Abstract
The role of the redox state of the apoplast in hormone responses, signaling cascades, and gene expression was studied in transgenic tobacco (Nicotiana tabacum) plants with modified cell wall-localized ascorbate oxidase (AO). High AO activity specifically decreased the ascorbic acid (AA) content of the apoplast and altered plant growth responses triggered by hormones. Auxin stimulated shoot growth only when the apoplastic AA pool was reduced in wild-type or AO antisense lines. Oxidation of apoplastic AA in AO sense lines was associated with loss of the auxin response, higher mitogen-activated protein kinase activities, and susceptibility to a virulent strain of the pathogen Pseudomonas syringae. The total leaf glutathione pool, the ratio of reduced glutathione to glutathione disulfide, and glutathione reductase activities were similar in the leaves of all lines. However, AO sense leaves exhibited significantly lower dehydroascorbate reductase and ascorbate peroxidase activities than wild-type and antisense leaves. The abundance of mRNAs encoding antioxidant enzymes was similar in all lines. However, the day/night rhythms in the abundance of transcripts encoding the three catalase isoforms were changed in response to the AA content of the apoplast. Other transcripts influenced by AO included photorespiratory genes and a plasma membrane Ca2+ channel-associated gene. We conclude that the redox state of the apoplast modulates plant growth and defense responses by regulating signal transduction cascades and gene expression patterns. Hence, AO activity, which modulates the redox state of the apoplastic AA pool, strongly influences the responses of plant cells to external and internal stimuli.
The concept that plants respond to the imposition of environmental stress by inducing defense pathways and slowing vegetative growth is widely accepted. Although the nature of the mechanisms that control these processes is poorly understood, it is considered to involve the coordinated regulation of plant hormones that modulate DELLA proteins and antioxidant defenses (Fath et al., 2002; Achard et al., 2006). Key changes in gene expression are engaged that decrease cell division and elongation growth and enhance pathogen resistance (Knight and Knight, 2001). In this regard, the apoplast and cell wall act as a reservoir of information on the biotic and abiotic environment surrounding the cell as well as a major conduit of information between cells. Similarly, the plasmalemma has major functions in stress perception and the subsequent appropriate control of growth and defense.
Many environmental and metabolic triggers, including pathogen attack, ozone, and physical and chemical assaults, alter the redox state of the apoplast by triggering an oxidative burst at the plasmalemma. Similarly, reactive oxygen species (ROS) produced by NADPH oxidases and other enzymes often act as second messengers in plant growth responses. For example, ROS generated by the RHD2 NADPH oxidase (atrbohC) are required for root hair cell growth in Arabidopsis (Arabidopsis thaliana; Foreman et al., 2003). Similarly, auxin promotes the generation of hydroxyl radicals (OH) in the apoplast, which mediates elongation growth in maize (Zea mays; Schopfer et al., 2001) as well as root gravitropism (Joo et al., 2001). Salicylic acid induces ROS accumulation during the acquisition of freezing tolerance (Mora-Herrera et al., 2005) and plant-pathogen responses (Chen et al., 1993). Abscisic acid (ABA)-mediated ROS generation is also considered important in mediating stomatal closure (Pei et al., 2000), a process regulated by an abundance of ascorbic acid (AA; Chen and Gallie, 2004). Signaling mediated by ROS involves heterotrimeric G proteins (Joo et al., 2005) and protein phosphorylation regulated by specific protein Tyr phosphatases and mitogen-activated protein kinases (MAPK; Kovtun et al., 2000; Gupta and Luan, 2003; Rentel et al., 2004). MAPK cascades are not only involved in the signal transduction of a variety of biotic and abiotic stresses, such as wounding and pathogen infection (Asai et al., 2002; Yamamizo et al., 2006), but also are involved in mediating the action of some plant hormones, such as ethylene and auxin (DeLong et al., 2002). A role for MAPK cascades in auxin signaling was first demonstrated in Arabidopsis by Mockaitis and Howell (2000). An increase in myelin basic protein (MBP) kinase activity was observed in Arabidopsis seedlings treated with auxin, suggesting that MAPK cascade activation positively contributes to the control of auxin-mediated growth responses and associated early gene transcription (Mockaitis and Howell, 2000).
Whereas much attention has focused on the roles of ROS as signals influencing plant responses to environmental stimuli and developmental cues, relatively little attention has been given to the redox buffering capacity of the apoplast and how it is modulated. Despite the presence of flavonoids and polyamines in the cell wall that can act as antioxidants, the redox buffering capacity of the apoplast is weak (Horemans et al., 2000; Pignocchi et al., 2003). The apoplast contains no NAD(P)H or glutathione, so AA is considered the major antioxidant (Foyer and Noctor, 2005a). Hence, controlled oxidation of the apoplastic AA pool by the action of ascorbate oxidase (AO) might be predicted to have a similar effect on the apoplastic redox state as an oxidative burst (Pignocchi and Foyer, 2003; Foyer and Noctor, 2005a).
Whereas most of the cellular AA pool is localized within the cytoplasm, up to 5% is transported across the plasma membrane and into the apoplast, where it constitutes the major sink for gaseous oxidants such as ozone (Barnes et al., 2002). In addition to providing the major redox buffering capacity of the apoplast, AA also participates in cell wall formation, controlling phenoxy radical-mediated cross-linking and other processes that affect cell growth (Smirnoff, 2000). The redox status of the extracellular AA pool is regulated by AO (Pignocchi et al., 2003; Sanmartin et al., 2003). This enzyme is considered the first step in the AA degradation pathway in the apoplast (Green and Fry, 2005). The presence of this enzyme may also explain why AA in the apoplast is markedly more oxidized than cytoplasmic AA (Pignocchi et al., 2003). AO has long been considered to influence cell enlargement via the modulation of redox control of the apoplast (Kato and Esaka, 2000; Smirnoff, 2000), although the exact mechanism is still not clear. The controlled oxidation of apoplastic AA by the action of AO might be predicted to have a similar effect on the apoplastic redox state as an oxidative burst (Pignocchi and Foyer, 2003; Foyer and Noctor, 2005a).
The AA pool in the apoplast is a key determinant of oxidant signal duration and strength as well as overall redox status of the apoplast (Foyer and Noctor, 2000, 2005b). Low levels of AA and antioxidant buffering would allow oxidative signals to persist and accumulate within the apoplast. This could be predicted to influence cell growth and expansion; however, apoplastic AA availability could also be important in limiting ROS signaling leading to programmed cell death. Little is known about plasma membrane AA transport systems (Horemans et al., 2000), but they are known to be too slow to maintain a highly reduced extracellular AA pool in situations of high oxidant production. Together with AO, AA transporters probably participate in a futile cycle that regulates the accumulation of both reduced AA and its oxidized forms (monodehydroscorbate [MDHA] and dehydroascorbate [DHA]) in the apoplastic environment for functions in metabolism and growth. Such a cycle has been suggested to be useful in photosynthetic energy dissipation (Nanasato et al., 2005). AA-based systems could also be important in driving plasmalemma and tonoplast electron transport chains. Both membranes contain an AA-dependent cytochrome b561, which is reduced on one face by AA and oxidized on the other by MDHA or other substrates for MDHA reductase (MDHAR; Preger et al., 2005). AA-based systems may also be crucial in controlling cell expansion and in facilitating the ROS-mediated signal transmission that occurs in response to atmospheric pollutants, pathogens, and hormones (Pignocchi et al., 2003).
