Abstract
The highly conserved small Rho G-protein, Cdc42p plays a critical role in cell polarity and cytoskeleton organization in all eukaryotes. In the yeast Saccharomyces cerevisiae, Cdc42p is important for cell polarity establishment, septin ring assembly, and pheromone-dependent MAP-kinase signaling during the yeast mating process. In this study, we further investigated the role of Cdc42p in the mating process by screening for specific mating defective cdc42 alleles. We have identified and characterized novel mating defective cdc42 alleles that are unaffected in vegetative cell polarity. Replacement of the Cdc42p Val36 residue with Met resulted in a specific cell fusion defect. This cdc42[V36M] mutant responded to mating pheromone but was defective in cell fusion and in localization of the cell fusion protein Fus1p, similar to a previously isolated cdc24 (cdc24-m6) mutant. Overexpression of a fast cycling Cdc42p mutant suppressed the cdc24-m6 fusion defect and conversely, overexpression of Cdc24p suppressed the cdc42[V36M] fusion defect. Taken together, our results indicate that Cdc42p GDP–GTP cycling is critical for efficient cell fusion.
INTRODUCTION
Eukaryotic cells respond to a range of different external signals by gene induction, protein recruitment and activation, and morphological changes. Such responses are critical during diverse developmental processes. The mating process in the yeast Saccharomyces cerevisiae is an ideal system for investigating external signal-mediated responses as exposure to mating pheromone triggers an ordered series of events, which terminates in the formation of a diploid zygote. During mating in S. cerevisiae, MATa and MATa cells respond to peptide pheromones, α- and a-factor, respectively, secreted by cells of the opposite mating type (for reviews see; Leberer et al., 1997; Dohlman and Thorner, 2001). These mating pheromones bind to specific G-protein–coupled receptors on each cell type and receptor activation results in cell cycle arrest, transcriptional induction of mating-specific genes, morphological changes leading to formation of a pear-shaped shmoo, and polarized growth toward a mating partner. In response to pheromone, the actin cytoskeleton and secretory apparatus polarize toward the tip of the mating projection (Baba et al., 1989; Read et al., 1992; Smith et al., 2001), and new cell wall and plasma membrane material is deposited at this unique location, which becomes the site of cell contact and ultimately fusion (Lipke et al., 1976; Tkacz and MacKay, 1979). In particular, polarized growth is necessary for positioning the cell fusion apparatus to the site of cell contact. During mating, two yeast cells physically attach to one another forming a prezygote with a continuous extracellular matrix. Specific degradation of the cell wall material at the prezygote septum is necessary so that the plasma membranes can become tightly apposed and fuse. Subsequently, nuclear migration and nuclear fusion result in the formation of a diploid zygote.
The binding of pheromone to the G-protein–coupled pheromone receptor results in release of the βγ subunits of the heterotrimeric G-protein. This heterodimer Gβγ is critical for activation of the mitogen-activated protein kinase (MAPK) cascade that is necessary for G1 cell cycle arrest and mating-specific gene transcription. In addition, released Gβγ is required for cell shape changes and oriented growth along a pheromone gradient (chemotropism) via interactions with the guanine nucleotide exchange factor Cdc24p and the cyclin-dependent kinase inhibitor Far1p (Valtz et al., 1995; Butty et al., 1998; Nern and Arkowitz, 1998, 1999). Recently, it has been shown that the released Gα subunit can bind the MAP kinase Fus3p, which subsequently phorphorylates the formin Bni1p (Metodiev et al., 2002; Matheos et al., 2004), both of which are necessary for cell fusion leading to a diploid zygote (Dorer et al., 1997).
Proteins involved in different signaling processes, such as osmotic balance (Philips and Herskowitz, 1997; Nelson et al., 2004), cell wall remodeling (Santos et al., 1997; Fitch et al., 2004), cell polarization (Valtz and Herskowitz, 1996; Dorer et al., 1997; Gammie et al., 1998), and pheromone response (Brizzio et al., 1996; Elia and Marsh, 1998) are important for cell fusion. In addition, several proteins that are highly induced by mating pheromone including the integral membrane protein Fus1p and the cytoplasmic protein Fus2p, which binds the yeast amphiphysin ortholog Rvs161p (Brizzio et al., 1998), are thought to have a specific function in cell fusion (Trueheart et al., 1987) and interact with a range of proteins implicated in cell fusion (Nelson et al., 2004). These proteins may serve as a scaffold or platform for proteins involved in pheromone signaling and polarity. Fus1p and Fus2p localize to the region of cell fusion before cell wall degradation (Trueheart et al., 1987; Elion et al., 1995; Brizzio et al., 1998), where clusters of vesicles have been observed by electron microscopy (Gammie et al., 1998). Fus1 mutants do not accumulate such vesicles at the zone of cell fusion, whereas fus2 mutants accumulate vesicles at this region (Gammie et al., 1998). For plasma membrane fusion the integral membrane protein, Prm1p is important for stabilizing membrane fusion after septum degradation (Heiman and Walter, 2000; Jin et al., 2004).
The highly conserved small GTPase Cdc42p and its guanine nucleotide exchange factor Cdc24p are critical for polarized growth during budding (Johnson, 1999). Cdc42p has been shown to also be required for pseudohyphal and invasive filamentous growth (Mosch et al., 2001). Temperature-sensitive cdc42 and cdc24 mutants are defective in pheromone-dependent FUS1 induction and G1 arrest at the nonpermissive temperature (Simon et al., 1995); however, these effects are likely to be due to an unresponsiveness to pheromone as a result of cells in an inappropriate cell cycle stage (Oehlen and Cross, 1998). Several α-factor resistant alleles of cdc42 have been isolated that are defective in PAK kinase interactions indicating that this G-protein functions in pheromone-dependent MAP-kinase signaling (Moskow et al., 2000). Mating-specific cdc24 mutants have been isolated that are chemotropism defective (Nern and Arkowitz, 1998), and more recently, cdc24 alleles that are defective in cell fusion have been isolated (Barale et al., 2004). These cdc24 alleles defective in cell fusion during mating have mutations in conserved residues of the catalytic exchange factor domain, raising the possibility that Cdc42p might also be important for cell fusion. The role of this GTPase module could be analogous to that of the guanine nucleotide exchange factor Myoblast city (a DOCK-180 homolog) and the small G-protein Rac1 during Drosophila myoblast fusion (Nolan et al., 1998; Hakeda-Suzuki et al., 2002). Consistent with this hypothesis, a recent study examining two-hybrid interactions of Fus1p showed that a GTP-locked Cdc42p mutant (G12V) interacts with Fus1p and Fus2p (Nelson et al., 2004).
In this study, we show that the highly conserved Rho G-protein Cdc42p is necessary for cell fusion in yeast. We identified mating-specific cdc42 mutants that had a valine-to-methionine change in position 36 in the Switch I region. This cdc42 mutant responds to mating pheromone but is defective in cell fusion and in localizing Fus1p to the site of polarized growth, similar to the previously isolated cdc24-m6 mutant. Overexpression of a fast cycling Cdc42p mutant suppresses the cdc24-m6 fusion defect, and conversely, overexpression of Cdc24p suppresses the cdc42[V36M] fusion defect. Together our results indicate that Cdc42p GDP–GTP cycling is critical for cell fusion.
MATERIALS AND METHODS
General Techniques
Standard techniques and media were used for yeast growth and genetic manipulation (Rose et al., 2004), and unless otherwise indicated yeast were grown at 30°C.
Strains and Plasmids
The strains used in this study are described in Table 1. CDC42 deletion strains were generated either with a knockout cassette (Δ1) or by polymerase chain reaction (PCR)-mediated gene replacement (Δ2; Nern and Arkowitz, 1999). The knockout cassette was constructed from pBSCDC42 (CDC42 ORF with 147 nucleotides 3′ of stop codon) with LEU2 replacing all but the first 62 and the last 15 codons. STE18 deletion strain was generated by PCR-mediated gene replacement (Nern and Arkowitz, 1999). Gene disruptions were confirmed by PCR and phenotype. A cdc24Δ cdc42Δ haploid strain with CDC24 and CDC42 on CEN plasmids (RAY1728) was obtained by crossing the corresponding single deletion strains (a derivative of RAY1052 with a pRS414CDC24 and RAY1556) followed by sporulation. Cdc24 cdc42 double mutant strains were generated by transformation and plasmid shuffling of RAY1728. For all experiments, with the exception of suppression experiments, the indicated CDC24, cdc24, CDC42, and cdc42 genes are the sole copies present, and their expression is driven by their endogenous promoters on CEN plasmids. Fusion defects were similar in all strain backgrounds (single and double deletion strains and both mating types).
Table 1.
Yeast strains used in this study
| Strain | Genotypea | Source |
|---|---|---|
| JY426 | MATa, leu2-3,-112, ura3-52, his4-34, fus1-Δ1, fus2-Δ3 | Cold Spring Harbor Laboratory |
| JY429 | MATα, trp1Δ1, ura3-52, cyh2, fus1-Δ1, fus2-Δ3 | Cold Spring Harbor Laboratory |
| SEY6210 | MATα, leu2-3,-112, ura3-52, his3-Δ200, trp1-Δ901, lys2-801, suc2-Δ9 | S. Emr |
| SEY6211 | MATa, leu2-3,-112, ura3-52, his3-Δ200, trp1-Δ901, ade2, suc2-Δ9 | S. Emr |
| RAY399 | MATα/a, leu2-3,-112/leu2-3,-112, ura3-52/ura3-52, his3-Δ200/his3-Δ200, trp1-Δ901/trp1-Δ901, LYS2/lys2-801, ADE2/ade2, suc2-Δ9/suc2-Δ9 | This studyb |
| RAY485 | RAY399 CDC42/cdc42-Δ1::LEU2 | This study |
| RAY513 | SEY6210 cdc42-Δ1::LEU2 with pRS424GALGFPCDC42 | This studyc |
| RAY563 | SEY6210 sph1-Δ1::HIS3 | Arkowitz and Lowe (1997) |
| RAY567 | SEY6211 sph1-Δ1::HIS3 | Arkowitz and Lowe (1997) |
| RAY950 | MATa, leu2-3,-112, ura3-52, his3-Δ200, trp1-Δ901, lys2-801, ade2, cdc24::LEU2 with pRS416GalHis6CDC24 | Nern and Arkowitz (1998) |
| RAY1042 | RAY950 with pRS414CDC24 instead of pRS416GalHis6CDC24 | Nern and Arkowitz (1998) |
| RAY1052 | SEY6211 cdc24-Δ1::LoxP with pEG(KT)CDC24 | Nern and Arkowitz (2000a) |
| RAY1142 | SEY6211 cdc24::TRP1 cdc24-m1, bud1Δ::LoxP HIS5Sp LoxP | Nern and Arkowitz (1999) |
| RAY1487 | SEY6211 cdc24::TRP1 CDC24, URA3::GFPBUD1 | Nern and Arkowitz (2000a) |
| RAY1556 | SEY6210 cdc42-Δ1::LEU2 with pRS416CDC42 | This studyd |
| RAY1565 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36M, P69T] | This studyd,e |
| RAY1635 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36M] | This studyd,f |
| RAY1638 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[F37I] | This studyd,f |
| RAY1640 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36A, A41G] | This studyd,f |
| RAY1685 | RAY950 with pRS414cdc24-m6 instead of pRS416GalHis6CDC24 | Barale et al. (2004) |
| RAY1728 | SEY6211 cdc24-Δ1::LoxP, cdc42-Δ1::LEU2 with pRS414CDC24 and pRS416CDC42 | This studyg |
| RAY1772 | SEY6211 cdc42-Δ2::HIS5Sp with pRS416CDC42 | This study |
| RAY1793 | SEY6211 cdc24-Δ1::LoxP, cdc42-Δ1::LEU2 with pRS414CDC24 and pRS416cdc42[V36V]i | This studyh |
| RAY1801 | SEY6211 cdc24-Δ1::LoxP, cdc42-Δ1::LEU2 with pRS414cdc24-m6 and pRS416cdc42[V36V]i | This studyj |
| RAY1830 | SEY6211 cdc24-Δ1::LoxP, cdc42-Δ1::LEU2 with pRS414CDC24 and pRS413GFPCDC42 | This studyh |
| RAY1833 | SEY6211 cdc24-Δ1::LoxP, cdc42-Δ1::LEU2 with pRS414cdc24-m6 and pRS413GFPCDC42 | This study |
| RAY1907 | SEY6210 sph1-Δ1::HIS3, URA3::GFPBUD1 | This study |
| RAY1912 | SEY6210 cdc42-Δ1::LEU2 with pRS414cdc42[V36V]i | This study |
| RAY1914 | SEY6210 cdc42-Δ1::LEU2 with pRS414cdc42[V36M] | This study |
| RAY1918 | SEY6211 cdc42-Δ2::HIS5Sp with pRS414cdc42[V36V]i | This study |
| RAY1920 | SEY6211 cdc42-Δ2::HIS5Sp with pRS414cdc42[V36M] | This study |
| RAY1926 | SEY6211 cdc42-Δ2::HIS5Sp with pRS416cdc42[V36M] | This study |
| RAY1951 | SEY6211 cdc24-Δ1::LoxP, cdc42-Δ1::LEU2 with pRS414CDC24 and pRS413GFPcdc42[V36M] | This study |
| RAY1952 | SEY6211 cdc24-Δ1::LoxP, cdc42-Δ1::LEU2 with pRS414CDC24 and pRS416cdc42[V36M] | This studyk |
| DMY13 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36W] | This studyd |
| DMY14 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36C] | This studyd |
| DMY15 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36F] | This studyd |
| DMY16 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36L] | This studyd |
| DMY17 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36I] | This studyd |
| DMY18 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36A] | This studyd |
| DMY19 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36T] | This studyd |
| DMY20 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36Y] | This studyd |
| DMY21 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36Q] | This studyd |
| DMY22 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36S] | This studyd |
| DMY23 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36H] | This studyd |
| DMY25 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36K] | This studyd |
| DMY26 | SEY6210 cdc42-Δ1::LEU2 with pRS416cdc42[V36R] | This studyd |
a HIS5Sp refers to HIS5 from Schizosaccharomyces pombe.
