Abstract
With 8–14C–styrene oxide as substrate, specific glutathione S-transferase and epoxide hydrase activities were determined in subcellular fractions of liver, lungs, kidney, and intestinal mucosa from rabbit, rat, and guinea pig. Liver had the highest enzyme activities in each species. Rat and guinea pig had higher glutathione S-transferase activity in both liver and kidney than rabbit. Rat testis also had appreciable glutathione S-transferase activity.
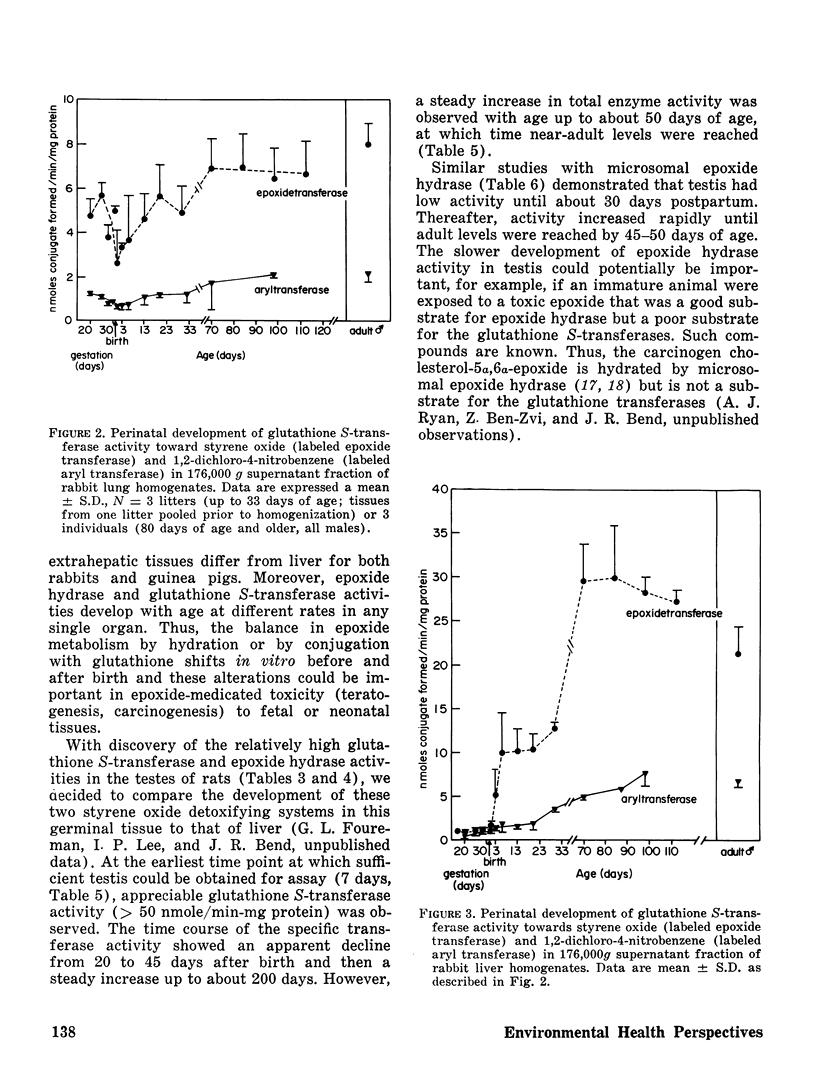
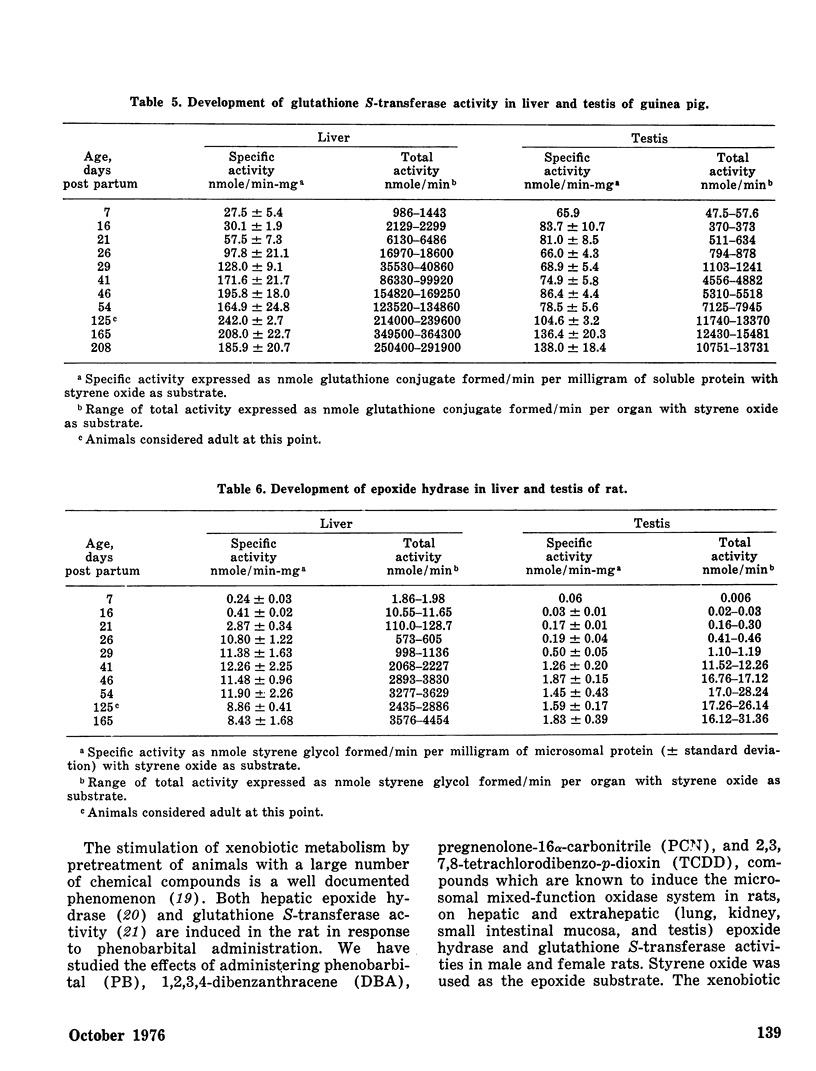
The perinatal development of epoxide hydrase and glutathione S-transferase was followed in liver and several extrahepatic tissues of fetal and neonatal guinea pigs and rabbits. The rates at which enzyme activities reached adult levels in the extrahepatic tissues differed from the liver in both species. Epoxide hydrase and glutathione S-transferases developed at different rates in each organ, demonstrating that the relative importance of these two detoxifying pathways for styrene oxide may shift before and after birth.
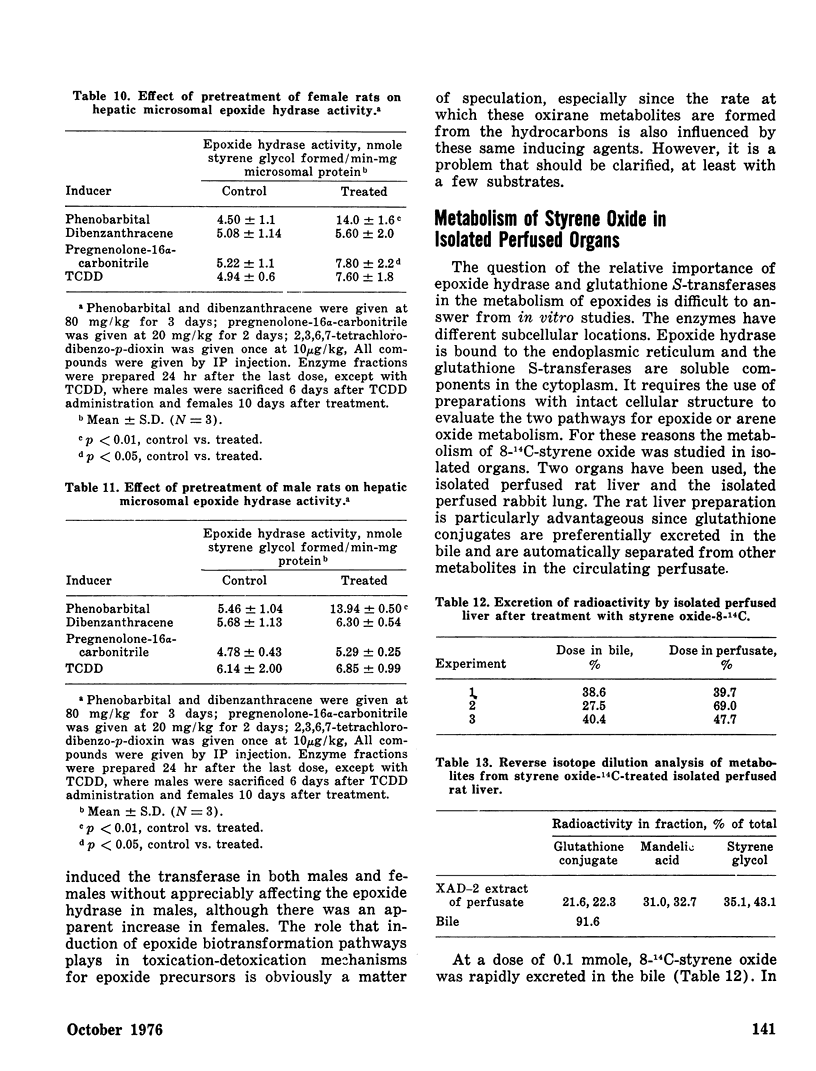
The effects of pretreating male and female rats with phenobarbital (PB), 1,2,3,4-dibenzanthracene (DBA), pregnenolone-16α-carbonitrile (PCN), or 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on hepatic and extrahepatic epoxide hydrase and glutathione