cells in these cultures.
Indeed, there is correlative evidence suggesting this possibility. The effect of ATP in increasing the number of MOG-positive oligodendrocytes after 7 days in mono-culture, but not at earlier times, parallels the increase in astrocytes from 3% to 8% and correlates with the amount of LIF released at these times, although the level of LIF is much lower than in pure astrocyte cultures. (4 week old DRG cultures contain 6.3% ± 0.804% GFAP+ cells, and oligodendrocyte monocultures contain 3.9% ± 0.454% GFAP+ cells after 2 days in culture.)
Another consideration favoring astrocytes as the primary source of LIF, rather than DRG neurons, is that the presence of LIF in DRG neurons under normal conditions is controversial. Although DRG neurons can express LIF after axotomy, there is little evidence that significant levels of LIF are expressed in DRG axons during myelination (Banner and Patterson, 1994). Similarly for oligodendrocytes, LIF has been reported in a transformed cell line derived from oligodendrocytes (Mey and Henkes, 2002), but the presence of LIF in oligodendrocytes under normal conditions is not well studied. In comparison with the large amount of LIF released from astrocytes after treatment with 2MeSATP, the possible contribution of LIF from DRG neurons or oligodendrocytes would appear minimal.
Astrocytes Promote Myelination by an LIF-Dependent Mechanism
Correlative data showing an association between astrocytes and myelination in culture and during development are consistent with the conclusions from the present series of studies. The increase in GFAP-positive cells seen during development correlates with the normal developmental period of myelination in spinal cord (Dziewulska et al., 1999), and experimental studies in cell culture show that astrocytes induce oligodendrocytes to align their processes with axons (Meyer-Franke et al., 1999). We therefore tested directly whether astrocytes promoted myelination of DRG axons by oligodendrocytes. Cocultures of DRG neurons and oligodendrocytes, which normally contain 3% astrocytes, were supplemented with 5% to 15% astrocytes (relative to total number of oligodendrocytes plated), and myelination was compared after 2-3 weeks. The results showed a potent effect of astrocytes in promoting myelination (Figure 7A). Cocultures made from O1-positive oligodendrocytes obtained by immunopanning to remove astrocytes and immature oligodendrocytes, showed no increase in myelination after treatment with 100 μM 2MeSATP (Figure 7B). This evidence showing that astrocytes are essential for the ATP effect on myelination is consistent with the evidence that ATP acts to increase myelination by stimulating release of LIF from astrocytes. This was verified by adding 8% astrocytes to the cocultures made from O1+ oligodendrocytes and finding that this rescued the effect of 2MeSATP treatment in promoting myelination (Figure 7B). In addition, this experiment with immunopurified O1+ stage oligodendrocytes provides further evidence that ATP acts by stimulating myelination of mature oligodendrocytes, rather than promoting differentiation of immature cells, because these cocultures were made with oligodendrocytes after maturation to the O1+ stage, and LIF promoted myelination of these cocultures lacking immature oligodendrocytes (Figure 7B).
Figure 7.

Astrocytes Promote Myelination in Response to ATP Receptor Stimulation by Releasing LIF (A) A positive effect of astrocytes on myelination was show by supplementing cocultures of DRG neurons and oligodendrocytes with astrocytes. Myelination after 2 weeks in coculture increased in proportion to the percentage of additional astrocytes added 3 hr after plating OPCs on DRG neurons (solid dots). Similar effects were seen in cultures supplemented with astrocytes from mice heterozygous for LIF (open circle). However, supplementing cultures with astrocytes derived from homozygous LIF-/- mice failed to increase myelination (solid triangle). The inability of astrocytes from LIF-/- mice to increase myelination could be restored by adding 0.1 ng/ ml LIF to the cultures (open triangle) (n = 1200 microscope fields). Error bars represent SEM. (B) Eliminating astrocytes from cultures also eliminates the positive effect of 2MeSATP on myelination. This was shown by making co-cultures from O1+ oligodendrocytes purified by immunopanning. Cultures made from mature O1+ oligodendrocytes were still responsive to LIF, as shown by the increase in myelination produced by adding 0.1 ng/ml LIF to these cultures. The positive effect of 100 μM 2MeSATP on myelination could be restored by supplementing these cocultures of O1-positive oligodendrocytes with 8% astrocytes, strongly implicating astrocytes as the primary source of LIF in response to electrical stimulation causing the release of ATP from axons. Double asterisks, p < 0.001; n = 400 microscope fields. Error bars represent SEM.