b Constructed by mating SEY6210 and SEY6211.
c Plasmid pRS424GALGFPCDC42 was transformed into RAY399 and tetrads were dissected.
d Indicated plasmid pRS416CDC42 or cdc42 mutant was transformed into RAY513 and pRS424GALGFPCDC42 was cured.
e Isolated in first cdc42 mutant screen.
f Isolated in second cdc42 mutant screen.
g RAY1052 was transformed with pRS414CDC24 and plasmid pEG(KT)CDC24 was cured. This strain was then crossed to RAY1556 followed by sporulation.
h Constructed by transformation of RAY1728 with pRS413GFPCDC42 and pRS416CDC42 was cured (RAY1830). Subsequently strain was transformed with indicated pRS416cdc42 plasmid and plasmid pRS413GFPCDC42 was cured.
i Cdc42[V36V] was generated by site-directed mutagenesis of Cdc42p replacing Val 36 with Val (in addition to a silent SnaBI site).
j Constructed by transformation of RAY1793 with pRS313CDC24 and pRS414CDC24 was cured. Subsequently strain was transformed with pRS414cdc24-m6 plasmid and plasmid pRS313CDC24 was cured.
k Constructed by transformation of RAY1793 with pRS413GFPCDC42 and pRS416cdc42[V36V] was cured. Subsequently the strain was transformed with pRS416cdc42[V36M] plasmid and plasmid pRS413GFPCDC42 was cured.
Plasmids used in this study are listed in Table 2. pRS414CDC24 contains the CDC24 ORF including 258 base pairs upstream of the ATG and 10 new unique restriction sites in CDC24 (Nern and Arkowitz, 1998). pRS313CDC24 was constructed by cloning a HindIII/SpeI fragment that contained the CDC24 ORF into pRS313. pRS414CDC42 and pRS416CDC42 contains the CDC42 ORF including 521 base pairs upstream of the ATG. Point mutations were generated by site-directed mutagenesis with the Pfu polymerase (Promega, Charbonnieres Les Bains, France) using the DpnI method (Weiner et al., 1994) and identified by the addition or removal of a silent restriction site. For Cdc24-m6p (R416G) an endogenous EcoRV site was removed 1247 base pairs after the ATG, for Cdc42[V36M]p (and all the V36 mutations) a SnaBI site was added 93 base pairs after the ATG, for Cdc42[C18A]p an EarI site was added 53 base pairs after the ATG, and for Cdc42[T35A]p a PstI site was added 102 base pairs after the ATG. All mutations generated were confirmed by sequencing (ABI [Courtaboeuf, France] PRISM big-dye terminator cycle sequencing kit).
Table 2.
Plasmids used in this study
| Plasmid | Vector | Insert | Source |
|---|---|---|---|
| pBS KS− II | Stratagene | ||
| pMal-c2 | New England Biolabs | ||
| pGEX 6P-2 | Amersham | ||
| pT7Tag | Pollock and Treisman (1990) | ||
| p3xHA-His5 | pBS | 3xHA-HIS5Sp | Rayner and Munro (1998) |
| pRS313 | CEN HIS3 | Sikorski and Hieter (1989) | |
| pRS413 | CEN HIS3 | Sikorski and Hieter (1989) | |
| pRS414 | CEN TRP1 | Sikorski and Hieter (1989) | |
| pRS416 | CEN URA3 | Sikorski and Hieter (1989) | |
| pRS424 | 2 μm TRP1 | Christianson et al., 1992 | |
| pRS424GAL | 2 μm TRP1 | GAL1 promoter | This study |
| p2μA-TPI | 2 μm ADE2 | TPI1 promoter | Nern and Arkowitz (2000b) |
| pYes | 2 μm URA3 | Invitrogen | |
| pYes263 | 2 μm URA3 | Melcher (2000) | |
| pGRHis | 2 μm TRP1 | HIS5Sp | This study |
| pRS424GALGFPCDC42 | pRS424 | GFPCDC42 | This study |
| pBSCDC42 | pBS | CDC42 | This study |
| pRS416CDC42 | pRS416 | CDC42 | This study |
| pRS414CDC42 | pRS414 | CDC42 | This study |
| pRS413GFPCDC42 | pRS413 | GFPCDC42 | This study |
| pYesHACDC42 | pYes | HACDC42 | This study |
| pT7CDC42 | pT7Tag | CDC42 | This study |
| pMalCDC42 | pMal-c2 | CDC42 | This study |
| pRS414CDC24 | pRS414 | CDC24 | Nern and Arkowitz (1998) |
| pRS313CDC24 | pRS313 | CDC24 | This study |
| pRS416GalHis6CDC24 | pRS416 | His6CDC24 | Nern and Arkowitz (1998) |
| pEG(KT)CDC24 | pEG(KT) | CDC24 | Nern and Arkowitz (1998) |
| p2μA-TPI-HACDC24 | p2μA-TPI | HACDC24 | This study |
| pT7CDC24 | pT7Tag | CDC24 | This study |
| pRS406GFPBud1 | pRS406 | GFPBud1 | Nern and Arkowitz (2000a) |
| pYes263RGA1 | pYes263 | RGA1 | This study |
| pGRHis-STE20HA | pGRHis | STE20HA | This study |
| Yep352FUS1/FUS2 | Yep352 | FUS1/FUS2 | Santos and Snyder (2003) |
| Yep352FUS3 | Yep352 | FUS3 | Santos and Snyder (2003) |
| pRS316FUS1GFP | pRS316 | FUS1GFP | Santos and Snyder (2003) |
| pRS413FUS1GFP | pRS413 | FUS1GFP | This study |
| pRS316-CDC3-GFP | pRS316 | CDC3-GFP | Caviston et al. (2003) |
| pGEX 6P-CRIB | pGEX 6P-2 | CRIBSTE20 | This study |
For suppression experiments p2μA-TPI-HACDC24, pYes263RGA1, pGRHis-STE20HA, pYesHACDC42, Yep352FUS1/FUS2 (Santos and Snyder, 2003), and Yep352FUS3 (Santos and Snyder, 2003) were used. CDC24 was cloned into p2μA-TPI (Nern and Arkowitz, 2000b), and subsequently an oligonucleotide encoding a hemagglutinin (HA) epitope was cloned 5′ of the ATG yielding p2μA-TPI-HACDC24. RGA1 was PCR amplified from genomic DNA using a 5′ primer with a NcoI site preceding the ATG and a 3′ primer with a NotI site after the stop codon, and a NcoI/NotI fragment was cloned into pYes263 (Melcher, 2000). A 3xHA epitope-tagged Ste20p was constructed by PCR-based gene replacement in SEY6210 using p3xHA-His5 (Rayner and Munro, 1998) as template and oligonucleotides with 60 nucleotides 5′ and 3′ of the termination codon. STE20HA was gap repaired from this strain using a pRS424 plasmid containing the STE20 promoter and HIS5 gene, resulting in pGRHis-STE20HA. pBSCDC42 was constructed by PCR amplification of CDC42 from genomic DNA using a 5′ primer with a HindIII site preceding the ATG and a 3′ primer 147 nucleotides 3′ of the stop codon. The HindIII/blunted CDC42 PCR product was cloned into a HindIII/SmaI-digested pBluescript KS− II (Stratagene, Amsterdam, Holland). To generate pYesHACDC42, the CDC42 ORF was amplified by PCR using a 5′ primer with an in-frame HA epitope preceded by a HindIII site and a 3′ primer with a BamHI site after the stop codon using pBSCDC42 as a template and cloned into pYes2 (Invitrogen, Cergy Pontoise, France).
For localization of Cdc42p, pRS413GFPCDC42, which contained a 521-nucleotide CDC42 promoter followed by GFP fused to CDC42, was used. GFP was PCR amplified using a 5′ primer with a HindIII site preceding the ATG and a 3′ primer with an EcoRI site 5′ of the stop codon. This HindIII/EcoRI-digested PCR product was cloned into appropriately digested pBluescript, yielding pBSGFP. Subsequently CDC42 was PCR amplified using a 5′ primer with an EcoRI site immediately 3′ of the ATG and a 3′ primer with a BamHI site after the stop codon using pBSCDC42 as a template and cloned into EcoRI/BamHI-digested pBSGFP, yielding pBSGFPCDC42. The promoter region of CDC42 (521 nucleotides 3′ of ATG) was PCR amplified from genomic DNA using 5′ primer with a XbaI site and a 3′ primer with a HindIII site that replaced 6 nucleotides 5′ of the ATG. This XbaI/HindIII-digested CDC42 promoter fragment together with a HindIII/SacI GFPCDC42 fragment from pBSGFPCDC42 were cloned into pRS413 digested with XbaI/SacI, yielding p413GFPCDC42. pRS424GALGFPCDC42 was constructed by cloning a BamHI/KpnI GFPCDC42 fragment from pBSGFPCDC42 into appropriately digested pRS424GAL. pRS424GAL was constructed by cloning a NgoMIV/SacII GAL1 fragment from pRS416GAL into appropriately digested pRS424. pRS416GAL was constructed by cloning a NgoMIV/XbaI GAL1 fragment from pYes into appropriately digested pRS416. For the localization of Fus1GFP, a 5.2-kb NgoMIV/ScaI FUS1GFP fragment from pRS316FUS1GFP (Santos and Snyder, 2003) was cloned into an appropriately digested pRS413 vector. For septin localization pRS316-CDC3-GFP (Caviston et al., 2003) was used.
