Abstract
In the adult hippocampus, gonadal steroids induce neural remodeling through cellular and molecular mechanisms that are largely unknown. The calcium-dependent cell adhesion molecule N-cadherin, which participates in the developmental organization of the nervous system, has recently been localized to hippocampal synapses and is suspected to participate in adult synaptic physiology. Little is currently known about the regulation of cadherins in the adult central nervous system, although posttranslational modifications are thought to account for variability in N-cadherin expression levels. To evaluate the possibility that gonadal steroids regulate N-cadherin in the adult hippocampus, we examined hippocampal N-cadherin mRNA levels and protein expression in castrated adult male rats treated with testosterone, or its metabolites 17β-estradiol or dihydrotestosterone. Northern blot analysis indicated increased hippocampal N-cadherin mRNA levels in the adult rat hippocampus after treatment with 17β-estradiol but not testosterone or dihydrotestosterone. Increased N-cadherin immunoreactivity was observed in CA1 and CA3 pyramidal cells after 17β-estradiol treatment. Additionally, both 17β-estradiol and testosterone treatment increased N-cadherin immunoreactivity in the neuropil of the stratum lacunosum-moleculare, which includes apical dendrites from pyramidal cells. In contrast, dihydrotestosterone treatment had no effect on levels of N-cadherin protein expression in CA1 or CA3 pyramidal cells or in the stratum lacunosum-moleculare. These results demonstrate that, in the hippocampus, expression levels of N-cadherin are dynamic in adulthood. To our knowledge, there have been no other demonstrations of steroid regulation of cadherin expression in neural populations. These results suggest a possible adhesive mechanism for steroid-induced plasticity of the adult nervous system.
Keywords: estrogens, androgens, neuronal plasticity, pyramidal cells, cell adhesion
Steroids can induce a highly plastic environment in the adult nervous system, comparable to that observed in earlier developmental stages. For example, gonadal steroids induce neurogenesis (1), reduce programmed cell death (2), and induce synaptic remodeling (3, 4) in the adult hippocampus. Little is currently known about the cellular and molecular mechanisms underlying this process. We investigated the possibility that the cell adhesion molecule N-cadherin (N-cad), which participates in the organization of the developing nervous system, is regulated by gonadal steroids in the adult hippocampus.
The cadherins are a diverse class of calcium-dependent cell adhesion molecules (5) that are widely expressed in the nervous system. Cadherins bind to one another both in cis and trans (6, 7), resulting in strong zipper-like punctate adhesions between apposing cell membranes. Cadherin-mediated cell adhesion is homophilic; i.e., cells expressing cadherins bind preferentially to, and sort with, cells expressing similar cadherin subtypes (8), and they additionally sort according to the expression levels of the same cadherin subtype (9). The regulated expression of cadherin subtypes is believed to provide a molecular mechanism for the segregation of distinct cell populations and the organization of tissue morphology during embryonic development (10, 11).
The cadherin subtype N-cad has been implicated in several developmental processes in the central nervous system, including governing differentiation of neuroepithelium (12), the sorting and segregation of discrete neural populations (13), and axon patterning (14, 15). During synaptogenesis, cadherin-mediated interactions are thought to promote synapsis between apposing neurons or neuronal processes, providing an adhesive mechanism for the formation and maintenance of functional connectivity (11, 13, 16). Whereas high expression levels of N-cad are observed in the developing nervous system, expression levels of N-cad are generally greatly reduced in adulthood (17).
In the adult brain, N-cad has been studied primarily in the context of chemical synapses. N-cad is abundant in isolated rat forebrain postsynaptic densities (18), and cadherins that are localized to chemical synapses border the transmitter release zone and are associated with cytoplasmic specializations typical of adherens junctions (16, 19). N-cad becomes restricted to glutamatergic synapses in the adult hippocampus (20), and molecular characteristics of N-cad are activity dependent (21), suggesting that N-cad expression is tightly regulated in the adult hippocampus, and lending further support to the hypothesis that cadherins serve to establish and maintain functional connectivity between neural populations. N-cad function is also required to initiate long term potentiation, an electrophysiological correlate of synaptic plasticity, between CA3 and CA1 pyramidal neurons (22), suggesting that cadherin function continues to participate in remodeling of the adult nervous system.