To further test whether the effect of astrocytes in stimulating myelination was due to LIF release from astrocytes, we performed experiments with astrocytes derived from mice with the LIF gene disrupted (Figure7A). The effect of astrocytes in promoting myelination of wild-type DRG axons was completely blocked by supplementing cocultures with astrocytes derived from homozygous LIF-/- mice (Stewart et al., 1992)(Figure 7A). The loss of activity in astrocytes from LIF-/- mice in promoting myelination could be rescued by supplementing the cultures with recombinant rat LIF (0.1 ng/ml) (Figure 7B).
Summary and Conclusions
These experiments reveal several important new interactions between different types of cells (neurons and two types of glia) and between different signaling systems (purinergic and cytokine signaling) in the CNS. They are the first to report that ATP can increase myeli-nation, that synthesis and release of LIF from astrocytes is stimulated by action potential firing, that interactions between purinergic receptor signaling and cytokine signaling occurs in astrocytes, and that this can involve astrocytes in the process of myelination according to functional activity in axons.
Taken together, these results reveal a new mechanism by which electrical activity promotes myelination of CNS axons at a later developmental stage and possibly into postnatal life. This process is mediated by ATP receptor activation, rather than adenosine receptor activation, and it acts through different molecular and biological mechanisms from the previously identified activity of adenosine in promoting myelination by stimulating OPC differentiation (Stevens et al., 2002). Electrical activity in premyelinated axons increases myelination after OPCs mature to a promyelinating stage by the activity-dependent release of ATP from axons, which acts in a paracrine manner on astrocytes to release LIF and stimulate myelination at this later stage of development.
The link between electrical activity and LIF release by activation of P2 receptors may have relevance to other developmental processes such as survival or differentiation of neurons and glia (e.g., Mayer et al., 1994; Barres and Raff, 1993) and possible interaction with immune system responses involving this cytokine, for example in multiple sclerosis (Butzkueven et al., 2002). Because high levels of LIF are detrimental to myelination, impulse activity could, under certain pathological conditions accompanied by high levels of cytokines (or ATP), inhibit myelination or remyelination rather than stimulate it.
These new findings may provide novel approaches to understanding and treating myelin disorders in the CNS after OPCs have matured to the promyelinating stage. Alexander disease, for example, is a fatal white matter disorder of childhood caused by mutations in the GFAP gene (Johnson 2002; Li et al., 2002). Astrocytes of these patients exhibit a characteristic abnormal morphology, but the reasons for white matter abnormalities in Alexander disease are not understood. A possible link between GFAP abnormalities in astrocytes and myelination is provided by the present findings implicating astrocytes in activity-dependent myelination.
These new findings help resolve previous unexpected observations from studies of genetically modified mice. Unexpectedly, myelin formation is impaired in LIF-/- mice in the absence of effects on neuronal survival (Bugga et al., 1998). An equally surprising phenotype is seen in mice carrying a null mutation in the cytoskeletal protein GFAP in astrocytes. These mice develop abnormal myelination (Liedtke et al., 1996), even though astrocytes do not form myelin. The physiological basis for this phenotype is not understood, but this result was interpreted by the authors as evidence that the cytoskeletal protein GFAP is essential for local secretion of an unknown factor by astrocytes that promotes myelination by oligodendrocytes (Liedtke et al., 1996). The present findings would suggest that the unknown substance could be LIF secreted in response to ATP liberated by axons firing action potentials. These findings unite purinergic and cytokine signaling through an activity-dependent mechanism, and they provide a reasonable explanation integrating these two previously separate observations on myelination in vivo from genetic manipulation of the cytokine LIF and the astrocytic protein, GFAP.
Experimental Procedures
Cell Culture and Electrical Stimulation
DRG neurons were dissected from the spinal cords of embryonic day (E) 13.5 mice as described (Stevens et al., 1998). Neurons were grown for w4 weeks in MEM medium supplemented with N3 containing 100 ng/ml of nerve growth factor and 5% heat-inactivated horse serum in the side compartment of three-compartment chambers equipped with stimulating electrodes (Fields et al., 1992). Mitosis of nonneuronal cells was inhibited by a 4 day treatment with 13 μg/ml fluoro-2’-deoxyuridine (FUDR) beginning 1 day after plating. These cultures can be maintained indefinitely with half-volume changes of medium every 3 days.
