Abstract
The vesicular stomatitis virus (VSV) G protein is a model transmembrane glycoprotein that has been extensively used to study the exocytotic pathway. A signal in the cytoplasmic tail of VSV G (DxE or Asp-x-Glu, where x is any amino acid) was recently proposed to mediate efficient export of the protein from the endoplasmic reticulum (ER). In this study, we show that the DxE motif only partially accounts for efficient ER exit of VSV G. We have identified a six-amino-acid signal, which includes the previously identified Asp and Glu residues, that is required for efficient exit of VSV G from the ER. This six-residue signal also includes the targeting sequence YxxØ (where x is any amino acid and Ø is a bulky, hydrophobic residue) implicated in several different sorting pathways. The only defect in VSV G proteins with mutations in the six-residue signal is slow exit from the ER; folding and oligomerization in the ER are normal, and the mutants eventually reach the plasma membrane. Addition of this six-residue motif to an inefficiently transported reporter protein is sufficient to confer an enhanced ER export rate. The signal we have identified is highly conserved among divergent VSV G proteins, and we suggest this reflects the importance of this motif in the evolution of VSV G as a proficient exocytic protein.
INTRODUCTION
In the secretory pathway, transport of newly synthesized membrane and secretory proteins is mediated by COPII-coated vesicles that package them in the endoplasmic reticulum (ER) for transport to the Golgi complex. An initial proposal suggested that this process was nonselective (Pfeffer and Rothman, 1987), such that in the absence of ER retention information a protein would progress by default to the Golgi complex. Currently, it is accepted that efficient transport from the ER is not a default process. Soluble and membrane proteins have been shown to be selectively sorted away from ER resident proteins during export from the ER (Mizuno and Singer, 1993; Balch et al., 1994). Although a bulk membrane traffic pathway from the ER is likely to exist, efficient exit must be mediated by information in the cargo proteins.
The study of the vesicular stomatitis virus glycoprotein (VSV G) provided much of the evidence in support of a selective transport mechanism for ER export. Truncation of the 29-amino-acid cytoplasmic domain of the VSV G protein to a single amino acid slows its rate of transport from the ER without affecting the folding or trimerization rates (Doms et al., 1988). In cells expressing VSV G, microinjection of antibodies against the cytoplasmic domain or the introduction of a 29-mer cytoplasmic tail peptide into permeabilized cells also slows the transport of the VSV G protein (Kreis, 1986; Nishimura and Balch, 1997). In addition, immunoelectron microscopy has demonstrated that VSV G is concentrated 5- to 10-fold in ER-derived vesicles and vesicular tubular clusters relative to its concentration in ER cisternae (Balch et al., 1994).
Despite the focus by many groups on the ER export process, the identification of a universal signal for the selective sorting and concentration of protein in the ER has remained elusive. Recently, two separate motifs for promoting ER export have been suggested. The p24 and ERGIC-53 proteins, both known to cycle within the ER–Golgi region, contain a di-phenylalanine motif in their cytoplasmic domain that is important for efficient ER exit (Fiedler et al., 1996; Kappeler et al., 1997; Dominguez et al., 1998) and is capable of binding COPII components in vitro (Kappeler et al., 1997; Dominguez et al., 1998). A di-acidic DxE motif (where x is any amino acid) found in the VSV G cytoplasmic domain and other transmembrane proteins has been proposed to act as the signal for efficient ER export of noncycling proteins (Nishimura and Balch, 1997).
We have found the di-acidic DxE motif only partially accounts for the efficient movement of VSV G from the ER. Mutation of the di-acidic signal in the VSV G tail only moderately slowed ER exit relative to the complete removal of the tail. We report that a larger region of the tail, residues Tyr-Thr-Asp-Ile-Glu-Met, is required for efficient ER export of VSV G. This six-amino-acid motif is sufficient to enhance the rate of ER export of a poorly exported reporter construct composed of Tac with a poly-glycine cytoplasmic domain.
MATERIALS AND METHODS
Cells and Transfection
BHK-21 cells were maintained in Dulbecco's modified Eagle's medium (DME) with 5% fetal calf serum at 37°C in 5% CO2. Thirty-five-millimeter dishes of BHK-21 cells (60–70% confluent) were infected with the recombinant vaccinia virus vTF7–3 encoding T7 RNA polymerase (Fuerst et al., 1986) in 0.25 ml of Opti-MEM (Life Technologies, Gaithersburg, MD) at a multiplicity of infection of 10–20. For metabolic labeling experiments, the inoculum was replaced after adsorption for 30 min at 37°C with Opti-MEM containing 15 μl of LipofectAMINE (Life Technologies) and 5 μg of a vector (pAR2529 or pBluescript; Stratagene, La Jolla, CA) encoding the appropriate gene behind the T7 promoter. Expression was analyzed at 3.5 h after infection. For indirect immunofluorescence experiments, the procedure was identical except cells were plated on coverslips, 10 μl of Lipofectin (Life Technologies) were used instead of LipofectAMINE, and expression was analyzed at 7 h after infection.
Mutagenesis
The genes encoding all VSV G cytoplasmic domain mutant proteins were generated using the Kunkel method of oligonucleotide-directed mutagenesis (Kunkel et al., 1987) as described by Swift and Machamer (1991). Mutant VSV G proteins were named with the number of the substituted cytoplasmic tail residues (1–29) appended with the single-letter code of the replacement amino acid. For example, VSV G 21A23A corresponds to the substitution of residues 21 and 23 of the cytoplasmic domain with alanine. Some mutations were constructed in the background of the G mutant TMB (which has a BamHI site introduced at nucleotide 1483 with no amino acid change; Puddington et al., 1986). The mutant protein CT1 has been previously described (Scullion et al., 1987).