Although little is known about the factors regulating cadherin expression in the adult nervous system, there are a number of lines of evidence that suggest that gonadal steroids may regulate N-cad expression in the adult hippocampus. Gonadal steroids are known to influence adult rat hippocampal synaptic architecture (3, 4), electrophysiology (23–25), and lesion-induced process outgrowth (26), all of which are neuroplastic events in which N-cad has been implicated. Furthermore, gonadal steroids are known to be key regulators of cadherin mRNA and protein expression levels in peripheral reproductive tissues (27–29). We therefore suspected that N-cad expression levels may also be regulated by these hormones in the adult hippocampus. To test this possibility, we determined the effects of testosterone (T) and its metabolites 17β-estradiol (E2) and dihydrotestosterone (DHT) on N-cad mRNA expression levels and protein distribution in the hippocampus of adult rats.
Methods
The animals used in these studies were 36 locally bred adult (60 days or older) male Sprague–Dawley rats housed individually and maintained in standard laboratory conditions. Animals were randomly assigned into four treatment groups: (i) animals receiving T, (ii) animals receiving E2, (iii) animals receiving DHT, and (iv) a no steroid control.
Steroid Manipulation.
Castrations were performed under halothane anesthesia. Animals were implanted with capsules fashioned from Silastic tubing (1.57-mm inner diameter × 3.18-mm outer diameter; Dow Corning) containing T (40 mm in length), E2 (10 mm in length), or DHT (30 mm in length). Implants of these sizes have previously been established to produce high serum titers of the respective steroids (30, 31). All steroids were obtained from Steraloids (Wilton, NH). Control capsules were empty silastic tubes (40 mm in length). Capsules were preincubated in PBS for 72 h and washed in ethanol before implantation (30). Capsules were implanted s.c. in the neck at the time of castration and remained in place for a duration of 4 wk, at which time the animals were killed and processed for Northern blot analysis or immunohistochemistry as described below.
Northern Blot Analysis.
Animals (n = 3 from each treatment condition) were anesthetized by CO2 inhalation and killed by decapitation. The hippocampus was removed rapidly and frozen on dry ice. Total RNA was prepared from hippocampal tissues by the phenol-chloroform method (32). The RNA species were resolved by electrophoresis in 1% wt/vol agarose gels containing 3.7% vol/vol formaldehyde. Approximately 20 μg of total RNA were loaded in each lane. The fractionated RNA species were then transferred onto charged nylon membranes (Amersham Canada, Oakville, ON) as previously described (33).
The Northern blots were incubated in a 3% BSA wt/vol solution dissolved in 5× SSPE (20× SSPE consists of 0.2 M sodium phosphate monobasic, pH 7.4, containing 25 mM EDTA and 3 M NaCl) at 37°C for 30 min. The Northern blots were then transferred to a prehybridization solution consisting of 50% vol/vol deionized formamide, 5× Denhardt's solution (5 Prime→3 Prime), 1% vol/vol SDS, 50 mM sodium phosphate dibasic, and 5 mM sodium phosphate monobasic. The blots were incubated in this solution at 37°C for 2 h. Heat-denatured salmon sperm DNA (final concentration 0.2 mg/ml; 5 Prime→3 Prime) and a radiolabeled rat N-cad cDNA probe (described in ref. 34) were then added to the prehybridization solution. The probe was radiolabeled according to the methods of Feinberg and Vogelstein (35) and heat-denatured, before being added to the prehybridization solution. The blots were incubated in the presence of the radiolabeled cDNA probe at 37°C for 16 h. The Northern blots were then washed twice with 2× SSPE at room temperature (10 min/wash), twice with 2× SSPE containing 1% vol/vol SDS at 55°C (30 min/wash) and twice with 0.2× SSPE at room temperature (30 min/wash). The Northern blots were subjected to autoradiography to detect the hybridization of the radiolabeled cDNA probe to the mRNA species.
To standardize the amounts of total RNA in each lane, the blots were then reprobed with a radiolabeled synthetic cDNA specific for 28S rRNA (described in ref. 34). The blots were again subjected to autoradiography to detect the hybridization of the radiolabeled cDNA probe to the 28S rRNA. The resulting autoradiograms were then scanned by using an LKB laser densitometer. The absorbance values obtained for the N-cad mRNA transcript were normalized relative to the corresponding 28S rRNA absorbance value.
