Abstract
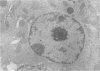
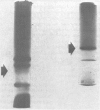
Lead-induced inclusion bodies in renal tubular cells of rats have been studied in vitro after isolation by differential centrifugation. The inclusion bodies are insoluble in physiological media but may be dissolved in denaturants like 6M urea and sodium deoxycholate. They contain about 40–50 μg of lead/mg protein, but only about 10% of this is tightly bound. They also contain calcium, iron, zinc, copper, and cadmium. The protein is rich in glutamic and aspartic acids, glycine and cystine. When dissolved in 6M urea, the protein migrates as a single band on acrylamide gel electrophoresis and has a molecular weight of 27,500. It is suggested that the inclusion bodies function as an intracellular depot of nondiffusible lead.
Further studies have been directed toward finding a free, unaggregated lead-containing protein fraction. Nuclear proteins from kidneys of lead-toxic rats were separated into NaCl-, Tris-, and NaOH-soluble fractions and an insoluble acidic fraction. A quantitatively small lead-containing protein was found in the 0.14M NaCl fraction. Amino acid composition, electrophoretic mobility, molecular weight, and ability to bind lead are similar to those of insoluble inclusion body protein. The possible role of this soluble lead-binding protein in the formation of nuclear inclusion bodies is at present time not certain. These studies do suggest, however, that protein-bound lead in renal tubular cells may be partitioned between insoluble and nondiffusible morphologically discrete inclusion bodies and a soluble, extractable fraction which is presumably diffusable.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goyer R. A., Leonard D. L., Moore J. F., Rhyne B., Krigman M. R. Lead dosage and the role of the intranuclear inclusion body. An experimental study. Arch Environ Health. 1970 Jun;20(6):705–711. doi: 10.1080/00039896.1970.10665647. [DOI] [PubMed] [Google Scholar]
- Goyer R. A., May P., Cates M. M., Krigman M. R. Lead and protein content of isolated intranuclear inclusion bodies from kidneys of lead-poisoned rats. Lab Invest. 1970 Mar;22(3):245–251. [PubMed] [Google Scholar]
- HASS G. M., BROWN D. V., EISENSTEIN R., HEMMENS A. RELATIONS BETWEEN LEAD POISONING IN RABBIT AND MAN. Am J Pathol. 1964 Nov;45:691–727. [PMC free article] [PubMed] [Google Scholar]
- Hsu F. S., Krook L., Shively J. N., Duncan J. R., Pond W. G. Lead inclusion bodies in osteoclasts. Science. 1973 Aug 3;181(4098):447–448. doi: 10.1126/science.181.4098.447. [DOI] [PubMed] [Google Scholar]
- Moore J. F., Goyer R. A., Wilson M. Lead-induced inclusion bodies. Solubility, amino acid content, and relationship to residual acidic nuclear proteins. Lab Invest. 1973 Nov;29(5):488–494. [PubMed] [Google Scholar]
- Patel G., Patel V., Wang T. Y., Zobel C. R. Studies of the nuclear residual proteins. Arch Biochem Biophys. 1968 Dec;128(3):654–662. doi: 10.1016/0003-9861(68)90075-1. [DOI] [PubMed] [Google Scholar]
- STEELE W. J., BUSCH H. STUDIES ON ACIDIC NUCLEAR PROTEINS OF THE WALKER TUMOR AND LIVER. Cancer Res. 1963 Sep;23:1153–1163. [PubMed] [Google Scholar]
- STEELE W. J., BUSCH H. STUDIES ON THE COMPOSITION OF NUCLEAR RESIDUAL PROTEINS FROM RAT LIVER AND WALKER 256 CARCINOSARCOMA. Exp Cell Res. 1964 Jan;33:68–73. doi: 10.1016/s0014-4827(64)81013-2. [DOI] [PubMed] [Google Scholar]
- Simpson C. F., Damron B. L., Harms R. H. Abnormalities of erythrocytes and renal tubules of chicks poisoned with lead. Am J Vet Res. 1970 Mar;31(3):515–523. [PubMed] [Google Scholar]
- Stowe H. D., Goyer R. A., Krigman M., Wilson M., Cates M. Experimental oral lead toxicity in young dogs. Clinical and morphologic effects. Arch Pathol. 1973 Feb;95(2):106–116. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]