The genes encoding poly-glycine cytoplasmic domains were constructed by annealing four overlapping oligonucleotides to create a double-stranded, 92-nucleotide DNA fragment with a 5′ BamHI site and a 3′ XbaI site. Equal amounts of a 5′ or a 3′ oligonucleotide pair were annealed in 2× SSC buffer (30 mM sodium citrate and 0.3 M NaCl, pH 7.0) by heating to 100°C for 5 min and cooling 1°C/min to 30°C. The doubled stranded 5′ and 3′ pieces were mixed together with T4 ligase (Life Technologies) in the buffer provided by the manufacturer for 2.5 h at 16°C. To create VSV G constructs with the poly-glycine tails, the resultant DNA fragment was ligated with vector containing the VSV G TMB mutant digested with BamHI and XbaI (removing the cytoplasmic domain). To create the Tac constructs, Tac-DKQTLL (received from Michael Marks, University of Pennsylvania Medical School, Philadelphia, PA) (Letourneur and Klausner, 1992) was subcloned from pCDM8.1 into pBluescript and mutagenized with the Quik Change kit (Stratagene) to create a BamHI site at the end of the transmembrane domain at nucleotide 789. An XhoI–BamHI fragment of Tac, containing the lumenal and transmembrane domains, was ligated with vector containing the appropriate cytoplasmic domain sequence.
Radiolabeling, Immunoprecipitation, and Oligosaccharide Processing
BHK-21 cells expressing VSV G or mutant G proteins were starved in serum-, cysteine-, and methionine-free DME for 15 min before labeling for 5 min with 0.5 ml of serum-, cysteine-, and methionine-free DME containing 200 μCi/ml l-[35S]in vitro cell labeling mix (Amersham, Arlington Heights, IL). Cells were chased for various times in DME containing 5% FCS and a threefold excess of unlabeled methionine. Cells were lysed in detergent solution (50 mM Tris, pH 8.0, 1% NP-40, 0.4% deoxycholate, and 62.5 mM EDTA) with 20 μg/ml aprotinin, 20 μg/ml leupeptin, and 2 μg/ml pepstatin A, and VSV G or Tac proteins were immunoprecipitated with a polyclonal anti-VSV (Swift and Machamer, 1991) or a polyclonal anti-Tac antibody (a generous gift from Michael Marks) and fixed Staphylococcus aureus (Calbiochem-Novabiochem, La Jolla, CA). The kinetics of oligosaccharide processing were determined as described previously, using 0.4 mU of endoglycosidase H (endo H; New England Biolabs, Beverly, MA) (Machamer et al., 1985, 1993). Proteins were separated on 10% polyacrylamide gels (Laemmli, 1970) and detected using a Fujifilm (Tokyo, Japan) BAS-1500 PhosphorImager. The percent of protein processed at each chase time was determined after quantitation using the MacBas program version 2.5 (Fuji Photo Film, Tokyo, Japan).
Indirect Immunofluorescence
Double-label indirect immunofluorescence staining of transiently transfected BHK-21 cells was performed essentially as described (Swift and Machamer, 1991). Fixed, nonpermeabilized cells were labeled with the I1 monoclonal anti-VSV G antibody (Lefrancois and Lyles, 1982) followed by Texas Red-conjugated, affinity-purified goat anti-mouse immunoglobulin G (Jackson ImmunoResearch, Avondale, PA) to detect protein at the plasma membrane. Cells were then permeabilized, and intracellular VSV G was detected by labeling with affinity-purified polyclonal anti-VSV G antibody followed by fluorescein-conjugated affinity-purified goat anti-rabbit immunoglobulin G (Jackson ImmunoResearch). The polyclonal anti-VSV G antibody was affinity purified from rabbit anti-VSV serum on an Affigel 10 column (Bio-Rad, Hercules, CA) conjugated with 0.6 mg/ml VSV G protein, following the manufacturer's instructions. The VSV G protein was isolated from purified VSV grown in BHK-21 cells (Cluett et al., 1997) by solubilization in 1% Triton X-100 and 10 mM Tris-HCl, pH 8.0, at 4°C followed by ultracentrifugation to remove other viral proteins. Images were collected on a Axioskop microscope (Zeiss, Thornwood, NY) equipped with epifluorescence and a Sensys charge-coupled device camera (Photometrics, Tucson, AZ) using IP Lab software (Signal Analytics, Vienna, VA).
Trimerization Assay
Oligomerization of VSV G and mutant VSV G proteins was analyzed by velocity gradient centrifugation in sucrose essentially as described (Doms et al., 1988). Continuous 5–20% sucrose gradients were poured over a 60% cushion. All solutions were in 20 mM Tris, 30 mM 2-[N-morpholino]ethanesulfonic acid, pH 5.8, and 100 mM NaCl (MNT buffer) containing 0.1% Triton X-100. BHK-21 cells expressing VSV G or mutant VSV G proteins were labeled with 250 μCi of l-[35S]in vitro cell labeling mix per dish (Amersham) for 5 min, chased for 10 min, and lysed at 0°C in MNT buffer containing 1% Triton X-100 and protease inhibitors. Postnuclear supernatants were loaded onto the gradients and spun in an SW50.1 rotor (Beckman Instruments, Palo Alto, CA) at 44,000 rpm for 18 h. Fifteen fractions per gradient were collected, immunoprecipitated with anti-VSV antibody, and analyzed on a 10% polyacrylamide gel (Laemmli, 1970). The percentage of monomer (4S) and trimer (8S) relative to the total VSV G or mutant VSV G was determined by phosphorimaging.
RESULTS
Substitution of Six Residues of the Cytoplasmic Domain of VSV G Slows Intracellular Transport
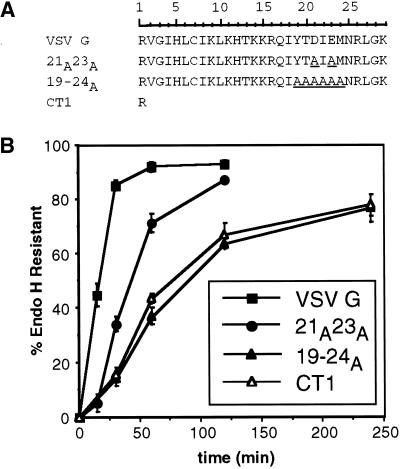
To determine the region of the VSV G tail required for efficient ER export, various amino acid substitutions, deletions, and truncations of the 29-amino-acid cytoplasmic domain were constructed. The rate of ER export of the mutant VSV G proteins was assessed by determining the rate of acquisition of resistance to endo H. The concerted action of mannosidase II and N-acetylglucosaminyltransferase I on N-linked oligosaccharides in the medial Golgi results in resistance to cleavage by endo H (reviewed by Kornfeld and Kornfeld, 1976). The rate of this carbohydrate-processing step includes the time required for maturation within the ER as well as ER to Golgi transport. Defects in folding and/or trimer assembly within the ER have not been observed for any VSV G proteins with mutations in the cytoplasmic tail (Doms et al., 1988). Thus, the rate of endo H processing has been used to infer the rate of exit of VSV G from the ER (Nishimura and Balch, 1997); see below.