S-transferase activities toward styrene oxide were determined. PB increased both enzyme activities in liver of both sexes. PCN induced only glutathione S-transferase activity in female liver. Extrahepatic epoxide hydrase and glutathione S-transferase activities were unaffected except that TCDD doubled female renal epoxide hydrase activity and PB increased intestinal epoxide hydrase activity in both sexes.
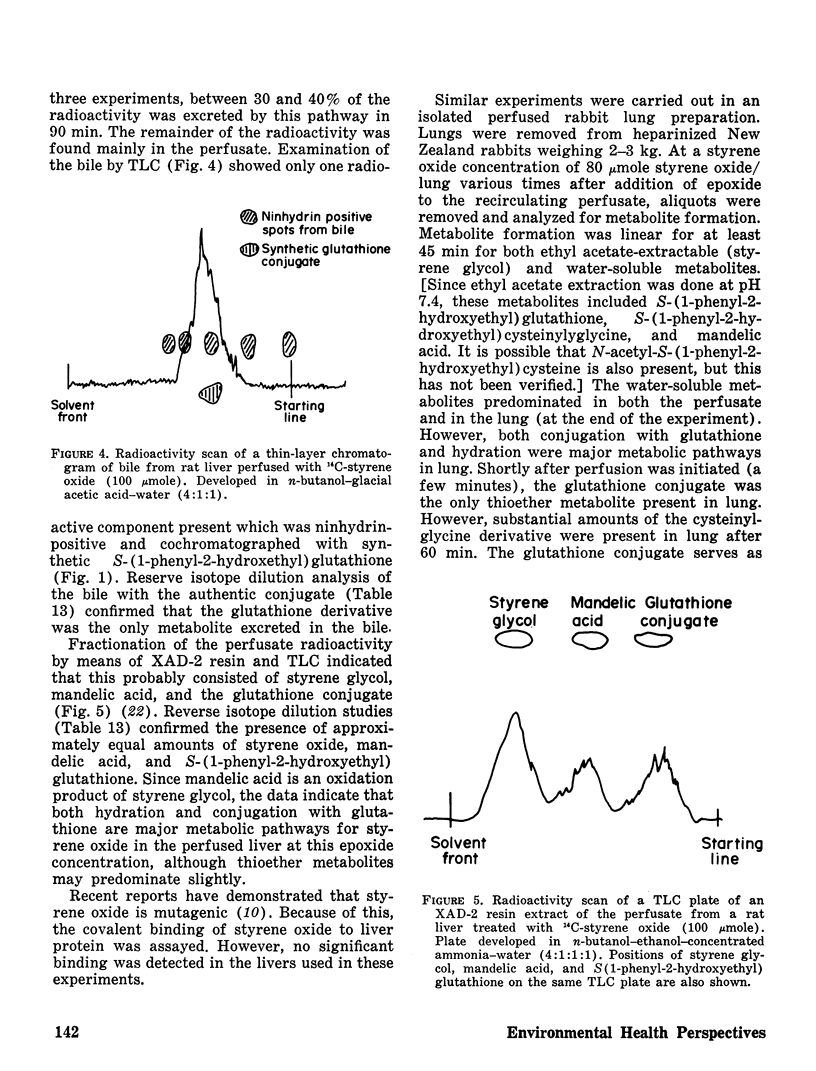
Styrene oxide biotransformation was studied in isolated, perfused rat liver and rabbit lung preparations. Conjugation with glutathione was a major metabolic pathway although significant amounts of diol were also formed in each instance. In rat liver, 27–40% of the administered styrene oxide was excreted via the bile mainly as S-(1-phenyl-2-hydroxyethyl)glutathione.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYLAND E., WILLIAMS K. AN ENZYME CATALYSING THE CONJUGATION OF EPOXIDES WITH GLUTATHIONE. Biochem J. 1965 Jan;94:190–197. doi: 10.1042/bj0940190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird W. M., Harvey R. G., Brookes P. Comparison of the cellular DNA-bound products of benzo(alpha)pyrene with the products formed by the reaction of benzo(alpha)pyrene-4,5-oxide with DNA. Cancer Res. 1975 Jan;35(1):54–57. [PubMed] [Google Scholar]
