Abstract
Many endocytosed proteins in yeast travel to the vacuole, but some are recycled to the plasma membrane. We have investigated the recycling of chimeras containing green fluorescent protein (GFP) and the exocytic SNARE Snc1p. GFP-Snc1p moves from the cell surface to internal structures when Golgi function or exocytosis is blocked, suggesting continuous recycling via the Golgi. Internalization is mediated by a conserved cytoplasmic signal, whereas diversion from the vacuolar pathway requires sequences within and adjacent to the transmembrane domain. Delivery from the Golgi to the surface is also influenced by the transmembrane domain, but the requirements are much less specific. Recycling requires the syntaxins Tlg1p and Tlg2p but not Pep12p or proteins such as Vps4p and Vps5p that have been implicated in late endosome–Golgi traffic. Subtle changes to the recycling signal cause GFP-Snc1p to accumulate preferentially in punctate internal structures, although it continues to recycle to the surface. The internal GFP-Snc1p colocalizes with Tlg1p, and immunofluorescence and immunoelectron microscopy reveal structures that contain Tlg1p, Tlg2p, and Kex2p but lack Pep12p and Sec7p. We propose that these represent early endosomes in which sorting of Snc1p and late Golgi proteins occurs, and that transport can occur directly from them to the Golgi apparatus.
INTRODUCTION
Endocytosis of extracellular molecules and plasma membrane proteins is required to internalize nutrients, to regulate the level of cell surface receptors, to remove damaged proteins from the membrane, and to recycle membrane proteins involved in exocytosis back to the secretory pathway. To accomplish these tasks the endocytic pathway must contain molecular machinery for the sorting of endocytosed proteins into different membrane-ound transport intermediates and the delivery of these transport intermediates to appropriate target membranes within the cell. In yeast, the internalization of plasma membrane proteins such as specific permeases and G-protein-oupled receptors is relatively well characterized, but little is known about how subsequent membrane fusion events within the cell generate, maintain,and allow transport between discrete endocytic compartments (Geli and Riezman, 1998; Wendland et al., 1998).
Morphological studies have focused on transport from the cell surface to the lysosome-like vacuolar compartment, in the main using one of three different markers. The lipid dye FM4-64 specifically accumulates in vacuolar membranes. Internalization to the vacuole is ATP dependent and can be blocked by mutations affecting membrane trafficking (Vida and Emr, 1995; Zheng et al., 1998). The plasma membrane receptors for mating pheromones are endocytosed to the vacuole both constitutively and at increased rates when bound to their ligands. Thus alpha factor and its receptor, Ste2p, have been used to follow endocytic protein transport (Singer-Kruger et al., 1993; Hicke et al., 1997). A third way of following endocytosis is to allow spheroplasted cells to take up positively charged nanogold particles to the vacuole. This allows examination of the membrane compartments involved by electron microscopy (Prescianotto-Baschong and Riezman, 1998). These approaches have shown that at least two classes of endosome are involved in transport to the vacuole. Early endosomes appear as scattered punctate structures throughout the cytosol. Before endocytosed protein and lipid reach the vacuole they pass through a relatively large late endosomal or prevacuolar compartment, often located adjacent to the vacuole. The final endosomal structures have the morphology of a multivesicular body, whereas early endosomes are tubulovesicular structures (Prescianotto-Baschong and Riezman, 1998).
The endocytic pathway to the vacuole is thought to converge with the delivery of newly synthesized vacuolar proteins at an endosomal or prevacuolar compartment. Evidence in support of this is provided by the phenotype of class E vps mutants, in which exit from a prevacuolar compartment is blocked. Class E vps mutants form an aberrant, enlarged prevacuolar compartment, which accumulates both endocytosed markers and vacuolar proteins (Davis et al., 1993; Rieder et al., 1996; Babst et al., 1997; Hicke et al., 1997; Bryant et al., 1998; Conibear and Stevens, 1998). However, this does not preclude transport between the endocytic and secretory pathways at other points.
Although most studies have concentrated on transport to the vacuole, some endocytosed proteins are thought to be recycled to the cell surface. The best studied example of a protein following this itinerary is Chs3p, a subunit of the cell wall biosynthetic enzyme chitin synthase. A proportion of Chs3p is found in an intracellular pool, the formation or maintenance of which depends on endocytosis (Ziman et al., 1996, 1998; Holthuis et al., 1998b). When transport through this pool is blocked, Chs3p is no longer correctly localized to the bud neck. Endosomes also play an important role, in both yeast and mammalian cells, in maintaining the steady-state distribution of late Golgi membrane proteins (reviewed by Conibear and Stevens, 1998). Well-characterized examples of such proteins are Kex2p and DPAPA, which act in the late Golgi to modify alpha mating pheromone as it is secreted. Specific signals on the cytoplasmic portion of these proteins are required for retrieval from a post-Golgi compartment that is likely to also function as an endosome. This conclusion is largely based on the observation that late Golgi localized chimeric membrane proteins are cleaved by vacuolar/late endosomal proteases and can be trapped in class E endosomes (Bryant and Stevens, 1997).
Membrane trafficking pathways have been elucidated in part by analysis of the SNARE proteins. These integral membrane proteins are central components of the intracellular membrane fusion machinery (reviewed by Rothman, 1994; Hay and Scheller, 1997; Nichols and Pelham, 1998; Pelham, 1999), fusion requiring the formation of SNARE complexes that span the two membranes (Nichols et al., 1997; Ungermann et al., 1998). Different SNARE proteins have different subcellular localizations and are involved in different membrane transport steps. Hence analysis of their locations, the complexes that they form, and their mutant phenotypes gives information about these steps. A subset of SNAREs form the syntaxin family, and all SNARE-dependent membrane fusion steps so far examined involve a member of this family.
Yeast contains eight syntaxins identifiable by sequence homology, of which four have been implicated in endocytic pathways. Vam3p is located on vacuoles and is required for fusion to them (Darsow et al., 1997; Nichols et al., 1997; Wada et al., 1997; Srivastava and Jones, 1998), whereas Pep12p is present in endosomes and is required for the bulk transport of proteins from either the Golgi or the cell surface to the vacuole (Becherer et al., 1996; Holthuis et al., 1998b). A separate specialized pathway from the Golgi to the vacuole that bypasses this Pep12p requirement also exists and is used by a few proteins such as alkaline phosphatase and Vam3p itself (Cowles et al., 1997; Piper et al., 1997; Stepp et al., 1997). Two other syntaxins, Tlg1p and Tlg2p, have been implicated in the recycling of both late Golgi proteins and Chs3p; their removal also slows endocytosis from the surface to the vacuole but does not prevent it (Abeliovich et al., 1998; Holthuis et al., 1998a,b; Seron et al., 1998). The locations and precise functions of these two syntaxins have been controversial, there being evidence for their presence in both a Golgi compartment and an endosomal one. However, the analysis of strains lacking multiple syntaxins clearly indicates their involvement in the pathway that recycles endocytosed Chs3p to the surface, a pathway that does not require the late endosomal syntaxin Pep12p and that remains largely uncharacterized (Holthuis et al., 1998b).
In this paper we have used a different and more convenient marker to characterize this recycling pathway, namely the SNARE Snc1p. Snc1p is the yeast equivalent of the synaptobrevin/VAMP proteins of animal cells, and together with its close relative Snc2p it mediates fusion of exocytic vesicles with the plasma membrane; an epitope-tagged version of Snc1p has previously been shown to be present largely on the plasma membrane (Protopopov et al., 1993). We show that green fluorescent protein (GFP)-tagged Snc1p is also mostly at the cell surface but undergoes rapid endocytosis and resecretion via the Golgi apparatus. Genetic and morphological analysis indicates that this pathway is distinct from the previously characterized route from late endosomes to the Golgi. Mutagenesis defines different sequence requirements for exocytosis, endocytosis, and diversion from the vacuolar pathway and provides evidence for a receptor-mediated sorting event in an early endosomal compartment. Analysis by immunofluorescence and immuno-electron microscopy (EM) suggests that Tlg1p, Tlg2p, and the late Golgi protein Kex2p are distributed between these early endosomes and Golgi membranes. Our results suggest that early endosomes constitute a major sorting site for proteins in yeast, as in animal cells.
MATERIALS AND METHODS
Yeast Strains
Table 1 lists the starting yeast strains used. Strains containing fluorescent SNC1 variants were in general made by integrating expression plasmids at URA3. Expression levels were screened by fluorescence microscopy, and low-level–expressing cells were checked by Western blotting with anti-Snc antibodies. The strains analyzed expressed the chimera at one to two times the level of the endogenous Snc proteins.
Table 1.
Yeast strains used in this work
| Strain | Genotype | Source |
|---|---|---|
| SEY6210 | MATα ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | S. Emr |
| SEY6211 | MATa ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 ade2-101 suc2-Δ9 | S. Emr |
| RH1597 | MATa end4-1, his4 leu2 ura3 bar1 | H. Riezman |
| RAY397 | MATα leu2-3, −112 ura3-52 his3-Δ200 sec6-4 | R. Arkowitz |
| RSY281 | MATα sec23-1 ura3-52 his4-619 | R. Schekman |
| RSY277 | MATα sec21-1 ura3-52 | R. Schekman |
| PC70 | MATα ura3 leu2 trp1 cop1-1 (previously ret1-1) | P. Cosson |
| JK740 | MATa ade2-1 can1-100 his3-11, −15 leu2-3, −112 trp1-1 ura3-1 cmd1-1 | J. Kilmartin |
| SEY4-1 | MATα ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 vps4-1 | M. Seaman |
| BHY152 | MATα ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 vps5::HIS3 | M. Seaman |
| MLY202 | MATα ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 vps5::HIS3 sec6-4::LEU2 | This work |
| KKY11 | MATα ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 vps17::HIS3 | M. Seaman |
| EMY18 | MATα ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 vps35::HIS3 | M. Seaman |
| SW14B3 | sec14-3 ura3-52 leu2-3, −112 his3-200 trp1-901 | Pelham lab |
| DB51 | MATα ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 sed5-1 | Pelham lab |
| JHY002 | MATα ura3-52 his3-Δ200 leu2-3, −112 trp1-Δ901 suc2-Δ9 lys2-801 tlg2::HIS5 (S. pombe) | Pelham lab |
| JH005 | MATα ura3-52 his3-Δ200 leu2-3, −112 trp1-Δ901 suc2-Δ9 lys2-801 pep12::HIS3 | Pelham lab |
| JHY016 | MATα ura3-52 his3-Δ200 leu2-3, −112 trp1-Δ901 suc2-Δ9 tlg1::TRP1 | Pelham lab |
| MLY201 | ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 suc2-Δ9 ade2-101 snc1:HIS5 (S. pombe) snc2::TRP1 + pSNC1[2μ URA3 TPI-SNC1] | This work |
The snc1Δ snc2Δ strain MLY201 was constructed by replacing SNC1 in SEY6211 with the Schizosaccharomyces pombe HIS5 gene. The SNC2 gene in SEY6210 was disrupted by inserting the TRP1 gene at the unique BglII site in the gene. The two disruption strains were subsequently crossed, transformed with a URA3 plasmid carrying wild-type SNC1 (pSNC1), and sporulated, and spores were selected that were disrupted for both of the genomic SNC genes but supported by the plasmid. Loss of the plasmid was lethal in this strain, as judged by inability to grow on plates containing 5-fluoroorotic acid. For some experiments a methionine-repressible MET3 promoter construct driving SNC1 was inserted at the LEU2 locus, and the URA3 plasmid was removed by selection on 5-fluoroorotic acid. These two strains were used to check the ability of SNC1 chimeras to support growth by testing for ability to lose the pSNC1 plasmid or to grow on methionine-containing plates, respectively.
