Abstract
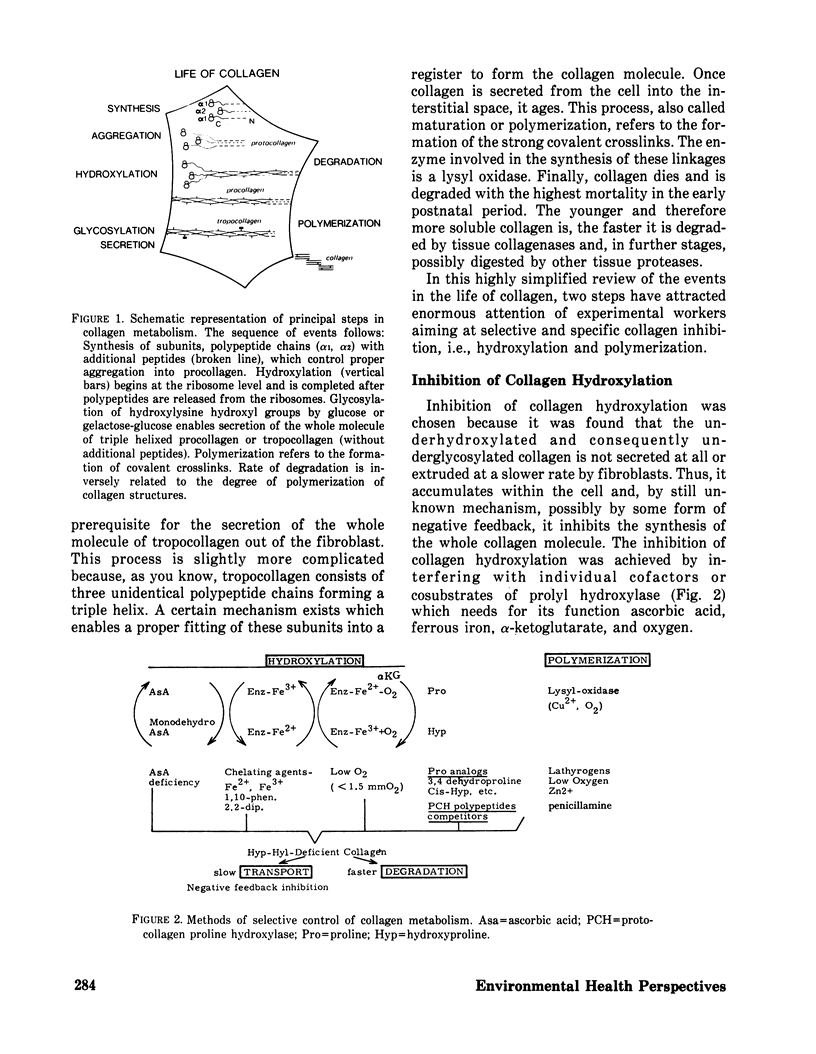
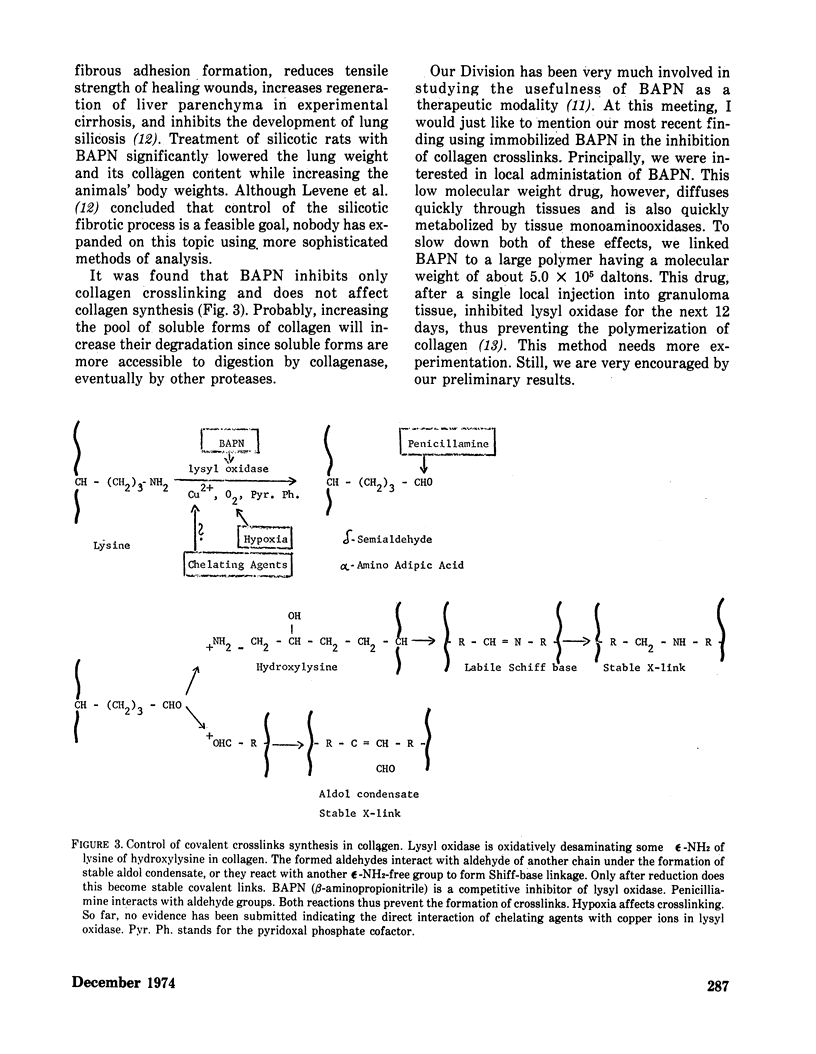
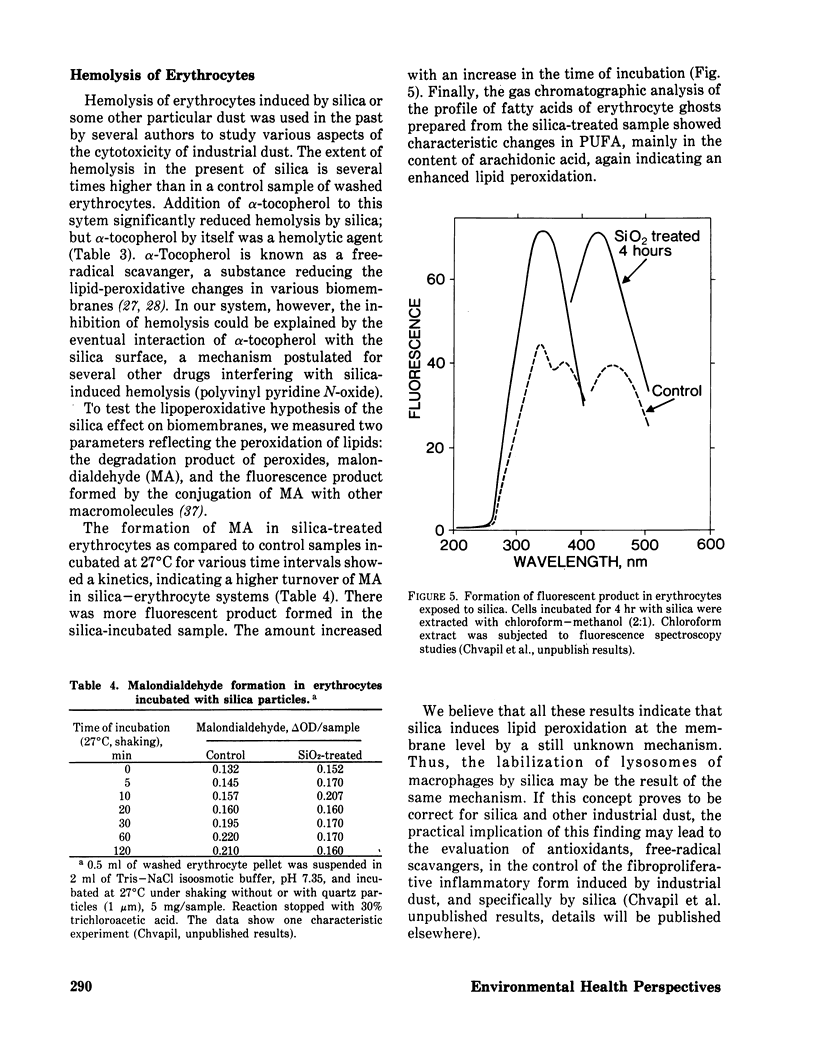
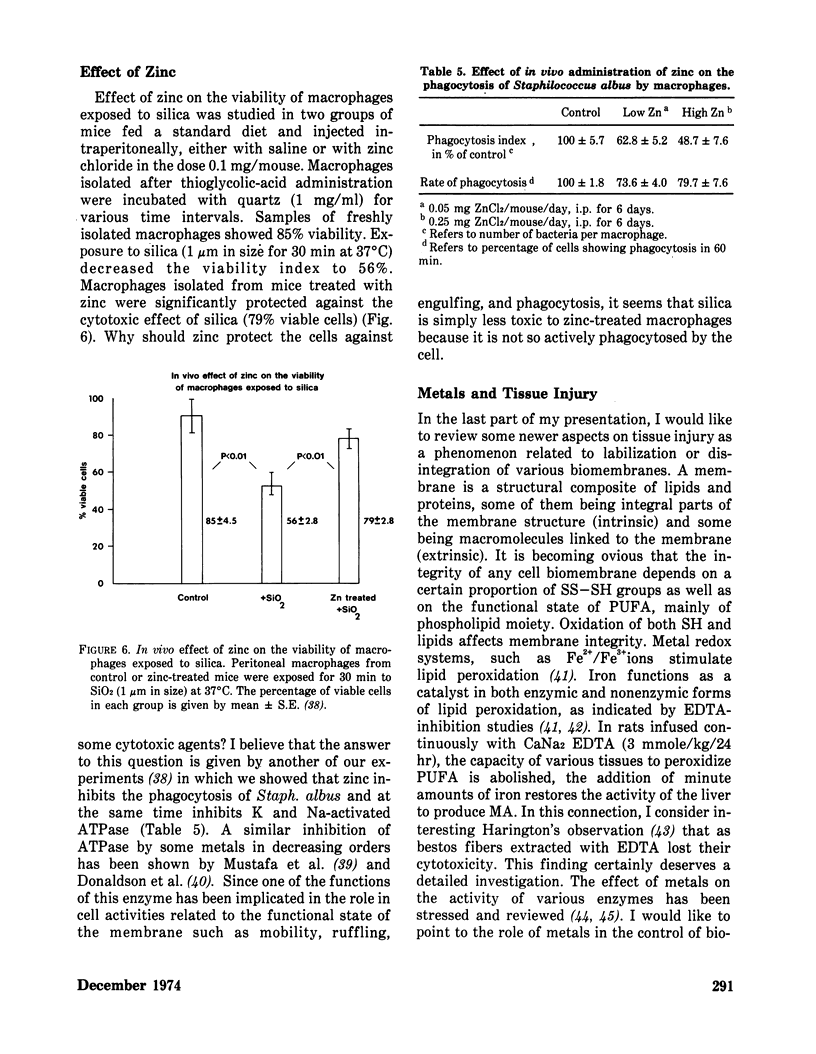
Methods controlling tissue fibrosis are classified into those specifically inhibiting various metabolic aspects of collagen selectively in the injured tissue (ascorbic acid deficiency, effect of agent chelating Fe2+, proline analogs, lathyrogens). The most promising method seems to be the blocking of crosslinks formation among collagen molecules by β-aminopropionitrile, a competitive inhibitor of a crosslinking enzyme, lysyl oxidase. The second group of methods is called nonspecific, as they affect any stage of inflammatory process preceding the activation of fibroblasts. The importance of activated macrophages in the stimulation of fibroblast is discussed. Finally, a new concept is proposed, indicating the function of zinc ions in the control of the integrity of biomembrances, tissue reactivity to noxious agents. It is suggested that zinc may control NADPH dependent lipid peroxidation at the membrane level by inhibiting NADPH oxidase activity. The implication of these ideas to lung fibrosis induced by silica or asbestos is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C. Lysosomes and the toxicity of particulate pollutants. Arch Intern Med. 1971 Jul;128(1):131–139. [PubMed] [Google Scholar]
- Barber A. A., Bernheim F. Lipid peroxidation: its measurement, occurrence, and significance in animal tissues. Adv Gerontol Res. 1967;2:355–403. [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Kodicek E. Studies in vivo on the biosynthesis of collagen and elastin in ascorbic acid-deficient guinea pigs. Evidence for the formation and