Published experiments analyzing the transport rates of truncated VSV G proteins directed our focus to the distal 11 amino acids of the cytoplasmic domain, residues 19–29 (Whitt et al., 1989). Substitution of amino acids 19–24 of the tail with alanine residues (19–24A) reduced the rate of transport relative to VSV G (Figure 1). The half-time for acquisition of endo H resistance of 19–24A was comparable with that of VSV G with a single-amino-acid tail (CT1), suggesting that no information for efficient export remained in the cytoplasmic domain. The half-time for transport of CT1 to the medial Golgi has been shown to be four- to eightfold slower than wild-type VSV G (Rose and Bergmann, 1983; Doms et al., 1988; Whitt et al., 1989). The variability may depend on the cell type and expression system used. Here, we found that CT1 was transported to the medial Golgi nearly fivefold more slowly than VSV G.
Figure 1.
Efficient intracellular transport of VSV G requires six residues in the cytoplasmic tail. (A) Amino acid sequence (single-letter code) of the entire cytoplasmic domain of wild-type and mutant VSV G proteins. The scale above the primary sequence of the VSV G tail indicates the residue number corresponding to each amino acid of the cytoplasmic domain. Residue 1 follows the last residue of the transmembrane domain, whereas residue 29 corresponds to the C-terminal amino acid of the tail. Substituted residues in mutant tails are underlined. (B) Kinetics of acquisition of endo H resistance of VSV G, 21A23A, 19–24A, and CT1. BHK-21 cells expressing VSV G or mutant VSV G proteins were pulse labeled for 5 min and chased for the times indicated. Immunoprecipitated proteins were subjected to endo H treatment as described in MATERIALS AND METHODS and separated by SDS-PAGE. The amount of endo H-resistant protein was quantitated by phosphorimaging. Each time point represents the mean of a minimum of three experiments ± SD.
The 19–24A mutation includes substitution of two acidic residues (Asp21 and Glu23) that were previously suggested to serve as a generalized signal for efficient ER export (Nishimura and Balch, 1997). To determine whether replacing the two acidic residues accounted for the slower transport of the 19–24A mutant, the Asp and the Glu were changed to alanines. This mutant (21A23A) was only partially slowed relative to 19–24A and CT1 (Figure 1). Thus, the two acidic residues contribute to efficient export yet must do so in concert with the other residues in the 19- to 24-amino-acid region.
Mutant VSV G Proteins Slow to Exit the ER Trimerize with Wild-Type Kinetics
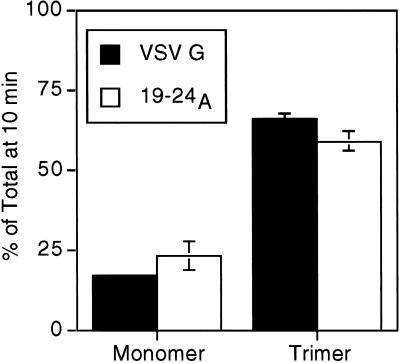
To confirm that the reduced carbohydrate-processing kinetics were not due to delayed maturation within the ER, trimerization of the mutant VSV G proteins was analyzed. Formation of homotrimers is the last maturation step known to occur before exit of VSV G from the ER (Doms et al., 1988). The CT1 and 21A23A proteins have previously been shown to fold and trimerize with normal kinetics (Doms et al., 1988; Nishimura and Balch, 1997). We measured trimer formation of the 19–24A protein by assaying the density shift on sucrose gradients associated with the transition from monomer to trimer (Doms et al., 1988). The 19–24A protein trimerized with kinetics similar to wild-type VSV G (Figure 2). These results confirm that the slow transport in the Golgi complex of the mutant proteins (Figure 1) does not reflect a defect in folding or assembly but a delay in ER export.
Figure 2.
VSV G proteins slow to exit the ER trimerize with wild-type kinetics. BHK-21 cells expressing VSV G or 19–24A were pulse labeled for 5 min and solubilized after a 10-min chase. Lysates were loaded onto 5–20% linear sucrose gradients and centrifuged as described in MATERIALS AND METHODS. Fractions were collected, immunoprecipitated with anti-VSV antibody, and analyzed by SDS-PAGE. The percentage of protein found as monomer (4S) or trimer (8S) relative to the total pool of VSV G or 19–24A protein was quantitated using phosphorimager analysis. Each time point represents the mean of two experiments ± SEM.
VSV G Proteins with Delayed ER Export Kinetics Ultimately Reach the Cell Surface
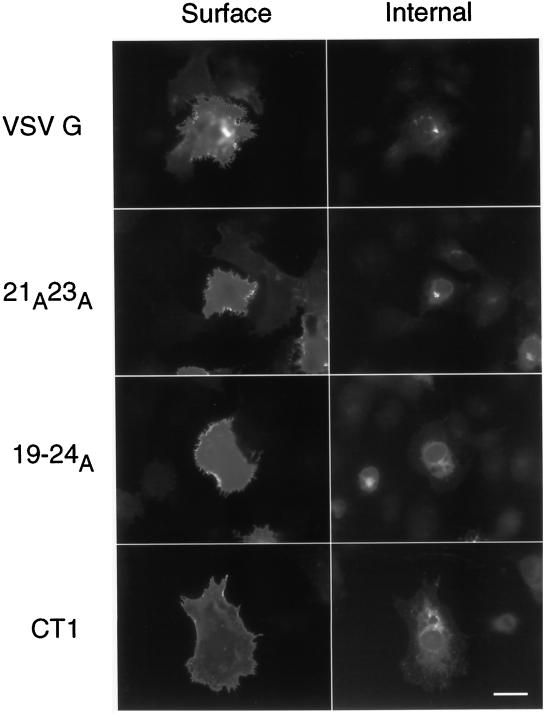
The steady-state distribution of the mutant VSV G proteins was examined by indirect immunofluorescence in BHK-21 cells. Surface labeling of nonpermeabilized cells was detected in cells expressing wild-type VSV G, 19–24A, 21A23A, and CT1 (Figure 3). At shorter expression times, the mutant proteins exhibited less cell surface staining than the wild-type protein (our unpublished results). Thus, the mutant proteins eventually reach the plasma membrane, even though their exit from the ER is less efficient than wild-type VSV G protein.