The vps5 sec6 strain (MLY202) was constructed from strain BHY152 by replacing the wild-type SEC6 gene with a cassette containing sec6-4 (in reverse orientation) and LEU2.
A derivative of SEY6210 in which sequences encoding three copies of the hemagglutinin (HA) epitope were inserted at the C terminus of the KEX2 ORF was kindly provided by J. Holthuis (Cambridge, United Kingdom). A strain in which GFP coding sequences were inserted into the chromosomal SEC7 gene was kindly provided by B. Glick (University of Chicago) and is described elsewhere (Seron et al., 1998).
Plasmids
SNC1 constructs were derived from a cDNA plasmid kindly donated by Julian Rayner (Cambridge, United Kingdom). The SNC1 cDNA or PCR-generated derivatives were cloned into pRS406 (Sikorski and Hieter, 1989) behind sequences expressing the mut2 GFP variant (Cormack et al., 1996) from the TPI promoter, as described by Wooding and Pelham (1998). Exchange of sequences encoding different transmembrane domains was achieved by PCR, using primers encoding the appropriate domains. The endocytosis-defective variant of SNC1 was made by introducing silent PstI and Blp1 sites by PCR at nucleotides 149 and 206 and inserting synthetic oligonucleotides between them encoding the mutations.
Mutagenic PCR was performed as described by Muhlrad et al. (1992), and libraries of mutants were cloned into a pRS416 version of the expression plasmid described above. Mutant plasmids were recovered from yeast and sequenced. Libraries of transmembrane domain (TMD) variants were made by PCR from SNC1 using degenerate PCR primers encoding random mixtures of phenylalanine, leucine, isoleucine, methionine, and valine. A plasmid expressing SNC1 from the methionine-repressible MET3 promoter (Cherest et al., 1987) was made by cloning the promoter (a gift from J. Holthuis) as an XhoI–EcoRI fragment in front of an SNC1 cDNA EcoRI–BamHI fragment in the vector pRS405. Alanine scanning mutants of SNC1 were made by PCR, which was facilitated by introducing silent ClaI, NarI and BglII sites at nucleotides 174, 229, and 264. All constructs and mutants were verified by chain termination sequencing (Oswell DNA Sequencing, Southampton, United Kingdom).
The plasmid for expressing Tlg2p from its own promoter was derived from a pRS316 (CEN, URA3; Sikorski and Hieter, 1989)-based plasmid expressing triple-myc Tlg2p from the TPI promoter (Holthuis et al., 1998a). The TPI promoter was removed and replaced with genomic sequence from the 400 bp immediately upstream of the TLG2 ORF.
Imaging of Live Cells
Cells were grown into early log phase in appropriate media. Where indicated, they were incubated with FM4-64 (Molecular Probes, Eugene, OR) in minimal complete medium supplemented with 32 μM FM4-64. To label vacuoles, cells were incubated with FM4-64 for 15 min and then washed (or in some cases not) and incubated for a further 1 h. For microscopy cells were placed onto slides coated with concanavalin A and allowed to attach. After sealing under coverslips they were imaged in a single plane on an MRC-600 confocal scanning laser microscope (Bio-Rad, Stevenage, United Kingdom) or on a Zeiss (Thornwood, NY) Axioskop microscope equipped with a Micromax charge-coupled device camera (Princeton Instruments, Trenton, NJ).
For following individual cells over short time courses, they were placed on agarose on a temperature-controlled stage (Wooding and Pelham, 1998), and imaging was performed in conjunction with temperature shifts. Because the small punctate structures in which some mutants were found moved rapidly, these cells were imaged using a single slow scan of the confocal microscope.
Quantitative analysis of time courses was performed on a Macintosh computer using the public domain NIH Image program (developed at the National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). Images of single optical sections at the midpoint of a cell were obtained, and total pixel intensity was calculated for the whole cell and for the plasma membrane region. Plasma membrane fluorescence as a fraction of the total was plotted, and half-times for the changes were estimated.
Affinity Purification of Tlg1p and Pep12p Antibodies
For immunofluorescence crude antisera against Tlg1p and Tlg2p (Holthuis et al., 1998a) were affinity purified using standard techniques. Briefly, the entire cytosolic domains of Pep12p and Tlg1p were expressed in Escherichia coli strain BL-21 as fusion proteins with glutathione S-transferase, using the vector pGEX4T-3 (Amersham Pharmacia Biotech, Uppsala, Sweden). Soluble glutathione S-transferase fusion proteins were purified on glutathione-Sepharose beads (Amersham Pharmacia Biotech) following the manufacturer's instructions and covalently coupled to cyanogen bromide-activated Sepharose beads (Amersham Pharmacia Biotech), again following the manufacturer's instructions. The coupled beads were washed alternately with 0.1 M glycine, pH 3.0, and 0.1 M ethanolamine, pH 9.5, several times before incubation with the appropriate crude antiserum diluted 1:1 in PBS at room temperature for 2 h. After repeated washes with PBS and PBS supplemented with 0.4 M KCl, bound antibody was eluted with 0.1 M glycine, pH 3.0, followed by 0.1 M ethanolamine, pH 9.5, and the pH of the combined eluates was adjusted to 7.0.
Immunofluorescence
Fixation, spheroplasting, and dehydration of yeast cells were carried out precisely as described (Kilmartin and Adams, 1984). The affinity-purified Tlg1p and Pep12p antisera were used at a dilution of 1:100. Use of tlg1Δ and pep12Δ cells as negative controls showed that the punctate staining observed with both antibodies was specific, with negligible background staining observed in the appropriate null strain. Myc-tagged Tlg2p was detected using the 9E10 monoclonal antibody, and HA-tagged Kex2p was detected using 3F10 monoclonal antibody (Boehringer Mannheim, Mannheim, Germany). Secondary antibodies coupled to Alexa fluorophores were from Molecular Probes. Fixed cells labeled for immunofluorescence were examined by confocal microscopy. All confocal images shown are of a single confocal plane.
Immuno-EM
Strain SEY6210 was grown in YPUAD medium to early logarithmic phase and then fixed overnight at 4°C by direct addition of glutaraldehyde (final, 0.2%) and formaldehyde (final, 3%) to the culture medium. The cells were washed in 50 mM HEPES, pH 7, and 3 mM Kcl and incubated in 1% NaIO4, and free aldehyde groups were quenched with 50 mM NH4Cl as described (van Tuinen and Riezman, 1987). Dehydration, infiltration, and polymerization in LR GOLD resin (London Resin, London, United Kingdom) was done according to the supplier's instructions. Thin sections of ∼50 nm were cut and mounted on 200-mesh nickel grids.
All primary antibodies used were raised in rabbits. The immunoglobulin G (IgG) fraction of each antiserum was purified on protein A-Sepharose columns by standard procedures and used for immunolabeling at dilutions determined empirically. Secondary IgG-colloidal gold conjugates (Bio Cell, Cardiff, United Kingdom) were diluted 1:50.
Grids were placed upside-down on 50-μl droplets of blocking solution (150 mM NaCl, 10 mM potassium phosphate, pH 7.5, 0.1% Tween 20, 2% fatty acid-free BSA [Sigma, St. Louis, MO]) for 20 min. The grids were then transferred to droplets containing appropriate dilutions of the primary antibodies in blocking buffer and incubated for 4 h at room temperature. The grids were then washed three times for 5 min with PBS solution and 0.2% BSA and then three times for 5 min in PBS solution. They were then washed by dipping 10 times in a 100-ml beaker of bidistilled water. The grids were incubated for 10 min in blocking buffer before transferring to droplets with secondary antibodies and incubation for 2 h. After washing as above, they were fixed for 10 min in 1% glutaraldehyde in PBS solution to preserve the immunolabeling and then washed by dipping 10 times in a 100-ml beaker of distilled water. Free aldehydes were again quenched with 50 mM NH4Cl and washed with distilled water. Labeling with the second primary antibodies was done essentially as described for the first primary antibodies, except the blocking solution contained 0.5% Tween 20 in PBS solution. After washing in water, the grids were stained in 6% uranyl acetate for 5 min and in Reynold's lead citrate for 30–60 s. Background controls were performed on sections of the same sample in which the incubation with the second primary antibody was omitted and were essentially negative. For quantitation of each double labeling, 31 cell sections that contained gold particles of either size were photographed, and all structures within these cells (∼150 total) were scored for the presence of each marker.
RESULTS
Recycling of Snc1p
To investigate the recycling pathway for a plasma membrane protein, we followed the transport of the exocytic SNARE Snc1p, tagged at its N terminus with GFP and expressed at a level no greater than twice that of the endogenous Snc protein (see MATERIALS AND METHODS). This GFP-tagged protein was functional in that it could support normal growth in a strain lacking both SNC1 and SNC2, which are an essential pair of genes in our yeast strain. In >90% of wild-type cells GFP-Snc1p was found on the plasma membrane, sometimes restricted to the bud or more generally to regions of polarized growth, although there was also some internal fluorescence (Figure 1). To test for its recycling, we expressed GFP-Snc1p in cells containing a temperature-sensitive allele of sec6. SEC6 is required for fusion of exocytic vesicles with the plasma membrane, and at the nonpermissive temperature (37°C) sec6 cells accumulate clusters of secretory vesicles, often localized to sites of secretion—the bud in small-budded cells and near the forming septum in large-budded cells (Novick et al., 1980). When sec6-4 cells were shifted to 37°C, GFP-Snc1p accumulated in patches in the expected locations of secretory vesicles, with correspondingly less being at the plasma membrane (Figure 1). This suggests that GFP-Snc1p is endocytosed and reenters the normal pool of late secretory vesicles.