Immunohistochemistry.
Animals (n = 6 from each treatment condition) were anesthetized deeply and perfused transcardially with PBS followed by 2% vol/vol acrolein PBS. The brains were then removed and cryoprotected in 20% wt/vol sucrose PBS for 24 h. Coronal sections (50 μm) through the extent of the hippocampus were prepared on a freezing microtome and stored in cryoprotectant (36) at −20°C until free floating indirect immunohistochemistry was performed. The wash buffer used was 0.05 M Tris-buffered saline (TBS, pH 7.2) unless otherwise specified. After three 5-min rinses, excess aldehydes were removed by treatment with 1% wt/vol sodium borohydride for 10 min. After another three washes, endogenous peroxidase was quenched with 0.03% vol/vol hydrogen peroxide for 30 min. Sections were rinsed three times for 5 min, and nonspecific binding was blocked by incubation with 5% vol/vol normal horse serum (Vector Laboratories) in 0.05% wt/vol gelatin, 0.1% vol/vol Triton X-100 (Sigma) in TBS for 30 min at 37°C. Sections were then incubated in the mouse monoclonal antibody 13A9 (kind gift from K. Knudsen, Lakenau Institute, Wynnewood, PA), generated against the cytoplasmic domain of human N-cad (37) and previously characterized in rat hippocampus (20), at a 1:500 dilution in TBS containing gelatin and Triton X-100 for 72 h at 4°C. After three 5-min washes in TBS-containing gelatin and Triton X-100, sections were incubated for 90 min with biotinylated rat-adsorbed horse anti-mouse antiserum (Vector Laboratories) at 1:200 dilution in TBS containing gelatin and Triton X-100. After three rinses, sections were incubated for 90 min with avidin-biotin complex (Vectastain Elite kit, Vector Laboratories) at 1:500 dilution in TBS. Sections were rinsed three times, and immunolabeling was visualized with 0.035 wt/vol 3,3′ diaminobenzidine (Sigma) as the chromagen.
Image Analysis.
All data analysis was conducted by an experimenter blind to condition on a Nikon Eclipse E-600 microscope. CA1 and CA3 fields were viewed at ×100 magnification and loaded into mcid software (ais-c; Imaging Research, St. Catherines, ON, Canada) using a Sony DXC-950 3CCD color video camera. The relative optical density (ROD) of CA3 and CA1 pyramidal cell layers as well as the stratum lacunosum-moleculare (SL-M) was determined as follows. The optical density target areas were determined, and the background optical density, determined by the unlabeled cortex overlying the hippocampus, was then subtracted from these scores. The SL-M was defined as the area between 300 μm from the CA1 pyramidal cell layer and the inner blade of the dentate gyrus.
Results
E2 Regulates N-cad mRNA Levels in the Adult Hippocampus.
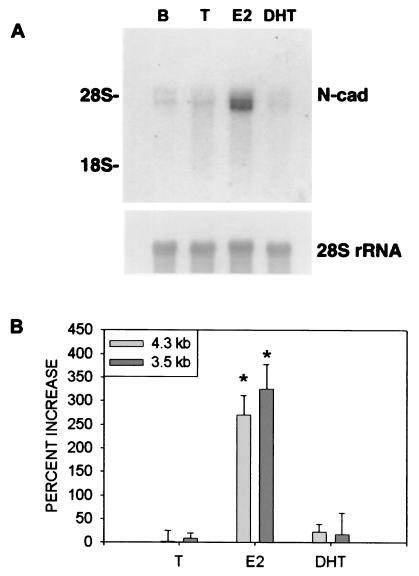
E2 treatment caused a marked increase in N-cad mRNA levels of both 4.3-kilobase (t6 = −6.19, P < 0.05) and 3.5-kilobase (t6 = −6.02, P < 0.05) species in the adult hippocampus, whereas treatment with T or the nonaromatizable androgen DHT had no effect on the levels of N-cad mRNA transcripts present in this tissue (Fig. 1). Both transcripts apparently originate from a single gene (38, 39). Although noncoordinate regulation of the 4.3-kilobase and 3.5-kilobase species has been described during rat testicular development (40), the significance of these two N-cad mRNA species is unclear.
Figure 1.