Because the redox buffering capacity of the apoplast is always low compared to the cytoplasm, a steep redox gradient will arise across the plasma membrane when the apoplast AA pool is oxidized. We proposed that this gradient could influence gene expression through altered calcium release and modified channel activity (Pignocchi and Foyer, 2003). One way that shifts in apoplast redox status could facilitate such effects is through interactions between receptor proteins containing oxidizable thiols that are situated in, or near, the membrane surface (Pignocchi and Foyer, 2003). To determine whether AO and apoplastic AA could exert such effects, we used sense and antisense expression of AO in tobacco (Nicotiana tabacum) to deliver plants in which the redox state of the AA pool in the apoplast was decreased or increased in a targeted manner (Pignocchi et al., 2003). The results presented here show that the redox state of the AA pool in the leaf apoplast specifically influences (1) plant growth responses to hormones and MAPK activity; (2) antioxidant enzyme activities without affecting the leaf glutathione pool; (3) the abundance of specific transcripts, particularly an important calcium channel, and genes involved in photorespiration; and (4) diurnal expression patterns of catalase (CAT), glycolate oxidase, and other genes.
RESULTS
We have previously shown that the whole leaf AA pool is 90% reduced in all lines used in this study. In contrast, the apoplast AA pool is only about 3% reduced in AO sense lines, 70% reduced in AO antisense lines, and 40% reduced in wild-type plants (Pignocchi et al., 2003).
Plant Response to Developmental Stimuli: Effects of Exogenous Auxin and GA3 on Seedling Growth
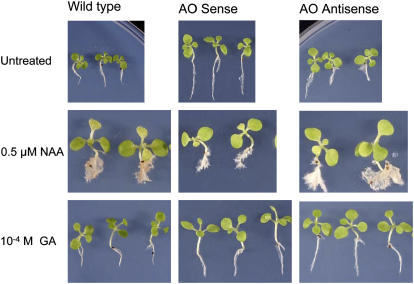
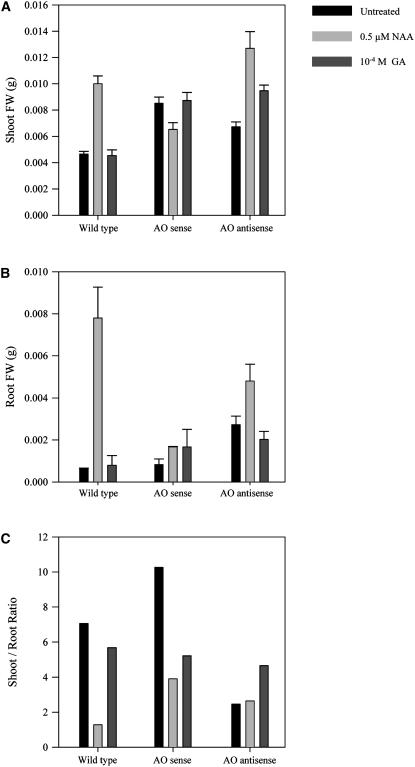
We chose to study the responses of these plants to auxin (naphthylacetic acid [NAA]) and GA3 because of the documented involvement of ROS in auxin responses (Joo et al., 2001; Schopfer et al., 2001, 2002) and the marked effect of AA on GA synthesis that we have observed (G. Kiddle and C.H. Foyer, unpublished data). Between 15 and 20 T2 seedlings per line were analyzed 10 d after spraying with water (untreated controls), NAA, or GA3. Several different independent transgenic lines were studied in each case. The effect of hormone treatments on plant phenotype is shown in Figure 1. For simplicity, the composite results of the analyses using different independent transgenic lines are shown in Figure 2. Application of auxin to wild-type seedlings enhanced shoot growth (i.e. advanced development of cotyledons and leaves; Fig. 1). Moreover, shoot fresh weight was increased compared with untreated controls (P < 0.01; Fig. 2A). A similar stimulatory effect of auxin was recorded in AO antisense seedlings (P < 0.01; Fig. 2A). In contrast, shoots of auxin-treated AO sense lines accumulated 25% to 30% less biomass following auxin treatment than untreated controls (P < 0.01; Fig. 2A). In all lines, auxin treatment resulted in the abundant proliferation of lateral roots (LR; Fig. 1) and had a stimulatory effect on root fresh weights, although this effect was much more evident in wild-type than in sense or antisense seedlings (Fig. 2B). Auxin treatment significantly decreased (P < 0.01) the shoot-to-root ratio in wild-type and sense plants, but had little effect on this parameter in antisense lines (P > 0.01; Fig. 2C). Conversely, whereas GA3 treatment had no significant effect (P > 0.05) on the shoot-to-root ratio of wild-type plants, this parameter was significantly decreased by GA3 treatment in the sense lines and increased in the antisense plants (P < 0.01; Fig. 2C).
Figure 1.
Comparison of the phenotype of untransformed controls (wild type), AO sense, and AO antisense plants 10 d after treatment with auxin or GA3. Wild-type, AO sense, and AO antisense seedlings were treated with either 0.5 μm−1 NAA, 10−4 m GA3, or water (untreated) and grown for a further 10 d after treatment.
Figure 2.
Comparison of shoot fresh weight (A), root fresh weight (B), and the shoot-to-root ratios (C) of untransformed controls (wild type), AO sense, and AO antisense plants 10 d after treatment with auxin or GA3. Shoots and roots were excised from the seedlings 10 d after treatment and the biomass (fresh weight) of each organ was measured. Results from between 15 to 20 T2 seedlings per line were evaluated 10 d after hormone treatment.
Effects of Altered AO on Leaf MAPK Activities and Responses to Pathogen Infection
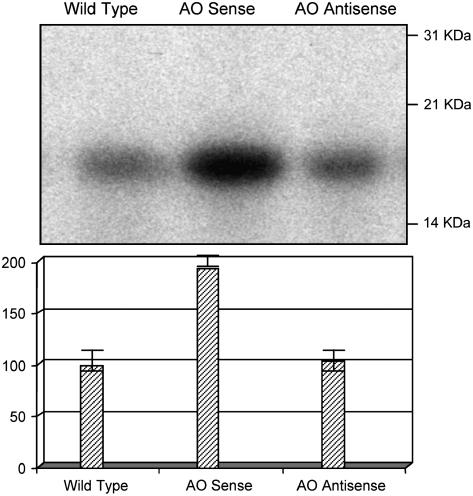
The insensitivity to auxin treatment observed in the AO sense lines could be due to constitutive activation of auxin signal transduction pathways via a conserved MAPK signaling cascade, as previously shown (Kovtun et al., 1998). To test this hypothesis, we measured in vitro MAPK activity in wild-type, AO sense, and AO antisense lines. In vitro total MBP phosphorylation activity was used as a measure of total MAPK activity because MBP constitutes the substrate of most MAPKs, including MAPKs involved in hormone and pathogen responses. MBP phosphorylation in AO sense plants was twice that found in wild-type and AO antisense plants (Fig. 3; P < 0.01).
Figure 3.