- Chan J. T., Black H. S. Skin carcinogenesis: cholesterol-5alpha,6alpha-epoxide hydrase activity in mouse skin irradiated with ultraviolet light. Science. 1974 Dec 27;186(4170):1216–1217. doi: 10.1126/science.186.4170.1216. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Holtzman J. L., Gillette J. R., Milne G. W. The incorporation of 18-O into naphthalene in the enzymatic formation of 1,2-dihydronaphthalene-1,2-diol. J Biol Chem. 1967 Oct 10;242(19):4386–4387. [PubMed] [Google Scholar]
- James M. O., Fouts J. R., Bend J. R. Hepatic and extrahepatic metabolism, in vitro, of an epoxide (8-(14) C-styrene oxide) in the rabbit. Biochem Pharmacol. 1976 Jan 15;25(2):187–193. doi: 10.1016/0006-2952(76)90289-6. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W. Arene oxides: a new aspect of drug metabolism. Science. 1974 Aug 16;185(4151):573–582. doi: 10.1126/science.185.4151.573. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B., Zaltzman-Nirenberg P., Udenfriend S. 1,2-naphthalene oxide as an intermediate in the microsomal hydroxylation of naphthalene. Biochemistry. 1970 Jan 6;9(1):147–156. doi: 10.1021/bi00803a019. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N., Kuhlekamp J., Clifton G. Drug induction of hepatic glutathione S-transferases in male and female rats*. Biochem J. 1975 Feb;146(2):351–356. doi: 10.1042/bj1460351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. Y., Somogyi A., West S., Kuntzman R., Conney A. H. Pregnenolone-16 -carbonitrile: a new type of inducer of drug-metabolizing enzymes. Arch Biochem Biophys. 