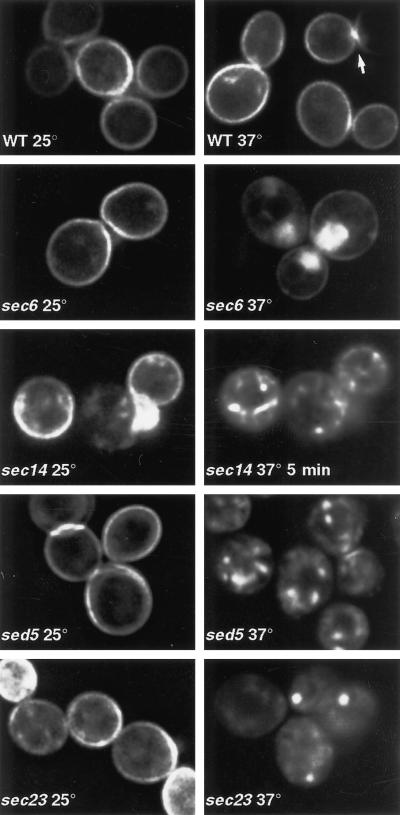
Figure 1.
GFP-Snc1p distribution in secretory mutants. Images of live cells, either wild type for secretory function (WT) or carrying the indicated mutations, are shown. The sec14 panels show the same cells imaged on a heated stage before and 5 min after temperature shift. WT, sec6, and sec23 cells were incubated for 1 h at 37°C; sed5 cells were incubated for 30 min. The arrow indicates an example of concentration of GFP-Snc1p in the bud (to the left of the arrow) relative to the mother cell (to the right).
The speed of GFP-Snc1p recycling was revealed most dramatically by continuous monitoring of individual cells of a sec14-3 strain, in which exit from the Golgi is blocked at high temperature (Novick et al., 1980; Franzusoff and Schekman, 1989). After a shift of the cells to 37°C, GFP-Snc1p moved from the cell surface to internal structures. Substantial redistribution occurred within 5 min (Figure 1). Quantitation of the plasma membrane fluorescence indicated approximate half-times of 7.8 and 9.7 min, respectively, for the internalization process in the left- and right-hand cells shown in Figure 1. Given the speed of this effect, it seems likely that Snc1p moves from the plasma membrane to a sec14-sensitive Golgi compartment before returning to the surface, although more indirect effects of the sec14 mutation are also possible.
Further evidence that a functional Golgi complex is required for the recycling pathway was provided by analyzing GFP-Snc1p in temperature-sensitive sed5 and sec23 mutants. Lesions in the Golgi syntaxin Sed5p block traffic to the early Golgi and cause vesiculation of the late Golgi (Wooding and Pelham, 1998); sec23 mutants block exit from the endoplasmic reticulum (ER), and under these circumstances Sed5p becomes trapped in the ER and the Golgi vesiculates (Morin-Ganet et al., 1998; Wooding and Pelham, 1998). In both cases there was a dramatic loss of fluorescence from the cell surface upon shift to the nonpermissive temperature, indicating that endocytosis could continue but redelivery to the plasma membrane was blocked (Figure 1). The internalized protein accumulated in structures of variable apparent sizes. We did not characterize them further, because the major disruption to the endomembrane system that occurs in these mutants makes interpretation of marker protein distributions difficult.
The redistribution of GFP-Snc1p seen in these experiments is most likely due to movement of existing molecules, rather than degradation of these and their replacement with newly synthesized ones. In all cases the effects were visible within 30 min, a time that we found was insufficient to restore GFP levels after photobleaching (our unpublished observations; also see Wooding and Pelham, 1998). This is borne out by the lack of obvious ER staining even after 60 min in a sec23 mutant (Figure 1), although in longer time courses (2 h) newly synthesized material did eventually accumulate there. Cycloheximide treatment was not used to eliminate newly synthesized protein, because it alone caused internalization of GFP-Snc1p. Previous studies have shown that such treatment has profound effects on the secretory and endocytic pathways, blocking alpha factor transport in late endosomes and also the retrograde transport of Sed5p from Golgi to ER (Hicke et al., 1997; Wooding and Pelham, 1998).
Retrieval of GFP-Snc1p Requires Tlg1p and Tlg2p but Not Late Endosomes
The pathway followed by Snc1p seemed similar to that followed by Chs3p, which we have previously shown depends on the late Golgi/early endosomal SNAREs Tlg1p and Tlg2p but not the late endosomal SNARE Pep12p (Holthuis et al., 1998b). In agreement with this, we found that when GFP-Snc1p was expressed in tlg1Δ or tlg2Δ cells, little if any cell surface fluorescence was evident (Figure 2). In both tlgΔ strains GFP-Snc1p was present in intracellular membranes, but these had different morphologies. Relatively large structures were detectable in tlg1Δ, whereas more scattered punctate labeling was evident in tlg2Δ, and in both cases there was an increased cytosolic haze, which may correspond to transport vesicles. The larger structures in tlg1Δ cells are presumably endocytic in nature, but the distribution of GFP-Snc1p did not entirely correspond to that of the endocytic tracer dye FM4-64 after 2 h of uptake, a time at which labeling of vacuoles is apparent (Figure 2). Because removal of Tlg1p or Tlg2p does not significantly impair exocytic membrane traffic (Holthuis et al., 1998a), the intracellular GFP-Snc1p in the deletion strains is unlikely to be newly synthesized material that has not yet reached the cell surface. Rather, it is likely to be endocytosed material that has reached neither the vacuole nor the secretory pathway. Further evidence for this is presented below.
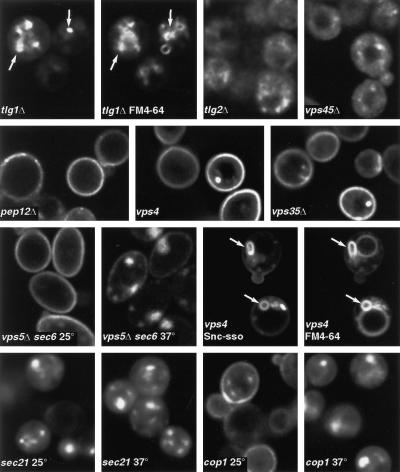
Figure 2.
Requirements for recycling. Except where indicated, cells of the indicated genotypes expressing GFP-Snc1p were imaged (live). The tlg1 cells were labeled with FM4-64 for 2 h before imaging in separate channels to reveal GFP and vacuolar membranes. In this pair of images, and in subsequent figures, arrows indicate identical positions in the two channels. For vps5 s6, sec21, and cop1 cells, incubation at 37°C was for 1 h. Snc-sso refers to a version of GFP-Snc1p whose transmembrane domain was replaced by that of Sso1p, a change that prevents retrieval of the protein from the endocytic pathway (see RESULTS). The vps4 cells expressing this protein were labeled with FM4-64, and both images are shown. Note the class E compartments containing both GFP and FM4-64 (arrows).
We also examined the role of Vps45p, a Sec1p homologue that binds to Tlg2p and is required for its stability and that is also implicated in Pep12p function (Burd et al., 1997; Webb et al., 1997; Nichols et al., 1998). In a vps45Δ strain Snc1p-GFP was seen predominantly in diffuse vesicular structures, as in tlg2Δ cells (Figure 2).
Pep12p is a syntaxin required for traffic through late endosomes to the vacuole (Becherer et al., 1996; Holthuis et al., 1998b). In contrast to the results with tlg1Δ and tlg2Δ strains, in a pep12Δ strain most cells localized GFP-Snc1p correctly, although in some (∼15%) intracellular staining was also apparent (Figure 2). Pep12p is thus not essential for the recycling of Snc1p. We also tested several genes that are required for retrieval of proteins, in particular the carboxypeptidase Y sorting receptor Vps10p, from a late endosomal compartment to the Golgi (reviewed by Conibear and Stevens, 1998). Mutation of VPS4 results in the formation of enlarged late endosomal/prevacuolar structures, the “class E compartment” to which proteins can be delivered but from which they cannot easily exit either to the vacuole or the Golgi. Vps5p and Vps35p are specifically required for retrieval of proteins from late endosomes to the Golgi. Figure 2 shows that GFP-Snc1p remained predominantly on the plasma membrane in vps4, vps5, and vps35 null mutants, and identical results were obtained with a vps17 mutant (our unpublished observations), whose properties are similar to those of vps5 and vps35.
To verify that recycling of GFP-Snc1p was occurring in these vps mutants, we performed two controls. First, we constructed a vps5Δ sec6-4 double mutant and found that GFP-Snc1p accumulated in vesicles at the nonpermissive temperature, just as in the sec6-4 single mutant (Figure 2). Second, we tested endocytosis in vps4 cells by expressing an altered form of GFP-Snc1p whose transmembrane domain had been replaced with that of Sso1p. As we show below, this altered protein is delivered to the plasma membrane and endocytosed normally but fails to be retrieved from the endocytic pathway and instead travels to the vacuole. In vps4 cells the Snc-sso chimera accumulated in enlarged class E compartments, often visible in optical sections as ring-shaped structures (Figure 2). These also labeled prominently with the endocytic tracer dye FM4-64 and could be seen to be adjacent to but distinct from the larger, more faintly stained vacuoles (Figure 2). Occasional bright dots, which may correspond to these structures, were seen in vps4 cells expressing the normal GFP-Snc1p (Figure 2), but these represented only a small fraction of the total GFP fluorescence. We conclude that GFP-Snc1p is mostly retrieved from the endocytic pathway before it arrives at the prevacuolar compartment and thus returns to the Golgi by a pathway distinct from that followed by Vps10p.
The cycling of GFP-Snc1p in vps5, vps17, and vps35 mutants has additional significance. These genes encode subunits of a putative coat complex termed retromer (Seaman et al., 1998), and the results imply that this coat is not required for retrieval of GFP-Snc1p from the endocytic pathway. Mutations that affect another coat, COPI, did have an effect: both sec21-1 (γ-COP) and cop1-1 (α-COP, originally called ret1-1) strains showed no GFP-Snc1p on the surface after incubation at 37°C (Figure 2). At 25°C the GFP-Snc1p distribution was normal in cop1-1 cells, but strikingly the sec21-1 cells showed an exclusively intracellular distribution even at this temperature, which is permissive for growth. These results are not easy to interpret, because COPI is implicated in intra-Golgi traffic, and as shown above, Golgi function is required for GFP-Snc1p recycling. However, cop1-1 cells show little defect in secretion or endocytosis to the vacuole even at 37°C, and at 25°C sec21-1 cells are also fully secretion competent (Letourneur et al., 1994; Hicke et al., 1997). We cannot therefore exclude the possibility that COPI is the coat responsible for mediating the retrieval of GFP-Snc1p from endosomes.