Comparison of MAPK activity of leaves from untransformed controls (wild type), AO sense, and AO antisense plant MAPK activity was determined by the relative phosphorylation (top) of MBP in vitro using extracts of leaf proteins from wild-type, AO sense, and AO antisense plants. Phosphorylation of each substrate band was quantified by phosphor-imaging analysis (bottom).
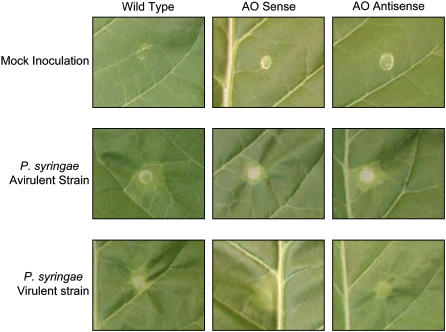
Because MAPK signaling has been shown to be involved in plant resistance to avirulent and virulent strains of Pseudomonas syringae (Desikan et al., 2001; Menke et al., 2004), we explored responses to pathogen challenge in the AO transgenic lines versus wild-type plants. Leaves of 7-week-old AO sense, AO antisense, and wild-type tobacco plants were inoculated with a suspension containing an equal volume of either a virulent or an avirulent strain of P. syringae (Fig. 4). A mock inoculation with phosphate buffer alone was used as a negative control. No lesions were detected up to 48 h after infection (data not shown). At 48 h, lesions appeared on the infected leaves. The lesions were confined to the inoculation area in all lines in response to infection with the avirulent strain. When the virulent strain of P. syringae was used, the lesions in AO sense plants showed a far more diffuse spread in infection compared with wild-type or AO antisense leaves (Fig. 4). The same results were observed in three independent repetitions.
Figure 4.
Comparison of pathogen-induced lesion formation of untransformed controls (wild type), AO sense, and AO antisense leaves 48 h after inoculation. The appearance of necrotic lesions was measured in leaves subjected to a mock inoculation (top sections, which represents the negative control) and in leaves inoculated with a P. syringae avirulent strain (middle sections) or a P. syringae virulent strain (bottom sections).
Effects on Antioxidant Enzyme Activities and Leaf Glutathione Content
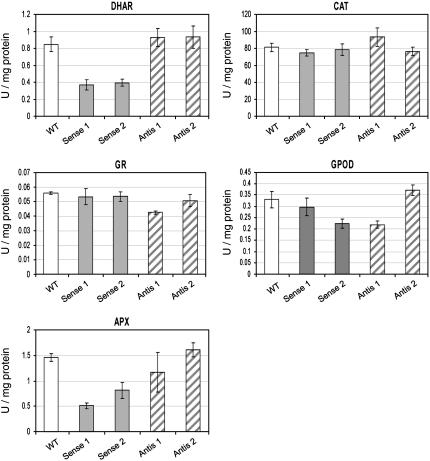
The activities of antioxidant enzymes were measured in leaves of wild-type, two AO sense (Sense 1 and Sense 2), and two AO antisense (Antis 1 and Antis 2) lines (Fig. 5). Whereas activities were similar in wild-type and antisense lines (P > 0.05), marked changes were observed in AO sense lines. In AO sense lines, significant (P < 0.01) decreases in DHA reductase (DHAR) and ascorbate peroxidase (APX) activities were recorded. No significant changes (P > 0.05) in glutathione reductase, CAT, or nonspecific peroxidase (guiacol peroxidase [GPOD]) activities were observed (Fig. 5). When glutathione was measured in the same leaves, the total amounts of the glutathione pools and relative amounts of reduced glutathione and glutathione disulfide were similar in all lines (data not shown).
Figure 5.
Activities of antioxidant enzymes in untransformed controls (wild type), AO sense, and AO antisense lines. Effects of varying AO activity on antioxidant enzyme activities were compared in leaves from untransformed controls (wild type), two AO sense lines (Sense 1 and Sense 2), and two AO antisense lines (Antis 1 and Antis 2).
Effects on Transcript Abundance
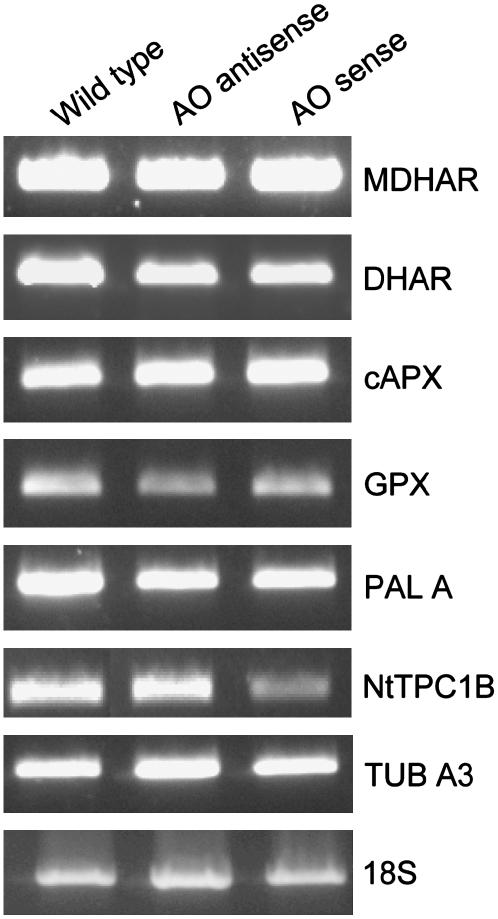
To investigate whether the changes in antioxidant enzyme activities observed in AO sense lines were the result of transcriptional or posttranscriptional regulation, we performed semiquantitative reverse transcription (RT)-PCR on AO sense and antisense plants. Little difference in the abundance of transcripts encoding antioxidant enzymes such as MDHAR, DHAR, APX, and glutathione peroxidase (GPX) were observed (Fig. 6). Phe ammonia-lyase (PAL) transcripts were also similar in all lines. However, there was a marked decrease in transcripts encoding the plasmalemma-localized two-pore Ca2+ channel-associated gene, NtTPC1B, in AO sense lines compared to antisense and wild-type lines. The housekeeping genes tubulin A3 and 18S were used as controls.
Figure 6.
Relative abundance of transcripts encoding a selection of antioxidant enzymes and the ion channel NtTPC1B. Transcript abundance was measured by semiquantitative RT-PCR in leaves from untransformed controls (wild type), AO sense, and AO antisense lines: Transcripts include MDHAR, DHAR, cytosolic ascorbate peroxidase (cAPX1), GPX, PAL, ion channel NtTPC1B, tubulin, and 18S (controls).