1972 Oct;152(2):457–462. doi: 10.1016/0003-9861(72)90239-1. [DOI] [PubMed] [Google Scholar]
- Oesch E., Jerina D. M., Daly J. A radiometric assay for hepatic epoxide hydrase activity with [7-3H] styrene oxide. Biochim Biophys Acta. 1971 Mar 10;227(3):685–691. doi: 10.1016/0005-2744(71)90017-9. [DOI] [PubMed] [Google Scholar]
- Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica. 1973 May;3(5):305–340. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- Otsuji H., Ikeda M. The metabolism of styrene in the rat and the stimulatory effect of phenobarbital. Toxicol Appl Pharmacol. 1971 Feb;18(2):321–328. doi: 10.1016/0041-008x(71)90123-2. [DOI] [PubMed] [Google Scholar]
- Sims P., Grover P. L. Epoxides in polycyclic aromatic hydrocarbon metabolism and carcinogenesis. Adv Cancer Res. 1974;20:165–274. doi: 10.1016/s0065-230x(08)60111-6. [DOI] [PubMed] [Google Scholar]
- Wood A. W., Goode R. L., Chang R. L., Levin W., Conney A. H., Yagi H., Dansette P. M., Jerina D. M. Mutagenic and cytotoxic activity of benzol[a]pyrene 4,5-, 7,8-, and 9,10-oxides and the six corresponding phenols. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3176–3180. doi: 10.1073/pnas.72.8.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]