Figure 3.
VSV G proteins slow to exit the ER ultimately reach cell surface. To detect protein at the cell surface, nonpermeabilized BHK-21 cells expressing wild-type or mutant VSV G proteins were fixed and stained with a monoclonal VSV G antibody and a Texas Red-conjugated secondary antibody. Cells were subsequently permeabilized, and intracellular VSV G was detected with an affinity-purified polyclonal anti-VSV G antibody followed by fluorescein-conjugated secondary antibody. Note the prominent Golgi staining present in the cells expressing VSV G and 21A23A that is absent in the cells expressing the 19–24A and CT1 proteins. Bar, 10 μM.
When intracellular labeling of the same cells was performed after permeabilization, strikingly different patterns were observed for several of the mutant proteins. A tight juxtanuclear staining pattern characteristic of the Golgi complex was frequently detected in cells expressing the VSV G and 21A23A proteins but not in those expressing the 19–24A and CT1 proteins (Figure 3). The latter mutants showed only ER-like intracellular staining, even when cell surface staining was prominent. One interpretation of the absence of Golgi staining is that the 19–24A and CT1 proteins were not able to concentrate during ER exit (and thus Golgi staining was not obvious). This would imply that concentration and efficient ER exit are linked.
Residues 19–24 of the VSV G Tail All Contribute to Efficient ER Export
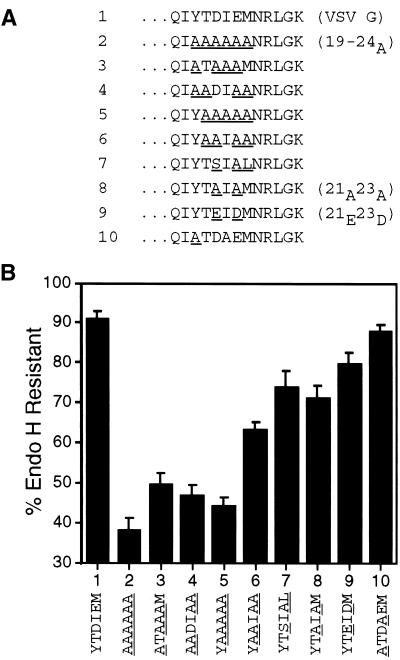
To determine whether each of the six amino acids in the 19–24 region contributed to the efficient ER export of VSV G, additional substitutions were made in the 19–24A mutant. This approach proved more sensitive than individual substitutions in an alanine scan (Nishimura and Balch, 1997). Analysis of these mutants demonstrated all six residues (Tyr19-Thr20-Asp21-Ile22-Glu23-Met24) contributed to efficient transport. The slow transport rate exhibited by the 19–24A protein was not reproduced in mutants with substitutions in any subset of the six residues (Figure 4).
Figure 4.
All six residues in the 19–24 region of the cytoplasmic domain contribute to efficient ER export of VSV G. (A) Amino acid sequence (single-letter code) of the C-terminal 13 residues of the cytoplasmic domain of wild-type and mutant VSV G proteins. Substituted residues in mutant tails are underlined. Numbers to the left of the amino acid sequence refer to lane numbers in the graph in B. Names of wild-type and mutant proteins discussed frequently in the text appear in parentheses to the right of the amino acid sequence. (B) BHK-21 cells expressing wild-type or mutant VSV G proteins were pulse labeled for 5 min and chased for 60 min. Immunoprecipitated proteins were subjected to endo H treatment as described in MATERIALS AND METHODS and separated by SDS-PAGE. The amount of endo H-resistant protein was quantitated by phosphorimaging. Each time point represents the mean of at least four experiments ± SD.
This six-amino-acid sequence contains the YxxØ motif (where Ø is a bulky hydrophobic residue and x is any amino acid) conserved in many signals including endocytosis and basolateral sorting (for summaries, see Thomas and Roth, 1994; Marks et al., 1997) as well as the di-acidic motif DxE proposed to enhance ER exit (Nishimura and Balch, 1997). We weighed the relative contributions of different pairs of residues in the six-residue sequence to efficient ER export by examining the affects of amino acid substitutions in the 19–24A background. The results of these experiments suggest that the Asp21-Glu23 and Tyr19-Ile22 pairs make similar contributions to the export signal, whereas the remaining two amino acids, Thr20 and Met24, contribute to a lesser extent. After 60 min of chase, the processing of a mutant protein with Asp21-Glu23 changed to Ala-Ala was decreased by 20% compared with the wild-type protein (Figure 4, compare lanes 1 and 8). Replacing the Tyr19-Ile22 pair in addition to Asp21-Glu23 decreased the processing by an additional 20% (Figure 4, lane 8 vs. 3), whereas mutating Thr20-Met24 as well as Asp21-Glu23 had a lesser effect on processing (Figure 4, lane 8 vs. 6). Nishimura and Balch (1997) substituted alanines at individual residues in this region and found only the Asp21 or Glu23 replacements slowed transport. We did not reproduce their point mutations; however, our mutant collection does suggest the relative contributions of several of the individual residues in this region. Ile22 appears to contribute more than Tyr19 (Figure 4, compare lane 6 vs. 5 with 5 vs. 2). In addition, the contribution of Tyr19 appears greater than that of Asp21 (Figure 4, lane 6 vs. 4).
The relative contributions of the Tyr19-Ile22 and Asp21-Glu23 pairs were also examined by directly substituting either pair with alanine residues. Substitution of the Tyr19-Ile22 pair with alanines did not dramatically alter the amount of endo H-resistant protein present relative to wild-type VSV G (Figure 4, compare lanes 1 and 10). By contrast, mutation of the Asp21-Glu23 pair to alanines moderately decreased ER export (Figure 4, lane 1 vs. 8). Assessing the relative contributions of the Tyr-Ile and Asp-Glu pairs in this manner (when the rest of the signal is present) suggests that the Tyr-Ile pair contributes to the ER export signal to a lesser extent than the Asp-Glu pair.