E2 regulates levels of N-cad mRNA transcripts. (A) Autoradiogram of a Northern blot containing total RNA extracted from the hippocampus of a control adult castrate rat implanted with an empty capsule (lane B), or adult castrate rats implanted with capsules containing T, E2, or DHT (lanes T, E2, and DHT, respectively). (Upper Left) The positions of the 28S and 18S rRNA species are shown. The blot was probed with radiolabeled N-cad cDNA (Upper) and then stripped and reprobed with another cDNA specific for 28S rRNA (Lower). (B) The areas under the curve for the laser densitometric scans are expressed as a percentage of that for vehicle-treated castrate controls. *, Significantly different from those of vehicle controls as determined by independent groups t test with α = 0.05.
E2 Increases N-cad Expression in Defined Areas of the Adult Rat Hippocampus.
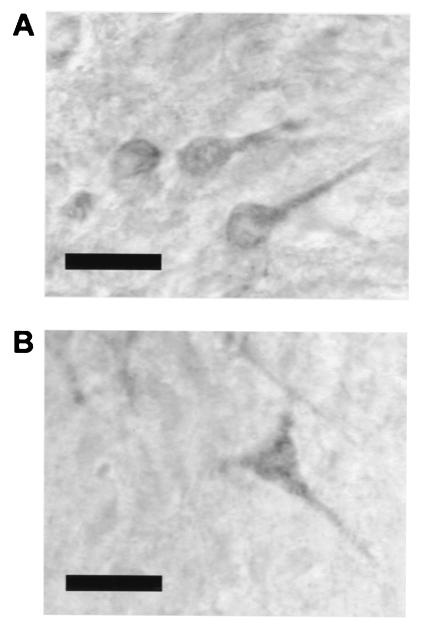
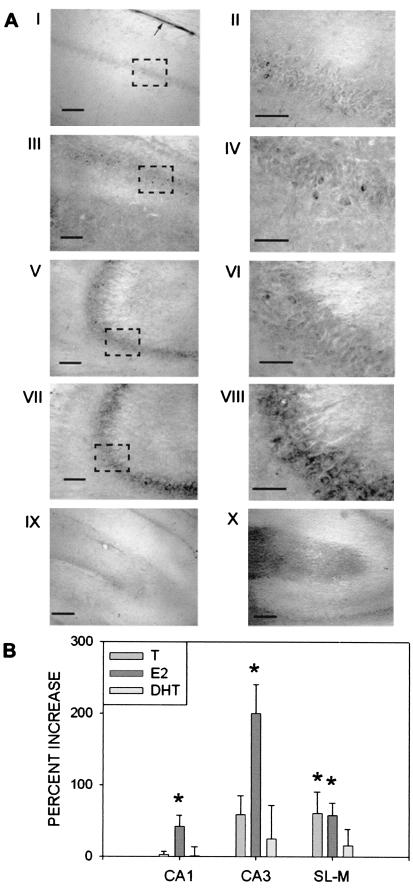
N-cad immunoreactivity was localized to CA3 pyramidal cell bodies, including the proximal apical dendrites as previously described (refs. 16 and 20; Fig. 2A), although significant CA1 pyramidal cell staining was also observed (Fig. 2B) after steroid treatment. Increased N-cad expression in CA1 and CA3 pyramidal neurons was observed after treatment with E2 (CA1 t12 = 2.24, CA3 t12 = 3.64) but not T or DHT (Fig. 3B). Ependymal glial cells were strongly labeled as previously described (refs. 17 and 41; Fig. 3AI), although steroid regulation of N-cad immunoreactivity was not observed in this cell population. Labeled neurons were also observed in the polymorphic layer of the dentate gyrus.
Figure 2.
Immunolocalization of N-cad in the pyramidal cell layers. Representative photomicrographs showing N-cad immunoreactivity in somata and proximal apical dendrites of (A) CA3 pyramidal cells and (B) CA1 pyramidal cells (original magnification, ×1,000; bar = 20 μm).
Figure 3.