Sorting Signals on Snc1p: Internalization from the Plasma Membrane
To gain further insight into the trafficking of Snc1p, we sought to define amino acid sequences required for each sorting event on its circular route. At least three distinct budding steps can be imagined, each of which is potentially signal mediated: exit from the Golgi in secretory vesicles, internalization from the plasma membrane, and segregation away from material bound for the vacuole, which presumably occurs in endosomes.
Initial experiments focused on internalization from the plasma membrane. Extensive studies of the Snc1p homologue Vamp2 in animal cells have defined a region required for this, with two residues being especially important (Grote et al., 1995). These residues are conserved in Snc1p, and we made the equivalent mutations (V40A and M43A). When tagged with GFP the resultant mutant (en−) showed a plasma membrane distribution in wild-type cells. More significantly, it also was present on the plasma membrane in tlg1Δ cells (Figure 3). This result has two implications. First, it shows that these two residues form part of an evolutionarily conserved endocytosis signal, because their alteration inhibits uptake of Snc1p from the cell surface. Second, it confirms that the mislocalization of GFP-Snc1p observed in tlg1Δ cells is not due to a defect in its transport to the plasma membrane but rather to a postendocytic sorting defect.
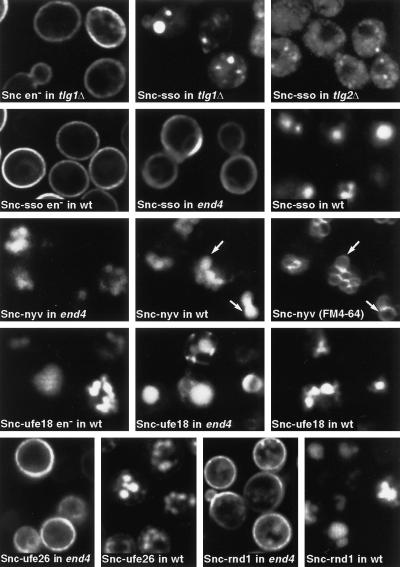
Figure 3.
Behavior of GFP-Snc1p chimeras with different transmembrane domains. The chimera TMD sequences and their locations are listed in Table 2; examples are shown here. Snc refers to GFP-Snc1p. Hyphenated names indicate chimeras with TMDs derived from Sso1p (sso), Nyv1p (nyv), Ufe1p with insertions to make the TMD 18 and 26 residues long (ufe18 and ufe26), or the sequence called random1 in Table 2 (rnd1). These were expressed in wild-type (wt), end4, or tlg mutants as indicated. Constructs labeled en− had in addition the point mutations that inhibit endocytosis (V40A and M43A). The wild-type cells expressing Snc-nyv were labeled with FM4-64 to reveal vacuoles, and both images are shown.
Sorting of GFP-Snc1p during Exit from the Golgi
To address specifically the sorting of GFP-Snc1p during exit from the Golgi, we assayed the effects of various sequence alterations either in the context of the V40A and M43A point mutations (en−) or in an endocytosis-defective end4 strain (Raths et al., 1993). This allowed the initial sorting of the altered proteins into the exocytic or vacuolar pathway to be determined without the complication of subsequent endocytosis. It has previously been shown that the destination of membrane proteins leaving the yeast Golgi is affected by the length and composition of their TMDs (Rayner and Pelham, 1997). Snc1p has a TMD of 20 residues, shorter than is typical for plasma membrane proteins in yeast (Munro, personal communication), and to investigate its importance for targeting we prepared a number of chimeras with altered TMDs. The results are summarized in Table 2, and examples are shown in Figure 3.
Table 2.
Fate of GFP-Snc1p constructs with altered transmembrane domains
| TMD sequence | Length | Origin | Location in end4 | Location (en−) | Location in wild type |
|---|---|---|---|---|---|
| KMCLALVIIILLVVIIVPIAVHFSR* | 20 | Snc1p | PM | PM | PM |
| KCWLIVFAIIVVVVVVVVVPAVVKTR* | 22 | Sso1p | PM | PM | Vac |
| KNITLLTFTIILFVSAAFMFFYLW* | 23 | Nyv1p | Vac | Vac | Vac |
| KLTTYGAIIMGVFILFLVLDYVG* | 18 | “Ufe1p” | Vac | Vac | Vac |
| KLTTYGAIIMGVFILFLVLVLDYVG* | 20 | “Ufe1p” | Vac | Vac | |
| KLTTYGAIIMGVFILFLVLVLVLDYVG* | 22 | “Ufe1p” | Vac | Vac | |
| KLTTYGAIIMGVFILFLVLVLVLVLVLDYVG* | 26 | “Ufe1p” | PM | PM | Vac |
| KVVVVVVVVVVVVVVVVVVVVVVVVVVHFSR* | 26 | Synthetic | PM | Vac | |
| KVFVIIFLVLLMVILLLVVLVHFSR* | 20 | Random 1 | PM | Vac | |
| KIVVVLILIFVIIIIMLLLILHFSR* | 20 | Random 2 | PM | Vac | |
| KLVLLLLFLLMFLLILLLVILHFSR* | 20 | Random 3 | PM | Vac | |
| KVLILIFVLFIIFILVIVLVIIL* | 22 | Random 4 | PM | Vac |
The TMD and following residues were exchanged for those shown. “Ufe1p” refers to lengthened versions of the TMD of Ufe1p, inserted residues being underlined in the sequence. PM, plasma membrane; Vac, vacuole.
In the first set of mutants, the normal TMD was replaced with those of other SNAREs. The TMD of the plasma membrane syntaxin Sso1p supported transport to the plasma membrane, as might be expected. In contrast, the TMD of Nyv1p (a vacuolar Snc1p homologue), although longer than that of Snc1p, was sufficient to redirect GFP-Snc1p to the vacuole.
The behavior of Snc1p contrasted with previous results obtained by altering the TMD length of the ER syntaxin Ufe1p, which suggested that a TMD of 26 residues was required for transport to the cell surface. To see whether this difference could be due to the presence of a dominant sorting signal in the cytoplasmic domain of Snc1p, we tested the Ufe1p-derived TMDs on GFP-Snc1p. These gave the expected results, with shorter TMDs (18–22 residues) resulting in accumulation in the vacuole, whereas the chimera with a 26-residue TMD was on the plasma membrane (Table 2 and Figure 3). Thus, the different length requirements relate to the specific TMD sequences rather than the nature of the attached cytoplasmic domain.
Finally, we tested Snc1 derivatives with a number of synthetic and random TMD sequences composed of F, I, V, L, and M and found that even random sequences of 20 residues can support exocytosis (listed in Table 2; an example is shown in Figure 3). We conclude that although the TMD does have an important influence on the sorting of Snc1p at this step, its length is not the only important feature. Other properties, such as overall hydrophobicity, may be equally significant. Indeed, the greater length required for the Ufe1p-derived TMD to reach the cell surface may reflect the relatively polar nature of its first few residues.
TMD-dependent Sorting of GFP-Snc1p in the Endocytic Pathway
We next tested the role of the Snc1p TMD in retrieval from the endocytic pathway by examining the same set of chimeras with a normal endocytic signal in wild-type cells. The results were striking: all the chimeras that accumulated on the surface when endocytosis was blocked were now found in the vacuole (Table 2; the Sso1, Ufe26, and random1 constructs are shown in Figure 3). This indicates that the nature of the TMD is crucial for the sorting of GFP-Snc1p into the retrieval pathway. Because the requirements for retrieval are clearly different from the requirements for exocytosis, this sorting event must occur in an organelle that is not itself on the exocytic pathway.
The properties of the TMD chimeras revealed several other features of endocytic sorting. For example, although the GFP domain was at the cytoplasmic N-terminal end of the GFP-Snc1p constructs, all chimeras that reached the vacuole showed a diffuse fluorescence that filled the organelle. This was true for the chimera bearing the Nyv1p TMD (Figure 3, compare the pattern of Snc-nyv with that of FM4-64), even though Nyv1p itself is present on the outer vacuolar membrane (Nichols et al., 1997). The most likely explanation is that the chimeric proteins enter the internal vesicles that are created as endosomes mature (Odorizzi et al., 1998). It seems that the Nyv1p TMD can target a protein to the endocytic pathway but is not sufficient to specify its precise location.
The ease with which the chimeras reached the vacuole contrasted with the inefficient delivery of GFP-Snc1p to the vacuole in tlg1 or tlg2 cells (Figure 2). Expression of the Snc1p-Sso1p chimera in tlg mutant cells resulted in similarly impaired transport (Figure 3). Thus the Tlg proteins are required for efficient passage of GFP-Snc1p from the plasma membrane through the endosomal system regardless of whether it carries a retrieval signal. This agrees broadly with previous findings obtained with other endocytic markers (Abeliovich et al., 1998; Holthuis et al., 1998a; Seron et al., 1998), although uptake of some markers, notably the dye FM4-64, is much less obviously affected by tlg mutations (Holthuis et al., 1998b).
Sequence Requirements for GFP-Snc1p Retrieval from Endosomes
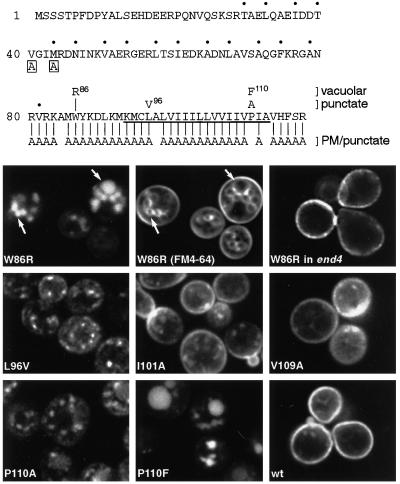
We investigated the requirements for retrieval in more detail using single amino acid substitutions. In one approach random mutants of the entire Snc1p sequence were prepared by error-prone PCR, and these were screened visually (in the form of GFP chimeras) for mislocalization. Two mutants were obtained whose altered distributions were due to single changes: W86R and L96V. The W86R mutation resulted in transport to the vacuole, and testing in end4 cells confirmed that it passed via the plasma membrane. In contrast, the L96V mutant protein was present in scattered, highly mobile punctate structures (Figure 4).
Figure 4.