The fact that Nishimura and Balch (1997) did not observe reduced ER exit rates for each individual substitution within the 19–24 region of the tail suggests that the signal is not dramatically affected by small perturbations. Similarly, we observed that changing the Tyr19-Ile22 pair to Ala-Ala did not appreciably affect the transport rate of VSV G (Figure 4, lane 10 vs. 1), yet restoring the Tyr19-Ile22 pair to the 19–24A mutant substantially altered (30% difference) the amount processed at 60 min of chase (Figure 4, compare lanes 6 and 2). Perhaps the overall composition or secondary structure of this region is recognized by cytoplasmic transport machinery. It is interesting to note that negative charge alone cannot explain the contribution of the Asp21-Glu23 pair to ER export. The simultaneous substitution of Asp21 with Glu and Glu23 with Asp (21E23D) did not significantly enhance the transport relative to the mutant with alanines in these positions (Figure 4, compare lanes 8 and 9).
The Tyr-Thr-Asp-Ile-Glu-Met Sequence but Not Asp-x-Glu Is Sufficient for Efficient ER Export
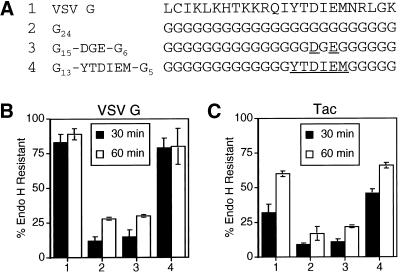
After confirming the contribution of amino acids 19–24 of the G tail toward the efficient ER export of VSV G, we determined whether these six amino acids were sufficient to promote ER exit. A poly-glycine tail was constructed to serve as a neutral background (i.e., lacking any ER export information) for the addition of the six-amino-acid sequence. VSV G with a poly-glycine cytoplasmic domain was slowly transported from the ER (Figure 5). When the six amino acid residues Tyr-Thr-Asp-Ile-Glu-Met were added to the poly-glycine tail, the kinetics of endo H resistance were comparable with that of the wild-type VSV G tail (Figure 5B). Thus, the six residues were sufficient for the efficient ER export rate exhibited by VSV G. In addition, we tested the ability of the di-acidic motif to restore efficient ER export to the slow VSV G poly-glycine tail mutant. The addition of the Asp-x-Glu motif to the poly-glycine sequence did not significantly affect the kinetics of ER exit (Figure 5B).
Figure 5.
Residues 19–24 of the VSV G tail are sufficient to mediate efficient ER export. (A) Amino acid sequence (single-letter code) of the C-terminal 24 residues of the cytoplasmic domain of the wild-type VSV G protein and poly-glycine mutant tails. Numbers to the left of the amino acid sequences refer to lane numbers in the graphs in B and C. Residues in the poly-glycine background that have been substituted with the wild-type VSV G sequence are underlined. BHK-21 cells expressing VSV G (B) or Tac (C) proteins containing the mutant tails described in A (with the additional sequence RVGIH or RIH, respectively, directly following the transmembrane domain) were pulse labeled for 5 min and chased for 30 or 60 min. Immunoprecipitated proteins were subjected to endo H treatment as described in MATERIALS AND METHODS and separated by SDS-PAGE. The amount of endo H-resistant protein was quantitated by phosphorimaging. Each point represents the mean of a minimum of two experiments ± SD.
To confirm that these six amino acids were sufficient to promote efficient ER export, the same cytoplasmic tails were tested on a reporter protein unrelated to VSV G. For these studies, we selected the well-characterized type I glycoprotein Tac (human interleukin-2 receptor alpha chain), which has been used extensively as a reporter to study various trafficking steps (Bonifacino et al., 1990; Letourneur and Klausner, 1992; Humphrey et al., 1993; Rajasekaran et al., 1994; Marks et al., 1995; Mallet and Maxfield, 1999). As demonstrated with the VSV G protein, the presence of the six-amino-acid sequence in a poly-glycine framework conferred a rate of transport from the ER that was enhanced relative to the unmodified poly-glycine tail (Figure 5C). The rate exhibited by the tail with the six-amino-acid signal was comparable with that achieved by Tac containing the entire VSV G cytoplasmic domain sequence (Figure 5C). Addition of the Asp and Glu residues to the poly-glycine tail did not enhance the rate of transport relative to Tac with the poly-glycine tail (Figure 5C).
Secondary Structure of the VSV G Tail Contributes to Efficient ER Export
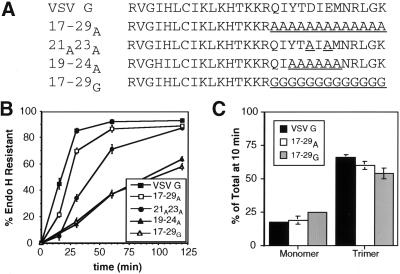
In the course of our mutagenesis, the alanine substitutions within the VSV G tail were extended to include residues surrounding amino acids 19–24 of the cytoplasmic domain. Surprisingly, the resulting mutant protein (17–29A) exhibited an essentially wild-type export rate from the ER (Figure 6B). This result suggests that the machinery responsible for enhanced ER export responds to the structure of the cytoplasmic domain in addition to primary sequence. The 17–29A tail is predicted to form a long alpha helix that would mimic the short alpha-helical structure predicted for the C-terminal region of the VSV G tail using the profile network prediction Heidelberg program (Rost and Sander, 1993, 1994). Unlike a long stretch of alanines, a cluster of glycine residues or a short alanine stretch (19–24A) is not predicted to adopt an alpha-helical structure (Rost and Sander, 1993, 1994). The 17–29G protein was exported from the ER slowly like the CT1 protein (Figure 6B). Like the other mutant proteins studied, the trimerization of 17–29A and 17–29G was similar to wild-type VSV G (Figure 6C).
Figure 6.