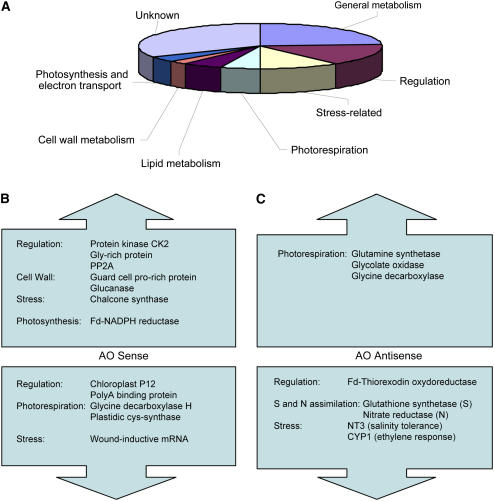
To study differentially expressed genes in AO transgenic lines on a larger scale, we performed subtractive hybridization screening of one AO sense and one AO antisense line against the wild type. A library comprising more than 300 clones was obtained from the leaves of 4-week-old wild-type, AO sense, and AO antisense plants grown in soil. Leaf samples were harvested 9 h after the beginning of the light period. Over 70 expressed sequence tags (ESTs), which showed high scores when aligned with database sequences, were altered in AO sense and antisense lines with respect to the wild type (Fig. 7A). Of these, 23% (18 ESTs) were involved in general metabolism, 16% (12 ESTs) constitute regulatory genes, 10% (eight ESTs) were stress related, 5% (four ESTs) were involved in photorespiration, and 4% (three ESTs) were involved in photosynthesis and electron transport (Fig. 7A). Interestingly, Gly decarboxylase transcripts were decreased in AO sense lines, whereas transcripts encoding for a Fd-NADPH reductase were increased (Fig. 7B). Moreover, Gln synthetase (GS-2), glycolate oxidase, and nitrate reductase were more highly expressed in AO antisense lines, whereas others, such as glutathione synthetase, were decreased (Fig. 7C). All transcript shifts indicated in Figure 7, B and C, were confirmed by real-time PCR (data not shown). For a full description of all gene expression changes measured in these experiments, see Supplemental Table I.
Figure 7.
Subtractive hybridization screening of leaves from untransformed controls (wild type), AO sense, and AO antisense lines reveals genes that are differentially expressed as a result of modified AO activity. A, Pie chart shows the total array of genes with modified expression in AO transgenic plants. B, Some of the key genes that are up- and down-regulated in AO sense lines. C, Some of the key genes that are up- and down-regulated in AO antisense plants.
Effects on Diurnal Patterns of Transcript Abundance
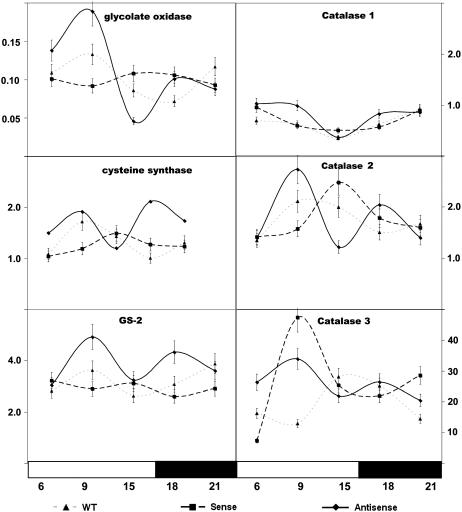
The above experiments indicated that key transcripts associated with photorespiration were modified by altered AO expression in transgenic plants. Because many of these transcripts show a day/night rhythm in transcript abundance, we analyzed by real-time PCR the effect of AO expression on the day/night abundance of transcripts associated with photorespiration, particularly those involved in photorespiratory hydrogen peroxide (H2O2) metabolism (Fig. 8). The abundance of actin transcripts was similar in all transgenic lines and was unaffected by the dark/light treatments employed here (data not shown). Actin was therefore used as an internal control. For consistency, primers for glycolate oxidase (U62485), GS-2 (X66940), and Cys synthase (AM087457) were designed on the basis of sequences identified in the subtractive screening. Real-time PCR data for these transcripts confirmed the findings shown in Figure 7, B and C. At the sampling time used for the subtractive screening (9 h in the light period), transcript abundance of glycolate oxidase and GS-2 were increased in AO antisense plants compared to wild type. In contrast, Cys synthase transcripts were found to be decreased in AO sense plants compared to wild type (Fig. 8). CAT 1, CAT 2, and CAT 3 all showed day/night rhythms in transcript abundance that were modified by altered AO expression.
Figure 8.
Comparison of the effects of modified AO abundance on the diurnal rhythms of key transcripts in the leaves of untransformed controls (wild type), AO sense, and AO antisense lines. Real-time PCR data for the day/night changes in transcript abundance are shown for CAT isoforms 1, 2, and 3, glycolate oxidase, Cys synthase, and GS-2 in leaves of wild-type (▴), AO sense (▪), and AO antisense (♦) plants.
DISCUSSION
The redox state of the AA pool in the apoplast has the potential to exert a profound influence on cellular redox signaling (Pignocchi and Foyer, 2003; Foyer and Noctor, 2005a, 2005b). The experiments discussed here were designed specifically to explore the influence of the redox state of the apoplast as defined by the abundance of the major antioxidant buffer AA on signal transduction and gene expression. We have focused on processes associated with plant growth and defense because apoplastic redox homeostasis is central to the perception and signaling related to environmental and hormonal cues. We have previously shown that the redox state of the AA pool in the apoplast can be modulated independently of the cytoplasmic pool, creating a large redox gradient across the plasma membrane (Pignocchi and Foyer, 2003; Sanmartin et al., 2003). Here, we provide evidence that oxidation of the AA pool in the apoplast specifically modulates signal transduction components, leading to changes in gene expression that alter plant responses to hormone signals and pathogen attack.
The concept that AA and AO regulate plant growth has long been accepted (Chinoy, 1984), but the mechanisms remain obscure. The data presented here confirm that AA and AO in the apoplast are involved in the regulation of growth via effects on sensitivity to hormone triggers that regulate growth. In particular, we demonstrate that auxin-mediated responses are lost when AA in the apoplast is highly oxidized due to enhanced AO activity in AO sense plants. Early work on AO demonstrated an induction of AO by auxin in pumpkin (Cucurbita maxima; Esaka et al., 1992) and the presence of a cis-acting region in the AO promoter that is responsible for auxin regulation has been suggested (Kisu et al., 1997). We have also previously demonstrated that AO expression is induced by auxin in tobacco leaves and this induction is associated with stimulation of plant growth (Pignocchi et al., 2003). The AO-induced auxin insensitivity observed here was tissue specific because stimulation of growth by exogenous auxin was only lost in the shoots of the AO overexpressers and did not decrease auxin-dependent root proliferation.
The loss of auxin responses in AO sense plants could be explained by three possible mechanisms. First, AO has been shown to catalyze the oxidative decarboxylation of auxin in maize roots (Kerk et al., 2000) and Arabidopsis shoots (Östin et al., 1998). Thus, the high AO activity in AO sense lines may contribute to loss of the auxin response in shoots, but not in roots, by auxin decarboxylation. Second, loss of auxin response in AO sense plants might be due to alteration of the sensitivity of auxin receptors by changes in the redox state of the apoplast. Plant responses to auxin are mediated by poorly characterized receptors that monitor the hormone concentrations in the apoplast (Löbler and Klämbt, 1985; Barbier-Brygoo et al., 1989). Such receptors often contain disulfide bridges that can undergo thiol-disulfide exchange reactions, and hence it is possible that their structure and function can be influenced by the redox state of the apoplast. Third, enhancement of AO-dependent accumulation of ROS in the apoplast of AO sense plants could mimic the mode of action of auxin, desensitizing the plants to further auxin stimulation. ROS are well-characterized secondary messengers in auxin-induced stimulation of growth (Schopfer et al., 2002) and hormone-mediated ROS generation has been shown to oxidize the AA pool in the apoplast (Takahama, 1994). Because the apoplast is already oxidized in AO-overexpressing plants, it is possible that further stimulation of ROS generation by auxin has minimal effects on oxidative signaling processes leading to auxin insensitivity. A constitutive auxin response pathway in the AO sense plants is also supported by the enhanced growth rate of AO sense plants, as previously observed (Pignocchi et al., 2003).