GFP-Snc1p point mutants. At the top the sequence of Snc1p is shown. Dots indicate the heptad repeat involved in SNARE complex formation, and the transmembrane domain is underlined. Boxed letters show the point mutations that affect endocytosis. For residues 80–117, the mutations and the distributions of the mutant proteins are summarized. All but one of the Ala substitutions gave plasma membrane staining, with variable amounts of punctate staining in addition. The lower panels show vacuolar staining of W96R in wild type (with FM4-64 double label) but not in end4 cells, and the patterns in wild-type cells of L96V, P110A, P110F, and two examples of the more variable Ala substitutions (I101A and V109A). Unmutated GFP-Snc1p (wt) was analyzed in parallel for comparison. The FM4-64-labeled cells were photographed in the presence of the dye and thus show plasma membrane as well as vacuolar staining.
Given the obvious importance of the TMD and adjacent residues, we systematically mutated individual amino acids throughout this region (Figure 4). Surprisingly, changing individual residues to Ala had little effect: no such mutant showed a vacuolar distribution. However, the mutants sometimes showed an increase in labeled punctate structures like those found in L96V. This phenotype was highly variable, showing sensitivity to minor differences in expression level and growth state. Punctate structures could also be observed in some cells expressing unmutated GFP-Snc1p (Figure 4, compare the examples of I101A and V109A with wild type grown under the same conditions). Because of this variability, we were unable in blind comparisons to distinguish reliably any of the alanine mutants from wild type, with the exception of P110A, which showed a consistently punctate pattern in >80% of the cells, together with traces of faint vacuolar staining (Figure 4). A more radical change, P110F, resulted in vacuolar fluorescence in all cells (Figure 4).
These results suggest that the TMD and adjacent sequence are recognized over an extended region. Introduction of very different amino acids or changes to the entire TMD can disrupt recognition, but no single residue is critical. A similar phenomenon has been observed in studies of the Rer1p-mediated sorting of Sec12p, in which the TMD is also involved (Sato et al., 1996).
The sequence requirements for Snc1p sorting were considerably more stringent than the requirements for its function, in terms of sustaining growth. Thus we found that mutants that could reach the cell surface, including ones in which the TMD was replaced with a completely different sequence (W86R, P110F, Snc-sso, and Snc-ufe26), were capable of supporting growth in the absence of endogenous Snc1p and Snc2p even though they were ultimately missorted to the vacuole. Thus the TMD changes are unlikely to disrupt SNARE complex formation in general.
Punctate Intermediates in the Recycling Pathway
A possible explanation for the punctate pattern exhibited by the more subtly altered versions of GFP-Snc1p, notably the L96V and P110A mutants, is that it corresponds to intermediates in the normal recycling pathway. These mutations could, by reducing the affinity for a sorting receptor, increase the average time spent in early endosomes and result in accumulation of the protein there. More radical changes would of course block recognition completely and allow passage to the vacuole by default.
If this interpretation is correct, it should be possible to chase the mutant proteins out of the punctate structures and back to the plasma membrane by blocking endocytosis. To test this we expressed the L96V mutant in cells carrying a thermosensitive mutation in the calmodulin gene, cmd1-1. This mutation has been shown to block alpha factor endocytosis rapidly after a temperature shift (Kubler et al., 1994).
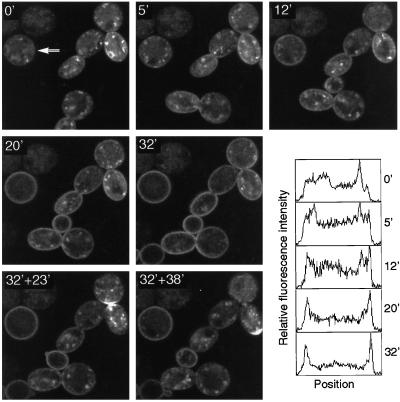
Figure 5 shows sequential confocal images of a group of such cells, taken before, during, and after a period of heating to the nonpermissive temperature (37°C). Before heating, the GFP fluorescence was mostly internal, with punctate structures clearly visible. After 12 min at high temperature a change was clearly detectable, as confirmed by intensity profiles across the diameter of one of the cells (Figure 5). By 20–30 min plasma membrane fluorescence predominated, with a corresponding decrease in punctate structures. This change was reversible: when heating was discontinued the cells cooled to 30°C over 10 min, and by 23 min the punctate pattern once again predominated in some of the cells, although others recovered more slowly. Quantitation of the images gave a 15-min half-time for the initial transition from punctate to plasma membrane pattern for the cell marked by an arrow in Figure 5. Because it may take up to 5 min for endocytosis to be affected by the temperature shift (Kubler et al., 1994), this is likely to be an overestimate. Nevertheless, a half-time of 10–15 min implies that exocytosis of the internal L96V protein occurs at a rate that is slightly slower than that of endocytosis of GFP-Snc1p in sec14 cells. This can account for the internal location of this mutant, assuming that it is endocytosed normally. Conversely, the predominantly surface location of the wild-type protein suggests that it is retrieved and exocytosed considerably more rapidly than the L96V mutant.
Figure 5.
Recycling of the GFP-Snc1p L96V mutant. cmd1-1 cells expressing the mutant protein were incubated on a heated stage at 28°C and imaged (0′). The temperature was then shifted to 37°C within the next 2 min, and images were taken at the indicated times (minutes) after the temperature shift. Heating was turned off at 32 min, and the cells were cooled slowly, reaching 30°C ∼10 min later. The last two images were taken 23 and 38 min after the heating was turned off. A polarized surface distribution of GFP-Snc1p can be seen at the forming septum (32′ + 23′) and at the incipient bud site (32′ + 38′) in the right cell. Fluorescence intensity profiles from a 12-pixel-wide horizontal strip across one cell, as indicated by the arrow (0′), are shown for each time point. These are normalized plots, which show the relative distribution of fluorescence across the cell, not absolute values. Some movement of cells on the slide occurred, and this accounts for the appearance of additional cells at 12 and 32 min.
Our conclusion is that the altered distribution of L96V and other subtly altered Snc1p mutants reflects a slowing of their retrieval from endosomes, but that the protein continues to cycle. Small changes in the rates of endocytosis and exocytosis would thus affect the steady-state pattern significantly, accounting for the variable phenotype of some of the mutants. A second conclusion is that the internal structures containing the L96V protein are intermediates in the recycling pathway. They are likely to be the early endosomes in which sorting of Snc1p occurs. We therefore sought to characterize them further using more conventional organelle markers.
Visualization of Intracellular Compartments
Because recycling of Snc1p involves the syntaxins Tlg1p and Tlg2p but not Pep12p, we sought morphological evidence for an endosomal compartment, distinct from the Golgi, containing one or both of the Tlg proteins. We have previously shown that the these proteins fractionate on a sucrose density gradient in two peaks, which we postulated to correspond to an endocytic and a late Golgi compartment (Holthuis et al., 1998a), but there has been some uncertainty as to their locations. Analysis has been complicated by the use of overexpression or GFP tagging, which may perturb the normal steady-state distribution of a recycling protein.
In an attempt to circumvent these problems we prepared affinity-purified antisera, which allowed detection of endogenous Tlg1p and the endosomal SNARE Pep12p by immunofluorescence. In addition, we used triple myc-tagged Tlg2p expressed from its own promoter on a CEN plasmid, in a strain from which TLG2 had been deleted. This tagged protein is fully functional, as judged from its ability to sustain GFP-Snc1p sorting, and fractionated in the same manner as endogenous Tlg2p on sucrose density gradients (our unpublished observations). As further markers we used Kex2p tagged at its C terminus with a triple HA epitope and the peripheral Golgi protein Sec7p tagged with GFP, the tag sequences being added to the normal chromosomal gene in each case. Sec7p-GFP has been shown to have a distribution similar to that of the wild-type protein (Seron et al., 1998), and HA-tagged Kex2p expressed in this manner had a fractionation profile on sucrose gradients indistinguishable from that of the normal protein.
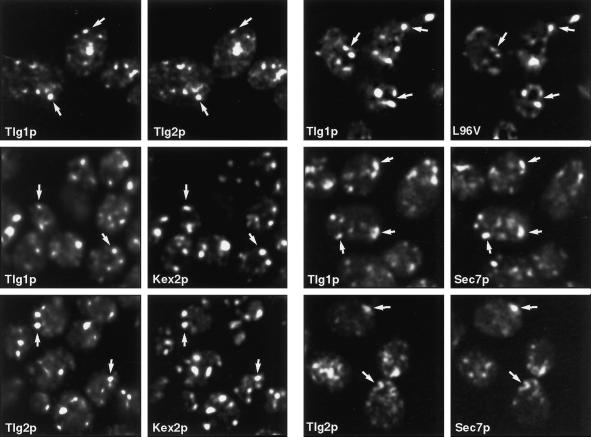
Using these reagents, we found by double-label immunofluorescence that Tlg1p, Tlg2p, and Kex2p have closely overlapping distributions. Furthermore, the L96V mutant of GFP-Snc1p was predominantly present in structures containing Tlg1p (Figure 6).
Figure 6.
Immunofluorescence of late Golgi/endosomal markers. Pairs of panels show fixed cells double labeled with appropriate antibodies or, in the case of Sec7p and the L96V mutant of GFP-Snc1p, with GFP. Arrows indicate equivalent positions. Note extensive colocalization of Tlg1p, Tlg2p, Kex2p, and the L96V mutant but limited overlap between Tlg1p or Tlg2p and Sec7p.
Kex2p, and by inference the similarly distributed Tlg proteins, would be expected to be present at least partially in Golgi compartments. To investigate this we performed double-label experiments with the Tlg proteins and the Golgi marker Sec7p. There was significant colocalization of both Tlg1p and Tlg2p with Sec7p-GFP, but the overlap was incomplete, with <50% of the discrete structures coinciding (Figure 6). This is consistent with the view that Tlg1p, Tlg2p, and Kex2p all cycle between late Golgi cisternae and an endosomal compartment and are distributed between these structures.
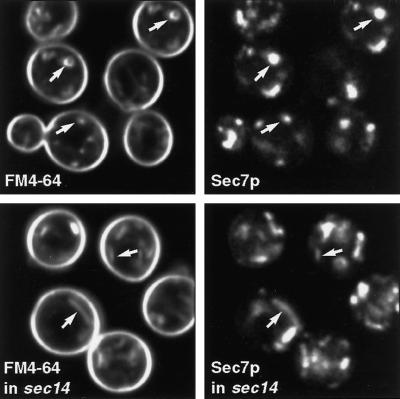
Tlg1p-positive structures that lack Sec7p are good candidates for recycling endosomes. We considered that one way to label these selectively, and to distinguish them from Golgi cisternae, might be to label them by brief exposure of cells to the endocytic tracer dye FM4-64. We therefore incubated cells expressing Sec7p-GFP with FM4-64 and imaged the live cells after brief periods of dye uptake. Figure 7 shows that there was substantial labeling of at least some of the Sec7p-containing structures with FM4-64. This was true as soon as internalization of the dye could be detected (after ∼5 min), although there were also FM4-64-containing structures that lacked Sec7p. We repeated the experiment in a sec14 strain at a nonpermissive temperature. Under these conditions late Golgi cisternae proliferate, forming large linear structures, which are revealed by the Sec7p-GFP, and these could also be labeled by FM4-64 (Figure 7). Thus, although FM4-64 eventually accumulates in vacuoles, a substantial proportion of the dye evidently follows the recycling pathway to the Golgi apparatus, as defined by the presence of Sec7p and sensitivity to the effects of sec14, and this occurs rapidly. As a consequence, we were not able to distinguish endosomal structures from Golgi cisternae by FM4-64 labeling alone.