For in vitro–coupled transcription-translation, the entire CDC24 ORF (a 2.6-kb BamHI/SalI fragment) from pEG(KT)CDC24 was cloned into a pT7Tag plasmid (Pollock and Treisman, 1990). Furthermore, the entire CDC42 ORF was amplified by PCR using a 5′ primer preceded by a BamHI site and a 3′ primer with an XbaI site after the stop codon using pBSCDC42 as a template and cloned into pT7Tag plasmid (Pollock and Treisman, 1990). For MBPCdc42p expression, the entire CDC42 ORF was PCR amplified using a 5′ primer with an EcoRI site immediately 3′ of the ATG and a 3′ primer with a BamHI site after the stop codon with pBSCDC42 as a template and cloned into EcoRI/BamHI-digested pMal-c2 (New England Biolabs, Saint Quentin en Yvelines, France). For GST-CRIB pulldown experiments, the CRIB domain of Ste20p was amplified by PCR with primers containing unique BglII and NotI sites. This PCR product, which encodes aa 304-346 of Ste20p, was then cloned into a pGEX 6P-2 plasmid (Amersham, Orsay, France). All constructs were verified by dye terminator sequencing (ABI prism).
Isolation of Mating Mutants
For the initial cdc42 mutant screen, the entire ORF was amplified with Taq polymerase using mutagenic PCR conditions with pBSCDC42 as a template and cloned into HindIII/XbaI-digested pRS416CDC42 as previously described (Nern and Arkowitz, 1998; Barale et al., 2004). This library was transformed into RAY513, and mating mutants were isolated (Nern and Arkowitz, 1998; Barale et al., 2004). The second screen was carried out in which the Switch I region (amino acids 31-41) was mutagenized using oligonucleotide mixtures. An oligonucleotide encompassing this region (GACTATGTTCCAACAGTGTTCGATAACTATGCG) with 15 base pairs on 5′ and 3′ ends was synthesized in which at each base there was a mixture of 91% indicated base and 3% of the three remaining bases. This frequency was chosen to introduce between two and three nucleotide changes per mutant copy. Ten bacterial clones of this library were sequenced, confirming that mutations were randomly located. In the third screen the valine 36 residue in Cdc42p was changed to 15 different amino acids (V36M, V36A, V36L, V36I, V36P, V36S, V36T, V36Y, V36W, V36Q, V36C, V36K, V36R, V36H, V36E) with the Pfu polymerase (Promega) using the DpnI method (Weiner et al., 1994). These mutants were transformed into RAY513, and viability was assessed by growth on glucose-containing media. Spot matings were also carried out with log phase–growing cells on a lawn of enfeebled tester RAY1142 on a YEPD plate incubated 3 h at 30°C before being replica plated.
Sequence Alignment and Structure Modeling
Sequence alignments were carried out using the BLAST algorithm (Altschul et al., 1990). Structural model threading was generated by homology modeling of the S. cerevisiae Cdc42p primary sequence using Swiss-Model service based on the transition state human Cdc42 complex for GTP hydrolysis structure pdb file 2NGR (Nassar et al., 1998).
Mating Assays, Pheromone Response Assays, and Phenotypic Analyses
Patch and quantitative mating were carried out as described (Nern and Arkowitz, 1998) with the indicated tester strain. Bud scars and actin cytoskeleton were visualized with formaldehyde-fixed cells as previously described (Nern and Arkowitz, 1998) except that Alexa-488 phalloidin (Molecular Probes, Cergy Pontoise, France) was used for the latter. Pheromone-induced cell cycle arrest (halo assays), induction of a Fus1LacZ reporter, and cell shape changes were assayed as described (Nern and Arkowitz, 1998, 1999). For quantitative cell fusion assays, matings were performed as described (Barale et al., 2004). Mating pairs included both prezygotes and zygotes, where prezygotes were identified as a mating pair in which only one cell was fluorescent and a septum was visible between the cells. The percentage of prezygotes is the number of prezygotes divided by the total number of mating pairs.
Microscopy
Actin cytoskeleton was imaged using a Deltavision deconvolution microscopy system (Applied Precision, Issaquah, WA) on an Olympus IX-70 microscope (Rungis, France) with an NA 1.4 60× objective. Images were deconvolved using softWoRX, and maximum intensity projections of Z-stacks were carried out. Cells were examined using a Leica DMR epifluorescence microscope (Rueil-Malmaison, France) with an NA 1.35 100× objective. Images were recorded with a Princeton Instruments Micromax CCD (Roper Scientific, Evry, France), using IPLab (Scanalytics, Rockville, MD) software. Scale bars represent 5 μm.
Immunoblot Analyses
Total yeast protein extracts were prepared (Nern and Arkowitz, 1999) from budding and shmooing cells using 107 cells and from mating mixtures using 106 cells. Extracts were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose membrane (Amersham), and probed with a polyclonal serum against Cdc42p (1:1000), a mouse monoclonal antibody (mAb) BP1FG (a kind gift from T. Lithgow, 1:10,000) against glyceraldehyde-3-phosphate dehydrogenase, a polyclonal serum against GFP (1:5000; Nern, 2000), or an mAb against HA (HA.11 Babco, Seraing, Belgium, 1:1000). Polyclonal serum against Cdc42p was either from Santa Cruz Biotechnology (Heidelberg, Germany, sc-7172) or from rabbits immunized with MBPCdc42. Immunoblots were visualized by enhanced chemiluminescence (luminol-coumaric acid) on a Fuji-Las3000 (Clichy, France). Equal amounts of cells were used in each experiment, and equal amounts of protein in each lane were confirmed by Ponceau S staining of nitrocellulose membranes.
In Vitro Binding Studies
In vitro–coupled transcription-translation was carried out using a Quick TNT rabbit reticulocyte lysate system (Promega) and 80 μCi [35S]methionine (ICN, Illkirch, France, 1175 Ci/mmol) in a reaction volume of 100 μl using either pT7CDC24, pT7CDC42, pT7cdc42[V36M], or pT7cdc42[T35A], following the manufacturer's instructions.
MBPCdc42 fusion proteins or GSTCRIB fusion protein were expressed in BL21 Escherichia coli cells grown at 37°C. After 4-h induction with 0.1 mM IPTG, cells were resuspended in 10% sucrose, 50 mM TrisCl, pH 8, and frozen in liquid nitrogen. All subsequent steps were carried out at 4°C. Cells were lysed by sonication in buffer A (phosphate-buffered saline containing 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 0.1% Triton X-100). Extracts were clarified by centrifugation (10,000 × g for 10 min), and fusion proteins were isolated using amylose resin (New England Biolabs) or glutathione Sepharose 4B (Amersham). Protein concentrations were estimated by comparing intensities of bands on Coomassie-stained SDS-PAGE gels.
For Cdc24p Cdc42p bindings amylose resin bound MBPCdc42 was incubated with buffer B (20 mM TrisCl, pH 7.5, 50 mM NaCl, 1 mM DTT, 5% glycerol, 0.1% Triton X-100, and Boehringer Mannheim protease inhibitor, Meylan, France) containing 10 mM EDTA, for 1 h at room temperature in order to strip nucleotides (nucleotide free). To GTPγS load nucleotide free fusion proteins, amylose resin–bound MBPCdc42 was incubated with buffer B containing 10 mM MgCl2 and 120 μM GTPγS (Boehringer Mannheim) for 30 min at room temperature (Hart et al., 1994). For binding assays, MBPCdc42 fusion proteins (∼500 ng) bound to amylose resin were incubated with [35S]Cdc24p in 100 μl of buffer B containing either 10 mM EDTA or 10 mM MgCl2 and 120 μM GTPγS for 2 h at 30°C. Amylose resin samples were then washed five times with 100 μl of the respective buffer. Proteins were eluted with SDS-PAGE sample buffer and analyzed by SDS-PAGE followed by Coomassie blue staining and autoradiography. A Fuji BAS1000 phosphorimager was used for quantification based on two input amounts.
For GSTCRIB pulldown experiments, EDTA (30 μM) was first added to 100 μl of the in vitro–translated [35S]Cdc42p; subsequently GTPγS (0.3 mM) was added, the reaction was incubated at room temperature for 15 min, and finally MgCl2 (200 μM) was added. Subsequently GSTCRIB bound to GSH agarose was added, and reactions were incubated for 1 h at 4°C. Glutathione Sepharose resin samples were then washed five times with 200 μl of GPL buffer (20 mM TrisCl, pH 7.4, 150 mM NaCl, 5 mM MgCl2, 5 mM glycerol phosphate, 1 mM DTT, Boehringer Mannheim protease inhibitor, 0.5% NP-40), and then proteins were eluted in SDS-PAGE sample buffer and analyzed by SDS-PAGE, followed by autoradiography and phosphorimager quantification.
RESULTS
Identification of cdc42 Mating Mutants
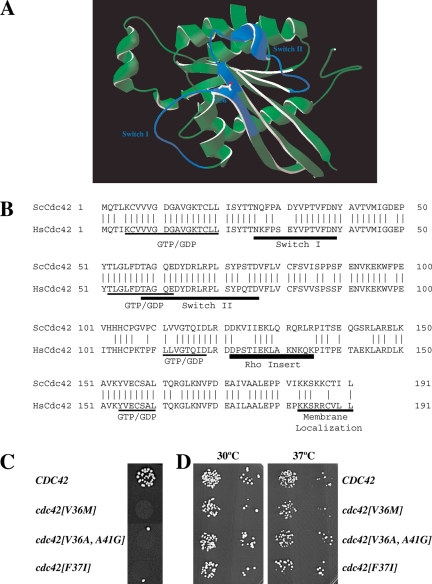
To elucidate the functions of the Rho G-protein Cdc42p in the yeast mating process, we screened a randomly mutagenized library for cdc42 mutants specifically defective in mating, as previously described (Nern and Arkowitz, 1998; Barale et al., 2004). Over 25,000 yeast colonies were tested for mating-specific defects and two mutants were isolated. Sequencing revealed that both mutants had the amino acid alteration V36M. One of these mutants had an additional P69T mutation. Recreation of each individual amino acid change indicated that V36M was responsible for the mating defect. Valine 36 is located in the Switch I region (Figure 1, A and B). Switch I and II regions undergo nucleotide-dependent conformational changes when the two hydrogen bonds between an amino acid from each Switch domain and the gamma phosphate of GTP are released after GTP hydrolysis (Vetter and Wittinghofer, 2001). Structural studies have revealed that these Switch regions show increased flexibility compared with the rest of the G-protein (Farrar et al., 1997; Ito et al., 1997). Hence we mutagenized the region encoding amino acids 31-41 using randomized oligonucleotide mixtures. These oligonucleotide mixtures were designed to introduce on average two to three nucleotide changes in each CDC42 copy. After screening 3000 yeast transformants, 14 mutants defective in mating were isolated. Sequencing revealed 9 mutants, which had only the V36M amino acid change. Two mutants had the V36M amino acid change in addition to a second change (either D38A or A41R), and two mutants had both the V36A and A41G amino acid alterations. Finally, one mating mutant had a F37I mutation. The cdc42[V36M], cdc42[V36A, A41G], and cdc42[F37I] mutants were all defective for mating when crossed with an enfeebled mating partner (Figure 1C) and did not exhibit any growth defects at 30 and 37°C (Figure 1D).