Effects of steroid treatment on hippocampal N-cad protein expression. (A) Representative photomicrographs of N-cad immunoreactivity. II, IV, VI, and VIII (original magnification, ×600; bar = 50 μm) are magnifications of the boxed areas in I, III, IV, and VII (original magnification, ×200; bar = 100 μm), respectively. Low or absent staining in area CA1 (I and II) and CA3 (V and VI) was typical of vehicle-, T-, or DHT-treated castrated males. Increased staining typical of castrated males treated with E2 was observed in areas CA1 (III and IV) and CA3 (VII and VIII). IX depicts low or absent staining in the SL-M typical of vehicle or DHT-treated males (original magnification, ×100; bar = 200 μm). X depicts increased staining in the SL-M typical of castrated males treated with T or E2. Arrow in I indicates the immunopositive ependymal glia layer overlying the alveus. (B) Percentage change in relative optical density of N-cad immunoreactivity by area and steroid treatment (bars represent SEM). Increase is relative to vehicle treated castrate males. T, Castrated males treated with testosterone; E2, castrated males treated with 17β-estradiol; DHT, castrated male treated with dihydrotestosterone. CA1, CA1 pyramidal cell layer; CA3, CA3 pyramidal cell layer; SL-M, stratum lacunosum-moleculare. *, Significantly different from vehicle-treated controls as indicated by independent groups t test with α = 0.05.
Punctate N-cad immunoreactivity was observed in the neuropil of the SL-M (Fig. 3 AIX–AX). Treatment with T (t12 = 2.2) or E2 (t12 = 2.56), but not the nonaromatizable androgen DHT, increased N-cad immunoreactivity in the neuropil of the SL-M but not the stratum radiatum (Fig. 3 AX and B).
Discussion
Gonadal Steroids Regulate Cadherin Expression in the Hippocampus.
In these studies, we found that gonadal steroids regulate N-cad expression in the hippocampus. Surprisingly, E2 but not T or DHT was capable of regulating the levels N-cad mRNA and N-cad immunoreactivity in CA1 and CA3 pyramidal neurons. The inability of T to regulate N-cad expression levels in the hippocampus may reflect negligible adult levels of aromatization of T to E2 in the adult rat hippocampus, consistent with aromatase activity assays (42, 43). Although both T and E2 have been shown to regulate N-cad expression levels in the testes (44), the inhibition of aromatase activity by 1,4,6-androstatrien-3,17-dione prevents T-mediated increases in N-cad expression levels in cultured Sertoli cells (45), suggesting that T regulates N-cad via conversion to E2.
Previous studies have generally shown coordinate regulation of cadherin mRNA transcripts and protein expression levels. In contrast, we found that, whereas T did not increase N-cad mRNA levels, both T and E2 increased immunoreactivity to N-cad protein in the SL-M. Although this appears to be noncoordinate N-cad mRNA and protein regulation, it is possible that T treatment does indeed increase N-cad mRNA transcripts, but this increase is undetectable by Northern blot analysis. For example, a localized increase in a relatively small area (such as in SL-M dendrites) could become diluted beyond detectable levels as a result of assaying the entire hippocampus. Alternatively, increased N-cad immunoreactivity in the SL-M after T treatment may reflect an increase in the levels of the N-cad mRNA transcripts present in cells originating outside of the hippocampus (e.g., in the entorhinal cortex), which then transport N-cad protein into the hippocampus.
E2-mediated increases in N-cad-immunoreactivity in pyramidal neurons may result from genomic actions of estrogen receptors within this cell population. Estrogen receptors have been detected in CA1 and to a lesser extent in CA3 by autoradiography (46), in situ hybridization histochemistry (47, 48), and immunohistochemistry (49, 50). Estrogen receptors are also localized to glia (50) and interneurons (51) in the CA1 and CA3 regions, raising the possibility that E2 acts on pyramidal neurons indirectly to increase N-cad expression levels.
The finding that gonadal steroids regulate N-cad expression in adult neural populations confirms the hypothesis that gonadal steroids regulate cadherin expression in the nervous system and includes an entire class of molecules as potential targets of neuroendocrine regulation.
Significance of Steroid Regulation of N-cad.
Cadherin function in the adult nervous system has been assumed to be regulated by posttranslational modifications such as phosphorylation (52), differential association with catenins (53), or proteolytic turnover of cadherins (54). In addition, it has been proposed that proteolytic turnover of N-cad is locally and posttranslationally regulated by synaptic transmission (21, 22). The present demonstration that E2 increases steady-state N-cad mRNA levels in the adult hippocampus provides a novel mechanism whereby cadherin function might be regulated in adulthood.