Primary cultures of OPCs were obtained from cerebral cortices of E19-E20 rats or mice and plated into 75 cm2 tissue culture flasks. The resulting cultures were maintained at 5% CO2 at 37°C in medium containing 10% fetal bovine serum (Hyclone). After 11 days in culture, the flasks were shaken at 37°C for 3 hr to kill nonglial cells, and then the medium was changed, and the flasks shaken overnight to lift OPCs from the flask. The cell suspension was pelleted, resus-pended, and incubated in an uncoated culture dish for 30 min to enrich the population for OPCs. Contaminating cells, primarily endothelial cells, astrocytes, macrophages, and microglia adhere strongly to the plastic and can be separated out by this panning method. For some experiments, O1-positive oligodendrocytes were obtained by immunopanning (Gard and Pfeiffer, 1989). Immunocytochemistry confirmed that these were mature oligodendrocytes without contaminating astrocytes or NG2-positive cells.
Astrocytes were obtained from cerebral cortices of E19-E20 rats or mice. After oligodendrocytes were isolated, the flasks (above) were shaken for 2 days to remove floating cells and cell debris. Adherent astrocytes were removed by 0.25% trypsin (5 min at 37°C) and plated in 35 mm dishes or multicompartment cultures as required.
For myelinated cocultures, purified OPCs (>90% OPCs) were counted and plated on 3 to 4 week old DRG cultures at a density of 40,000 cells per side compartment. After 3-4 hr, the media was removed and replaced with differentiating media (DMEM + N1 + 0.5% FBS [Stevens et al., 2002]). 7 days later, cultures were treated with purinergic agonists and antagonists.
Action potentials were induced in DRG axons by 200 ms 5 V biphasic pulse through platinum electrodes in three-compartment chambers (Fields et al., 1992). Only those axons growing into the central compartment beneath the high-resistant barriers are stimulated by this method. 10 Hz phasic (0.5 s at 10 Hz every 2 s) stimulus patterns were used.
Pharmacological Treatment
ATP, adenosine, 2MeSATP, α-β methyl ATP, and UTP were obtained from Tocris. Adenosine, N'-Benzyl-5’-N-ethylcarboxamidoadenosine (NECA), apyrase, and TTX were obtained from Sigma. Antibody against mouse LIF was from R&D Systems, and human LIF was from Alomone. Cultures were treated with fresh agonist or antagonist solutions every 2-3 days for 2 weeks.
Sudan Black Stain for Compact Myelin
After 4% paraformaldehyde fixation, cultures were washed with PBS and postfixed for 1 hr with 0.1% osmium tetroxide. After two washes with PBS, the cultures were dehydrated with an ethanol series (25%, 50%, 70%) for 10 min each and incubated for 2 hr in a 0.5% Sudan black solution in 70% ethanol and washed three times in 3% ethanol and rehydrated in PBS. Nuclei were stained with Hoechst stain (1:2000) in PBS for 10 min.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 5 min and washed three times in PBS. Nonspecific protein binding was blocked with 3% NGS, 0.1% Triton X-100 for 1 hr at RT. The following primary antibodies were used in overnight incubations at 4°C: anti-NG2 (Chem-icon, 1:1000), anti-O4 (Chemicon, 1:1000), anti-O1 (Chemicon, 1:500), anti-GFAP (Dako, Carpinteria, CA, 1:2000), anti-MBP (Sternberger Monoclonals, 1:1000), anti-OX-42 (Serotec, Oxford, UK), and anti-mouse LIF (R&D Systems, 1:200). Anti-MOG antibody was kindly provided by Dr. Barres (Stanford University). FITC- and Rhodamine-conjugated secondary antibodies against the appropriate species were applied for 30 min at RT and viewed by epifluorescence or confocal microscopy. Similar procedures were used for immunocytochemical staining of mouse optic nerve after 4% paraformaldehyde fixation and cryosectioning. Cultures were rinsed in PBS, treated with 0.02% sodium azide in PBS to prevent internalization of lectin, and incubated in 0.02 mg/ml FITC labeled tomato lectin (Sigma) for 30 min at RT and fixed with 4% paraformaldehyde to visualize microglia.
Quantification of Myelin and Cell Numbers
The number of Sudan-black-positive or MOG-positive myelin profiles was counted by observers without knowledge of the treatment condition. Ten or more random fields were selected from each culture dish or side compartment of the Campenot chamber, and the total number of myelin profiles and Hoechst-positive nuclei in each field was recorded. Statistical analysis was performed by using Mini-tab software with the significance of differences tested by ANOVA and errors reported as SEM.