Figure 1.
Characterization of cdc42 mating mutants. (A) Three-dimensional structure model of S. cerevisiae Cdc42p. Model generated by homology modeling of S. cerevisiae primary sequence using Swiss-Model service based on the transition state Cdc42 complex for GTP hydrolysis structure pdb file 2NGR (Nassar et al., 1998). The amino acid residue Val 36 identified in this study is indicated, and Switch I and Switch II domains are colored in blue. (B) Sequence alignment of S. cerevisiae Cdc42p (ScCdc42) and human Cdc42 (HsCdc42). Vertical lines indicate identical residues. Guanine nucleotide-binding domain (GDP/GTP), Switch I and II regions, Rho insert, and membrane localization domain are indicated. (C) Spot mating of cdc42 mutants isolated from screen of aa 31-41. Matings using strains derived from RAY513 with indicated CDC42 or cdc42 gene (as sole copy behind its endogenous promoter on a CEN plasmid) are shown. Matings were carried out with the enfeebled tester RAY1142. (D) Cdc42 mutants grow normally. Serial dilutions of indicated mutants (same strains as above) were spotted onto YEPD plates and incubated for 2 d at the indicated temperatures.
As the results of these two screens demonstrated that the amino acid residue 36 of Cdc42p is critical for yeast mating, we changed this valine individually to 15 different amino acids in a strain where the mutant cdc42 was the sole copy (Table 3). Of these 15 different mutants, only the cdc42[V36E] was inviable. The other mutants could be grouped into three classes based on budding cell morphology and budding pattern: class I cells (L, I, S, Q, and C) exhibited normal morphology, class II cells (A, F, K, and R) were heterogeneous in morphology and in general more elongated, and class III cells (Y and W) were markedly larger than wild-type cells. In addition, three mutants had an intermediate phenotype with cdc42[V36M] mutant cells somewhat elongated and cdc42[V36T] and cdc42[V36H] cells both elongated and larger compared with wild-type cells. Mutants in class I budded predominantly with an axial pattern (70–90% axial), class II mutants had a reduced percentage of cells with axial budding (60–70%), and class III mutants budded predominantly with a random pattern (20–50% axial). We examined the ability of the mutants with a normal morphology to mate with an enfeebled tester. Of these mutants (V36I, V36L, V36M, V36S, V36Q, and V36C) only cdc42[V36M] was substantially defective in mating. These results indicate that residue 36 in Cdc42p is necessary for both vegetative growth and mating and that the nature of the amino acid residue at this position is critical for these functions. We focused on the cdc42[V36M] mutant as it had the strongest mating defect and its vegetative growth was similar to wild-type cells.
Table 3.
Morphology, budding pattern, and mating efficiency of cdc42 mutants
| Amino acid 36 | Viability | Morphologya | Axial bud site selectionb | Mating efficiencyc |
|---|---|---|---|---|
| Val | + | I | 89 | ++ |
| Ala | + | II | 65 | + |
| Leu | + | I | nd | ++ |
| Ile | + | I | nd | ++ |
| Met | + | I/II | 75 | − |
| Phe | + | II | nd | nd |
| Ser | + | I | 70 | ++ |
| Thr | + | II/III | nd | + |
| Tyr | + | III | nd | ++ |
| Trp | + | III | 49 | − |
| Gln | + | I | nd | + |
| Cys | + | I | 89 | ++ |
| Lys | + | II | 58 | + |
| Arg | + | II | 70 | nd |
| His | + | II/III | 22 | + |
| Glu | − | nd | nd | nd |
a Wild-type morphology (I), elongated cells (II), and large cells (III).
b Percentage axial budding pattern (n = 100).
c Plate mating efficiency with an enfeebled tester; ++, wild-type efficiency; +, slightly defective; and −, strongly defective.
Cdc42[V36M] Cells Respond to Mating Pheromone by Cell Cycle Arrest, Gene Induction, and Cell Polarization
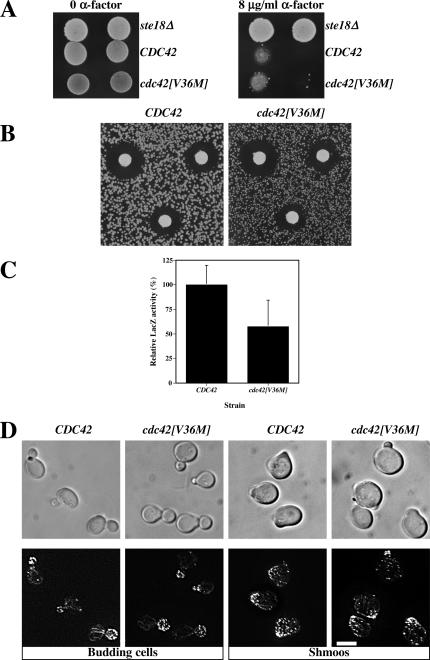
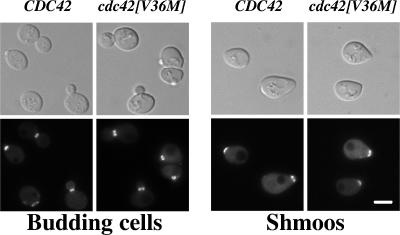
Several previously isolated cdc42 mutants were resistant to mating pheromone arrest, including cdc42[V36A] (Moskow et al., 2000); hence we examined whether cdc42[V36M] cells were able to continue to divide in the presence of α-factor. MATa CDC42 wild-type and cdc42[V36M] mutant cultures were spotted on YEPD plates containing or lacking 8 μg/ml α-factor. Both wild-type and mutant cells were unable to grow in the presence of mating pheromone compared with a control ste18Δ mutant that does not respond to pheromone (Whiteway et al., 1989; Figure 2A). Similarly, mating pheromone halo assays were carried out to analyze the pheromone concentration dependence of growth arrest. Figure 2B shows that the growth arrest halos of CDC42 and cdc42[V36M] cells were identical, indicating that this cdc42 mutant is fundamentally different from the cdc42[V36A] mutant previously described. Pheromone-dependent gene induction was examined using a FUS1-lacZ reporter and Figure 2C shows that, at saturating α-factor concentration, the induction level of FUS1 in cdc42[V36M] cells was reduced only twofold compared with CDC42 cells. The same result was obtained during mating. We next examined the morphology of cdc42[V36M] budding cells and shmoos and their actin distribution (Figure 2D). cdc42[V36M] budding cells had a similar morphology compared with a wild-type control; however, the mother cell appeared somewhat rounder in cdc42[V36M] mutants (Figures 2D and 5), suggesting that these cells are slightly less polarized than wild-type cells. In response to α-factor, cdc42[V36M] cells formed pear-shaped shmoos that were less polarized, with a larger mating projection than wild-type shmoos. Quantitation of the number of cells forming shmoos in the presence of α-factor revealed an identical percentage of shmoos in wild-type and cdc42[V36M] strains. Nevertheless, the actin cytoskeleton in wild-type and cdc42[V36M] shmoos was similarly polarized. We investigated whether cdc42[V36M] cells were able to orient growth toward a pheromone gradient and observed that mating projections of cdc42[V36M] cells were not restricted to a location adjacent to the previous bud scar as in chemotropism mutants. Together, these results demonstrate that cdc42[V36M] cells respond to mating pheromone.
Figure 2.
cdc42[V36M] cells respond to mating pheromone. (A) cdc42[V36M] cells are not resistant to mating pheromone. CDC42 (RAY1772), cdc42[V36M] (RAY1926), and ste18Δ strains were spotted on YEPD plates containing or lacking 8 μg/ml α-factor and incubated for 5 d. (B) cdc42[V36M] cells arrest growth in the presence of mating pheromone similar to wild-type cells. α-factor (1, 0.5, and 0.2 μg) was spotted on filters placed on a lawn of the indicated strain. Plates were incubated for 3 d. Measurements of the halo diameter indicated ≤5% difference between CDC42 and cdc42[V36M] halos. (C) cdc42[V36M] cells induce the mating-specific FUS1 gene in a pheromone-dependent manner. Cells containing the FUS1-lacZ plasmid pSG231 were incubated with 10 μM α-factor for 1 h, and LacZ activity was determined. The means of four determinations from two independent experiments are shown. Error bars, SD. LacZ activity for CDC42 cells (21.0 Miller units) was set at 100%. (D) cdc42[V36M] cells polarize their actin cytoskeleton. DIC and fluorescence images of budding cells and cells treated with 12 μM α-factor for 2 h are shown. Actin cytoskeleton is visualized with Alexa-488 phalloidin. Fluorescence images are maximum intensity projections of Z-sections (8–12 × 0.1 μm).
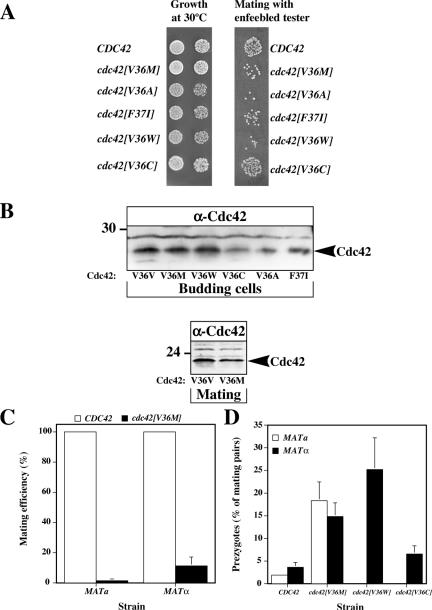
Cdc42 Mutants Are Defective in Cell Fusion
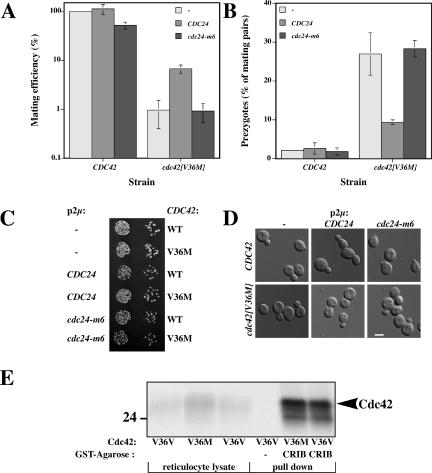
We next compared the mating efficiency and growth of several cdc42 mutants: cdc42[V36M], cdc42[V36A], and cdc42[F37I] strains isolated in our screen with cdc42[V36W] strain (class III morphology) and cdc42[V36C] cells (class I morphology). All strains grew at 30°C with cdc42[V36A], cdc42[F37I], and cdc42[V36W] cells forming slightly smaller colonies than wild-type cells. These three mutants together with cdc42[V36M] were all defective in mating with an enfeebled tester and formed ∼10-20-fold fewer diploid colonies than wild-type and cdc42[V36C] cells (Figure 3A). The expression levels of Cdc42p, Cdc42[V36M]p, and Cdc42[V36W]p were similar in budding cells, whereas the expression levels of Cdc42[V36C]p, Cdc42[V36A]p, and Cdc42[F37I]p were reduced (Figure 3B). Furthermore, the expression level of Cdc42[V36M]p in mating cells was similar to that of Cdc42p, indicating that the observed mating defect is not due to an altered expression level. Quantitative matings with an enfeebled mating partner (Figure 3C) demonstrated that the cdc42[V36M] mating defect is observed in both mating types, with a 60-fold reduction in mating efficiency in MATa cells and a 10-fold reduction in MATα cells. In addition, the cdc42[V36M] mutation behaves recessively because its mating defect was complemented by a wild-type copy. We tested whether these mutants were defective in cell fusion, as was previously observed for cdc24-m6 mating mutant (Barale et al., 2004). The percentage of mating pairs that were prezygotes was quantified after 4-h mating with a wild-type tester whose plasma membrane was labeled with GFP-Bud1. Prezygotes were identified as mating pairs in which GFP fluorescence was observed only in one cell. Figure 3D shows that both mating types of cdc42[V36M] cells accumulate prezygotes. In addition, the cdc42[V36W], cdc42[V36A], and cdc42[F37I] mutants also accumulate prezygotes during mating but not cdc42[V36C] (Figure 3D and unpublished data), indicating that this fusion defect is allele specific. Collectively, these results show that Cdc42p is required for cell fusion during mating, similar to Cdc24p.