Figure 7.
Uptake of FM4-64 to the Golgi. Live cells expressing Sec7p-GFP were incubated for 15 min with FM4-64 before imaging. The sec14 cells were incubated at 37°C for 10 min before addition of FM4-64. Note the elongated structures in these cells that are labeled both for Sec7p and the endocytic tracer.
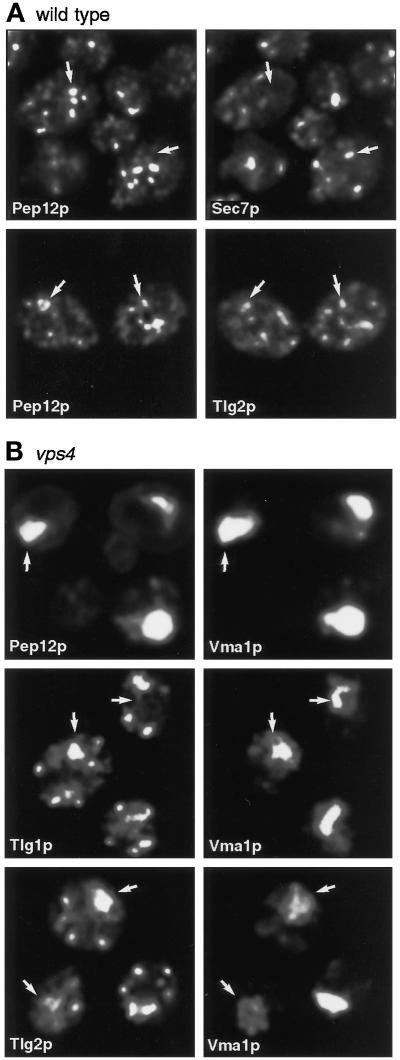
We next asked whether the Tlg-containing endosomal membranes were distinct from those containing Pep12p. Double labeling showed that Pep12p was present in scattered punctate structures, which as expected showed little overlap with those containing Sec7p (Figure 8A). A small amount of apparent overlap was detectable between Pep12p and Tlg2p (Figure 8A). We could not compare Tlg1p and Pep12p directly by immunofluorescence, because both were detected with rabbit antisera.
Figure 8.
Distribution of Pep12p relative to other markers. (A) Double label of fixed cells showing dissimilar distributions of Pep12p and Sec7p and limited overlap between Pep12p and Tlg2p. (B) Double label of vps4 cells showing restriction of Pep12p but not Tlg1p or Tlg2p to the class E compartment marked by Vma1p.
To investigate the potential colocalization of Pep12p and the Tlg proteins further, we examined a vps4Δ strain. In this mutant, as in other class E vps mutants, both vacuolar and prevacuolar markers accumulate in enlarged late endosomes, the class E compartment, which can be detected with antibodies against the vacuolar ATPase subunit Vma1p (reviewed by Conibear and Stevens, 1998). Figure 8B shows that Pep12p was confined to the class E compartment in vps4 cells, whereas much of the Tlg1p and Tlg2p remained in discrete punctate structures, as in wild-type cells.
Localization of Tlg1p by Immuno-EM
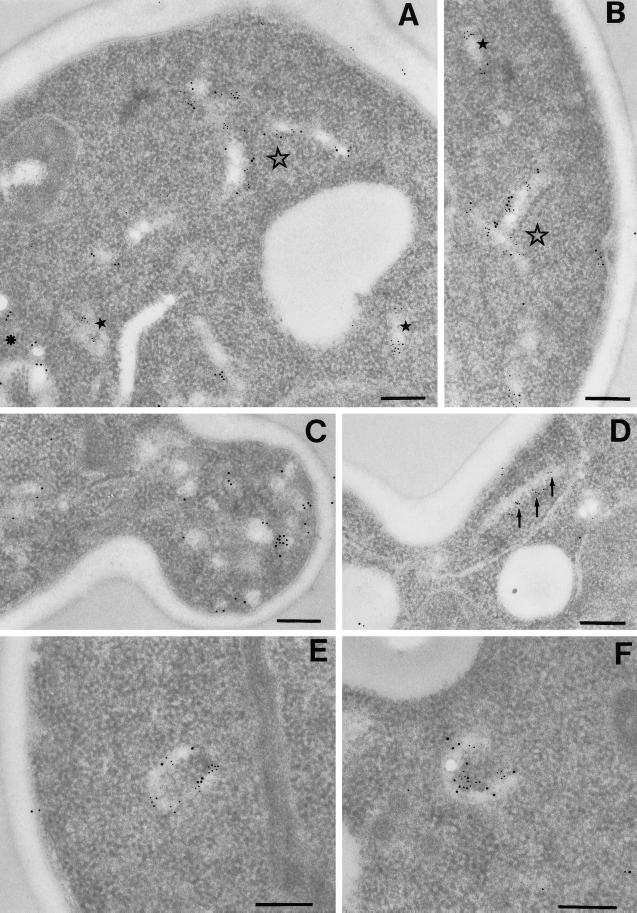
To further characterize the membranes marked by Tlg1p and their relationship to those containing Pep12p, we performed double-label immuno-EM with antibodies coupled to gold particles of different sizes (Figure 9). Tlg1p labeling was observed in numerous heterogeneous structures, as was Pep12p labeling. As expected from the immunofluorescence results, most of the structures could be labeled for only one of the proteins. However, membranes containing both could also be detected, as shown by the examples in Figure 9, A and B. In addition to elongated cisternae, Tlg1p was commonly observed in a subset of the vesicle-like structures (∼100 nm in diameter) found in small buds (Figure 9C), although labeling of the plasma membrane was rare. Pep12p was not observed in these structures.
Figure 9.
Immuno-EM of SNAREs. Immunolocalization of SNAREs on thin sections was performed as described in MATERIALS AND METHODS. Pep12p and Sed5p were detected using 5 nm IgG-colloidal gold, and Tlg1p was detected using 10 nm IgG-colloidal gold. The contrast of the gold particles has been digitally enhanced for clarity. (A and B) Localization of Pep12p and Tlg1p. Filled stars denote structures with only Pep12p labeling; the asterisk marks structures with only Tlg1p labeling; and the unfilled star marks structures showing colocalization of Pep12p and Tlg1p. (C–F) Localization of Sed5p and Tlg1p. The arrows mark a structure labeled only with Sed5p. Note the 100-nm vesicles with only Tlg1p labeling in C and structures showing colocalization of Sed5p and Tlg1p in E and F. Bars, 200 nm.
We also performed double labeling with antibodies to Sed5p. Sed5p itself was found largely in elongated membranes, often with a curved or circular cross section. The Tlg proteins are mostly absent from early Golgi compartments, as judged both by immunofluorescence (Holthuis et al., 1998a) and by immuno-EM, but we did observe cisternae that labeled with both anti-Tlg1p and anti-Sed5p (Figure 9, E and F). As with Pep12p, Sed5p was not observed in the Tlg1p-positive vesicular structures in small buds.
Counting of labeled organelles revealed that in these cells 28% of the Tlg1p-positive membranes also contained Pep12p, and 15% of them contained Sed5p. These results indicate that Tlg1p can reach both Pep12p-containing structures and compartments marked by Sed5p but is largely present in the steady state in heterogeneous structures that are likely components of the late Golgi/early endosome recycling pathway.
DISCUSSION
In this paper we have used a GFP-tagged version of the exocytic SNARE Snc1p to probe the recycling pathway from the plasma membrane and the sorting events that are involved. Because the fusion protein is functional, we assume that its behavior broadly reflects that of its untagged counterpart. However, our conclusions are derived from the behavior of the chimera and do not require its transport kinetics to be identical to those of the wild-type protein.
It is generally assumed that SNARE proteins that travel on vesicles are recycled for reuse, and it is clear that GFP-Snc1p is endocytosed and reenters exocytic vesicles. Because mutations that block traffic through the Golgi lead to rapid removal of GFP-Snc1p from the cell surface, it seems likely that recycling occurs via the Golgi rather than by a direct endosome–plasma membrane route. It is difficult to exclude completely the possibility that Golgi function is required only indirectly for recycling, as it is to some extent for the later stages of the endocytic pathway (Hicke et al., 1997), but if internalized Snc1p is to be used for subsequent rounds of exocytic traffic, it must return to the Golgi. Independent evidence for such a route is provided by our observation that FM4-64 dye is efficiently transferred to Golgi structures, as defined by the presence of Sec7p and their morphological alteration in a sec14 mutant. One consequence of recycling via the Golgi is that redelivery to the surface occurs in a polarized manner, a feature that may be important for the specific targeting of some recycling proteins such as the chitin synthase subunit Chs3p (Holthuis et al., 1998b).
GFP-Snc1p recycling is independent of the late endosome/prevacuolar compartment that has been characterized previously. Thus, although it requires the presence of the syntaxins Tlg1p and Tlg2p, it does not require the late endosomal SNARE Pep12p. Furthermore, it is unaffected by vps4, a mutation that inhibits exit from the prevacuolar compartment, or by removal of Vps5p, Vps17p, and Vps35p, which are components of the retromer coat that mediates the retrieval of proteins from this compartment (Seaman et al., 1998). There must therefore be two distinct routes from the endocytic pathway to the Golgi complex, one from early endosomes and one from later ones. Given the involvement of Tlg1p and Tlg2p in Chs3p trafficking (Holthuis et al., 1998b), it is likely that Chs3p follows a cycling itinerary similar to that of Snc1p.
The choice that faces an endocytosed protein is best illustrated by comparing the fate of GFP-Snc1p with that of a mutant version in which the TMD has been exchanged for that of Sso1p. Both versions are endocytosed in an END4-dependent manner, and both require an endocytosis signal that is similar to that on the animal cell versions of Snc1p (Grote et al., 1995). This in itself is striking, because the requirements for endocytosis seem to differ somewhat in yeast and animal cells.
