Abstract
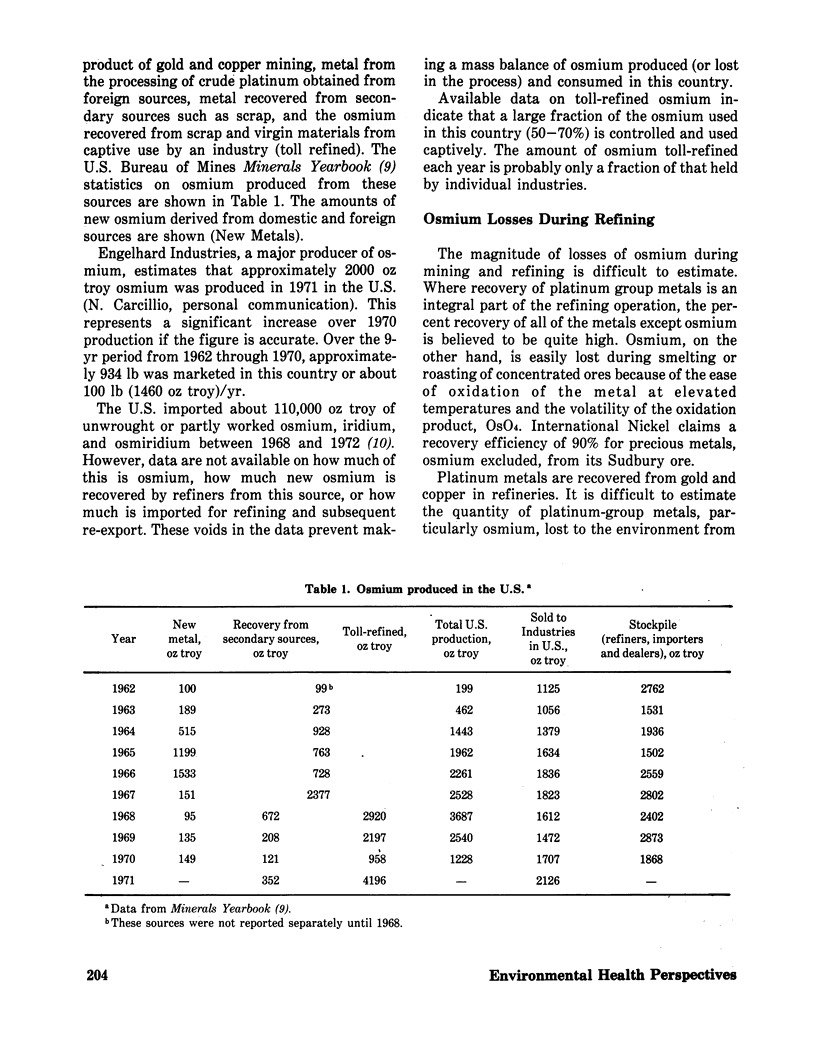
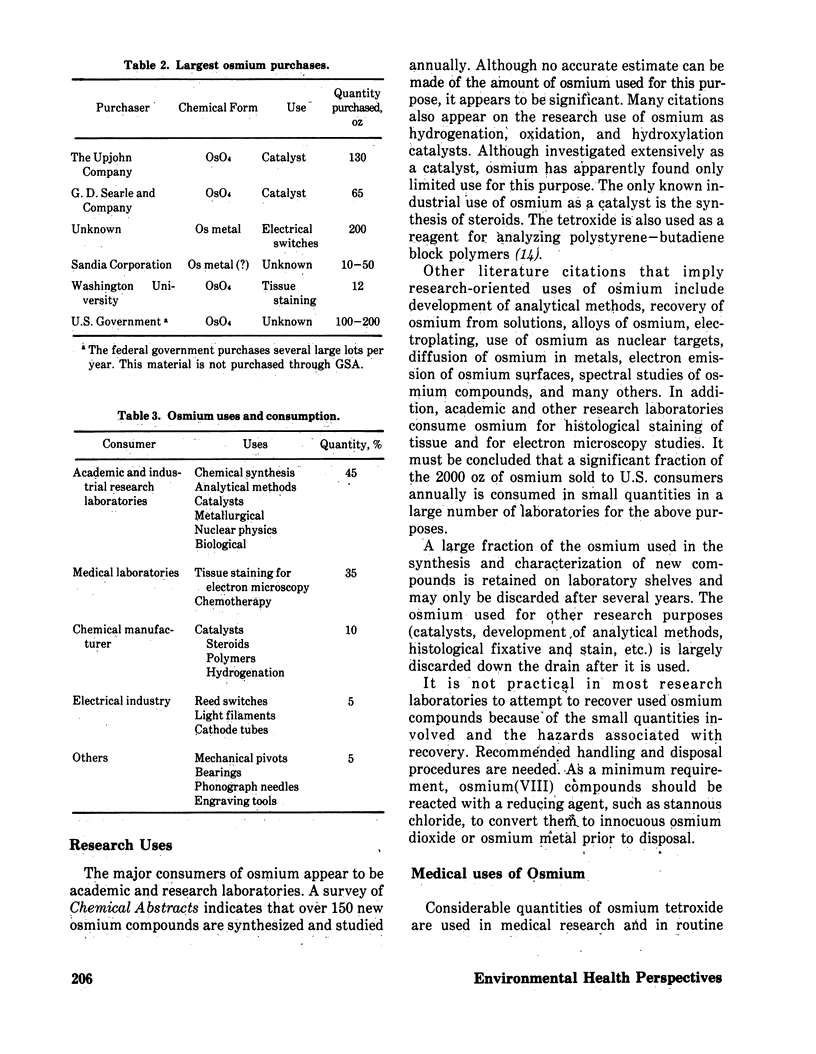
In the U.S., the chief source of new osmium is copper refining, where this metal is produced as a byproduct. Probably less than 10% of the osmium in the original copper ore is recovered, and 1000–3000 oz troy of osmium is lost each year to the environment as the toxic, volatile tetroxide from copper smelters. In 1971, about 2000 oz troy of osmium was domestically refined, most of which was from secondary sources. An additional 4169 oz troy of osmium was toll-refined. Major uses for osmium tetroxide identified are for catalysis, especially in steroid synthesis, and for tissue staining. Minor uses of osmium metal are for electrical contacts and for imparting hardness to alloys for mechanical pivots, etc. Unreclaimed osmium tetroxide that reaches wastewater streams is probably rapidly reduced by organic matter to nontoxic osmium dioxide or osmium metal, which would settle out in the sediment of the water course. Waste osmium metal, itself innocuous and chemically resistant, would be oxidized to the toxic tetroxide if incinerated.
Because of the small amounts used and their wide dispersal, the amounts of osmium tetroxide in wastewater and air should pose no hazard to man or the environment. The chief acute toxic effects of osmium tetroxide are well known and include eye and respiratory-tract damage. Few data are available that provide information on possible effects of nonacute exposure resulting from environmental contamination by osmium. However, workers