Figure 3.
cdc42[V36M] cells have a reduced mating efficiency and accumulate prezygotes. (A) Spot matings and growth of cdc42 mutants. Serial dilutions of mutants derived from RAY513 with indicated CDC42 or cdc42 gene (as sole copy) were spotted onto YEPD plates and incubated for 2 d. Spot matings with indicated strains are shown. Matings were carried out with the enfeebled tester JY426. (B) The expression level of the Cdc42[V36M]p is similar to that of wild type in budding and mating cells. Strains were derived from RAY1772 (MATa) and RAY513 (MATα) with indicated CDC42 or cdc42 gene (as sole copy behind its endogenous promoter on a CEN plasmid). Cell extracts of budding and mating cells were analyzed by SDS-PAGE, followed by immunoblotting and probing with anti-Cdc42p polyclonal sera. Similar results were observed in three independent experiments. (C) The cdc42[V36M] mating defect is independent of the mating type. Strains in which CDC42 or cdc42[V36M] is the sole copy behind its endogenous promoter on a CEN plasmid (derived from MATa strain RAY1772 or MATα strain RAY513) were used for quantitative matings with a wild-type tester. Values shown are the means of five or six independent experiments with SD indicated and mating efficiencies for MATa and MATα CDC42 strains (1.4 and 3.0%, respectively) set to 100%. (D) cdc42[V36M] mating mixtures accumulate prezygotes. Strains with indicated CDC42 or cdc42 as sole copy behind its endogenous promoter on a CEN plasmid derived from RAY1772 (MATa) or RAY513 (MATα) were mated with a GFP-Bud1 expressing strain (RAY1907 or RAY1487). The percentage of prezygotes formed after 4 h was determined as the number of prezygotes (fluorescence in only one cell) divided by the number of mating pairs (prezygotes and zygotes), with values representing the means of 5 to 10 determinations (n = 200–300 mating pairs) from four to eight independent matings. Error bars, SD.
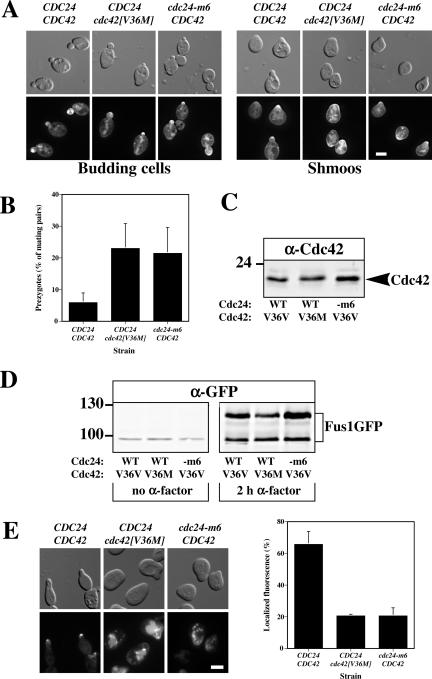
Cdc42p Is Required for Localization of Fus1p to the Site of Cell Fusion
We compared the phenotype of cdc42[V36M] cells to that of cdc24-m6 cells. To investigate whether the fusion defect in these two mutants was the result of an inappropriate Cdc42p localization, we determined the localization of a functional GFP-Cdc42 fusion whose expression level was twice that of endogenous Cdc42p. GFP-Cdc42 was expressed in MATa cdc24Δ cdc42Δ cells that carried a plasmid copy of CDC24 or cdc24-m6. GFP-Cdc42[V36M] was expressed in MATa cdc24Δ cdc42Δ cells that carried a plasmid copy of CDC24, and this strain exhibited a fusion defect similar to other cdc42[V36M] strains. These GFP-Cdc42 and GFP-Cdc42[V36M] fusions localized similarly to the sites of polarized growth (bud and shmoo tips) in wild-type and cdc24-m6 cells (Figure 4A). Shmoos expressing GFP-Cdc42[V36M] were less polarized than wild-type cells as observed in Figure 2D; however, measurement of the plasma membrane perimeter to which GFP-Cdc42 was enriched revealed that the size of this region was indistinguishable between wild-type and mutant strains (unpublished data). In this isogenic strain cdc24-m6 and cdc42[V36M] mutants accumulate prezygotes to the same extents, consistent with the notion that they have the same molecular defect (Figure 4B). Furthermore, expression levels of Cdc42p and Cdc42[V36M]p were similar in wild-type and mutant cells (Figure 4C). Previously, we demonstrated that Cdc24p was required for localizing the cell fusion protein Fus1p to the site of cell contact and the shmoo tip. In response to α-factor, Fus1-GFP was highly induced in both cdc24 and cdc42 mutants despite the approximately twofold lower level of Fus1p in cdc42[V36M] cells (Figures 2C and 4D). Figure 4E shows that cdc42[V36M] and cdc24-m6 cells are similarly defective in localizing Fus1GFP to the shmoo tip. Overexpression of Fus1p and Fus2p did not suppress the cdc42[V36M] fusion defect, indicating that decreased levels of Fus1p are not responsible for this defect. As other cdc42 mutants in which valine 36 was changed to a threonine, alanine, or glycine had defects in septin organization (Gladfelter et al., 2001, 2002; Caviston et al., 2003), we examined the septin cytoskeleton in wild-type and cdc42[V36M] cells growing vegetatively and responding to mating pheromone. Figure 5shows that the septin cytoskeleton visualized using Cdc3-GFP was identical in wild-type and mutant budding and shmooing cells. The defect in Fus1p localization is therefore not the result of an altered actin or septin cytoskeleton. Thus, two mutants, one in the G-protein Cdc42p and another in its exchange factor Cdc24p, are specifically defective in cell fusion, behave similarly and are both defective in restricting the localization of the cell fusion protein Fus1p to the site of contact.
Figure 4.
Cdc42p and Cdc24p are required for correct localization of the cell fusion protein Fus1. (A) GFP-Cdc42[V36M] localizes to the bud and shmoo tips. Indicated strains (RAY1830, RAY1951, and RAY1833), which are MATa cdc24Δ cdc42Δ cells carrying a plasmid copy of CDC24 and GFP-Cdc42 or CDC24 and GFP-Cdc42[V36M] or cdc24-m6 and GFP-Cdc42, each behind their respective endogenous promoter on a CEN plasmid, were either grown in the absence (left panels) or presence (right panels) of 12 μM α-factor for 2 h, and fluorescence and DIC images were taken. (B) cdc24-m6 and cdc42[V36M] mating mixtures accumulate similar amounts of prezygotes. Indicated strains (RAY1793, RAY1801, and RAY1952) were mated with RAY1487 (GFP-Bud1) and analyzed as in 3D. Values are the means of four independent matings (n = 200 mating pairs). Error bars, SD. (C) Expression levels of Cdc42p in CDC42/CDC24, cdc42[V36M]/CDC24, and CDC42/cdc24-m6 cells. Extracts of logarithmically growing cells (RAY1793, RAY1801, and RAY1952) were analyzed by immunoblot and probed with anti-Cdc42p polyclonal sera. Similar results were observed in three independent experiments. (D) cdc42[V36M] cells induce Fus1-GFP in the presence of mating pheromone. Extracts of cells from the same experiment as above were analyzed by immunoblot either in the absence or presence of α-factor. The lower band is an unglycosylated form of Fus1-GFP. Similar results were observed in three independent experiments. (E) Fus1-GFP is not correctly localized in cdc24-m6 and cdc42[V36M] shmoos. Indicated strains (RAY1793, RAY1801, and RAY1952) carrying a Fus1-GFP plasmid were incubated with 12 μM α-factor for 2 h, and fluorescence images were taken. The mean percentage of shmoos with an observable fluorescence signal at the tip of the mating projection was determined from two independent experiments (n = 200). Bars indicate values.
Figure 5.
The septin cytoskeleton is unaffected in cdc42[V36M] budding and shmooing cells. Indicated strains (RAY1918 and RAY1920) carrying a plasmid copy of Cdc3-GFP behind its endogenous promoter on a CEN plasmid were either grown in the absence (left) or presence (right) of 12 μM α-factor for 2 h, and fluorescence and DIC images were taken.
Cycling of Cdc42p between GDP and GTP States Is Critical for Cell Fusion
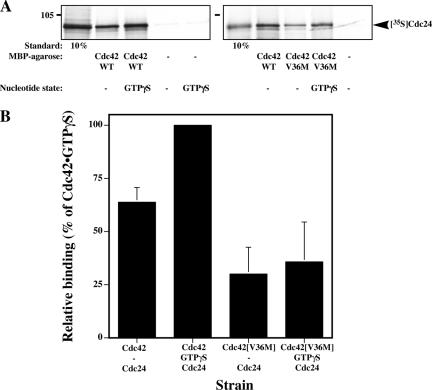
One explanation for both the Cdc42p and Cdc24p requirement in cell fusion is that this G-protein needs to be specifically activated by Cdc24p during this process. We first verified that Cdc42[V36M]p was still able to bind Cdc24p in vitro using reticulocyte lysate–translated [35S]Cdc24p and MBPCdc42 or MBPCdc42[V36M] immobilized on amylose resin. Cdc24p binds to both nucleotide free and GTPγS loaded MBPCdc42 and little to no binding was observed with MBP alone (Figure 6, A and B), indicating the binding is specific (Figure 6, A and B). In vitro, Cdc24p binds to a higher extent to Cdc42p•GTPγS compared with the nucleotide free form Cdc42p, similar to results previously observed in vivo (Bose et al., 2001). MBPCdc42[V36M] is able to bind Cdc24p, although to a lower extent than wild-type Cdc42p. This binding was independent of the nucleotide state of Cdc42[V36M]p. If Cdc42[V36M]p was activated to a lower extent than wild-type Cdc42p during mating, we would expect that overexpression of the exchange factor Cdc24p should suppress the fusion defect. Similarly, if Cdc24-m6p was defective in Cdc42p activation, then overexpression of this mutated exchange factor should not affect the fusion defect of cdc42[V36M] cells. To address these possibilities, we overexpressed either Cdc24p or Cdc24-m6p in both CDC42 and cdc42[V36M] cells and examined mating and fusion efficiencies. Overexpression of Cdc24p but not Cdc24-m6p partially suppressed both the mating and fusion defect of cdc42[V36M] cells (Figure 7, A and B). Figure 7, C and D, shows that overexpression of Cdc24p and Cdc24-m6p did not affect the growth nor the morphology of wild-type and cdc42[V36M] cells, indicating that these levels of exchange factor were not deleterious to the cells. Conversely, overexpression of the Cdc42p GTPase activating protein (GAP), Rga1p, resulted in an increase in the number of prezygotes that accumulate in cdc42[V36M] mating mixtures (27% prezygotes accumulated in cdc42[V36M] cells carrying an empty vector compared with 40% prezygotes accumulated in cdc42[V36M] cells overexpressing Rga1p). These opposing effects of GEF and GAP overexpression are not due to an indirect effect via the MAP kinase cascade as overexpression of the PAK kinase Ste20p and the MAP kinase Fus3p did not alter the percentage of prezygotes that accumulated in cdc42[V36M] mating mixtures. Furthermore, Cdc42[V36M]p can still bind the CRIB domain of Ste20p as illustrated by a GSTCRIB pulldown (Figure 7E). Our results rather suggest that the activation or cycling of Cdc42p is critical for efficient cell fusion.