Plant growth is finely regulated by interactions between hormones (Steffens et al., 2005). For example, whereas auxin is required for the initiation of LRs, further development of LRs is regulated by ABA-dependent components (Signora et al., 2001; De Smet et al., 2003). GA3 is a known regulator of elongation growth and acts in opposition to ABA and via degradation of the DELLA proteins that limit growth (Sun and Gubler, 2004; Achard et al., 2006). GA3 also increases the extensibility of the cell wall by preventing reactions that otherwise cause stiffening (Métraux, 1987), for example, via inhibition of apoplastic peroxidase activity (De Souza and MacAdam, 2001). AA prevents lignification and formation of cross-linking in the apoplast because it prevents phenolic- and H2O2-dependent oxidation reactions (Takahama, 1994, 1998; Takahama and Oniki, 1994). Therefore, higher AA/DHA content of the apoplast in AO antisense plants might be predicted to enhance GA3 action in the manner observed here. GA3 receptors on the external face of the plasma membrane (Sun and Gubler, 2004) may also be redox sensitive.
Taken together, the above results suggest that AO-mediated oxidation of the apoplast desensitizes plants to hormone cues. One explanation for this effect is that constitutive AO expression mimics the action of auxin via oxidation of the apoplast. ROS generated by the activity of Rboh-encoded NADPH oxidases participate in a wide range of hormone-induced developmental responses, as well as in plant-pathogen interactions and abiotic stress responses (Torres and Dangl, 2005). The NADPH oxidase-catalyzed oxidative burst, coupled to the activation of Ca2+ channels, has been suggested to represent a signaling link that is common to many plant growth and defense responses (Torres and Dangl, 2005). The results presented here suggest that AO activity, like NADPH oxidase, can trigger decreased abundance of Ca2+ channel transcripts and enhanced MAPK activities by causing oxidation of the apoplast.
The auxin-mediated signal transduction cascade is mediated by a conserved signaling cascade consisting of three protein kinases: MAPK, MAPK kinase (MAPKK), and MAPKK kinase (MAPKKK). Transient increases in protein kinase activity with characteristics of mammalian extracellular signal-regulated kinase-like MAPKs are induced by auxin in Arabidopsis roots (Mockaitis and Howell, 2000). MAPKs are also involved in redox signal transduction (Kyriakis and Avruch, 1996). A 44-kD MAPK-like activity, which is increased after exposure to auxin (Mockaitis and Howell, 2000), is considered to be involved in the repression of early auxin response genes (Kovtun et al., 1998). Here, we have used an in vitro kinase assay involving total MBP phosphorylation to detect MAPK family activity in the leaves of wild-type, AO sense, and AO antisense plants. The insensitivity to auxin observed in AO sense plants was linked to increased MBP phosphorylation. Because protein phosphorylation is involved in the regulation of both auxin responses and polar auxin transport (De Long et al., 2002), and down-regulation of auxin-inducible gene expression via MAPK activity is stimulated by ROS (Kovtun et al., 2000), it is logical to suggest that enhanced MAPK-type activity triggered by AO-mediated oxidation of the apoplast causes insensitivity to auxin.
The constitutive activation of the MAPK signaling cascades was coupled to the decreased expression of the calcium channel NtTPC1B in AO sense plants. NtTPC1B encodes a voltage-operated calcium channel, which is the major Ca2+-permeable channel activated by H2O2 (Kadota et al., 2005). The lower expression level of NtTPC1A/B is also consistent with altered resistance to a virulent strain of P. syringae. We have previously shown that the Arabidopsis low vitamin c mutants (vtc1 and vtc2) express pathogenesis-related proteins and exhibit enhanced resistance to infection by P. syringae (Pavet et al., 2005). The vtc leaves exhibit nuclear localization of NPR1 related to enhanced tissue glutathione and higher reduced glutathione:oxidized glutathione ratios. Such results demonstrate that the abundance of AA in foliage modifies the threshold for activation of plant innate defense responses via redox mechanisms (Barth et al., 2004; Pavet et al., 2005). The leaf glutathione pool was not affected by altered AO expression and innate immune defense responses were not enhanced. Moreover, diffuse spread of pathogen-induced lesions in leaves of AO sense plants may suggest that a decreased level of NtTPC1A/B transcripts enhances susceptibility to the virulent P. syringae. Because the NtTPC1A/B-encoded voltage-operated calcium channel is important to the orchestration of pathogen resistance responses (Kadota et al., 2005), its decreased abundance in AO sense plants might decrease the capacity of the plants to activate defense-associated enzymes, such as GPX. The results presented here concerning failure of the leaves of AO overexpressers to contain the spread of virulent P. syringae infection are purely qualitative. Hence, these results can only be considered as a possible indication that pathogen responses are altered in AO-overexpressing lines. However, it should be noted that AO overexpression has also been reported to increase sensitivity to infection by Botrytis cinerea (V. Fotopoulos, M. Sanmartin, and A.K. Kanellis, unpublished data). In this case, enhanced sensitivity to the pathogen is associated with decreased expression of TPC1BR, an important plasma membrane-associated Ca2+ channel (I. Hernandez, A.K. Kanellis, and J.D. Barnes, unpublished data).
We have previously shown that light-mediated control of AO expression is modified by oxidation of AA in the apoplast. Positive regulation of native AO transcript abundance by light in tobacco was completely lost when apoplastic AA was highly oxidized in transgenic plants (Pignocchi et al., 2003). The data presented here demonstrate that other transcripts that show pronounced day/night rhythms in transcript abundance are also influenced by AO-induced oxidation of AA in the apoplast. The three tobacco CAT genes show complex temporal expression patterns that are modified by AO expression in a manner that is unique to each transcript. The day/night fluctuations in CAT transcripts are similar to those observed in other species where CAT gene expression has been shown to be under circadian control (Zhong et al., 1994; Polidoros and Scandalios, 1998). The day/night patterns in CAT transcripts are, however, sensitive to metabolic and environmental influences; for example, they are modified by drought (Luna et al., 2005) and by changes in the cellular redox state caused by altered mitochondrial electron transport capacity (Dutilleul et al., 2003). Perhaps this is not surprising given that the promoters of some CAT genes contain antioxidant-responsive elements (Polidoros and Scandalios, 1998) that are sensitive to changes in the cellular redox state. The results presented here demonstrate that the redox state of the apoplast has a strong influence not only on the day/night patterns in CAT transcripts, but also on the day/night rhythms of other transcripts, such as glycolate oxidase and GS-2. Whereas glycolate oxidase is linked to redox metabolism through photorespiration, the effect on the pattern of GS-2 transcripts is less easy to explain. Although the oxidative cleavage of the GS-2 protein in situations of stress is well documented, this enzyme serves functions in ammonia assimilation, in primary nitrogen assimilation, and in photorespiration. These results suggest that the redox state of the apoplast influences clock control in circadian regulation as well as promoters that are sensitive to ROS and the cellular redox state.
