Abstract
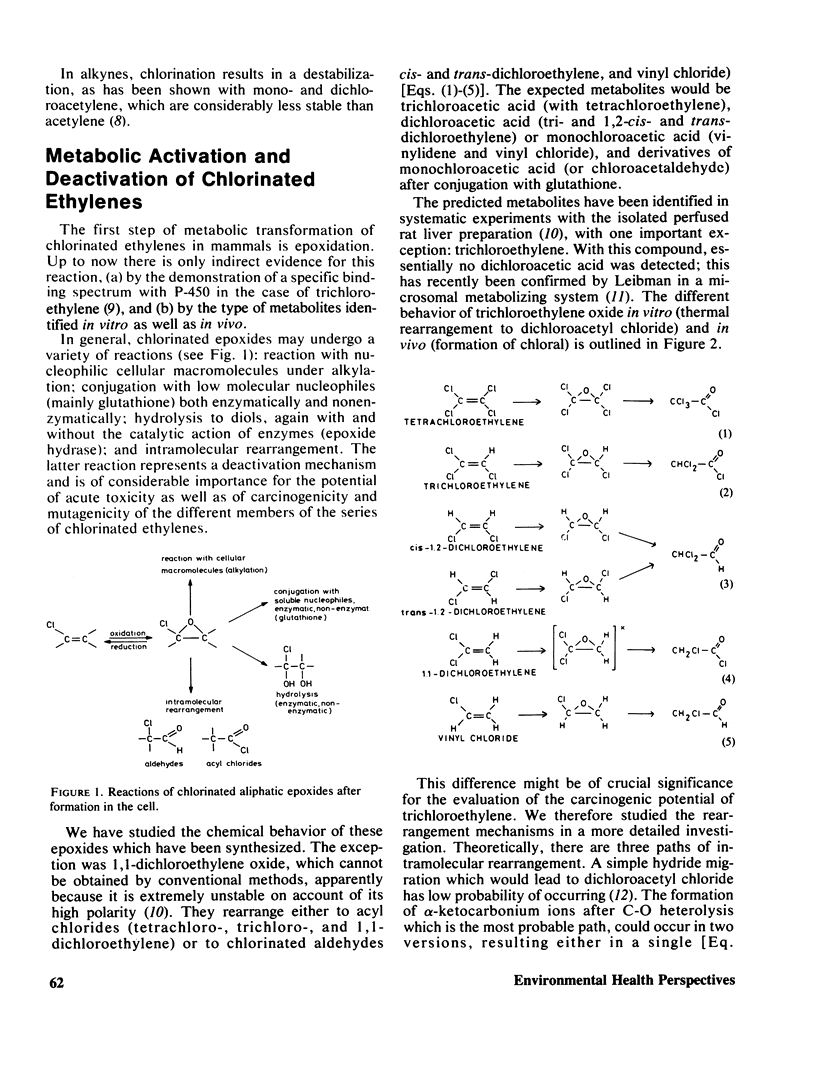
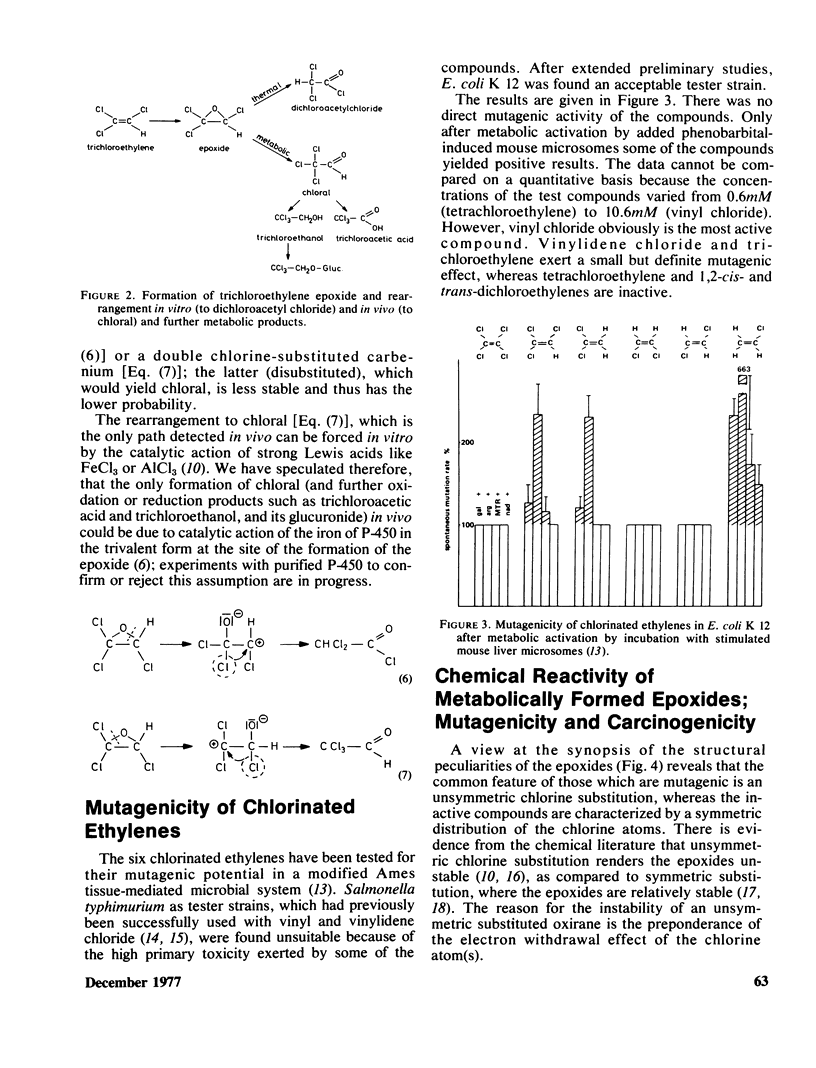
Chlorinated ethylenes are metabolized in mammals, as a first step, to epoxides. The fate of these electrophilic intermediates may be reaction with nucleophiles (alkylation), hydrolysis, or intramolecular rearrangement. The latter reaction has been studied in the whole series of chlorinated epoxiethanes. The rearrangement products found were: acyl chlorides (tetrachloro-, trichloro-, and 1,1-dichloroethylenes), or chlorinated aldehydes (1,2-dichloroethylenes, cis- and trans-, vinyl chloride). The metabolities found in vivo are identical with, or further derivatives of these rearrangment products, with one important exception: trichloroethylene. With this compound, in vivo rearrangement yields chloral exclusively. The mechanism of the different rearrangement has been identified as a Lewis acid catalysis. All chlorinated ethylenes have been investigated in a tissue-mediated mutagenicity testing system. The prominent molecular feature of those with mutagenic effects (trichloro-, 1,1-dichloro-, and monochloroethylene) is unsymmetric chlorine substitution which renders the epoxides unstable, whereas symmetric substitution confers relative stability and nonmutagenic property.