Figure 6.
Cdc42[V36M]p can bind Cdc24p in vitro. (A) In vitro–translated [35S]Cdc24p was incubated with resin-immobilized MBP, MBPCdc42, or MBPCdc42[V36M]. Where indicated, MBP fusions were loaded with GTPγS before binding. Binding reactions were analyzed by SDS-PAGE followed by autoradiography. Standard indicates the percentage of [35S]Cdc24p used in the binding reaction. The two different panels are from two independent experiments. (B) Quantitation of Cdc42p–Cdc24p in vitro bindings. A phosphoimager was used for quantitation. Values are the mean of four independent binding experiments. Error bars, SD. Binding of [35S]Cdc24p to Cdc42p•GTPγS (6.8% of input [35S]Cdc24p) was set to 100%.
Figure 7.
Overexpression of CDC24 but not cdc24-m6 partially suppresses fusion defect in cdc42[V36M] matings. (A) Overexpression of CDC24 suppresses mating defect of cdc42[V36M] cells. Strains in which CDC42 or cdc42[V36M] is the sole copy behind its endogenous promoter on a CEN plasmid (derived from RAY1918 or RAY1920) and indicated CDC24 is overexpressed were analyzed for mating efficiency and prezygote formation as described in Figure 3, C and D. Values represent the means of two independent matings, with bars indicating actual values. Matings were carried out with the enfeebled tester JY429. (B) Overexpression of CDC24 suppresses the fusion defect of cdc42[V36M] cells during mating. Values represent the means of two independent matings (n = 200–300 mating pairs), with bars indicating actual values. (C) Overexpression of CDC24 and cdc24-m6 does not affect growth of cdc42[V36M] cells. Cells were spotted on −Ade plates as described in Figure 3A. (D) Overexpression of CDC24 or cdc24-m6 does not affect the morphology of cdc42[V36M] cells. DIC images of indicated cells used for above matings grown in −Ade-containing media. (E) Cdc42[V36M]p•GTPγS binds the Ste20p CRIB domain similar to wild-type Cdc42p. [35S]Cdc42p loaded with GTPγS was incubated with GSTCRIB immobilized on amylose resin and bound radioactivity was analyzed by SDS-PAGE followed by autoradiography. Input [35S]Cdc42p reticulocyte lysate (5%) is shown. Quantitation by phosphorimager revealed no difference between Cdc42p and Cdc42[V36M]p GSTCRIB binding, whereas binding of Cdc42[T35A]p was dramatically reduced (unpublished data).
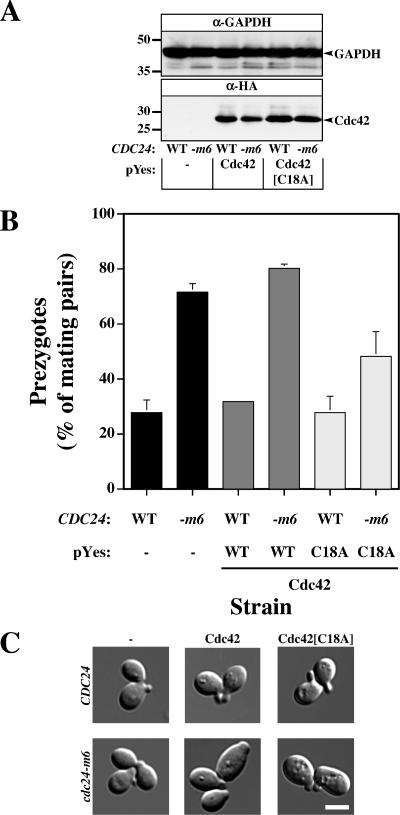
Therefore, we examined if increased levels of wild-type Cdc42p, a GTP-locked Cdc42p mutant (Q61L), or Cdc42p fast cycling mutant such as Cdc42[C18A]p, which has been shown to mimic constitutive activation by the exchange factor (Rossman et al., 2002), could suppress the cdc24-m6 fusion defect. When Cdc42[Q61L]p was overexpressed, cells grew more slowly and had an abnormal morphology, consistent with previous studies (Ziman et al., 1991). Overexpression of Cdc42[Q61L]p did not markedly affect cdc24-m6 mating efficiencies (mating efficiency of 8.3 ± 3.2 and 12.8 ± 6.3%, for Cdc42p and Cdc42[Q61L]p overexpression, respectively), nor did it affect the percentage of prezygotes (78 and 75% mating pairs are prezygotes for Cdc42p and Cdc42[Q61L]p overexpression, respectively). These results suggest that an increase in the level of the GTP-bound state is not sufficient for efficient cell fusion, and hence we examined whether overexpression of the fast cycling Cdc42[C18A]p mutant could suppress the fusion defect. As illustrated in Figure 8A, the level of overexpressed Cdc42p and Cdc42[C18A]p were similar in both strains (∼20-fold greater than the level of endogenous Cdc42p; unpublished data). Overexpression of Cdc42p did not alter the amount of prezygotes in cdc24-m6 cells; yet overexpression of Cdc42[C18A]p substantially reduced this amount indicative of a reduction in the fusion defect of cdc24-m6 matings (Figure 8B). This reduction in cell fusion defect was accompanied by a partial recovery of the cdc24-m6 mating defect (unpublished data). Budding cells, shmoos, and mating pairs had a similar morphology in all strains (Figure 8C and unpublished data). Interestingly, an increase in Cdc24p levels in CDC42 cells or Cdc42[C18A]p in CDC24 cells did not reduce the number of prezygotes, suggesting that in wild-type cells the GDP–GTP cycling is not limiting during the mating. Taken together these results indicate that Cdc42p GDP–GTP cycling is important for cell fusion during mating.
Figure 8.
Overexpression of a fast-cycling Cdc42p mutant partially suppresses the cdc24-m6 cell fusion defect. (A) Expression levels of different Cdc42p versions in CDC24 and cdc24-m6 cells. Extracts of logarithmically growing cells (RAY1042, and RAY1685) carrying pYes plasmid with indicated Cdc42p grown in galactose/fructose containing media were analyzed by immunoblot and probed with anti-HA mAb or anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mAb. Similar results were observed in three independent experiments. (B) Overexpression of fast-cycling Cdc42p partially suppresses the cdc24-m6 cell fusion defect. Cells were grown as described above, mated on galactose/fructose containing media with RAY1487 (GFP-Bud1), and analyzed as in 3D. Values are the means of two independent matings (n = 400 mating pairs). Error bars, SD. (C) Morphology of CDC24 and cdc24-m6 budding cells is unaffected by Cdc42p or Cdc42[C18A]p overexpression. DIC Images of indicated cells used for above matings grown in galactose/fructose containing media.
DISCUSSION
We have isolated several cdc42 mutants, which have recessive defects in yeast mating. Our results indicate that the Cdc42p Switch I residue valine 36 is critical for efficient mating. Substitution of this valine by different amino acids indicates that the cdc42[V36M] mutant is specifically defective in mating, despite responding to mating pheromone by G1 arrest, FUS1 transcription, and shmoo formation. Two-thirds of the viable cdc42 mutants with valine 36 amino acid substitutions had morphological defects of varying degrees. However, the mating defect of cdc42[V36M] cells is not likely to be an indirect result of its weak morphology defect because cdc42[V36H], cdc42[V36Y], and cdc42[V36K] mutants with more severe morphological defects all mated with efficiencies similar to the wild-type strain. The cdc42[V36M] mutant is defective in cell fusion and accumulates prezygotes during the mating process. This mutant is defective in localizing the component of the fusion machinery, Fus1p to shmoo tip in response to pheromone, similar to the previously isolated cdc24-m6 mutant. Such a role of Cdc42 in cell fusion is unlikely to be mediated by the MAP kinase cascade because overexpression of Ste20p or Fus3p did not diminish the fusion defect. Furthermore, Cdc42[V36M]p bound the Ste20p CRIB domain identical to wild-type Cdc42p, indicating that this mutant is able to bind CRIB domain–containing effectors. Overexpression of a fast cycling Cdc42p, but not Cdc42p or a GTP-locked mutant, partially suppressed the cdc24-m6 fusion defect. In addition, overexpression of Cdc24p reduced the fusion defect of cdc42[V36M] cells, whereas overexpression of Rga1p enhanced this fusion defect. Together our results strongly suggest that Cdc42p GDP–GTP cycling is necessary for efficient cell fusion during mating, perhaps in activating or localizing the fusion machinery at the site of cell contact.
Roles of Cdc42p in Mating
Different studies have examined the role of the small GTPase Cdc42p and its exchange factor Cdc24p in yeast mating and suggest that these proteins function in different steps of this process. Cdc24p interacts with Gβγ and Far1p, and this complex is required for chemotropic growth toward a mating partner (Valtz et al., 1995; Butty et al., 1998; Nern and Arkowitz et al., 1998, 1999). More recently, cdc24 mutants that are defective in cell fusion during mating have been isolated, indicating that this exchange factor functions also in a later step of the mating process (Barale et al., 2004). Cdc42p has been shown to be required for pheromone signaling and Ste20p localization (Moskow et al., 2000). Our work provides evidence for a role of Cdc42p in the cell fusion process. Together, these different analyses of CDC24 and CDC42 mutants indicate that this GTPase module is important for chemotropism (cdc24-m1), pheromone signaling (cdc42-md1), and cell fusion (cdc24-m6 and cdc42[V36M]). The functions of Cdc24p and Cdc42p in these processes are likely to be distinct events as cdc24-m6 and cdc42[V36M] are not defective in pheromone signaling nor chemotropism. Furthermore, Cdc42p interacts with different proteins during pheromone signaling and cell fusion, and these processes are likely to be temporally distinct.
Cdc42p Valine 36 Mutations
Three cdc42 mutants in which valine 36 is mutated (V36A, V36T, V36G) have been previously characterized. Moskow et al., 2000 isolated a cdc42-md1 in which residues Val36, Lys149, and Leu177 were altered to Ala, Gln, and Ser, respectively. This mutant is pheromone resistant and defective in FUS1 induction. In a mutant that contained only the Val36Ala substitution, both the pheromone resistance and the gene induction defect were reduced compared with cdc42-md1. The Cdc42p[V36A] mutant also had a mild defect in binding the Ste20p CRIB domain, in contrast to Cdc42p[V36M], which binds the CRIB domain similar to the wild type.
In cdc42[V36T], cdc42[V36A], and cdc42[V36G] mutants, cells are elongated with wide and misshaped mother bud necks and defects in septin organization, yet the actin cytoskeleton was correctly polarized (Galdfelter et al., 2001, 2002; Caviston et al., 2003). Overexpression of Cla4p suppressed the septin organization defect in cdc42[V36T] and cdc42[V36A] cells (Gladfelter et al., 2001, 2002). In vitro, Cdc42[V36T]p has reduced intrinsic GTPase activity when compared with wild-type Cdc42p, and overexpression of Rga1p suppressed the septin organization defect in this cdc42 mutant (Gladfelter et al., 2002). Both Cdc42[V36T]p and Cdc42[V36A]p mutants were shown to be impaired in binding the Cla4p, Ste20p, Gic1p, and Gic2p CRIB domains (Gladfelter et al., 2001).
