Abstract
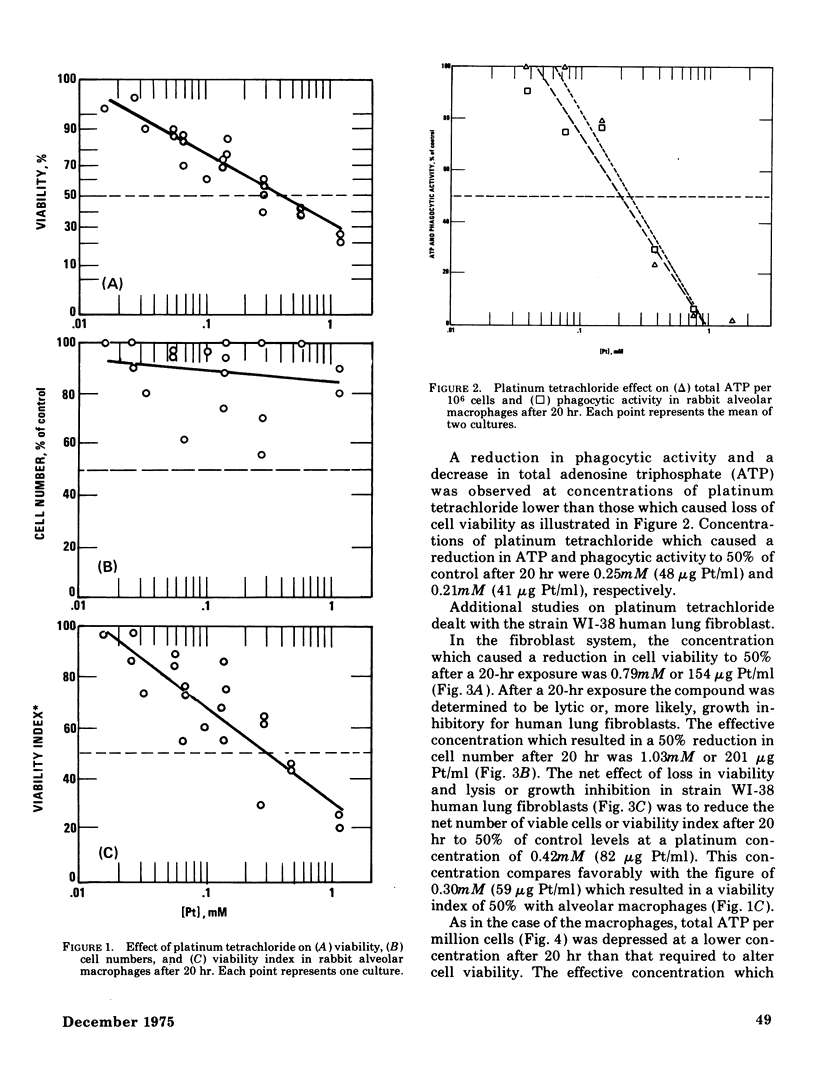
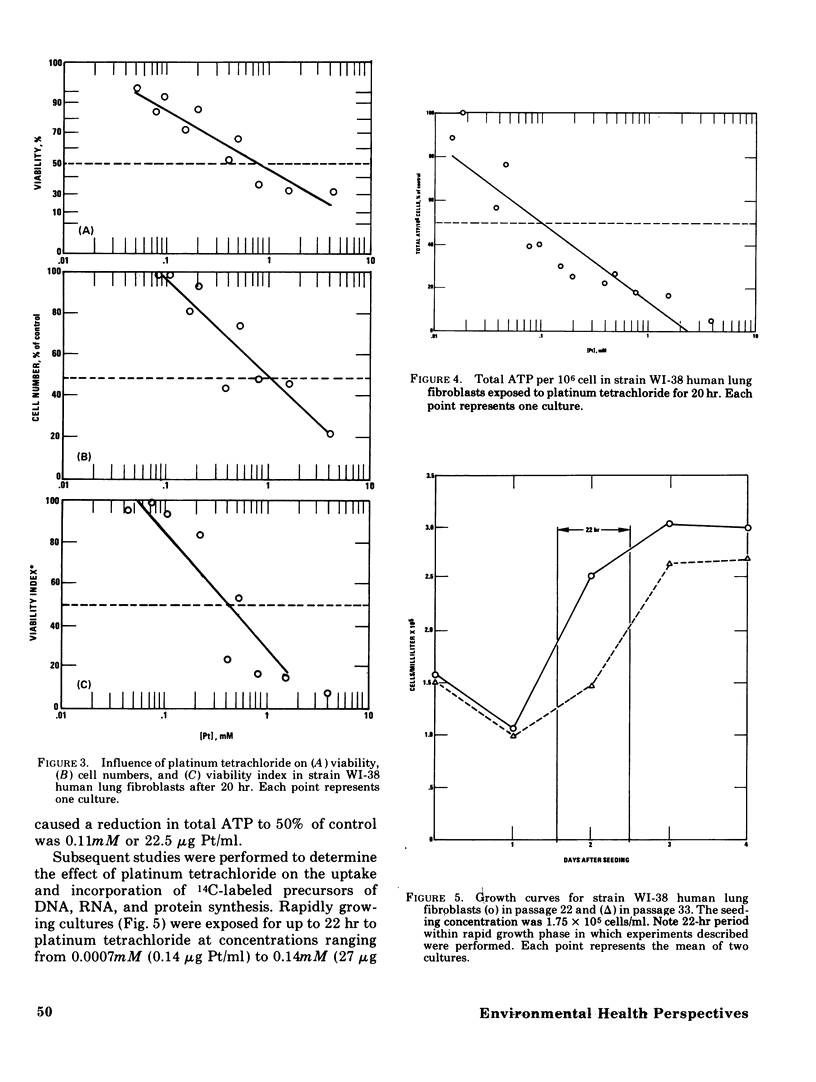
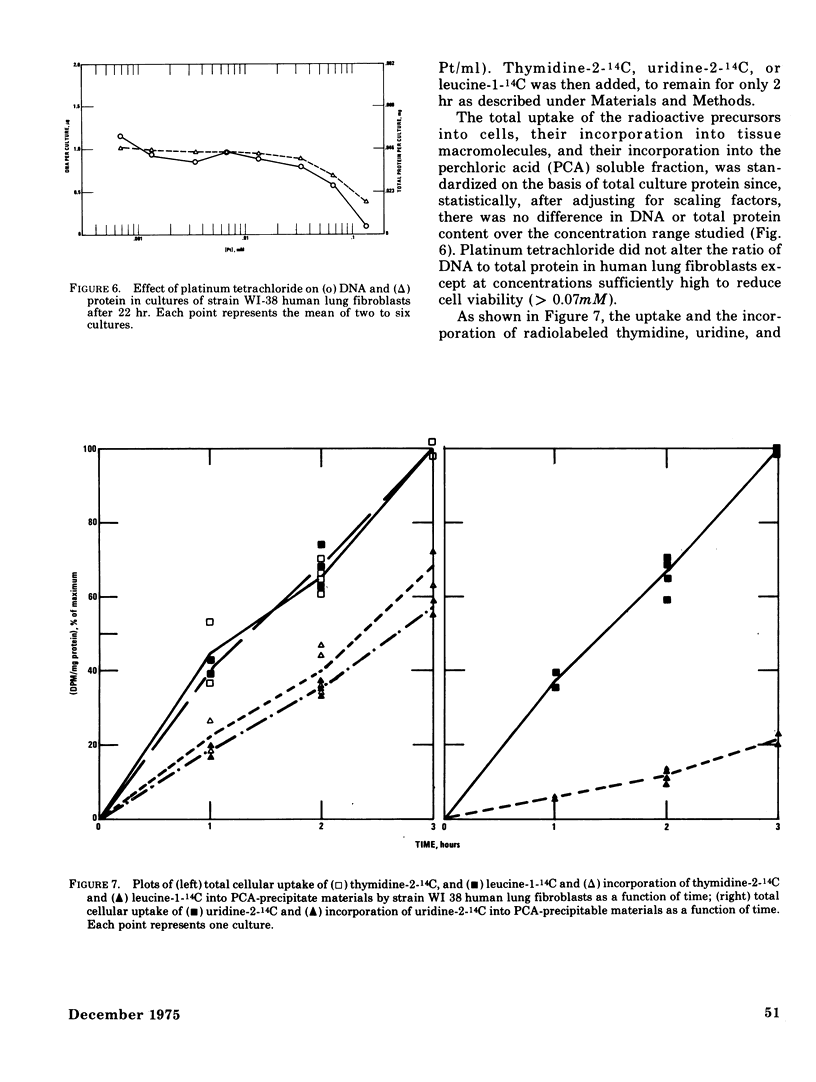
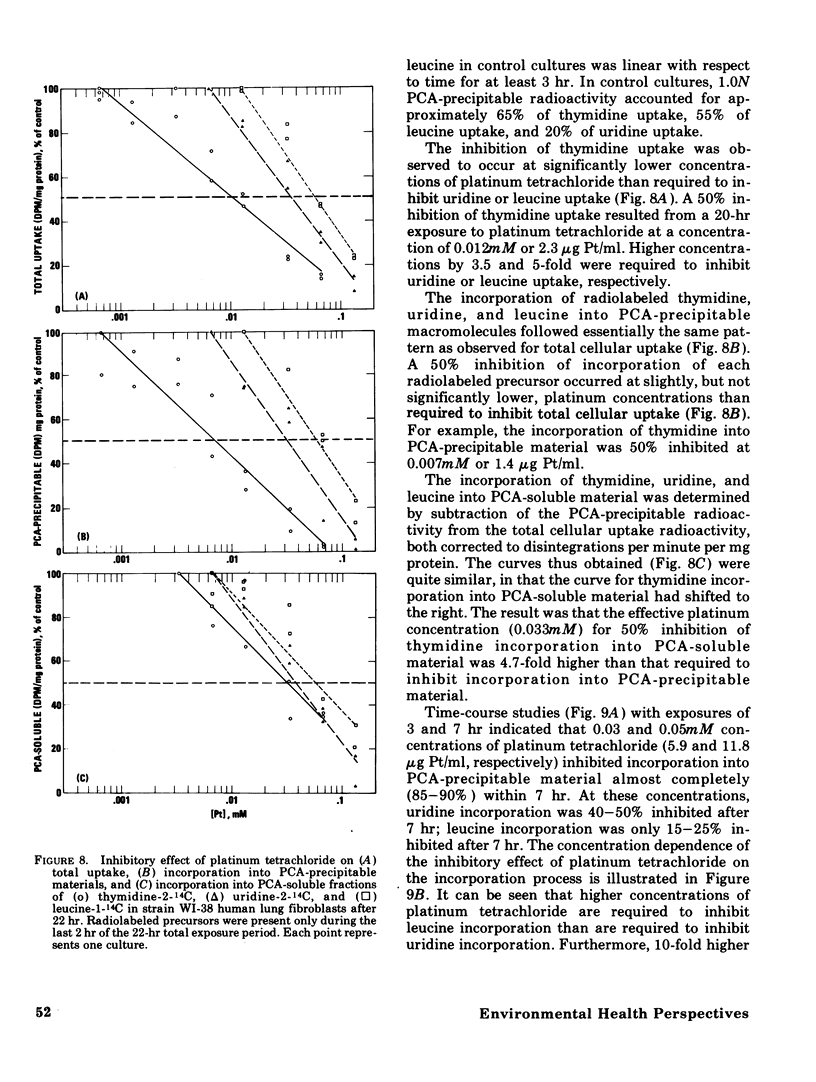
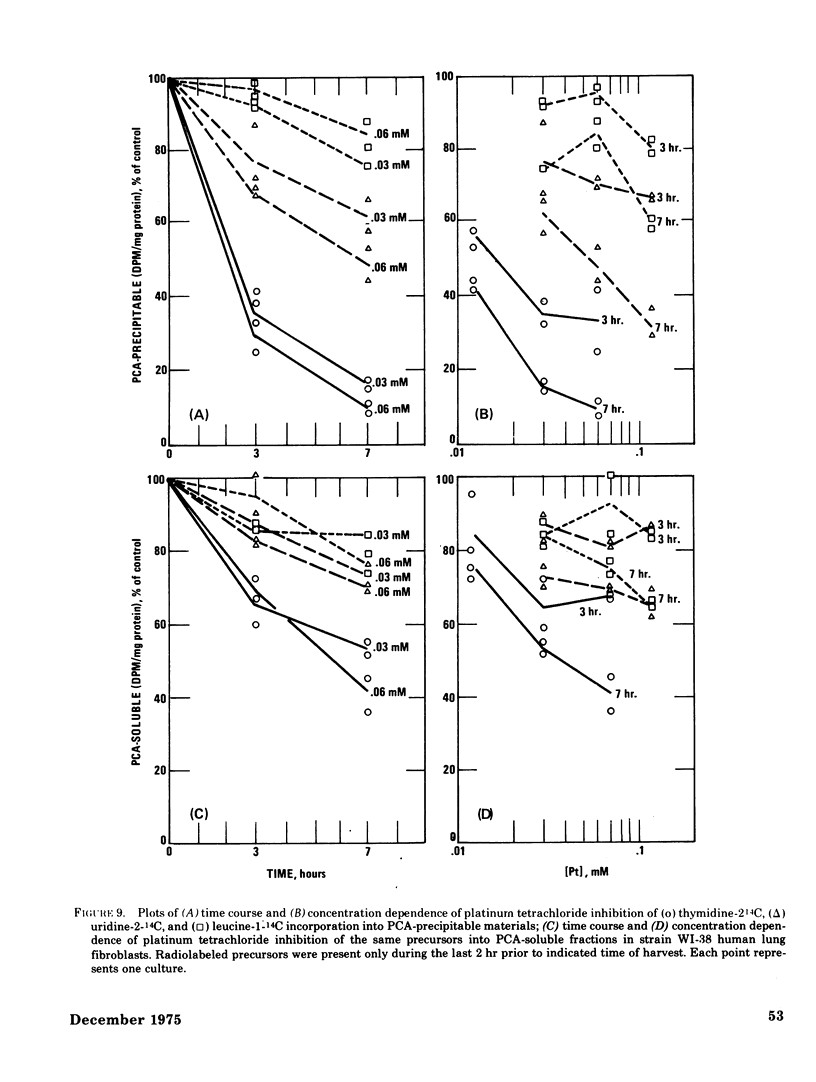
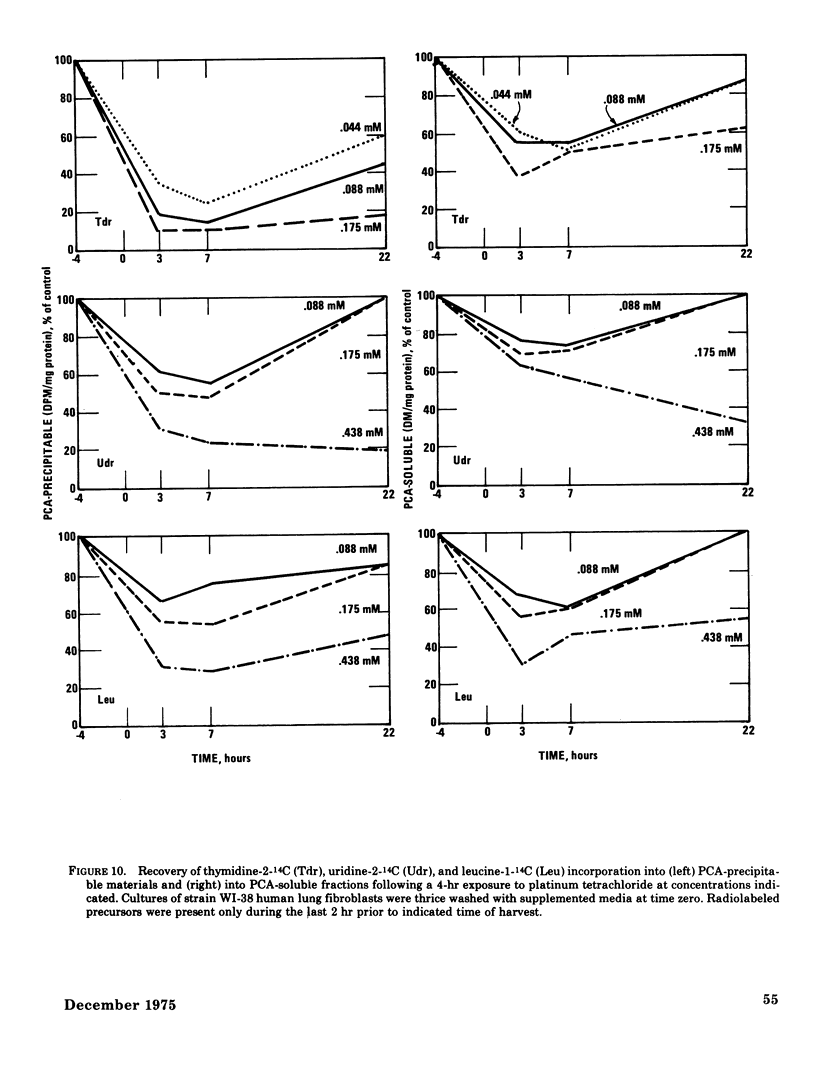
The acute toxicity of tetravalent platinum was studied in vitro by use of rabbit alveolar macrophages and human lung fibroblasts (strain WI-38). Alveolar macrophages were exposed in tissue culture for 20 hr to platinum dioxide (PtO2) or platinum tetrachloride (PtCl4). There was no evidence of dissolution of PtO2 and no decrease in viable cells at concentrations as high as 500 mug/ml. PtCl4 was soluble in the macrophage system and after a 20-hr exposure, resulted in loss of viability in 50% of the cells originally present at a concentration of 0.30mM (59 mug Pt/ml). After a 20-hr exposure, rapidly growing human lung fibroblasts were rendered nonviable by PtCl4 at comparable