Once internalized, the altered form of GFP-Snc1p proceeds to the vacuole, apparently by default. In contrast, the version with its normal TMD is transferred to the Golgi in a step that is crucially dependent on sequences within and adjacent to the TMD. This sorting event is likely to occur in a compartment marked by Tlg1p and Tlg2p, for several reasons. First, the Tlg proteins are required for recycling, whereas Pep12p is not. Second, although Tlg1p is not present on the plasma membrane Snc1p can readily be found in complexes containing Tlg1p (Holthuis et al., 1998a), suggesting that fusion occurs between endocytic vesicles containing Snc1p and a Tlg1p-bearing membrane. Third and most importantly, subtle alterations to the Snc1p TMD such as the L96V mutation result in the accumulation of the protein in Tlg1p-positive structures. Because Snc1p can be chased reversibly from these structures to the cell surface when endocytosis is blocked by the cmd1-1 mutation, they evidently comprise a station on the recycling pathway. We interpret the accumulation of the mutants there as being due to the slowing of their retrieval when recognition of the sorting signal is impaired, which would imply that these Tlg1p-positive structures are where sorting occurs. This conclusion fits well with our previous finding that Tlg1p and Tlg2p are sufficient to mediate recycling of Chs3p even in the absence of Pep12p and Vam3p, and that in wild-type cells the internal pool of Chs3p cofractionates with Tlg1p and Tlg2p (Holthuis et al., 1998b).
The sensitivity of GFP-Snc1p retrieval to mutations within the TMD suggests that this domain is recognized by a transmembrane receptor and actively recruited into carriers destined for the Golgi. It also provides strong evidence that the endosomes in which sorting occurs are physically distinct from the late Golgi compartment (the trans-Golgi network [TGN] equivalent). This is because the TMD requirements for transport from Golgi to cell surface are much less specific than the requirements for retrieval. If endocytosis occurred directly to the TGN, then Snc1p chimeras with heterologous TMDs would simply return to the surface, rather than pass to the vacuole.
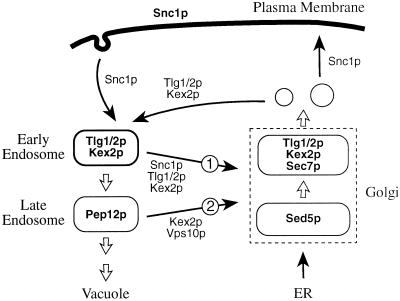
Figure 10 illustrates a simple model for the recycling pathway. In this model, late Golgi (TGN) membranes segregate into exocytic vesicles (containing Snc1p) and others containing the resident TGN proteins, including Tlg1p and Tlg2p, which do not progress to the plasma membrane. The Tlg1p detected in small buds by immuno-EM may be in these nonexocytic carriers, because only small amounts of Tlg1p are found on the cell surface even when endocytosis is blocked (Holthuis et al., 1998b; our unpublished observations). Late Golgi-derived vesicles subsequently fuse with Snc1p-bearing endocytic vesicles to form the earliest endosomes, which may continue to receive vesicles from both sources. From these structures most TGN proteins, and others from the plasma membrane such as Snc1p and Chs3p, are selectively returned to the Golgi. The remaining proteins reach a later endosome marked by Pep12p. Subsequent steps involve retromer-dependent removal of proteins such as the carboxypeptidase Y (CPY) receptor Vps10p in vesicles bound for the Golgi and budding of membranes into the lumen of the endosome to form a multivesicular body, which eventually fuses with the vacuole (Odorizzi et al., 1998; Seaman et al., 1998).
Figure 10.
Proposed outline model for endocytic traffic. Bold names indicate major locations of the indicated proteins; thin arrows represent vesicular transport steps; thick arrows show “default” pathways, which may correspond to compartment maturation or direct fusion. The two retrieval pathways are numbered: 1 is the route followed by GFP-Snc1p, dependent on Tlg1p and Tlg2p; 2 is the later retromer-dependent and vps4-sensitive pathway followed by Vps10p. Exactly how Vps10p reaches late endosomes—via early endosomes or by a more direct pathway—is not yet known. Although the two retrieval pathways are drawn as completely separate, they may have overlapping origins and/or a common destination. See DISCUSSION for details.
Recruitment of Snc1p and other recycling proteins into Golgi-bound vesicles is likely to be mediated ultimately by a cytoplasmic coat. We have shown that the retromer coat is not required for retrieval of GFP-Snc1p. Also, retrieval is not affected by deletion of any of the known adaptin homologues in yeast (APL1-6, APM1-4, and APS1-3; Van Horssen and Pelham, unpublished observations), although this does not rule out the possibility that clathrin is involved. The COPI coat is a good candidate, because retrieval appears more sensitive to mutations in coatomer subunits than is secretion itself. This would fit with results in animal cells that implicate COPI in endosomal sorting (see Daro et al., 1997, and references therein). However, the requirement for Golgi function for Snc1p recycling and the known involvement of COPI in this makes it difficult to draw a firm conclusion.
Our model predicts that organelles containing Tlg1p and other TGN proteins are physically and functionally heterogeneous. There is indeed evidence for such heterogeneity: the proteins are found in membranes of two different densities, which we have previously suggested might correspond to Golgi and early endosomes (Holthuis et al., 1998a), and immunofluorescence shows that only a subset of the structures containing Tlg1p or Tlg2p carry the Golgi marker Sec7p. A more specific prediction is that the earliest endocytic structures should contain markers destined both for recycling and transport to the vacuole, but this has proved hard to demonstrate: FM4-64 appears to be rapidly transferred to the Golgi, and a GFP-tagged version of the alpha factor receptor Ste2p rapidly reaches Pep12p-containing endosomes after uptake is stimulated with alpha factor (our unpublished observations; also see Holthuis et al., 1998b). We note that passage through Tlg1p-containing endosomes may not always be obligatory for endocytosed material and vacuolar hydrolases. Even in the absence of both Tlg1p and Tlg2p, FM4-64 can reach endosomes, probably by direct fusion of primary endocytic vesicles with membranes bearing Pep12p, and a substantial proportion of newly synthesized CPY can reach the vacuole (Holthuis et al. 1998a,b).
How proteins present in Tlg1p-containing endosomes are transferred to later ones marked by Pep12p is an interesting question. This appears to be the default pathway once retrieval signals are removed, which argues against a highly selective mechanism. The structures containing both Tlg1p and Pep12p that we observe by immuno-EM are plausible intermediates in the process and could in principle arise by fusion of the two types of endosome (or of membranes derived from them). However, there is another possibility. The later stages of endocytosis seem to occur by maturation and fusion of membranes to the vacuole, and this requires Pep12p to be removed, because it does not accumulate on the vacuole. Furthermore, vps mutations that block recycling of Vps10p to the Golgi also trap Pep12p in prevacuolar structures. Thus, it may be that Pep12p recycles through the Golgi (Figure 10, route 2) and is delivered to early endosomes, promoting their maturation into later structures and becoming concentrated as other proteins are removed.
The existence of two distinct pathways back from endosomes to the Golgi explains several previous observations. For example, although Snc1p, Chs3p, Tlg1p, and Tlg2p seem to recycle mainly from early endosomes, there is good evidence that the late Golgi proteins Kex2p and DPAPA contain cytoplasmic signals that mediate their retromer-dependent retrieval from later endosomes, and that they can, like the CPY receptor Vps10p, reach the class E compartment (an abnormal prevacuolar structure) in appropriate vps mutants (Voos and Stevens, 1998; Nothwehr et al., 1999). However, both DPAPA and Kex2p have a second signal, which slows their entry into this compartment and which has been interpreted as a TGN retention signal (Brickner and Fuller, 1997; Bryant et al., 1997). We suggest that these are in fact signals for retrieval from early endosomes, and that these proteins can follow both routes. The model also explains why, although GFP-Snc1p sorting is severely disrupted in a tlg2 mutant, CPY sorting is barely affected and no more than half of the DPAPA protein is lost to the vacuole: in this mutant, the late endosome retrieval pathway should remain functional (Abeliovich et al., 1998; Holthuis et al., 1998a; Nichols et al., 1998; Seron et al., 1998).
The precise roles of Tlg1p and Tlg2p in these retrieval pathways remain to be worked out. Both are required for the route from early endosomes, but their mutant phenotypes are different—the pattern of GFP-Snc1p is qualitatively different in tlg1 and tlg2 cells. As discussed previously (Holthuis et al., 1998b; Nichols et al., 1998), Tlg1p has the unusual property of binding to other syntaxins (Tlg2p and Sed5p) and thus could in part help target vesicles (on either route) to the Golgi. Tlg2p is a more typical syntaxin and might serve as a vesicle acceptor in early endosomes, the late Golgi, or both. However, because removal of either can potentially alter the location of the other, it is very difficult to discern their individual functions from these genetic experiments.
The two routes from endosomes to the Golgi use different machinery and most likely originate in distinct organelles, but whether they have different endpoints is less clear. Returning Golgi proteins following route 1 in Figure 10 might be delivered selectively to the late Golgi, perhaps using Tlg2p. However, the relatively efficient sorting of CPY in tlg mutants argues that the Vps10p recycling pathway (route 2) can use Sed5p. Other possibilities are that both routes use early and late Golgi interchangeably, or that all traffic to the Golgi uses Sed5p.
The concept of traffic from endosomes to the early Golgi may help explain the recent finding that in a different yeast strain, W303, Tlg1p is essential for transport of CPY from the ER to the Golgi. In these cells the distribution of Tlg1p is reported to overlap substantially with that of Sed5p (Coe et al., 1999). Our immuno-EM studies confirm that Tlg1p is capable of reaching membranes that contain Sed5p, although we found double-labeled structures to be infrequent. Together, the evidence suggests that Tlg1p helps vesicles derived from the endocytic pathway fuse with the Sed5p compartment, and that delivery of some component via this route is necessary for normal Golgi function. Evidently in the W303 strain this route is more dependent on Tlg1p, or more important for Golgi function, than in the SEY6210 strain that we have used. Why this should be is not obvious, but W303 cells are also more sensitive to disruption of YPT6, a gene whose mutant phenotype is strikingly similar to that of tlg1, and this has been shown to be due to mutation of the SSD1 locus in W303 (Li and Warner, 1996; Tsukada and Gallwitz, 1996). The differences in Tlg1p distribution presumably reflect different rate-limiting steps in its itinerary and illustrate the limitations of using circulating integral membrane proteins as compartment markers. Peripheral proteins that dissociate when a compartment matures or fragments may be more useful, and we are currently seeking early endosomal markers of this type.