Secondary structure of the tail may substitute for specific amino acid sequence. (A) Amino acid sequence (single-letter code) of wild-type and mutant VSV G proteins. Substituted residues are underlined. (B) Kinetics of acquisition of endo H resistance of VSV G, 17–29A, 21A23A, 19–24A, and 17–29G. BHK-21 cells expressing these proteins were pulse labeled for 5 min and chased for the times indicated. Immunoprecipitated proteins were subjected to endo H treatment as described in MATERIALS AND METHODS and separated by SDS-PAGE. The amount of endo H-resistant protein was quantitated by phosphorimaging. Each time point represents the mean of at least three experiments ± SD. (C) Oligomerization of VSV G, 17–29A, and 17–29G. BHK-21 cells expressing VSV G, 17–29A, or 17–29G were pulse labeled for 5 min and solubilized after a 10-min chase. Lysates were loaded onto 5–20% linear sucrose gradients and centrifuged as described in MATERIALS AND METHODS. Fractions were collected, immunoprecipitated with anti-VSV antibody, and analyzed by SDS-PAGE. The percentage of protein found as monomer (4S) or trimer (8S) relative to the total pool of VSV G or mutant proteins was quantitated using phosphorimager analysis. Each time point represents the mean of two experiments ± SEM.
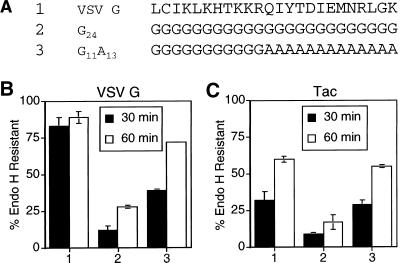
To rule out any effects of the upstream portion of the tail on the ability of the 13 alanines to promote efficient ER export, the 13 alanines were examined in the background of a poly-glycine tail. As described above, VSV G containing a 24-glycine cytoplasmic domain is transported slowly from the ER (Figures 5 and 7). When the last 13 residues of the 24 were changed from glycines to alanines (G11A13), the resultant mutant protein was transported more efficiently (Figure 7B). The effect of the 13 alanines was also transferable to the Tac protein (Figure 7C). It should be noted that although the 13-alanine sequence does enhance the rate of export from the ER relative to a poly-glycine sequence, the alanine stretch is not as effective as the full-length VSV G cytoplasmic domain containing the six-amino-acid signal (Figure 7).
Figure 7.
Poly-alanine sequence is sufficient to enhance the ER export rate of Tac. (A) Amino acid sequence (single-letter code) of the C-terminal 24 residues of the cytoplasmic domain of the wild-type VSV G protein and poly-glycine mutant tails. Numbers to the left of the amino acid sequences refer to lane numbers in the graphs in B and C. BHK-21 cells expressing VSV G (B) or Tac (C) proteins containing the mutant tails described in A (with the additional sequence RVGIH or RIH, respectively, directly following the transmembrane domain) were pulse labeled for 5 min and chased for 30 or 60 min. Immunoprecipitated proteins were subjected to endo H treatment as described in MATERIALS AND METHODS and separated by SDS-PAGE. The amount of endo H-resistant protein was quantitated by phosphorimaging. Each point represents the mean of a minimum of two experiments ± SD.
DISCUSSION
Signal for Efficient ER Exit of VSV G Localized to Six Residues of Cytoplasmic Tail
In this study, we have demonstrated that the efficient export of VSV G from the ER requires six amino acids in the distal portion of the 29-amino-acid cytoplasmic domain. This signal consists of the amino acids Tyr-Thr-Asp-Ile-Glu-Met, which correspond to residues 19–24 of the cytoplasmic tail. When this six-amino-acid sequence was replaced with alanine residues (19–24A), the mutant protein was transported to the Golgi nearly fivefold more slowly than the wild-type protein (Figure 1). The transport rate of the 19–24A mutant was equivalent to VSV G with a single-amino-acid cytoplasmic tail, CT1 (Figure 1). The slow rate of transport of 19–24A could not be attributed to a delay in maturation within the ER, because the mutant protein trimerized at a rate similar to the wild-type VSV G (Figure 2). Thus, the decreased rate of transport of 19–24A to the Golgi is likely to reflect a decrease in the rate of ER exit compared with wild-type VSV G protein. The slow rate of export for CT1 and 19–24A may represent the rate of bulk flow of membrane from the ER.
Further mutagenesis within residues 19–24 of the VSV G tail failed to identify a subset of the amino acids that affected ER export to the same extent as mutation of all six residues. Substituting any of the six alanines in the 19–24A mutant with the wild-type sequence improved transport (Figure 4). Because residues 19–24 were clearly important in the context of the wild-type tail, the six residues were tested for their ability to direct efficient export from the ER. Addition of this motif to a VSV G protein with a slowly transported poly-glycine cytoplasmic tail conferred a rate of transport equal to that of VSV G with the full cytoplasmic domain (Figure 5). Importantly, these six residues were also sufficient to confer efficient ER exit kinetics on Tac, a protein unrelated to VSV G (Figure 5).
Nishimura and Balch (1997) suggested that the aspartic acid at position 21 and the glutamic acid at position 23 of the VSV G cytoplasmic tail are sufficient for selective export from the ER. Our results demonstrate that the two acidic residues only partially contribute to the efficient ER export of VSV G. When the acidic pair of residues was replaced with alanines, the decrease in the half-time of transport was less than half that seen for 19–24A (Figures 1 and 4). Moreover, we found that addition of the Asp-x-Glu sequence to the slowly transported poly-glycine tail on VSV G or Tac was not sufficient to significantly alter the ER export rate of either protein (Figure 5).
Although our observations disagree with the conclusions of Nishimura and Balch (1997), they do not conflict with their primary data. Both studies used identical expression systems and cell types. To allow comparison of half-times for endo H processing in the Nishimura and Balch (1997) study to our results, we added half of the pulse label time (10 min for their study vs. our 5-min pulse) to the half-time of endo H resistance acquisition. Nishimura and Balch (1997) found that replacing both of the acidic residues with alanines slowed the half-time for acquisition of endo H resistance from the wild-type rate of ∼20 to ∼60 min (threefold). We found that the same replacement decreased the half-time of processing from 20 to 45 min (2.3- fold). Importantly, Nishimura and Balch (1997) showed that a truncated VSV G containing only the first three residues of the tail exhibited an even slower transport rate than the di-acidic to alanine replacement, with an endo H resistance half-time of much greater than 60 min, but this was not discussed.