We consider it unlikely that the cell fusion defect in cdc42[V36M] mutants is due to an indirect effect on septin organization for the following reasons. First, both cdc42[V36T] and cdc42[V36A] cells have a more pronounced morphology defect than cdc42[V36M] cells, yet a weaker mating defect. Second, we did not observe any alterations in the septin cytoskeleton in cdc42[V36M] cells either during budding or shmooing. Third, overexpression of Rga1p, instead of suppressing the fusion defect in cdc42[V36M] cells, increased the fusion defect in this mutant. In summary, our results strongly suggest that the fusion defect in cdc42[V36M] cells is not the result of perturbations of the septin cytoskeleton nor pheromone signaling, indicating that alterations in residue 36 in Switch I affect interactions with different effectors, depending on the amino acid substitution.
The Switch I together with the Switch II regions undergo nucleotide-dependent conformational changes. Release of the γ-phosphate upon hydrolysis of GTP allows the Switch regions to relax into the GDP-specific conformation. The Switch I region overlaps with an effector-binding region that includes the adjacent β2 strand and binds, for example, CRIB domain–containing effectors. Targeted molecular dynamics computational techniques have identified the Ras residue equivalent to valine 36 in Cdc42 as a critical hinge in the movements of the Switch I region (Diaz et al., 1997). Replacement of this residue in Ras (Ile 36) with an amino acid with a small side chain, such as glycine, increases the flexibility of this loop and hence accelerates the conformational changes between inactive and active states (Kuppens et al., 1999, 2003). Conversely, it is likely that replacement of valine 36 with either the bulky side chains of methionine or tryptophan residues decreases the flexibility of the Switch I loop and results in the conformational changes between inactive and active states becoming rate limiting in Cdc42, and we speculate that this conformational change is also rate limiting in vivo, in particular during cell fusion. Alternatively, an association of Cdc42[V36M]p with Cdc24p or another protein that stabilizes the interaction with this exchange factor may be reduced in vivo. This affect could be due to alterations in the dynamic properties of the Switch I region as has been suggested for the case of Ras (Spoerner et al., 2004). In either case, overexpression of Cdc24p would be expected to suppress the cell fusion defect as observed.
GDP–GTP Cycling of Cdc42p
Several studies have revealed that Cdc42p GTP hydrolysis is important for the function of this critical G-protein. In budding yeast, using mutants locked in the GTP-bound state (Cdc42[Q61L]p) or deletion of GAPs, it has been suggested that GTP hydrolysis is necessary for septin ring assembly (Gladfelter et al., 2002; Caviston et al., 2003) and establishment of cell polarity (Irazoqui et al., 2003). The inability of this GTP-locked Cdc42p mutant to function in septin ring assembly and cell polarity establishment has led to the suggestion that GDP–GTP cycling is important in these two processes. In mammalian cells a fast cycling version of Cdc42p, which undergoes a rapid GDP–GTP exchange without affecting GTPase activity induces cellular transformation (Lin et al., 1997, 1999). The replacement of alanine for cysteine in the Cdc42[C18A]p fast cycling mutant results in the loss of a hydrogen bond to the nucleotide α-phosphate, a concomitant reduction in GDP affinity and an ∼20-fold increase in the GDP–GTP exchange rate in vitro (Rossman et al., 2002). The increased intrinsic exchange rate of this mutant is further enhanced ∼10-fold by the presence of the exchange factor Dbs (Rossman et al., 2002). Our results demonstrate that the Cdc42[C18A]p fast cycling mutant can partially suppress the fusion defect of the cdc24-m6 cells. Neither overexpression of Cdc42p, nor Cdc42[Q61L]p were sufficient to suppress the cdc24-m6 fusion defect, indicating that the suppression by the fast cycling Cdc42p mutant was specific. Interestingly, overexpression of the Cdc42[C18A]p fast cycling mutant did not affect the fusion efficiency of wild-type cells, suggesting that GDP–GTP cycling is not limiting in this context.
Conversely, it is likely that in cdc42[V36M] mutants GDP–GTP exchange is limiting, because overexpression of the exchange factor Cdc24p partially suppresses the fusion defect in these cells. In contrast, overexpression of Cdc24-m6p had no effect on this fusion defect, suggesting that Cdc24-m6p has an altered exchange factor activity, consistent with structure function studies of analogous mutations in the Trio and Tiam1 exchange factors (Liu et al., 1998; Worthylake et al., 2000). It is unlikely that Cdc42[V36M]p GAP interactions are limiting during cell fusion because overexpression of the Rga1p GAP, which functions in yeast mating, did not decrease the fusion defect but rather increased the number of prezygotes that accumulate during mating. Furthermore, two-hybrid interactions between Cdc42[Q61L]p and Rga1p are not affected by the presence of the V36M mutation (S. Barale and R. Arkowitz, unpublished observations), suggesting that Cdc42[V36M]p interacts normally with this GAP. Together, our results strongly suggest that cycling of Cdc42p between GDP and GTP bound forms is important for efficient fusion, perhaps playing a role in correctly localizing and/or activating the fusion machinery.
Cdc42p and Fus1p
Recently, interactions between proteins required for cell fusion and components of the pheromone response pathway and cell polarity proteins were examined in a two-hybrid matrix (Nelson et al., 2004). A number of positive interactions were identified; among them GTP-locked Cdc42p was shown to interact with both Fus1p and Fus2p and Cdc24p with Fus2p (Nelson et al., 2004). These two-hybrid interactions together with the cdc24 and cdc42 mutants specifically defective in Fus1p localization that we have isolated suggest that this GTPase module may directly interact with the fusion machinery. We suggest that this specific interaction is necessary for restricting the localization of the fusion machinery, which is required for efficient fusion. We have attempted to coimmunoprecipitate Fus1p using epitope-tagged Cdc42p and conversely Cdc42p using a Fus1GFP fusion in cells treated with α-factor; however, we were unable to detect a stable coimmunoprecipitation (S. Barale and R. Arkowitz, unpublished observations). Hence, although Cdc42p GTP appears to be important for binding Fus1p and Fus2p as measured by two-hybrid assays (Nelson et al., 2004), we favor the notion that Cdc42p GDP–GTP cycling is necessary for assembly and maintenance of the fusion machinery to the site of cell contact and fusion. We propose that assembly of the protein complexes involved in cell fusion requires Cdc42p GTP binding and that GDP–GTP cycling mediated by GEFs and GAPs is critical for cell fusion. The fusion of other cell types as such as myoblast fusion requires the G-protein Rac and the guanine nucleotide exchange factor DOCK180 family member Myoblast city in Drosophila (Nolan et al., 1998; Hakeda-Suzuki et al., 2002), suggesting that the regulation of G-protein GDP–GTP cycling is conserved in range of cell fusion processes.
ACKNOWLEDGMENTS
We thank M. Bassilana for constructive criticism, C. Matthews for aid with microscopy, S. Clement for technical assistance, and E. Bi and M. Snyder for plasmids. This work was supported by the Centre National de la Recherche Scientifique, Fondation pour la Recherche Médicale-BNP-Paribas, and La Ligue Contre le Cancer. S.B. was supported by Fellowships from La Ligue Contre le Cancer and Association pour la Recherche sur le Cancer.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-11-1040) on March 29, 2006.
REFERENCES
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arkowitz R. A., Lowe N. A small conserved domain in the yeast Spa2p is necessary and sufficient for its polarized localization. J. Cell Biol. 1997;138:17–36. doi: 10.1083/jcb.138.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., Baba N., Ohsumi Y., Kanaya K., Osumi M. Three-dimensional analysis of morphogenesis induced by mating pheromone alpha factor in Saccharomyces cerevisiae. J. Cell Sci. 1989;94:207–216. doi: 10.1242/jcs.94.2.207. [DOI] [PubMed] [Google Scholar]
- Barale S., McCusker D., Arkowitz R. A. The exchange factor Cdc24 is required for cell fusion during yeast mating. Eukaryot. Cell. 2004;3:1049–1061. doi: 10.1128/EC.3.4.1049-1061.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose I. I., Irazoqui J. E., Moskow J. J., Bardes E. S., Zyla T. R., Lew D. J. Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle regulated phosphorylation of Cdc24p. J. Biol. Chem. 2001;11:7176–7186. doi: 10.1074/jbc.M010546200. [DOI] [PubMed] [Google Scholar]
- Brizzio V., Gammie A. E., Nijbroek G., Michaelis S., Rose M. D. Cell fusion during yeast mating requires high levels of a-factor mating pheromone. J. Cell Biol. 1996;135:1727–1739. doi: 10.1083/jcb.135.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzio V., Gammie A. E., Rose M. D. Rvs161p interacts with Fus2p to promote cell fusion in Saccharomyces cerevisiae. J. Cell Biol. 1998;141:567–584. doi: 10.1083/jcb.141.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butty A. C., Pryciak P. M., Huang L. S., Herskowitz I., Peter M. The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science. 1998;282:1511–1516. doi: 10.1126/science.282.5393.1511. [DOI] [PubMed] [Google Scholar]
- Caviston J. P., Longtine M., Pringle J. R., Bi E. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol. Biol. Cell. 2003;14:4051–4066. doi: 10.1091/mbc.E03-04-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Diaz J. F, Wroblowski B, Schlitter J, Engelborghs Y. Calculation of pathways for the conformational transition between the GTP- and GDP-bound states of the Ha-ras-p21 protein: calculations with explicit solvent simulations and comparison with calculations in vacuum. Proteins. 1997;28:434–451. [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J. W. Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu. Rev. Biochem. 2001;70:703–754. doi: 10.1146/annurev.biochem.70.1.703. [DOI] [PubMed] [Google Scholar]
- Dorer R., Boone C., Kimbrough T., Kim J., Hartwell L. H. Genetic analysis of default mating behavior in Saccharomyces cerevisiae. Genetics. 1997;146:39–55. doi: 10.1093/genetics/146.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia L., Marsh L. A role for a protease in morphogenic responses during yeast cell fusion. J. Cell Biol. 1998;142:1473–1485. doi: 10.1083/jcb.142.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion E. A., Trueheart J., Fink G. R. Fus2 localizes near the site of cell fusion and is required for both cell fusion and nuclear alignment during zygote formation. J. Cell Biol. 1995;130:1283–1296. doi: 10.1083/jcb.130.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar C. T., Halkides C. J., Singel D. J. The frozen solution structure of p21 ras determined by ESEEM spectroscopy reveals weak coordination of Thr35 to the active site metal ion. Structure. 1997;5:1055–1066. doi: 10.1016/s0969-2126(97)00257-8. [DOI] [PubMed] [Google Scholar]