The protein sorting events in the prevacuolar compartment in yeast are relatively well characterized, because defects at this point have an easily scored vps phenotype, and many mutants have been isolated. In contrast, sorting defects in early endosomes have much more subtle phenotypes, and this process has been harder to detect and study. The convenience of GFP-Snc1p as a marker should facilitate future studies, including the identification of the receptor and other machinery responsible for its retrieval.
ACKNOWLEDGMENTS
We thank Joost Holthuis, Ben Glick, Rob Arkowitz, John Kilmartin, and Matthew Seaman for yeast strains and plasmids. The work of C. P.-B. and H.R. was supported by a grant from the Swiss National Science Foundation.
REFERENCES
- Abeliovich H, Grote E, Novick P, Ferro-Novick S. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J Biol Chem. 1998;273:11719–11727. doi: 10.1074/jbc.273.19.11719. [DOI] [PubMed] [Google Scholar]
- Babst M, Sato TK, Banta LM, Emr SD. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 1997;16:1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer KA, Rieder SE, Emr SD, Jones EW. Novel syntaxin homologue, Pep12p, required for the sorting of lumenal hydrolyses to the lysosome-like vacuole in yeast. Mol Biol Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner JH, Fuller RS. SOI1 encodes a novel, conserved protein that promotes TGN-endosomal cycling of Kex2p and other membrane proteins by modulating the function of two TGN localization signals. J Cell Biol. 1997;139:23–36. doi: 10.1083/jcb.139.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Piper RC, Gerrard SR, Stevens TH. Traffic into the prevacuolar/endosomal compartment of Saccharomyces cerevisiae: a VPS45 dependent intracellular route and a VPS45 independent exocytic route. Eur J Cell Biol. 1998;76:43–52. doi: 10.1016/S0171-9335(98)80016-2. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Stevens TH. Two separate signals act independently to localize a yeast late Golgi membrane protein through a combination of retrieval and static retention. J Cell Biol. 1997;136:287–297. doi: 10.1083/jcb.136.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CG, Peterson M, Cowles CR, Emr SD. A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol Biol Cell. 1997;8:1089–1104. doi: 10.1091/mbc.8.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H, Kerjan P, Surdin-Kerjan Y. The Saccharomyces cerevisiae MET3 gene: nucleotide sequence and relationship of the 5′ noncoding region to that of. MET25. Mol Gen Genet. 1987;210:307–313. doi: 10.1007/BF00325699. [DOI] [PubMed] [Google Scholar]
- Coe JG, Lim AC, Xu J, Hong W. A role for Tlg1p in the transport of proteins within the Golgi apparatus of Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:2407–2423. doi: 10.1091/mbc.10.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear E, Stevens TH. Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim Biophys Acta. 1998;1404:211–230. doi: 10.1016/s0167-4889(98)00058-5. [DOI] [PubMed] [Google Scholar]
- Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- Cowles CR, Snyder WB, Burd CG, Emr SD. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J. 1997;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daro E, Sheff D, Gomez M, Kreis T, Mellman I. Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J Cell Biol. 1997;139:1747–1759. doi: 10.1083/jcb.139.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T, Rieder SE, Emr SD. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NG, Hoerexka JL, Sprague GF. Cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff A, Schekman R. Functional compartments of the yeast Golgi apparatus are defined by the sec7 mutation. EMBO J. 1989;8:2695–2702. doi: 10.1002/j.1460-2075.1989.tb08410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli M, Riezman H. Endocytic internalization in yeast and animal cells. J Cell Sci. 1998;111:1031–1037. doi: 10.1242/jcs.111.8.1031. [DOI] [PubMed] [Google Scholar]
- Grote E, Hao JC, Bennet MK, Kelly RB. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Hay JC, Scheller RH. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- Hicke L, Zanolari B, Pypaert M, Roher J, Riezman H. Transport through the yeast endocytic pathway occurs via morphologically distinct compartments and requires an active secretory pathway and Sec18p/N-ethylmaleimide-sensitive fusion protein. Mol Biol Cell. 1997;8:13–31. doi: 10.1091/mbc.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Dhruvakumar S, Pelham HRB. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 1998a;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Pelham HRB. The syntaxin Tlg1p mediates trafficking of chitin synthase III to polarized growth sites in yeast. Mol Biol Cell. 1998b;9:3383–3397. doi: 10.1091/mbc.9.12.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Adams AE. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984;98:922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler E, Schimmoller F, Riezman H. Calcium-independent calmodulin requirement for endocytosis in yeast. EMBO J. 1994;13:5539–5546. doi: 10.1002/j.1460-2075.1994.tb06891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Li B, Warner JR. Mutation of the Rab6 homologue of Saccharomyces cerevisiae, YPT6, inhibits both early Golgi function and ribosome biosynthesis. J Biol Chem. 1996;271:16813–16819. doi: 10.1074/jbc.271.28.16813. [DOI] [PubMed] [Google Scholar]
- Muhlrad D, Hunter R, Parker R. A rapid method for localized mutagenesis of yeast genes. Yeast. 1992;8:79–82. doi: 10.1002/yea.320080202. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Holthuis JC, Pelham HRB. The Sec1p homologue Vps45p binds to the syntaxin Tlg2p. Eur J Cell Biol. 1998;77:263–268. doi: 10.1016/s0171-9335(98)80084-8. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Pelham HRB. SNAREs and membrane fusion in the Golgi apparatus. Biochim Biophys Acta. 1998;1404:9–31. doi: 10.1016/s0167-4889(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HRB, Wickner W, Haas A. Homotypic vacuolar fusion mediated by v- and t-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Nothwehr SF, Bruinsma P, Strawn LA. Distinct domains within Vps35p mediate the retrieval of two different cargo proteins from the yeast prevacuolar/endosomal compartment. Mol Biol Cell. 1999;10:875–890. doi: 10.1091/mbc.10.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for posttranslational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- Pelham HRB. SNAREs and the secretory pathway—lessons from yeast. Exp Cell Res. 1999;247:1–8. doi: 10.1006/excr.1998.4356. [DOI] [PubMed] [Google Scholar]
- Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–545. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescianotto-Baschong C, Riezman H. Morphology of the yeast endocytic pathway. Mol Biol Cell. 1998;9:173–179. doi: 10.1091/mbc.9.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopov V, Govindan B, Novick P, Gerst JE. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell. 1993;74:855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Morin-Ganet MN, Rambourg A, Clermont Y, Kepes F. Role of endoplasmic reticulum-derived vesicles in the formation of Golgi elements in sec23 and sec18 Saccharomyces cerevisiae mutants. Anat Rec. 1998;251:256–264. doi: 10.1002/(SICI)1097-0185(199806)251:2<256::AID-AR15>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Raths S, Rohrer J, Crausaz F, Riezman H. End3 and end4—2 mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner J, Pelham HRB. Transmembrane domain dependent sorting of proteins to the ER and plasma membrane in yeast. EMBO J. 1997;16:1832–1841. doi: 10.1093/emboj/16.8.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder SE, Banta LM, Kohrer K, McCaffery JM, Emr SD. Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuolar sorting mutant. Mol Biol Cell. 1996;7:985–999. doi: 10.1091/mbc.7.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Sato M, Sato K, Nakano A. Endoplasmic reticulum localization of Sec12p is achieved by two mechanisms: Rer1p-dependent retrieval that requires the transmembrane domain and Rer1p-independent retention that involves the cytoplasmic domain. J Cell Biol. 1996;134:279–293. doi: 10.1083/jcb.134.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seron K, et al. A yeast t-SNARE involved in endocytosis. Mol Biol Cell. 1998;9:2873–2889. doi: 10.1091/mbc.9.10.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Kruger B, Frank R, Crausaz F, Riezman H. Partial purification and characterization of early and late endosomes from yeast. J Biol Chem. 1993;268:14376–14386. [PubMed] [Google Scholar]
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Jones EW. Pth1/Vam3p is the syntaxin homolog at the vacuolar membrane of Saccharomyces cerevisiae required for the delivery of vacuolar hydrolases. Genetics. 1998;148:85–98. doi: 10.1093/genetics/148.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada M, Gallwitz D. Isolation and characterization of SYS genes from yeast, multicopy suppressors of the functional loss of the transport GTPase Ypt6p. J Cell Sci. 1996;109:2471–2481. doi: 10.1242/jcs.109.10.2471. [DOI] [PubMed] [Google Scholar]
- Ungermann C, Nichols BJ, Pelham HRB, Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated organelles, is disassembled and activated for docking and fusion. J Cell Biol. 1998;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tuinen E, Riezman H. Immunolocalization of glyceraldehyde-3-phosphate dehydrogenase, hexokinase and carboxypeptidase Y in yeast cells at the ultrastructural level. J Histochem Cytochem. 1987;35:327–333. doi: 10.1177/35.3.3546482. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr S. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos W, Stevens TH. Retrieval of resident late-Golgi membrane proteins from the prevacuolar compartment of Saccharomyces cerevisiae is dependent on the function of Grd19p. J Cell Biol. 1998;140:577–590. doi: 10.1083/jcb.140.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Nakamura N, Ohsumi Y, Hirata A. Vam3p, a new member of syntaxin related protein, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae. J Cell Sci. 1997;110:301–306. doi: 10.1242/jcs.110.11.1299. [DOI] [PubMed] [Google Scholar]
- Webb GC, Hoedt M, Poole LJ, Jones EW. Genetic interactions between a pep7 mutation and the PEP12 and VPS45 genes: evidence for a novel SNARE component in transport between the Saccharomyces cerevisiae Golgi complex and endosome. Genetics. 1997;147:467–478. doi: 10.1093/genetics/147.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B, Emr SD, Riezman H. Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr Opin Cell Biol. 1998;10:513–522. doi: 10.1016/s0955-0674(98)80067-7. [DOI] [PubMed] [Google Scholar]
- Wooding S, Pelham HRB. The dynamics of Golgi protein traffic visualized in living yeast cells. Mol Biol Cell. 1998;9:2667–2680. doi: 10.1091/mbc.9.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Wu JN, Schober W, Lewis DE, Vida T. Isolation of yeast mutants defective for localization of vacuolar vital dyes. Proc Natl Acad Sci USA. 1998;95:11721–11726. doi: 10.1073/pnas.95.20.11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, Chuang JS, Schekman RW. Chs1p and Chs3p, two proteins involved in chitin synthesis, populate a compartment of the Saccharomyces cerevisiae endocytic pathway. Mol Biol Cell. 1996;7:1909–1919. doi: 10.1091/mbc.7.12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, Chuang JS, Tsung M, Hamamoto S, Schekman R. Chs6p-dependent anterograde transport of Chs3p from the chitosome to the plasma membrane in Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:1565–1576. doi: 10.1091/mbc.9.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]