It is presently unclear what roles N-cad plays in the adult hippocampus, although the finding that N-cad gene expression continues to be dynamic in the adult hippocampus is surprising, as alterations in cadherin expression levels are typically associated with cellular differentiation. For example, the spatio-temporal expression of cadherin subtypes in the developing mammalian nervous system has been associated with cellular differentiation, as is seen in the transition from epithelium to neuroepithelium, or with the onset of neural migration (10). Similarly, N-cad expression is ubiquitous in the developing mammalian central nervous system, but, as the adult phenotype emerges, expression levels of this cell adhesion molecule become less intense and restricted to specific cell populations (17). Activity-dependent regulation of dorsal root ganglion N-cad expression (52) occurs in the context of synaptic reorganization, before the emergence of the mature neural phenotype.
Steroid regulation of cadherin expression levels is observed in adult reproductive tissues and is associated with terminal cellular differentiation and structural remodeling (27, 29). Whereas steroid treatment does influence the turn-over of new hippocampal cells (1, 2), we did not observe a pattern of N-cad protein expression consistent with a role for steroid-induced N-cad expression in this process. We did, however, observe increased N-cad protein expression in cells that undergo structural remodeling in response to steroid treatment.
N-cad as a Participant in Estrogen-Induced Plasticity in the Adult Hippocampus.
Estrogen induces a number of morphological and functional alterations in the adult hippocampus. Apical dendritic spine density of hippocampal CA1 pyramidal neurons is increased after acute exogenous E2 administration in both males (55) and females (56). Associated with this increased spine density, an increase in synaptic density is also observed in response to E2 treatment within the apical dendrite field of CA1 pyramidal neurons, comprising the stratum radiatum and SL-M (55). The electrophysiology of CA3-CA1 Schaffer collaterals is similarly influenced by both E2 and T (23, 25).
The correspondence between areas in which E2 induces neural remodeling and those in which increased N-cad expression is observed after E2 treatment leads us to speculate that N-cad may be involved in mediating steroid-induced alterations in neural morphology and connectivity. Specifically, the observed E2-mediated increase in N-cad immunoreactivity in CA1 and CA3 pyramidal cells, as well as the neuropil of the SL-M, where Schaffer collateral synapsis between CA1 and CA3 has been described, may potentiate the formation of CA3-CA1 synapses in the SL-M. However, this interpretation is complicated by a lack of information concerning the effects of chronic steroid treatment on synaptic remodeling of CA1 Schaffer collaterals, raising the possibility that the steroid treatment regimen used here does not in fact result in the same type of neuroplasticity observed after acute steroid treatment.
Along with Schaffer collaterals from CA3, the neuropil of the SL-M contains perforant pathway afferents to CA1 and to dentate gyrus neurons. Steroid regulation of N-cad expression in the SL-M may therefore also reflect effects on perforant pathway afferents to the hippocampus. It is known, for example, that E2 has growth-promoting actions on septal and forebrain afferents (57) and that both T and E2 promote sprouting in hippocampal receptive fields after entorhinal lesions (26). Studies where N-cad function is disrupted may provide insights into the possible roles of steroid-induced N-cad expression in hippocampal physiology.
In summary, we found that gonadal steroids are capable of regulating N-cad in the adult rat hippocampus. E2, but not T or DHT, regulated N-cad mRNA levels in the adult hippocampus and increased N-cad immunoreactivity in CA1 and CA3 pyramidal cells. Both T and E2 increased N-cad immunoreactivity in the SL-M, whereas DHT had no effect on N-cad immunoreactivity in either of these regions of the adult rat hippocampus. To our knowledge, there have been no other studies that demonstrate that steroids regulate neuronal cadherin expression. The present findings further add to our understanding of the factors that serve to coordinate cadherin expression in the central nervous system. Moreover, our findings indicate that cadherin-gene expression continues to be dynamic in the adult brain. We suggest that steroid regulation of N-cad in the adult hippocampus may participate in structural remodeling of hippocampal neurons.
Acknowledgments
This work was funded by operating grant 0194522 (to N.V.W.) from the Natural Sciences and Engineering Research Council. D.A.M. is the recipient of a Natural Sciences and Engineering Research Council postgraduate scholarship. C.D.M. is the recipient of operating and scholarship funding from the Medical Research Council. S.G. is the recipient of a Medical Research Council doctoral research award.