degradation of a partially hydroxylated collagen. Biochem J. 1970 Sep;119(3):575–585. doi: 10.1042/bj1190575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar R. S., Liu T. Z. Evidence for free radical involvement in the hydroxylation of proline: inhibition by nitro blue tetrazolium. FEBS Lett. 1972 Oct 1;26(1):32–34. doi: 10.1016/0014-5793(72)80535-0. [DOI] [PubMed] [Google Scholar]
- Bidlack W. R., Tappel A. L. A proposed mechanism for the TPNH enzymatic lipid peroxidizing system of rat liver microsomes. Lipids. 1972 Aug;7(8):564–565. doi: 10.1007/BF02533027. [DOI] [PubMed] [Google Scholar]
- Castor C. W. Connective tissue activation. V. The flux of connective tissue activating peptide during acute inflammation. J Lab Clin Med. 1973 Jan;81(1):95–104. [PubMed] [Google Scholar]
- Castor C. W., Yaron M. Leukocyte-connective tissue cell interaction. II. The specificity, duration, and mechanism of interaction effects. Arthritis Rheum. 1969 Aug;12(4):374–386. doi: 10.1002/art.1780120405. [DOI] [PubMed] [Google Scholar]
- Chvapil M., Aronson A. L., Peng Y. M. Relation between zinc and iron and peroxidation of lipids in liver homogenate in CaEDTA-treated rats. Exp Mol Pathol. 1974 Apr;20(2):216–227. doi: 10.1016/0014-4800(74)90056-2. [DOI] [PubMed] [Google Scholar]
- Chvapil M., Hurych J., Ehrlichová E. The influence of various oxygen tensions upon proline hydroxylation and the metabolism of collagenous and non-collagenous proteins in skin slices. Hoppe Seylers Z Physiol Chem. 1968 Feb;349(2):211–217. doi: 10.1515/bchm2.1968.349.1.211. [DOI] [PubMed] [Google Scholar]
- Chvapil M., Hurych J., Mirejovská E. Effect of long-term hypoxia on protein synthesis in granuloma and in some organs in rats. Proc Soc Exp Biol Med. 1970 Dec;135(3):613–617. doi: 10.3181/00379727-135-35106. [DOI] [PubMed] [Google Scholar]