concentrations. A decrease in total cellular ATP was observed at lower concentrations in macrophages and fibroblasts along with a reduction in phagocytic activity of macrophages as compared to controls. With the fibroblasts, a 50% decrease in incorporation of 14C-thymidine was observed after a 22-hr exposure to PtCl4 at a concentration of 0.007mM; higher concentrations were required to inhibit the incorporation of 14C-uridine and 14C-leucine. Time-course studies indicated that the inhibition of 14C-thymidine incorporation was nearly complete (90%) after 7 hr in the presence of 0.06mM PtCl4. Under the same conditions, there was little inhibition (15%) of 14C-leucine incorporation and moderate inhibition (50%) of 14C-uridine incorporation. Higher concentrations of PtCl4 were required to inhibit 14C-thymidine incorporation into the acid-soluble fraction than were required to inhibit incorporation into the acid-precipitable fraction. Hence, the preferential inhibition of DNA synthesis by PtCl4 may result from an impairment of the incorporation process.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gardner D. E., Graham J. A., Miller F. J., Illing J. W., Coffin D. L. Technique for differentiating particles that are cell-associated or ingested by macrophages. Appl Microbiol. 1973 Mar;25(3):471–475. doi: 10.1128/am.25.3.471-475.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder H. C., Rosenberg B. Inhibitory effects of anti-tumor platinum compounds on DNA, RNA and protein syntheses in mammalian cells in virtro. Int J Cancer. 1970 Sep 15;6(2):207–216. doi: 10.1002/ijc.2910060207. [DOI] [PubMed] [Google Scholar]
- Howle J. A., Gale G. R. Cis-dichlorodiammineplatinum (II). Persistent and selective inhibition of deoxyribonucleic acid synthesis in vivo. Biochem Pharmacol. 1970 Oct;19(10):2757–2762. doi: 10.1016/0006-2952(70)90102-4. [DOI] [PubMed] [Google Scholar]