Glycolate oxidase produces H2O2 at very high rates in the peroxisomes of C3 plants as a result of photorespiratory carbon flow (Noctor et al., 2002). The robustness of plant cells to withstand H2O2 and other oxidants is due in part to the effective control of metabolic ROS production as well as to effective control of oxidant levels by a versatile leaf antioxidative system. The activity of CAT crucial for the removal of H2O2 formed by photorespiration is unchanged by modified AO expression. However, leaves of AO sense lines exhibit significantly less APX and DHAR activity than the wild type, although transcript abundance for these genes is unchanged. This suggests that the capacity to eliminate H2O2 within the cytoplasm may be impaired as a result of a decreased apoplastic redox state. This hypothesis is supported by evidence that suppressed expression of AO leads to enhanced salt tolerance (Yamamoto et al., 2005). In this regard, it is of interest to note that the amplitude of day/night changes in glycolate oxidase transcripts was increased in AO sense lines and dampened in AO antisense lines (Fig. 8). Modulation of the day/night abundance of transcripts associated with photorespiration suggests that the capacity for intracellular H2O2 generation may also be affected by the redox state of the apoplast. Because photorespiration is by far the biggest producer of H2O2 within photosynthetic cells, increased flux through this pathway facilitated by a higher abundance of component enzymes, such as glycolate oxidase and Gly decarboxylase, in the light would perhaps favor increased rates of H2O2 production. Whereas further experiments are required to corroborate this hypothesis, the observations reported here suggest that the redox networks in the apoplast and cytoplasm do not operate in isolation. Global regulation of cellular redox homeostasis may thus be achieved by adjustments of processes that produce and consume H2O2 both in the cytoplasm and in the extracellular matrix.
The results presented in this study allow a new perspective of cellular redox metabolism and homeostasis in which the extracellular redox environment has a marked influence on cellular signaling cascades that influence development, antioxidant defense, and biotic interactions. To date, only apoplastic NAPDH oxidase-catalyzed production of ROS has been considered in terms of a broad role in signaling responses to infections, the abiotic environment, programmed cell death, and developmental cues (Torres and Dangl, 2005). The results presented here show that AO-catalyzed AA oxidation in the apoplast can serve a similar function. Changes in apoplastic AO activity altered responses to hormone triggers and environmental cues. Hence, decreased AA abundance in the apoplast alone, independent of the cytoplasmic AA pool, is able to mimic the effects of ROS accumulation in the apoplast. Because the antioxidant defense capacity of the apoplast is decreased as a result of AA depletion in AO sense plants, H2O2 accumulates more readily than in the apoplast of wild-type or AO antisense plants (Yamamoto et al., 2005). These observations not only demonstrate the exquisite sensitivity of plant cytoplasm to the extracellular redox environment, but also show that significant changes in plant growth and defense can be brought about by regulation of AO activity. Moreover, these results demonstrate that redox changes in the apoplast alter sensitivity to pathogen infection in a manner that is distinct from that observed when symplastic AA levels are modified (Pavet et al., 2005). In the case of the symplast, low AA enhances pathogen resistance, whereas low AA in the apoplast decreases pathogen resistance.
These results emphasize the crucial importance of redox homeostasis in the apoplast in controlling signal transduction processes. Regulation of the AA pool in the apoplast could be used to modulate cross talk between different defense and growth pathways in a similar manner to that already described for ROS (Torres and Dangl, 2005). Taken together, these results show that the redox state of the apoplast and/or the redox gradient across the plasmalemma has a major effect on plant responses to a wide range of environmental and developmental triggers. In this way, required growth and defense responses could be influenced in a coordinated manner by redox-mediated changes in receptor sensitivity, calcium signaling, and protein phosphorylation cascades.
MATERIALS AND METHODS
Materials
Wild-type and transgenic (T2 and T3 generation) tobacco (Nicotiana tabacum; SR1 ecotype) plants expressing pumpkin (Cucurbita maxima) AO in the sense orientation (AO sense lines P221 and P372) and partial tobacco AO in antisense orientation (AO antisense lines T161 and T271) were grown in pots (containing 3 dm3 of John Innes no. 2 compost) in controlled environment chambers at 22°C with a 16-h photoperiod and a photosynthetic photon flux density of 250 μmol m−2 s−1 at plant height or on agar plates, as described in Pignocchi et al. (2003). For all experiments, unless otherwise specified, 5-week-old leaf samples were harvested at 9 h in the light period. Samples for real-time PCR were harvested at 6, 9, and 15 h (day), and 18 and 21 h (night).
Cultures of Pseudomonas syringae employed in this study were tomato (Lycopersicon esculentum) pv DC3000 pLAFR (virulent strain) and pv pLAFRavrRpt2 (avirulent strain).
Hormone Treatments
Ten-day-old seedlings, raised in petri dishes containing Murashige and Skoog agar culture medium (Sigma Chemicals) were sprayed to run off (using an aerosol spray bottle supplied by Nalgene) with 0.5 μm−1 NAA and 10−4 m GA3. Biomass was quantified after a further 10 d.
Preparation of Protein Extracts
Frozen tissue was ground to powder under N2. One hundred-milligram samples in Eppendorf tubes were homogenized for 1 min in 0.1 mL of lysis buffer containing 20 mm HEPES-KOH, pH 7.6, 100 mm KCl, 1.5 mm EGTA, 1.0 mm EDTA, 10% glycerol, 20 mm β-glycerophosphate, 50 mm NaF, 10 mm β-mercaptoethanol, and 1 mm phenylmethylsulfonyl fluoride. Extracts were centrifuged at 14,000g at 4°C for 45 min. The supernatant was used for all assays and quantified by Bradford assay (Bio-Rad).
In Vitro Kinase Assays
MAPK assays were performed in 25 μL of K buffer (25 mm HEPES, pH 7.9, 5 mm MgCl2, 0.1% 2-mercaptoethanol, 0.1 mm EDTA) with added 0.2 mg mL−1 substrate protein, MgCl2 (30 mm final), ATP (100 μm final), and 2.5 μCi [γ-32P]ATP. Substrates used were bovine MBP (Sigma-Aldrich). Five micrograms of protein extract were used for each assay. Reactions were incubated for 30 min at 30°C, then terminated by the addition of 5× SDS-PAGE loading buffer, and resolved by SDS-PAGE. Unincorporated [γ-32P]ATP (running with the bromphenol blue) was discarded from the gels and the gels were fixed in a methanol/acetic acid solution for 15 min prior to exposure to film. The degree of protein phosphorylation was quantified using a phosphor imager.
Pathogen Inoculations
Bacterial cultures were grown overnight at 28°C with centrifuging in King's B medium (peptone 20 g L−1; glycerol 1% v/v, K2HPO4 1.5 g L−1, pH 7.2) supplemented with tetracycline (10 μg mL−1) and rifampicin (100 μg mL−1). Cells from overnight cultures were washed and then resuspended in 10 mm MgCl2 to obtain a cell density of 0.5 × 10−6 cells mL−1; 0.1 mL of cell suspension was used for each inoculation. Inoculations were performed by infiltration into the underside of the leaves, using a 1-mL syringe without a needle. Three repetitions for each infection were performed.