- Chvapil M., Madden J. W., Carlson E. C., Peacock E. E., Jr Effect of cis-hydroxyproline on collagen and other proteins in skin wounds, granuloma tissue, and liver of mice and rats. Exp Mol Pathol. 1974 Jun;20(3):363–373. doi: 10.1016/0014-4800(74)90066-5. [DOI] [PubMed] [Google Scholar]
- Chvapil M. New aspects in the biological role of zinc: a stabilizer of macromolecules and biological membranes. Life Sci. 1973 Oct 16;13(8):1041–1049. doi: 10.1016/0024-3205(73)90372-x. [DOI] [PubMed] [Google Scholar]
- Chvapil M., Ryan J. N., Brada Z. Effects of selected chelating agents and metals on the stability of liver lysosomes. Biochem Pharmacol. 1972 Apr 15;21(8):1097–1105. doi: 10.1016/0006-2952(72)90103-7. [DOI] [PubMed] [Google Scholar]
- Chvapil M., Ryan J. N., Elias S. L., Peng Y. M. Protective effect of zinc on carbon tetrachloride-induced liver injury in rats. Exp Mol Pathol. 1973 Oct;19(2):186–196. doi: 10.1016/0014-4800(73)90078-6. [DOI] [PubMed] [Google Scholar]
- Chvapil M., Ryan J. N., Zukoski C. F. The effect of zinc and other metals on the stability of lysosomes. Proc Soc Exp Biol Med. 1972 Jun;140(2):642–646. doi: 10.3181/00379727-140-36521. [DOI] [PubMed] [Google Scholar]
- Chyapil M., Ryan J. N. Effect of 1,10-phenanthroline on drug oxidizing enzymes of rat liver microsomes. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1292–1299. doi: 10.1016/s0006-291x(71)80226-7. [DOI] [PubMed] [Google Scholar]