- Howle J. A., Gale G. R., Smith A. B. A proposed mode of action of antitumor platinum compounds based upon studies with cis-dichloro-((G- 3 H)dipyridine)platinum(II). Biochem Pharmacol. 1972 May 15;21(10):1465–1475. doi: 10.1016/0006-2952(72)90371-1. [DOI] [PubMed] [Google Scholar]
- Howle J. A., Thompson H. S., Stone A. E., Gale G. R. Cis-dichlorodiammineplatinum [II]: inhibition of nucleic acid synthesis in lymphocytes stimulated with phytohemagglutinin. Proc Soc Exp Biol Med. 1971 Jul;137(3):820–825. doi: 10.3181/00379727-137-35675. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LeRoy A. F. Interactions of platinum metals and their complexes in biological systems. Environ Health Perspect. 1975 Apr;10:73–83. doi: 10.1289/ehp.751073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrot J. L., Hébert R., Saindelle A., Ruff F. Platinum and platinosis. Allergy and histamine release due to some platinum salts. Arch Environ Health. 1969 Nov;19(5):685–691. doi: 10.1080/00039896.1969.10666910. [DOI] [PubMed] [Google Scholar]
- Pascoe J. M., Roberts J. J. Interactions between mammalian cell DNA and inorganic platinum compounds. I. DNA interstrand cross-linking and cytotoxic properties of platinum(II) compounds. Biochem Pharmacol. 1974 May 1;23(9):1359–1365. doi: 10.1016/0006-2952(74)90355-4. [DOI] [PubMed] [Google Scholar]
- Pascoe J. M., Roberts J. J. Interactions between mammalian cell DNA and inorganic platinum compounds. II. Interstrand cross-linking of isolated and cellular DNA by platinum(IV) compounds. Biochem Pharmacol. 1974 May 1;23(9):1345–1357. doi: 10.1016/0006-2952(74)90354-2. [DOI] [PubMed] [Google Scholar]
- Waters M. D., Gardner D. E., Aranyi C., Coffin D. L. Metal toxicity for rabbit alveolar macrophages in vitro. Environ Res. 1975 Feb;9(1):32–47. doi: 10.1016/0013-9351(75)90047-x. [DOI] [PubMed] [Google Scholar]