Inoculated plants were incubated under growth conditions for 48 h. Leaf discs of the infected leaves were harvested 2, 4, 6, 8, and 48 h after inoculation.
Determination of AA and Glutathione
Glutathione was measured as described by Noctor and Foyer (1998).
Enzyme Assays
CAT activity was determined in a reaction mixture containing 66 mm potassium phosphate (pH 7), 0.7 mm H2O2, and 2% (v/v) extract. The reaction was followed at 240 nm, employing an extinction coefficient for H2O2 of 0.039 mm−1 cm−1. Control assays for each replicate were performed in the presence of aminotriazole.
GPOD activity was determined in a reaction mixture containing 66 mm potassium phosphate (pH 6.1), 8 mm Guiacol, 10% (v/v) extract, and 2 mm H2O2. GPOD activity was determined as the rate of formation of tetraguiacol at 470 nm (extinction coefficient of 26.6 mm−1 cm−1).
Glutathione reductase activity was measured in a reaction mixture containing 66 mm potassium phosphate buffer (pH 7.8), 150 μm NADPH, 500 μm GSSG, and 5% (v/v) extract. The reaction was followed at 340 nm (extinction coefficient for NADPH of 6.22 mm−1 cm−1). Controls were performed in the absence of GSSG to assess nonenzymatic oxidation of NADPH.
DHAR activity was measured in a reaction mixture containing 66 mm potassium phosphate buffer (pH 6.5), 3 mm GSH, 250 μm DHA, and 5% (v/v) extract. Reaction was followed at 265 nm (extinction coefficient for AA of 14 mm−1 cm−1). Two control reactions were performed: one without GSH to assess non-GSH-dependent production of AA, and the other one, without extract, to assess non-DHAR production of AA.
APX activity was determined by monitoring oxidation of AA at 290 nm (ɛ = 2.88 mm−1 cm−1), as described by Nakano and Asada (1981).
All enzyme activities were measured using a Pye-Unicam SP8700 UV/visible spectrophotometer (Pye-Unicam). All reactions were developed at 25°C in 1-mL quartz cuvettes. Data were expressed as specific activity, with protein content determined using the method of Bradford.
Total RNA Extraction and Gene Expression Analysis
Total RNA was extracted by using the RNeasy plant mini kit (Qiagen), according to the supplier's recommendation. Residual DNA was removed with DNase I, amp grade (Gibco-BRL). The absence of DNA contamination in the samples was confirmed by a saturating PCR of 40 cycles using actin-specific (X63603) primers (5′-CGCGAAAAGATGACTCAAATC-3′ and 5′-AGATCCTTTCTGATATCCACG-3′), which give a 687-bp product with genomic DNA and a 533-bp product with cDNA. One microgram of total RNA was reverse transcribed using 0.5 μg oligo(dT)12–18 (Gibco-BRL) and 200 units SuperScript II (Gibco-BRL), following the supplier's recommendation. cDNA samples were standardized by PCR for actin and tubulin content using the gene-specific primers. On the basis of the published sequences, the following gene-specific primers were designed and used for amplification: MDHAR, 5′-GACAGAAACTTCAAATAGCCG-3′ and 5′-GAACATGTTGATCATTCTCGC-3′; DHAR (AY074787), 5′-ATCTGTGTCAAGGCTGCTG-3′ and 5′-ACTTCCTGCGAAACAACGG-3′; cAPX (U15933), 5′-CTGGAGGACCTGATGTTC-3′ and 5′-CGTCTAATAACAGCTGCC-3′; GPX (AB041518), 5′-CCAATCTAGCAAGCCTCAA-3′ and 5′-ATGCAGACAAATCCAGAGC-3′; PAL (X78269), 5′-GCGATAGACTTGAGGCATT-3′ and 5′-GATCCTGTTGTTTGGAGAACC-3′; NtTPC1B (AB124647), 5′-CCAACGGAGAATGGATTCG-3′ and 5′-CAGCATGGAGAAAGGAGCA-3′; tubulin A3 (AJ421413), 5′-TCCTCATATGCTCCtGTC-3′ and 5′-AGCAGACAAGCATTCTAC-3′; and 18S, 5′-GACGAACAACTGCGAAAG-3′ and 5′-CATCTAAGGGCATCACAG-3′.
For semiquantitative RT-PCR, the cycle number was kept within the linear range (30 cycles) and the conditions were 3 min at 94°C, a cycle of 45 s at 94°C, 30 s at 52°C, 45 s at 72°C, followed by 10 min at 72°C, using 0.5 μL of the RT reaction and 0.2 μm of each oligonucleotide primer in a total volume of 25 μL. The identity of the PCR products was verified by single-strand sequencing (ABI PRISM, 310 genetic analyzer; Perkin-Elmer). RT-PCR products were loaded on 2% (w/v) agarose gel containing 0.5 μg mL−1 ethidium bromide and the band intensities quantified with the Eagle Eye II (Stratagene).
Subtractive Library Construction
Total RNA was extracted using Tri reagent (Helena Biosciences). mRNA was isolated from total RNA using the PolyAtract kit (Promega). Subtractive hybridization was carried out using the PCR-select cDNA subtraction kit (BD Biosciences, CLONTECH), following the manufacturer's instructions. Forward and reverse subtractions were carried out using wild-type mRNA as the tester and each transformant line as the driver and vice versa to recover up- and down-regulated genes. The amplified, differentially expressed cDNAs were cloned using a TOPO-TA cloning system (Invitrogen). Plasmids bearing DNA insertions were extracted using a kit (Qiagen) and sequenced using M13 forward and reverse promoters (Macrogen). Sequence identification was performed using a BLAST search of the National Center for Biotechnology Information (NCBI) gene bank data resource.
Real-Time PCR
Leaves were harvested from 5-week-old plants throughout the day/night cycle at 6, 9, and 15 h (day), and 18 and 21 h (night). Leaf samples were frozen in liquid N2, homogenized into a fine powder, and RNA was extracted from each sample and cDNA synthesized as described by Pastori et al. (2003). Real-time PCR was performed using the following primers: actin 2/7 (U60495), 5′-TGAGCAAGGAAATCACCGCT-3′ and 5′-GATTAAGGTTGTTGCTCCACCG-3′; GAPDH (M14419), 5′-TGTGGACCTTACCGTAAGACTAGAGA-3′ and 5′-TCAAGGAGGAATCGGAGGG-3′; glycolate oxidase (U62485), 5′-CATGAGCACCACAGTCGTAGGA-3′ and 5′-ATCATGATTGCACCAACAGCC-3′; chloroplast partial Cys synthetase (AM087457), 5′-AGCTGTTGAAACTGCGAAGC-3′ and 5′-TGGTTGGGATTTCTTCCGG-3′; tubulin BA1 (AJ421411), 5′-TGATCCTCGCCATGGAAAGT-3′ and 5′-GATGTTGTGCCAAAGGATGTCA-3′; chloroplast GS-2 (X66940), 5′-GTGCGCCAGATACATCCTTGA-3′ and 5′-TGCCGGATGCCACACTAACTA-3′; CAT 1 (U93244), 5′-ACCAGGAGATAGGTACCGCTC-3′ and 5′-TTATTCGTCGGTGGGTGGA-3′; CAT 2 (U07627), 5′-TCGTGATGCAAAATCGTTCC-3′ and 5′-GAGTTTGCATACTTTTGCCTGG-3′; and CAT 3 (Z36977), 5′-TGCTAACAATTCCCCCTTCTGG-3′ and 5′-TGGTGGAGAAGCTTGCCAATT-3′. SYBR green RT-PCR amplification of each cDNA was performed using the JumpStart Taq ready-mix kit and the Applied Bio-Systems 7500 real-time PCR machine, according to the manufacturer's instructions The efficiency of each primer was calculated and the target gene data normalized to actin 2/7, according to the method described by Pfaffl (2001). Zero cDNA controls disassociation curves.