Slowed Export from the ER Correlates with Lack of Concentration
The selective export of proteins from the ER involves their sorting, as demonstrated by the concentration of proteins in ER-derived vesicles and the Golgi apparatus relative to the ER (Mizuno and Singer, 1993; Balch et al., 1994). Our finding that mutation of the complete 19–24 region of the tail slows export nearly fivefold suggests that the six-amino-acid signal could fully account for the concentration of VSV G during ER export. When cells expressing VSV G mutants that are slow to exit the ER (19–24A and CT1) were analyzed by indirect immunofluorescence, we did not detect Golgi staining, even though these proteins clearly move through the Golgi complex on their way to the plasma membrane (Figure 3). The absence of obvious Golgi staining could indicate that these mutant proteins fail to concentrate during export from the ER. Cells expressing the 21A23A protein (transported at a moderately slowed rate) had clear Golgi staining, suggesting that this protein is still capable of being concentrated (Figure 3). The 21A23A mutant may concentrate less efficiently than the wild-type VSV G protein, which would be difficult to detect using this assay. Indeed, using a different assay, Nishimura et al. (1999) recently demonstrated that the 21A23A mutant is less concentrated during export from the ER relative to wild-type VSV G. If the 19–24A mutant were examined in their assay, we would predict the absence of detectable concentration. Quantitative analysis will be required to directly compare the di-acidic motif and the larger six-amino-acid signal in cargo concentration.
Secondary Structure of the VSV G Tail May Contribute to Efficient ER Export
Our finding that replacing the last 13 residues of the VSV G tail with alanines restores the ER export rate to nearly that of wild-type VSV G protein was surprising, because this mutant completely lacks the six-residue signal (Figure 6). Equally unexpected is that substitution of the last 13 residues of a reporter construct with alanines enhanced transport, although not as efficiently as the Tyr-Thr-Asp-Ile-Glu-Met signal (Figure 7). The ability of both the six-amino-acid motif and the poly-alanine sequence to promote efficient export from the ER suggests that more than one polypeptide sequence can be recognized by the cellular ER export machinery. This may account for the inability of researchers to identify a universal ER export motif. This also could explain how many proteins can move efficiently from the ER in the absence of the Tyr-Thr-Asp-Ile-Glu-Met sequence.
It is not known how the poly-alanine sequence and the six-amino-acid signal can both mediate efficient transport. The poly-alanine mutant proteins may exit the ER using a separate pathway and/or machinery from that used by the slow 19–24A mutant. Alternatively, the poly-alanine and the wild-type VSV G tails both could be accommodated by the same transport components. Perhaps the conformation of the 19–24 region of the tail is important for ER exit. The alpha-helical structure predicted to form by a long stretch of alanines may mimic the secondary structure of the region defined by the six-amino-acid signal. Consistent with this idea, a VSV G protein containing 13 glycine residues at its C terminus (not predicted to form an alpha helix by the profile network prediction Heidelberg program) was transported as slowly as CT1 and 19–24A (Figure 5).
ER Exit Motif Is Part of a Larger Family of Sorting Signals
It is intriguing that the region promoting efficient ER export of VSV G includes the YxxØ motif implicated in many different sorting events, including targeting to the trans-Golgi network, early endosomes, and lysosomes (reviewed in Kirchhausen et al., 1997; Marks et al., 1997). Work on the role of tyrosine-based signals in the late secretory and endocytic pathways has demonstrated that the variability in the x and Ø positions of the YxxØ signal determines the affinity and specificity of the interaction of the signal with transport components, such as adaptors (Ohno et al., 1995, 1996, 1998; Boll et al., 1996). Accordingly, the affinity of the different adaptor complexes for certain tyrosine-based sequences influences the probability that proteins will undergo sorting at a particular intracellular compartment. The recent crystal structure of the μ2 adaptor subunit complexed with peptides corresponding to the epidermal growth factor receptor internalization motif or the TGN38 trans-Golgi targeting signal provides elegant structural confirmation of this process (Owen and Evans, 1998).
In VSV G, the tyrosine-containing signal involved in efficient ER export is also important for the basolateral sorting of VSV G in polarized epithelial cells (Thomas and Roth, 1994). For ER exit, the Thr, Asp, Glu, and Met residues in addition to the Tyr and Ile are important (Figure 4), whereas an upstream arginine contributes to the recognition of the basolateral sorting signal (Thomas and Roth, 1994). Interestingly, this tyrosine-containing signal in the VSV G tail is not involved in endocytosis, which has been shown to occur at only a low rate from the plasma membrane (Thomas et al., 1993). This could reflect selective pressures placed on the vesicular stomatitis virus. In virus-infected cells, the VSV G protein must accumulate quickly and efficiently at the plasma membrane to allow virus assembly and release. Toward this end, the evolution of the VSV G protein most likely included the acquisition of sorting signals that would result in enhanced movement to the cell surface. The ER exit motif we have identified may be the result of modification over time of signals for different trafficking steps found in cellular proteins. Because robust endocytosis of VSV G would not be advantageous for virus assembly, selection for a tail sequence that minimizes internalization while still allowing basolateral targeting and efficient ER exit would have been favored. The Glu and Asp residues embedded within the YxxØ signal may represent the most efficient way to accomplish this goal. A number of isolates of two serotypes of VSV from different geographical locations have been sequenced. Despite a large number of substitutions present in the cytoplasmic tails of the G proteins from these viruses, the Tyr-Thr-Asp-Ile-Glu-Met motif is strongly conserved (Nichol et al., 1989; Bilsel and Nichol, 1990). These data support the importance of the transport signal identified here.
The DxE motif is present in the cytoplasmic tails of a number of plasma membrane proteins, although it has been shown to enhance ER export for only two of these proteins (Nishimura and Balch, 1997; Bannykh et al., 1998). Interestingly, all of the proteins with the DxE motif identified by Bannykh et al. (1998) also possess an upstream YxxØ signal. It will be interesting to determine whether the DxE signal plays any role in ER exit for these proteins or whether instead it is involved in some aspect of endocytosis. Finally, it is clear that the DxE sequence in the VSV G cytoplasmic tail is not a universal signal for efficient ER export. It is not present in a number of membrane proteins that are efficiently transported from the ER to the plasma membrane and does not confer efficient export kinetics to a Tac reporter construct. Further understanding of the recognition of signals for efficient ER exit will require identification and structural studies of the transport machinery.
ACKNOWLEDGMENTS
We are grateful to Michael Marks (University of Pennsylvania Medical School, Philadelphia, PA) for the generous gifts of Tac cDNA and polyclonal anti-Tac antibody and patient advice on their use. We thank Lisa Grim and Angela McFillin for assistance with some of the mutagenesis. We also thank Ann Hubbard, Michael Maceyka, and Emily Corse for helpful comments on the manuscript. This work was supported by National Institute of Health grant ROI-GM-42522.