LIF Assay
Conditioned media was collected from cultures of DRG neurons, oligodendrocytes, astrocytes, and cocultures of DRG neurons and oligodendrocytes. The conditioned media from approximately eight cultures was collected, flash frozen, and subsequently concentrated with glycerine-coated Microsep tubes (Pall Life Sciences) to a volume of approximately 100 ml. At least two replicate 50 ml aliquots from each sample were assayed for LIF content on ELISA plates according to the manufacturer’s instructions (R&D Systems). LIF concentration values were obtained by linear regression of absorbance readings made with a microtiter plate reader, against known concentrations of LIF measured in serial dilution on each microtiter plate. The ELISA does not cross react with other cytokines, and our tests confirmed no cross reactivity with CNTF, a closely related cytokine to LIF.
Western Blot Analysis
Western blot was performed for the analysis LIF in DRG neurons, astrocytes, and oligodendrocytes. The cells were washed with PBS and lysed by adding M-PER mammalian protein extraction reagent (Pierce, Rockford, IL). Protein concentration was determined by Coomassie protein assay kit (Pierce, Rockford, IL). Electrophoresis was performed on 14% or 10%-20% gradient polyacrylamide gels (Invitrogen, Carlsbad, CA). As a positive control, recombinant mouse LIF (Chemicon) was used. Proteins were transferred to Immobilon transfer membranes (Millipore, Bedford, MA), and blots were blocked with 5% skim milk in 0.1% tween-20 tris buffer (TBST) overnight at 4°C. The blots were probed with anti-mouse LIF antibody (R&D Systems, Inc., Minneapolis, MN, 1:5,000) and anti-AnkyrinG antibody (Zymed Lab, Inc., 1:1,000) as a loading control overnight at 4°C, followed by horseradish peroxidase (HRP)-coupled donkey anti-goat secondary antibody (Chemicon) and sheep anti-mouse (Amersham Biosciences Buckinghamshire, UK). Blots were analyzed by ECL plus Western blotting detection system (Amersham Biosciences).
RNA Preparation and RT-PCR
Rat astrocytes were prepared as described above and plated onto axons of 3 to 4 week old cultures of DRG neurons 3 days prior to electrical stimulation in Campenot chambers. Medium was replaced with serum and NGF-free media 12 hr prior to electrical stimulation; 30 U/ml of apyrase was added if appropriate. DRG axons were electrically stimulated with a phasic pattern at 10 Hz for 24 hr. Total RNA was prepared by the TRIzol (Invitrogen) extraction method with modifications (Lee et al., 2004). cDNA was prepared from 2 mg total RNA with Superscript II (Invitrogen) according to manufacturer’s instructions. PCR reactions were carried out by Platinum PCR super-mix high fidelity (Invitrogen) for 35 cycles, and reaction products analyzed on a 2% TAE agarose gel, visualized with SYBR I green staining (Invitrogen).
Acknowledgments
We thank Ben Barres for monoclonal antibody against O1 and MOG, Peter Wadeson for assistance with cell culture, Daniel Abebe for assistance with experimental animals, and Trent Watkins for critical comments on an earlier draft of this manuscript. This work was supported by the intramural research program at National Institutes of Health, National Institute of Child Health and Human Development.
