Abstract
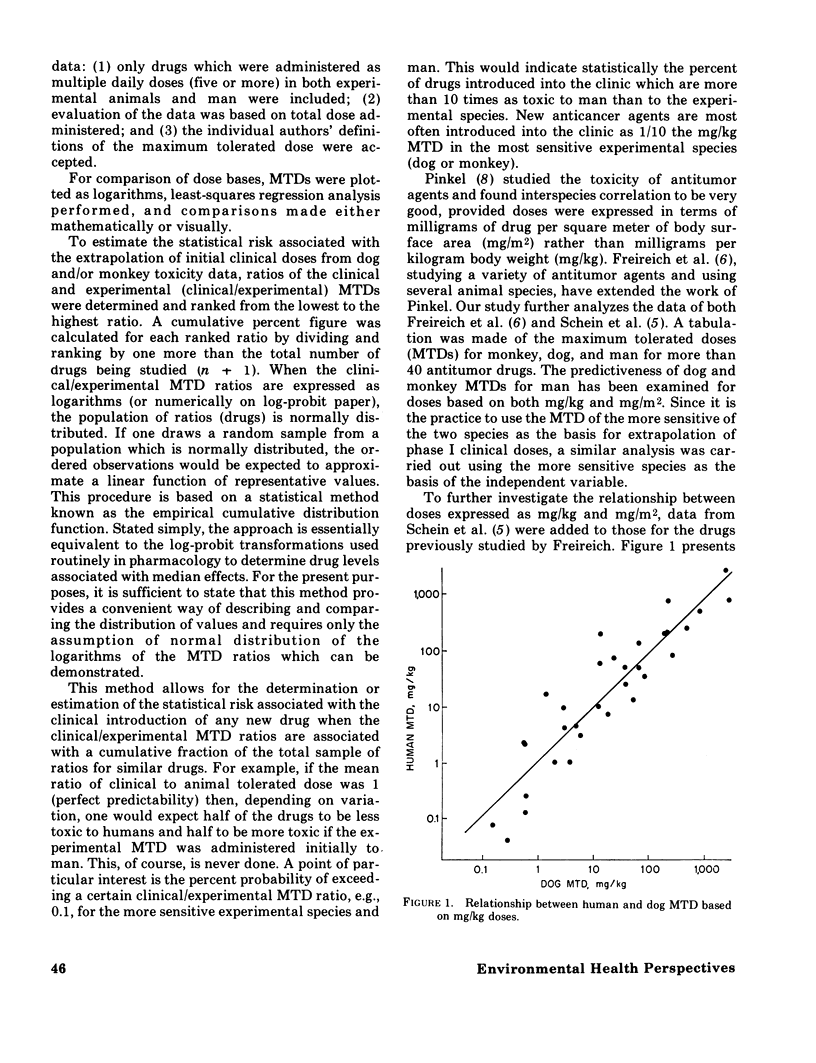
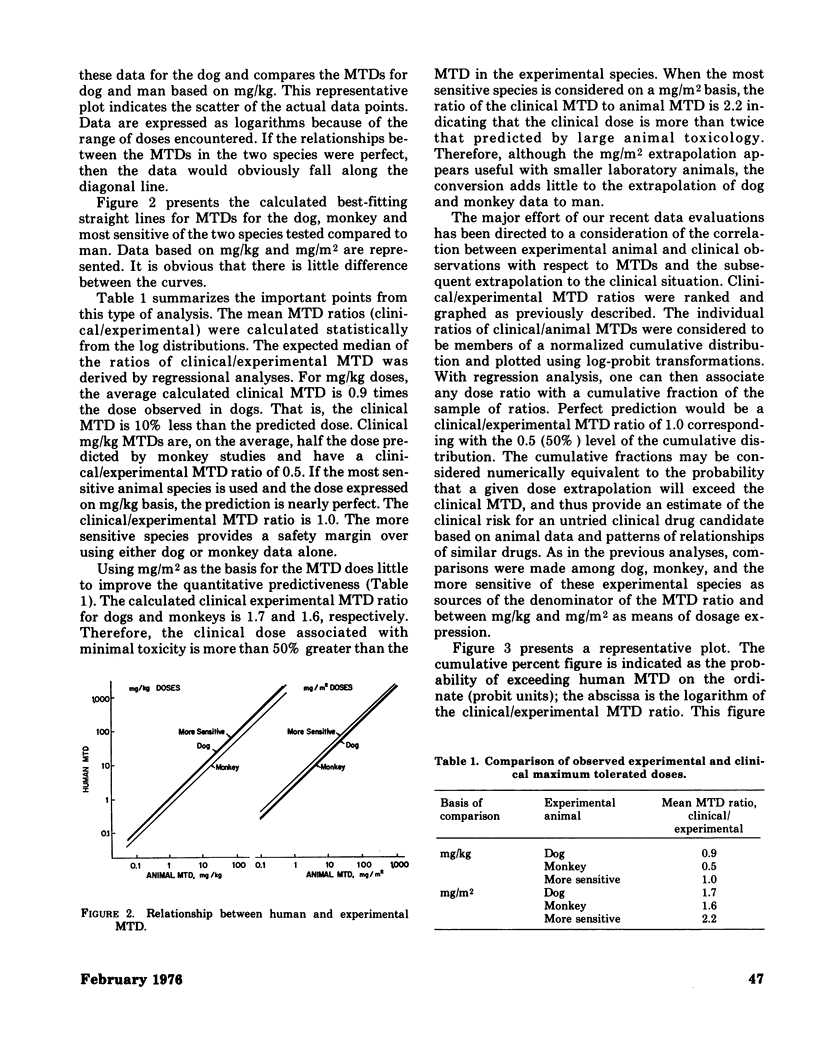
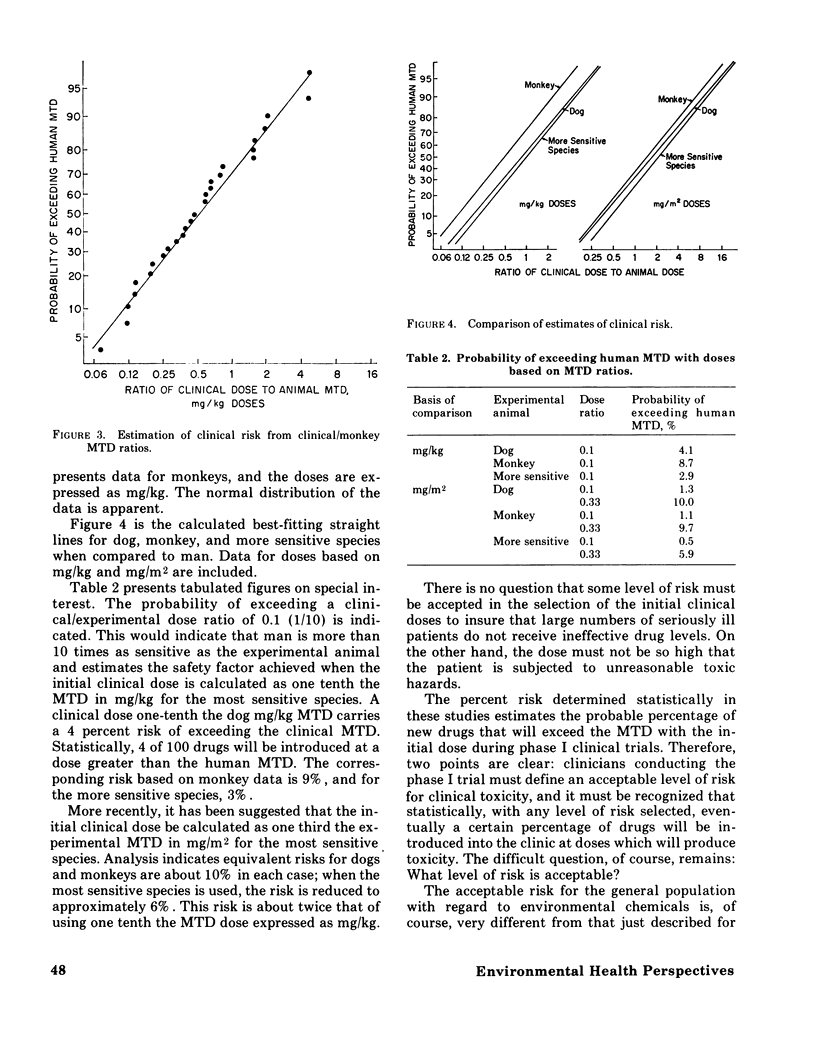
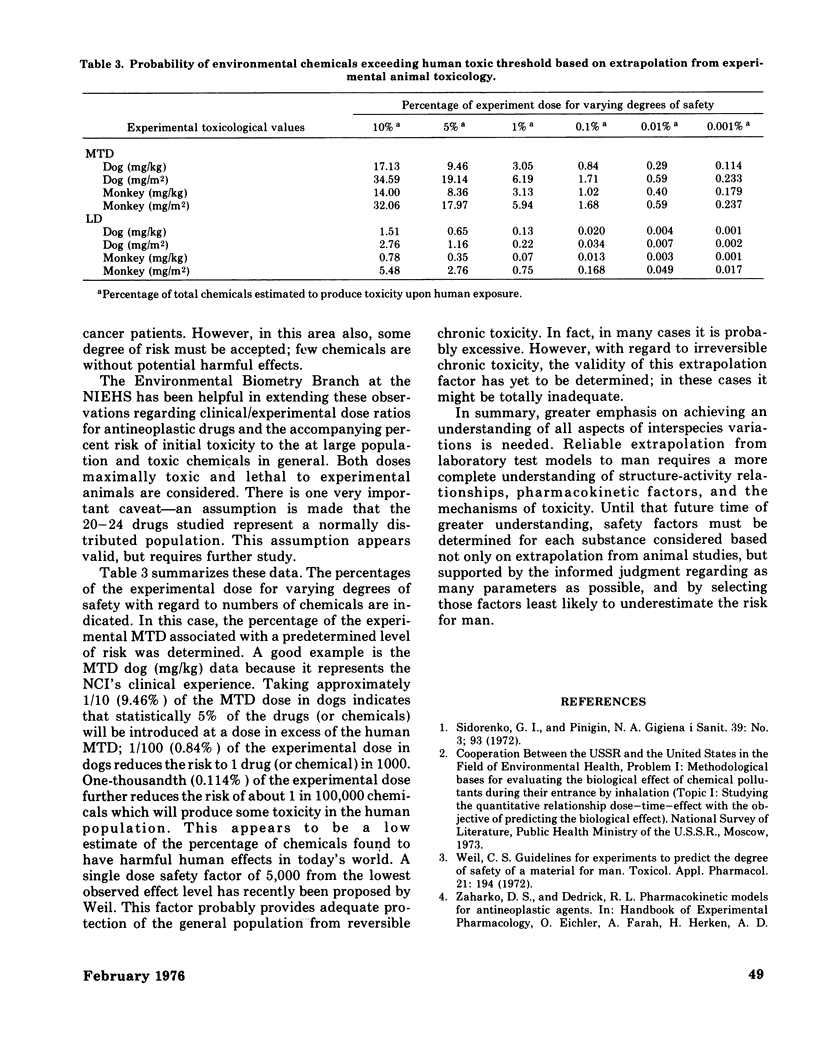
Some of the problems in extrapolating laboratory animal toxicity data to man are considered. The quantitative predictiveness of preclinical studies of anticancer drugs using dogs and monkeys for man has also been examined. The relationship between the maximum tolerated dose (MTD) in the dog, monkey, and the more sensitive of the two species and clinical observations are discussed. The effectiveness of using doses expressed on the basis of body weight (mg/kg) and body surface area (mg/m2) are compared. A method is introduced to assess the "statistical risk" associated with the extrapolation of the initial clinical (phase I) dose