Abbreviations
- N-cad
N-cadherin
- T
testosterone
- E2
17β-estradiol
- DHT
dihydrotestosterone
- SL-M
stratum lacunosum-moleculare
- TBS
Tris-buffered saline
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031562798.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031562798
References
- 1.Tanapat P, Hastings N B, Reeves A J, Gould E. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frye C A, McCormick C M. Brain Res. 2000;855:166–170. doi: 10.1016/s0006-8993(99)02208-8. [DOI] [PubMed] [Google Scholar]
- 3.Lewis C, McEwen B S, Frankfurt M. Brain Res Dev Brain Res. 1995;87:91–95. doi: 10.1016/0165-3806(95)00052-f. [DOI] [PubMed] [Google Scholar]
- 4.Woolley C S, McEwen B S. J Neurosci. 1993;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geiger B, Ayalon O. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro L, Fannon A M, Kwong P D, Thomson A, Lehmann M S, Grubel G, Legrand J F, Als-Nielsen J, Colman D R, Hendrickson W A. Nature (London) 1995;375:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 7.Tamura K, Shan W S, Hendrickson W A, Colman D R, Shapiro L. Neuron. 1998;20:1153–1163. doi: 10.1016/s0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- 8.Nose A, Nagafuchi A, Takeichi M. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg M S, Takeichi M. Proc Natl Acad Sci. USA. 1994;91:206–209. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeichi M. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 11.Redies C. Prog Neurobiol. 2000;61:611–648. doi: 10.1016/s0301-0082(99)00070-2. [DOI] [PubMed] [Google Scholar]
- 12.Ganzler-Odenthal S I, Redies C. J Neurosci. 1998;18:5415–5425. doi: 10.1523/JNEUROSCI.18-14-05415.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redies C, Takeichi M. Dev Biol. 1996;180:413–423. doi: 10.1006/dbio.1996.0315. [DOI] [PubMed] [Google Scholar]
- 14.Inoue A, Sanes J R. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- 15.Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T. Neuron. 1997;19:77–89. doi: 10.1016/s0896-6273(00)80349-9. [DOI] [PubMed] [Google Scholar]
- 16.Fannon A M, Colman D R. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 17.Redies C, Takeichi M. Dev Dyn. 1993;197:26–39. doi: 10.1002/aja.1001970104. [DOI] [PubMed] [Google Scholar]
- 18.Beesley P W, Mummery R, Tibaldi J. J Neurochem. 1995;64:2288–2294. doi: 10.1046/j.1471-4159.1995.64052288.x. [DOI] [PubMed] [Google Scholar]
- 19.Uchida N, Honjo Y, Johnson K R, Wheelock M J, Takeichi M. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benson D L, Tanaka H. J Neurosci. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka H, Shan W, Phillips G R, Arndt K, Bozdagi O, Shapiro L, Huntley G W, Benson D L, Colman D R. Neuron. 2000;25:93–107. doi: 10.1016/s0896-6273(00)80874-0. [DOI] [PubMed] [Google Scholar]
- 22.Tang L, Hung C P, Schuman E M. Neuron. 1998;20:1165–1175. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, Skinkle K L, Hicks T P. J Physiol (London) 1999;515:209–220. doi: 10.1111/j.1469-7793.1999.209ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakata K, Tokue A, Kawai N. J Urol. 2000;163:1333–1338. [PubMed] [Google Scholar]
- 25.Teyler T J, Vardaris R M, Lewis D, Rawitch A B. Science. 1980;209:1017–1018. doi: 10.1126/science.7190730. [DOI] [PubMed] [Google Scholar]
- 26.Morse J, Scheff S W, DeKosky S T. Exp Neurol. 1986;94:649–658. doi: 10.1016/0014-4886(86)90244-x. [DOI] [PubMed] [Google Scholar]
- 27.Blaschuk O W, Farookhi R. Dev Biol. 1989;136:564–567. doi: 10.1016/0012-1606(89)90283-2. [DOI] [PubMed] [Google Scholar]
- 28.MacCalman C D, Farookhi R, Blaschuk O W. Dev Genet. 1995;16:20–24. doi: 10.1002/dvg.1020160106. [DOI] [PubMed] [Google Scholar]