- Fitch P. G., Gammie A. E., Lee D. J., de Candal V. B., Rose M. D. Lrg1p Is a Rho1 GTPase-activating protein required for efficient cell fusion in yeast. Genetics. 2004;168:733–746. doi: 10.1534/genetics.104.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie A. E., Brizzio V., Rose M. D. Distinct morphological phenotypes of cell fusion mutants. Mol. Biol. Cell. 1998;9:1395–1410. doi: 10.1091/mbc.9.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter A. S., Bose I., Zyla T. R., Bardes E. S., Lew D. J. Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J. Cell Biol. 2002;156:315–326. doi: 10.1083/jcb.200109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter A. S., Moskow J. J., Zyla T. R., Lew D. J. Isolation and characterization of effector-loop mutants of CDC42 in yeast. Mol. Biol. Cell. 2001;12:1239–1255. doi: 10.1091/mbc.12.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeda-Suzuki S., Ng J., Tzu J., Dietzl G., Sun Y., Harms M., Nardine T., Luo L., Dickson B. J. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- Hart M. J., Eva A., Zangrilli D., Aaronson S. A., Evans T., Cerione R. A., Zheng Y. Cellular transformation and guanine nucleotide exchange activity are catalyzed by a common domain on the dbl oncogene product. J. Biol. Chem. 1994;269:62–65. [PubMed] [Google Scholar]
- Heiman M. G., Walter P. Prm1p, a pheromone-regulated multispanning membrane protein, facilitates plasma membrane fusion during yeast mating. J. Cell Biol. 2000;151:719–730. doi: 10.1083/jcb.151.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazoqui J. E., Gladfelter A. S., Lew D. J. Scaffold-mediated symmetry breaking by Cdc42p. Nat. Cell Biol. 2003;5:1062–1070. doi: 10.1038/ncb1068. [DOI] [PubMed] [Google Scholar]
- Ito Y. Regional polysterism in the GTP-bound form of the human c-Ha-Ras protein. Biochemistry. 1997;36:9109–9119. doi: 10.1021/bi970296u. [DOI] [PubMed] [Google Scholar]
- Jin H., Carlile C., Nolan S., Grote E. Prm1 prevents contact–dependent lysis of yeast mating pairs. Eukaryot. Cell. 2004;3:1664–1673. doi: 10.1128/EC.3.6.1664-1673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. I. Cdc 42, An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 1999;63:54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens S., Diaz J. F., Engelborghs Y. Characterization of the hinges of the effector loop in the reaction pathway of the activation of ras-proteins. Kinetics of binding of beryllium trifluoride to V29G and I36G mutants of Ha-ras-p21. Protein Sci. 1999;8:1860–1866. doi: 10.1110/ps.8.9.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens S., Hellings M., Jordens J., Verheyden S., Engelborghs Y. Conformational states of the switch I region of Ha-ras-p21 in hinge residue mutants studied by fluorescence lifetime and fluorescence anisotropy measurements. Protein Sci. 2003;12:930–938. doi: 10.1110/ps.0236303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E., Thomas D. Y., Whiteway M. Pheromone signalling and polarized morphogenesis in yeast. Curr. Opin. Genet. Dev. 1997;7:59–66. doi: 10.1016/s0959-437x(97)80110-4. [DOI] [PubMed] [Google Scholar]
- Lin R., Bagrodia S., Cerione R., Manor D. A novel Cdc42Hs mutant induces cellular transformation. Curr. Biol. 1997;7:794–797. doi: 10.1016/s0960-9822(06)00338-1. [DOI] [PubMed] [Google Scholar]
- Lin R., Cerione R. A., Manor D. Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J. Biol. Chem. 1999;274:23633–23641. doi: 10.1074/jbc.274.33.23633. [DOI] [PubMed] [Google Scholar]
- Lipke P. N., Taylor A., Ballou C. E. Morphogenic effects of alpha-factor on Saccharomyces cerevisiae a cells. J. Bacteriol. 1976;127:610–618. doi: 10.1128/jb.127.1.610-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio. Cell. 1998;95:269–277. doi: 10.1016/s0092-8674(00)81757-2. [DOI] [PubMed] [Google Scholar]
- Matheos D., Metodiev M., Muller E., Stone D., Rose M. D. Pheromone-induced polarization is dependent on the Fus3p MAPK acting through the formin Bni1p. J. Cell Biol. 2004;165:99–109. doi: 10.1083/jcb.200309089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher K. A modular set of prokaryotic and eukaryotic expression vectors. Anal. Biochem. 2000;277:109–120. doi: 10.1006/abio.1999.4383. [DOI] [PubMed] [Google Scholar]
- Metodiev M. V., Matheos D., Rose M. D., Stone D. E. Regulation of MAPK function by direct interaction with the mating-specific Galpha in yeast. Science. 2002;296:1483–1486. doi: 10.1126/science.1070540. [DOI] [PubMed] [Google Scholar]
- Mosch H. U., Kohler T., Braus G. H. Different domains of the essential GTPase Cdc42p required for growth and development of Saccharomyces cerevisiae. Mol. Cell. Biol. 2001;21:235–248. doi: 10.1128/MCB.21.1.235-248.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskow J. J., Gladfelter A. S., Lamson R. E., Pryciak P.M., Lew D. J. Role of Cdc42p in pheromone-stimulated signal transduction in Saccharomyces cerevisiae. Mol. Cell. Biol. 2000;20:7559–7571. doi: 10.1128/mcb.20.20.7559-7571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar N., Hoffman G. R., Manor D., Clardy J. C., Cerione R. A. Structures of Cdc42 bound to the active and catalytically compromised forms of Cdc42GAP. Nat. Struct. Biol. 1998;5:1047–1052. doi: 10.1038/4156. [DOI] [PubMed] [Google Scholar]
- Nelson B., Parsons A. B., Evangelista M., Schaefer K., Kennedy K., Ritchie S., Petryshen T. L., Boone C. Fus1p interacts with components of the Hog1p mitogen-activated protein kinase and Cdc42p morphogenesis signaling pathways to control cell fusion during yeast mating. Genetics. 2004;166:66–77. doi: 10.1534/genetics.166.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nern A. The role of the GTP/GDP exchange factor Cdc24p in the control of cell polarity during yeast cell mating. Ph.D. Thesis. Frankfurt am Main: Johann Wolfgang Goethe-Universität. 2000 [Google Scholar]
- Nern A., Arkowitz R. A. A GTP-exchange factor required for cell orientation. Nature. 1998;391:195–198. doi: 10.1038/34458. [DOI] [PubMed] [Google Scholar]
- Nern A., Arkowitz R. A. A Cdc24p, Far1p, Gβγ protein complex required for yeast orientation during mating. J. Cell Biol. 1999;144:1187–1202. doi: 10.1083/jcb.144.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nern A., Arkowitz R. A. G proteins mediate changes in cell shape by stabilizing the axis of polarity. Mol. Cell. 2000a;5:853–864. doi: 10.1016/s1097-2765(00)80325-1. [DOI] [PubMed] [Google Scholar]
- Nern A., Arkowitz R. A. Nucleocytoplasmic shuttling of the Cdc42p exchange factor Cdc24p. J. Cell Biol. 2000b;148:1115–1122. doi: 10.1083/jcb.148.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan K. M., Barrett K., Lu Y., Hu K. Q., Vincent S., Settleman J. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 1998;12:3337–3342. doi: 10.1101/gad.12.21.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlen L. J., Cross F. R. The role of Cdc42 in signal transduction and mating of the budding yeast Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:8556–8559. doi: 10.1074/jbc.273.15.8556. [DOI] [PubMed] [Google Scholar]
- Philips J., Herskowitz I. Osmotic balance regulates cell fusion during mating in Saccharomyces cerevisiae. J. Cell Biol. 1997;138:961–974. doi: 10.1083/jcb.138.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock R., Treisman R. A sensitive method for the determination of protein-DNA binding specificities. Nucleic Acids Res. 1990;18:6197–6204. doi: 10.1093/nar/18.21.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner J. C., Munro S. Identification of the MNN2 and MNN5 mannosyltransferases required for forming and extending the mannose branches of the outer chain mannans of Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:26836–26843. doi: 10.1074/jbc.273.41.26836. [DOI] [PubMed] [Google Scholar]
- Read E. B., Okamura H. H., Drubin D. G. Actin- and tubulin-dependent functions during Saccharomyces cerevisiae mating projection formation. Mol. Biol. Cell. 1992;3:429–444. doi: 10.1091/mbc.3.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Winston F., Hieter P. Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1991. [Google Scholar]
- Rossman K. L., Worthylake D. K., Snyder J. T., Cheng L., Whitehead I. P., Sondek J. Functional analysis of cdc42 residues required for Guanine nucleotide exchange. J. Biol. Chem. 2002;277:50893–50898. doi: 10.1074/jbc.M208580200. [DOI] [PubMed] [Google Scholar]
- Santos B., Duran A., Valdivieso M. H. CHS5, a gene involved in chitin synthesis and mating in Saccharomyces cerevisiae. Mol. Cell. Biol. 1997;17:2485–2496. doi: 10.1128/mcb.17.5.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos B., Snyder M. Specific protein targeting during cell differentiation: polarized localization of Fus1p during mating depends on Chs5p in Saccharomyces cerevisiae. Eukaryot. Cell. 2003;2:821–825. doi: 10.1128/EC.2.4.821-825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. N., De Virgilio C., Souza B., Pringle J. R., Abo A., Reed S. I. Role for the Rho-family GTPase Cdc42 in yeast mating-pheromone signal pathway. Nature. 1995;376:702–705. doi: 10.1038/376702a0. [DOI] [PubMed] [Google Scholar]
- Smith M. G., Swamy S. R., Pon L. A. The life cycle of actin patches in mating yeast. J. Cell Sci. 2001;114:1505–1513. doi: 10.1242/jcs.114.8.1505. [DOI] [PubMed] [Google Scholar]
- Spoerner M., Wittinghofer A., Kalbitzer H. R. Perturbation of the conformational equilibria in Ras by selective mutations as studied by 31P NMR spectroscopy. FEBS Lett. 2004;578:305–310. doi: 10.1016/j.febslet.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Sprague G.F.J., Thorner J. W. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae. In: Jones E. W., Pringle J. R., Broach J. R., editors. The Molecular and Cellular Biology of the Yeast Saccharomyces. Vol. 2. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1992. pp. 657–744. [Google Scholar]
- Tkacz J. S., MacKay V. L. Sexual conjugation in yeast. Cell surface changes in response to the action of mating hormones. J. Cell Biol. 1979;80:326–333. doi: 10.1083/jcb.80.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueheart J., Boeke J. D., Fink G. R. Two genes required for cell fusion during yeast conjugation: evidence for a pheromone-induced surface protein. Mol. Cell. Biol. 1987;7:2316–2328. doi: 10.1128/mcb.7.7.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtz N., Herskowitz I. Pea2 protein of yeast is localized to sites of polarized growth and is required for efficient mating and bipolar budding. J. Cell Biol. 1996;135:725–739. doi: 10.1083/jcb.135.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtz N., Peter M., Herskowitz I. FAR1 is required for oriented polarization of yeast cells in response to mating pheromones. J. Cell Biol. 1995;131:863–873. doi: 10.1083/jcb.131.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter I. R., Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- Weiner M. P., Costa G. L., Schoettlin W., Cline J., Mathur E., Bauer J. C. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene. 1994;151:119–123. doi: 10.1016/0378-1119(94)90641-6. [DOI] [PubMed] [Google Scholar]
- Whiteway M., Hougan L., Dignard D., Thomas D. Y., Bell L., Saari G. C., Grant F. J., O'Hara P., MacKay V. L. The STE4 and STE18 genes of yeast encode potential beta and gamma subunits of the mating factor receptor-coupled G protein. Cell. 1989;56:467–477. doi: 10.1016/0092-8674(89)90249-3. [DOI] [PubMed] [Google Scholar]
- Worthylake D. K., Rossman K. L., Sondek J. Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature. 2000;408:682–688. doi: 10.1038/35047014. [DOI] [PubMed] [Google Scholar]
- Ziman M., O'Brien J. M., Ouellette L. A., Church W. R., Johnson D. I. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol. Cell. Biol. 1991;11:3537–3544. doi: 10.1128/mcb.11.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]