from experimental animal data. The best clinical prediction is obtained when one uses the experimental MTD expressed in mg/kg for the more sensitive of the large animal species (dogs or monkeys). The clinical introduction of a new anticancer agent at a dose 1/10 the MTD in the more sensitive species carries a statistical risk of about 3%; that is, the initial doses of about 3 of every 100 new drugs introduced into the clinic will produce some toxic effects in man. These same data have been extended theoretically to the total population and toxic chemicals in general. Reliable extrapolation from laboratory test models to man requires a much more complete understanding of structure--activity relationships, pharmacokinetic factors, and mechanisms of toxicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freireich E. J., Gehan E. A., Rall D. P., Schmidt L. H., Skipper H. E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966 May;50(4):219–244. [PubMed] [Google Scholar]
- PINKEL D. The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res. 1958 Aug;18(7):853–856. [PubMed] [Google Scholar]
- Schein P. S., Davis R. D., Carter S., Newman J., Schein D. R., Rall D. P. The evaluation of anticancer drugs in dogs and monkeys for the prediction of qualitative toxicities in man. Clin Pharmacol Ther. 1970 Jan-Feb;11(1):3–40. doi: 10.1002/cpt19701113. [DOI] [PubMed] [Google Scholar]
- Weil C. S. Guidelines for experiments to predict the degree of safety of a material for man. Toxicol Appl Pharmacol. 1972 Feb;21(2):194–199. doi: 10.1016/0041-008x(72)90062-2. [DOI] [PubMed] [Google Scholar]