References
- Banner LR, Patterson PH. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc. Natl. Acad. Sci. USA. 1994;91:7109–7113. doi: 10.1073/pnas.91.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullé;n F. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bugga L, Gadient RA, Kwan K, Stewart CL, Patterson PH. Analysis of neuronal and glial phenotypes in brains of mice deficient in leukemia inhibitory factor. J. Neurobiol. 1998;36:509–524. doi: 10.1002/(sici)1097-4695(19980915)36:4<509::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Introduction: P2 receptors. Curr. Top. Med. Chem. 2004;4:793–803. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- Butzkueven H, Zhang JG, Soilu-Hanninen M, Hochrein H, Chionh F, Shipham KA, Emery B, Turnley AM, Petratos S, Ernst M, et al. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat. Med. 2002;8:613–619. doi: 10.1038/nm0602-613. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci. USA. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree JL, Mason JL, Marcus JR, Stull M, Levinson R, Matsushima GK, Popko B. Oligodendrocytes assist in the maintenance of sodium channel clusters independent of the myelin sheath. Neuron Glia Biol. 2004;1:179–192. doi: 10.1017/S1740925X04000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziewulska D, Jamrozik Z, Podlecka A, Rafalowska J. Do astrocytes participate in rat spinal cord myelination? Folia Neuropathol. 1999;37:81–86. [PubMed] [Google Scholar]
- Fields RD. The other half of the brain. Sci. Am. 2004;290:54–61. doi: 10.1038/scientificamerican0404-54. [DOI] [PubMed] [Google Scholar]
- Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528–531. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. Purinergic Signaling in Neuron-Glia Interactions, Novartis Foundation Symposium; New York, NY: John Wiley and Sons. 2006. [Google Scholar]
- Fields RD, Stevens B. ATP in signaling between neurons and glia. Trends Neurosci. 2000;23:625–633. doi: 10.1016/s0166-2236(00)01674-x. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Yu C, Neale EA, Nelson PG, Kettenmann H. Chronic electrical stimulation of multicompartment cell cultures. In: Grantyn R, editor. Practical Electrophysiological Methods. Wiley Press; New York: 1992. pp. 67–76. [Google Scholar]
- Gard AL, Pfeiffer SE. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989;106:119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magentic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Itoh K, Stevens B, Schachner M, Fields RD. Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 1995;270:1369–1372. doi: 10.1126/science.270.5240.1369. [DOI] [PubMed] [Google Scholar]
- Johnson AB. Alexander disease: a review and the gene. Int. J. Dev. Neurosci. 2002;20:391–395. doi: 10.1016/s0736-5748(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Lee PR, Cohen JE, Tendi EA, Farrer R, DeVries GH, Becker KG, Fields RD. Transcriptional profiling in an MPNST-derived cell line and normal human Schwann cells. Neuron Glia Biol. 2004;1:135–148. doi: 10.1017/s1740925x04000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Messing A, Goldman JE, Brenner M. GFAP mutations in Alexander disease. Int. J. Dev. Neurosci. 2002;20:259–268. doi: 10.1016/s0736-5748(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996;17:607–615. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- Markham J, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1:351–364. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M, Bhakoo K, Noble M. Ciliary neurotrophic factor and leukemia inhibitory factor promote the generation, maturation and survival of oligodendrocytes in vitro. Development. 1994;120:143–153. doi: 10.1242/dev.120.1.143. [DOI] [PubMed] [Google Scholar]
- Mey J, Henkes L. Retinoic acid enhances leukemia inhibitory factor expression in OLN-93 oligodendrocytes. Cell Tissue Res. 2002;310:155–161. doi: 10.1007/s00441-002-0624-x. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Shen S, Barres BA. Astrocytes induce oligodendrocyte processes to align with and adhere to axons. Mol. Cell. Neurosci. 1999;14:385–397. doi: 10.1006/mcne.1999.0788. [DOI] [PubMed] [Google Scholar]
- Park SK, Solomon D, Vartanian T. Growth factor control of CNS myelination. Dev. Neurosci. 2001;23:327–337. doi: 10.1159/000048716. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in normal pediatric population: a diffusion tensor MRI study. Hum. Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolding NJ, Frith S, Linington C, Morgan BP, Campbell AK, Compston DA. Myelin-oligodendrocyte glycoprotein (MOG) a surface marker of oligodendrocyte maturation. J. Neuroimmunol. 1989;22:169–176. doi: 10.1016/0165-5728(89)90014-3. [DOI] [PubMed] [Google Scholar]
- Stankoff B, Aigrot MS, Noel F, Wattilliaux A, Zalc B, Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. J. Neurosci. 2002;22:9221–9227. doi: 10.1523/JNEUROSCI.22-21-09221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. J. Neurosci. 1998;18:9303–9311. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakuni H, Kawaguchi N, Ohtani Y, Nakamura J, Katayama T, Nakagawa T, Minami M, Satoh M. ATP induces leukemia inhibitory factor mRNA in cultured rat astrocytes. J. Neuroimmunol. 2002;129:43–50. doi: 10.1016/s0165-5728(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours A-R. The myelogenic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Blackwell Scientific; Oxford, UK: 1967. pp. 3–65. [Google Scholar]
- Zalc B, Fields RD. Do action potentials regulate myelination? Neuroscientist. 2000;6:5–13. doi: 10.1177/107385840000600109. [DOI] [PMC free article] [PubMed] [Google Scholar]