- Comstock J. P., Udenfriend S. Effect of lactate on collagen proline hydroxylase activity in cultured L-929 fibroblasts. Proc Natl Acad Sci U S A. 1970 Jun;66(2):552–557. doi: 10.1073/pnas.66.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J., St Pierre T., Minnich J., Barbeau A. Seizures in rats associated with divalent cation inhibition of NA + -K + -ATP'ase. Can J Biochem. 1971 Nov;49(11):1217–1224. doi: 10.1139/o71-175. [DOI] [PubMed] [Google Scholar]
- Goscin S. A., Fridovich I. The role of superoxide radical in a nonenzymatic hydroxylation. Arch Biochem Biophys. 1972 Dec;153(2):778–783. doi: 10.1016/0003-9861(72)90398-0. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 1. N Engl J Med. 1972 Jan 27;286(4):194–199. doi: 10.1056/NEJM197201272860406. [DOI] [PubMed] [Google Scholar]
- Harington J. S. Fibrogenesis. Environ Health Perspect. 1974 Dec;9:271–279. doi: 10.1289/ehp.749271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harington J. S. Investigative techniques in the laboratory study of coal workers' pneumoconiosis: recent advances at the cellular level. Ann N Y Acad Sci. 1972 Dec 29;200:816–834. doi: 10.1111/j.1749-6632.1972.tb40243.x. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G., Styles J. A. Activity of a macrophage factor in collagen formation by silica. Nature. 1967 Apr 29;214(5087):521–522. doi: 10.1038/214521a0. [DOI] [PubMed] [Google Scholar]
- Hochstein P., Nordenbrand K., Ernster L. Evidence for the involvement of iron in the ADP-activated peroxidation of lipids in microsomes and mitochondria. Biochem Biophys Res Commun. 1964;14:323–328. doi: 10.1016/s0006-291x(64)80004-8. [DOI] [PubMed] [Google Scholar]
- Hunt T. K., Zederfeldt B., Goldstick T. K. Oxygen and healing. Am J Surg. 1969 Oct;118(4):521–525. doi: 10.1016/0002-9610(69)90174-3. [DOI] [PubMed] [Google Scholar]