Abbreviations used:
- DME
Dulbecco's modified Eagle's medium
- endo H
endoglycosidase H
- ER
endoplasmic reticulum
- VSV G
vesicular stomatitis virus glycoprotein
REFERENCES
- Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Bannykh SI, Nishimura N, Balch WE. Getting into the Golgi. Trends Cell Biol. 1998;8:21–25. doi: 10.1016/s0962-8924(97)01184-7. [DOI] [PubMed] [Google Scholar]
- Bilsel PK, Nichol ST. Polymerase errors accumulating during natural evolution of the glycoprotein gene of vesicular stomatitis virus indiana serotype isolates. J Virol. 1990;64:4873–4883. doi: 10.1128/jvi.64.10.4873-4883.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W, Ohno H, Songyang Z, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Suzuki CK, Klausner RD. A peptide sequence confers retention and rapid degradation in the endoplasmic reticulum. Science. 1990;247:79–82. doi: 10.1126/science.2294595. [DOI] [PubMed] [Google Scholar]
- Cluett EB, Kuismanen E, Machamer CE. Heterogeneous distribution of the unusual phospholipid semilysobisphosphatidic acid through the Golgi complex. Mol Biol Cell. 1997;8:2233–2240. doi: 10.1091/mbc.8.11.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez M, Dejgaard K, Fullekrug J, Dahan S, Fazel A, Paccaud JP, Thomas DY, Bergeron JJ, Nilsson T. gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J Cell Biol. 1998;140:751–765. doi: 10.1083/jcb.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Ruusala A, Machamer C, Helenius J, Helenius A, Rose JK. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988;107:89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JS, Peters PJ, Yuan LC, Bonifacino JS. Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993;120:1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler F, Klopfenstein DR, Foguet M, Paccaud JP, Hauri HP. The recycling of ERGIC-53 in the early secretory pathway. ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J Biol Chem. 1997;272:31801–31808. doi: 10.1074/jbc.272.50.31801. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Bonifacino JS, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Kreis TE. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefrancois L, Lyles DS. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. Virology. 1982;121:168–174. doi: 10.1016/0042-6822(82)90126-x. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Machamer CE, Florkiewicz RZ, Rose JK. A single N-linked oligosaccharide at either of two normal sites is sufficient for transport of vesicular stomatitis virus G protein to the cell surface. Mol Cell Biol. 1985;5:3074–3083. doi: 10.1128/mcb.5.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer CE, Grim MG, Esquela A, Chung SW, Rolls M, Ryan K, Swift AM. Retention of a cis Golgi protein requires polar residues on one face of a predicted alpha-helix in the transmembrane domain. Mol Biol Cell. 1993;4:695–704. doi: 10.1091/mbc.4.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet WG, Maxfield FR. Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J Cell Biol. 1999;146:345–359. doi: 10.1083/jcb.146.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Ohno H, Kirchhausen T, Bonifacino J. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- Marks MS, Roche PA, van Donselaar E, Woodruff L, Peters PJ, Bonifacino JS. A lysosomal targeting signal in the cytoplasmic tail of the beta chain directs HLA-DM to MHC class II compartments. J Cell Biol. 1995;131:351–369. doi: 10.1083/jcb.131.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Singer SJ. A soluble secretory protein is first concentrated in the endoplasmic reticulum before transfer to the Golgi apparatus. Proc Natl Acad Sci USA. 1993;90:5732–5736. doi: 10.1073/pnas.90.12.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol ST, Rowe JE, Fitch WM. Glycoprotein evolution of vesicular stomatitis New Jersey. Virology. 1989;168:281–291. doi: 10.1016/0042-6822(89)90268-7. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Balch WE. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Bannykh S, Slabough S, Matteson J, Altschuler Y, Hahn K, Balch WE. A di-acidic (DXE) code directs concentration of cargo during export from the endoplasmic reticulum. J Biol Chem. 1999;274:15937–15946. doi: 10.1074/jbc.274.22.15937. [DOI] [PubMed] [Google Scholar]
- Ohno H, Aguilar RC, Yeh D, Taura D, Saito T, Bonifacino JS. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J Biol Chem. 1998;273:25915–25921. doi: 10.1074/jbc.273.40.25915. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier M-C, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier M-C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR, Rothman JE. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi complex. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Puddington L, Machamer CE, Rose JK. Cytoplasmic domain of cellular and viral integral membrane proteins substitute for the cytoplasmic domain of the vesicular stomatitis virus glycoprotein in transport to the plasma membrane. J Cell Biol. 1986;102:2147–2157. doi: 10.1083/jcb.102.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran AK, Humphery JS, Wagner M, Miesenbock G, Le Bivic A, Bonifacino JS, Rodriguez-Boulan E. TGN38 recycles basolaterally in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1994;5:1093–1103. doi: 10.1091/mbc.5.10.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Bergmann JE. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983;34:513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Rost B, Sander C. Prediction of protein secondary structure at better than 70% accuracy. J Mol Biol. 1993;232:584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- Rost B, Sander C. Combining evolutionary information and neural networks to predict protein secondary structure. Proteins. 1994;19:55–72. doi: 10.1002/prot.340190108. [DOI] [PubMed] [Google Scholar]
- Scullion BF, Hou Y, Puddington L, Rose JK, Jacobson K. Effects of mutations in three domains of the vesicular stomatitis viral glycoprotein on its lateral diffusion in the plasma membrane. J Cell Biol. 1987;105:69–75. doi: 10.1083/jcb.105.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift AM, Machamer CE. A Golgi retention signal in a membrane-spanning domain of coronavirus E1 protein. J Cell Biol. 1991;115:19–30. doi: 10.1083/jcb.115.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DC, Brewer CB, Roth MG. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- Thomas DC, Roth MG. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J Biol Chem. 1994;269:15732–15739. [PubMed] [Google Scholar]
- Whitt MA, Chong L, Rose JK. Glycoprotein cytoplasmic domain sequences required for rescue of a vesicular stomatitis virus glycoprotein mutant. J Virol. 1989;63:3569–3578. doi: 10.1128/jvi.63.9.3569-3578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]