continually exposed to osmium tetroxide vapors (refiners and histologists) and rheumatoid arthritis patients who have received intra-articular injections of osmic acid solutions have shown no apparent damage from exposure to low levels of osmium.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUNYAN J., EDWIN E. E., GREEN J. Protective effect of trace elements other than selenium against dietary necrotic liver degeneration. Nature. 1958 Jun 28;181(4626):1801–1801. doi: 10.1038/1811801a0. [DOI] [PubMed] [Google Scholar]
- Collan Y., Servo C., Winblad I. An acute immune response to intra-articular injection of osmium tetroxide. Acta Rheumatol Scand. 1971;17(3):236–242. doi: 10.3109/rhe1.1971.17.issue-1-4.31. [DOI] [PubMed] [Google Scholar]
- Jacobs G. F., Liggett S. J. An oxidation-distillation procedure for reclaiming osmium tetroxide from used fixative solutions. Stain Technol. 1971 Jul;46(4):207–208. doi: 10.3109/10520297109067855. [DOI] [PubMed] [Google Scholar]
- Lörincz G., Isomäki H. A., Martio J. Changes in the synovial fluid caused by osmic acid. Acta Rheumatol Scand. 1970;16(3):217–222. [PubMed] [Google Scholar]
- Möttönen M., Pantio M., Nevalainen T. Effects of osmium tetroxide on the rabbit knee joint normal synovial membrane. Acta Rheumatol Scand. 1970 Apr;16(2):121–129. [PubMed] [Google Scholar]
- SCHWARZ K., ROGINSKI E. E., FOLTZ C. M. Ineffectiveness of molybdenum, osmium and cobalt in dietary necrotic liver degeneration. Nature. 1959 Feb 14;183(4659):472–473. doi: 10.1038/183472a0. [DOI] [PubMed] [Google Scholar]