- Karl L., Chvapil M., Zukoski C. F. Effect of zinc on the viability and phagocytic capacity of peritoneal macrophages. Proc Soc Exp Biol Med. 1973 Apr;142(4):1123–1127. doi: 10.3181/00379727-142-37190. [DOI] [PubMed] [Google Scholar]
- Levene C. I., Bye I., Saffiotti U. The effect of beta-aminopropionitrile on silicotic pulmonary fibrosis in the rat. Br J Exp Pathol. 1968 Apr;49(2):152–159. [PMC free article] [PubMed] [Google Scholar]
- Madden J. W., Chvapil M., Carlson E. C., Ryan J. N. Toxicity and metabolic effects of 3,4-dehydroproline in mice. Toxicol Appl Pharmacol. 1973 Nov;26(3):426–437. doi: 10.1016/0041-008x(73)90279-2. [DOI] [PubMed] [Google Scholar]
- Marasas L. W., Harington J. S. The in vitro hydroxylation of proline by silica powder. Environ Res. 1970 Jul;3(3):212–217. doi: 10.1016/0013-9351(70)90017-4. [DOI] [PubMed] [Google Scholar]
- Mustafa M. G., Cross C. E., Munn R. J., Hardie J. A. Effects of divalent metal ions on alveolar macrophage membrane adenosine triphosphatase activity. J Lab Clin Med. 1971 Apr;77(4):563–571. [PubMed] [Google Scholar]
- Richards R. J., Wusteman F. S. The effects of silica dust and alveolar macrophages on lung fibroblasts grown in vitro. Life Sci. 1974 Jan 16;14(2):355–364. doi: 10.1016/0024-3205(74)90066-6. [DOI] [PubMed] [Google Scholar]
- Simpson D. M., Ross R. Effects of heterologous antineutrophil serum in guinea pigs. Hematologic and ultrastructural observations. Am J Pathol. 1971 Oct;65(1):79–102. [PMC free article] [PubMed] [Google Scholar]
- Speer D. P., Chvapil M., Brendel K., Peacock E. E., Jr Use of large-molecular-weight compounds to produce local lathyrism in healing wounds. Surg Forum. 1973;24:37–39. [PubMed] [Google Scholar]
- Tappel A. L. Lipid peroxidation damage to cell components. Fed Proc. 1973 Aug;32(8):1870–1874. [PubMed] [Google Scholar]