- 29.Getsios S, Chen G T, Stevenson M D, Leclerc P, Blaschuk O W, MacCalman C D. Dev Dyn. 1998;211:238–247. doi: 10.1002/(SICI)1097-0177(199803)211:3<238::AID-AJA5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Smith E R, Damassa D A, Davidson J M. In: Methods in Psychobiology. Myer R D, editor. Vol. 3. New York: Academic; 1977. pp. 262–269. [Google Scholar]
- 31.Abdelgadir S E, Resko J A, Ojeda S R, Lephart E D, McPhaul M J, Roselli C E. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- 32.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 33.MacCalman C D, Bardeesy N, Holland P C, Blaschuk O W. Dev Dyn. 1992;195:127–132. doi: 10.1002/aja.1001950207. [DOI] [PubMed] [Google Scholar]
- 34.Chen B, Blaschuk O W, Hales B F. Teratology. 1991;44:581–590. doi: 10.1002/tera.1420440511. [DOI] [PubMed] [Google Scholar]
- 35.Feinberg A P, Vogelstein B. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 36.Watson R E, Weigand S J, Clough R W, Hoffman G E. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 37.Sacco P A, McGranahan T M, Wheelock M J, Johnson K R. J Biol Chem. 1995;34:20201–20206. doi: 10.1074/jbc.270.34.20201. [DOI] [PubMed] [Google Scholar]
- 38.Hatta K, Nose A, Nagafuchi A, Takeichi M. J Cell Biol. 1988;106:873–881. doi: 10.1083/jcb.106.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallis J, Fox M F, Walsh F S. Genomics. 1994;22:172–179. doi: 10.1006/geno.1994.1358. [DOI] [PubMed] [Google Scholar]
- 40.Cyr D G, Blaschuk O W, Robaire B. Endocrinology. 1992;131:139–145. doi: 10.1210/endo.131.1.1611992. [DOI] [PubMed] [Google Scholar]
- 41.Lagunowich L A, Schneider J C, Chasen C, Grunwald G B. J Neurosci Res. 1992;32:202–208. doi: 10.1002/jnr.490320209. [DOI] [PubMed] [Google Scholar]
- 42.MacLusky N J, Walters M J, Clark A S, Toran-Allerand C D. Mol Cell Neurosci. 1994;5:691–698. doi: 10.1006/mcne.1994.1083. [DOI] [PubMed] [Google Scholar]
- 43.Roselli C E, Horton L E, Resko J A. Endocrinology. 1985;117:2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- 44.MacCalman C D, Blaschuk O W. Endocr J. 1994;2:157–163. [Google Scholar]
- 45.MacCalman C D, Getsios S, Farookhi R, Blaschuk O W. Endocrinology. 1997;138:41–48. doi: 10.1210/endo.138.1.4831. [DOI] [PubMed] [Google Scholar]
- 46.Loy R, Gerlach J L, McEwen B S. Dev Brain Res. 1988;39:245–251. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- 47.Simerly R B, Chang C, Muramatsu M, Swanson L W. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 48.Shugrue P J, Lane M V, Merchenthaler I. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Schwartz P E, Rissman E F. Neuroendocrinology. 1997;66:63–67. doi: 10.1159/000127221. [DOI] [PubMed] [Google Scholar]
- 50.Azcoitia I, Sierra A, Garcia-Segura L M. Glia. 1999;26:260–267. [PubMed] [Google Scholar]
- 51.Weiland N G, Orikasa C, Hayashi S, McEwen B S. J Comp Neurol. 1997;388:603–612. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 52.Itoh K, Ozaki M, Stevens D, Fields R D. J Neurobiol. 1997;33:735–748. doi: 10.1002/(sici)1097-4695(19971120)33:6<735::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 53.Provost E, Rimm D L. Curr Opin Cell Biol. 1999;11:567–572. doi: 10.1016/s0955-0674(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 54.Lee M, Fink B D, Grunwald G B. Dev Genet. 1997;20:224–234. doi: 10.1002/(SICI)1520-6408(1997)20:3<224::AID-DVG5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 55.Woolley C S, Gould E, Frankfurt M, McEwen B S. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gould E, Woolley C S, Frankfurt M, McEwen B S. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibbs R B. Exp Neurol. 1998;151:289–302. doi: 10.1006/exnr.1998.6789. [DOI] [PubMed] [Google Scholar]