Full text
PDF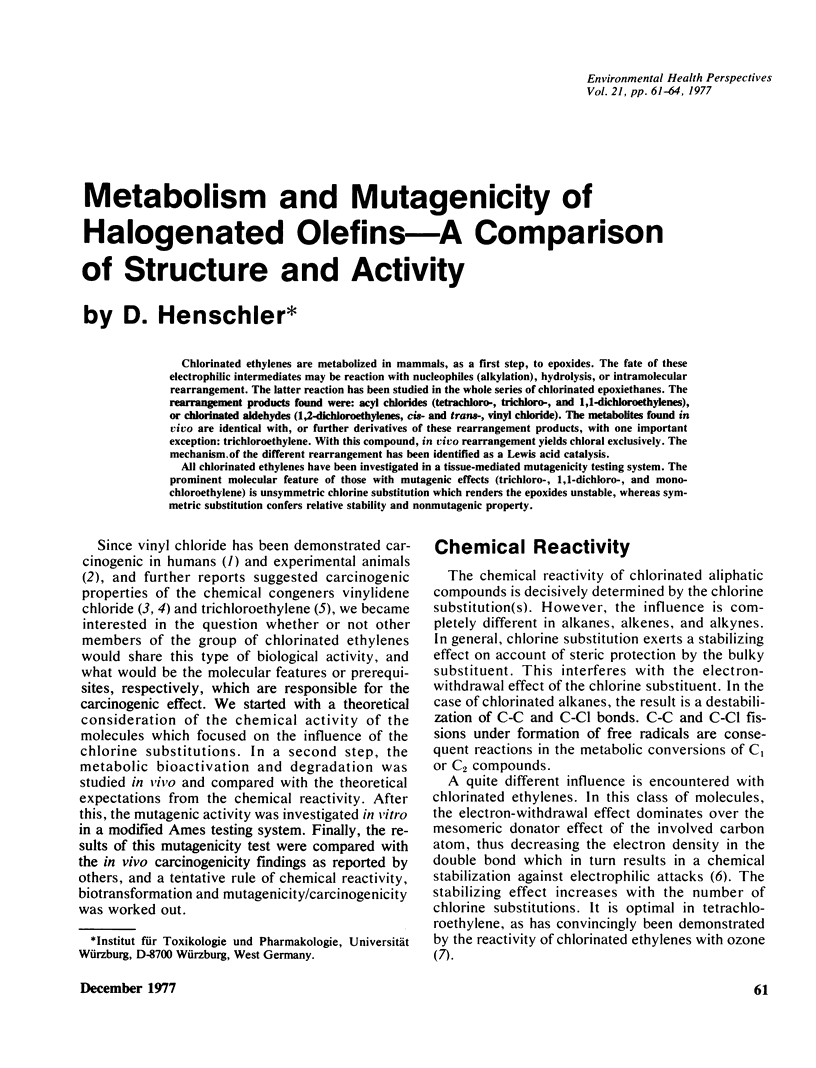

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch H., Malaveille C., Montesano R. Human, rat and mouse liver-mediated mutagenicity of vinyl chloride in S. typhimurium strains. Int J Cancer. 1975 Mar 15;15(3):429–437. doi: 10.1002/ijc.2910150309. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Malaveille C., Montesano R., Tomatis L. Tissue-mediated mutagenicity of vinylidene chloride and 2-chlorobutadiene in Salmonella typhimurium. Nature. 1975 Jun 19;255(5510):641–643. doi: 10.1038/255641a0. [DOI] [PubMed] [Google Scholar]
- Bonse G., Henschler D. Chemical reactivity, biotransformation, and toxicity of polychlorinated aliphatic compounds. CRC Crit Rev Toxicol. 1976 Oct;4(4):395–409. doi: 10.1080/10408447609164019. [DOI] [PubMed] [Google Scholar]
- Bonse G., Urban T., Reichert D., Henschler D. Chemical reactivity, metabolic oxirane formation and biological reactivity of chlorinated ethylenes in the isolated perfused rat liver preparation. Biochem Pharmacol. 1975 Oct 1;24(19):1829–1834. doi: 10.1016/0006-2952(75)90468-2. [DOI] [PubMed] [Google Scholar]
- Greim H., Bonse G., Radwan Z., Reichert D., Henschler D. Mutagenicity in vitro and potential carcinogenicity of chlorinated ethylenes as a function of metabolic oxiran formation. Biochem Pharmacol. 1975 Nov 1;24(21):2013–2017. doi: 10.1016/0006-2952(75)90396-2. [DOI] [PubMed] [Google Scholar]
- Henschler D., Eder E., Neudecker T., Metzler M. Carcinogenicity of trichloroethylene: fact or artifact? Arch Toxicol. 1977 Jul 19;37(3):233–236. doi: 10.1007/BF00355492. [DOI] [PubMed] [Google Scholar]