Statistical Analyses
All datasets were subjected to ANOVA and significant (P < 0.05) differences between individual means established using a Student's t test employing GENSTAT 5 (Payne et al., 1993).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers U60495, M14419, U62485, AM087457, AJ421411, X66940, U93244, U07627, and Z36977.
Supplementary Material
Acknowledgments
The authors are grateful to Prof. Muneharu Esaka (Hiroshima University, Higashi-Hiroshima, Japan) for kindly providing the AO cDNAs. We also thank David D'Haese and Anne Borland (Institute for Research on Environment and Sustainability, Newcastle University, UK) for constructive discussions.
This work was supported by a combination of funding from the Biotechnology and Biological Sciences Research Council, the National Environmental Research Council, and the European Union (Marie Curie training site HPMT–CT–2001–00219).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Christine H. Foyer (christine.foyer@bbsrc.ac.uk).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.078469.
References
- Achard P, Chaeng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phyto hormone signals. Science 322: 91–94 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Barbier-Brygoo H, Ephritikhine G, Klämbt D, Ghislain M, Guern J (1989) Functional evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proc Natl Acad Sci USA 86: 891–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JD, Zheng Y, Lyons TM (2002) Plant resistance to ozone: the role of ascorbate. In K Omasa, H Saji, S Youssefian, N Kondo, eds, Air Pollution and Plant Biotechnology. Springer-Verlag, Tokyo, pp 235–252
- Barth C, Moeder W, Klessig DF, Conklin PL (2004) The timing of senescence and response to pathogens is altered in the ascorbate-deficient mutant vitamin C-1. Plant Physiol 134: 178–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gallie DR (2004) The ascorbic acid redox state controls guard cell signalling and stomatal movement. Plant Cell 16: 1143–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Silva H, Klessig DF (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262: 1883–1886 [DOI] [PubMed] [Google Scholar]
- Chinoy NJ (1984) Ascorbic acid in plant growth and development. In W Junk, ed, The Role of Ascorbic Acid in Growth, Differentiation and Metabolism of Plants. Martinus Nijhoff, The Hague, The Netherlands, pp 68–195
- De Long A, Mockaitis K, Christensen S (2002) Protein phosphorylation in the delivery of and response to auxin signals. Plant Mol Biol 49: 285–303 [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Ichimura K, Shinozaki K, Neill SJ (2001) Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol 126: 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Signora L, Beeckman T, Inzé D, Foyer CH, Zhang H (2003) An ABA-sensitive lateral root developmental checkpoint in Arabidopsis. Plant J 33: 543–555 [DOI] [PubMed] [Google Scholar]
- De Souza IRP, MacAdam JW (2001) Gibberellic acid and dwarfism effects on the growth dynamics of B73 maize (Zea mays L.) leaf blades: a transient increase in apoplastic peroxidase activity precedes cessation of cell elongation. J Exp Bot 52: 1673–1682 [DOI] [PubMed] [Google Scholar]
- Dutilleul C, Garmier M, Mathieu C, Chetrit P, Noctor G, Foyer CH, De Paepe R (2003) Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. Plant Cell 15: 1212–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaka M, Fujisawa K, Goto M, Kisu Y (1992) Regulation of ascorbate oxidase expression in pumpkin by auxin and copper. Plant Physiol 100: 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath A, Bethke P, Beligni V, Jones R (2002) Active oxygen and cell death in cereal aleurone cells. J Exp Bot 53: 1273–1282 [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2000) Tansley Review No 112. Oxygen processing in photosynthesis: regulation and signalling. New Phytol 146: 359–388 [Google Scholar]
- Foyer CH, Noctor G (2005. a) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2005. b) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28: 1056–1071 [Google Scholar]
- Green MA, Fry SC (2005) Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-L-threonate. Nature 433: 83–87 [DOI] [PubMed] [Google Scholar]
- Gupta R, Luan S (2003) Redox control of protein tyrosine phosphatases and mitogen-activated protein kinases in plants. Plant Physiol 132: 1149–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horemans N, Foyer CH, Asard H (2000) Transport and action of ascorbate at the plant plasma membrane. Trends Plant Sci 5: 263–267 [DOI] [PubMed] [Google Scholar]
- Joo JH, Bae YS, Lee JS (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126: 1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV (2005) Different signaling and cell death roles of heterotrimeric G protein a and b subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y, Furuichi T, Sano T, Kaya H, Gunji W, Murakami Y, Muto S, Hasezawa S, Kuchitsu K (2005) Cell-cycle-dependent regulation of oxidative stress responses and Ca2+ permeable channels NtTPC1A/B in tobacco BY-2 cells. Biochem Biophys Res Commun 336: 1259–1267 [DOI] [PubMed] [Google Scholar]
- Kato N, Esaka M (2000) Expansion of transgenic tobacco protoplasts expressing pumpkin ascorbate oxidase is more rapid that that of wild type protoplasts. Planta 210: 1018–1022 [DOI] [PubMed] [Google Scholar]
- Kerk NM, Jiang K, Feldman LJ (2000) Auxin metabolism in the root apical meristem. Plant Physiol 122: 925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisu Y, Harada Y, Goto M, Esaka M (1997) Cloning of the pumpkin ascorbate oxidase gene and analysis of a cis-acting region involved in induction by auxin. Plant Cell Physiol 38: 631–637 [DOI] [PubMed] [Google Scholar]
- Knight H, Knight MR (2001) Abiotic stress signalling pathways: specificity and cross-talk. Trends Plant Sci 6: 262–267 [DOI] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 14: 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Zeng W, Sheen J (1998) Suppression of auxin signal transduction by a MAPK cascade in higher plants. Nature 395: 716–720 [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J (1996) Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem 271: 24313–24316 [DOI] [PubMed] [Google Scholar]
- Löbler M, Klämbt D (1985) Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). J Biol Chem 260: 9854–9859 [PubMed] [Google Scholar]
- Luna CM, Pastori GM, Driscoll S, Groten K, Bernard S, Foyer CH (2005) Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot 56: 417–423 [DOI] [PubMed] [Google Scholar]
- Menke FL, van Pelt JA, Pieterse CM, Klessing DF (2004) Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell 16: 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux JP (1987) Gibberellins and plant cell elongation. In PJ Davies, ed, Plant Hormones and Their Role in Plant Growth and Development. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 296–317
- Mockaitis K, Howell SH (2000) Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J 24: 785–796 [DOI] [PubMed] [Google Scholar]
- Mora-Herrera ME, Lopez-Delgado H, Castillo-Morales A, Foyer CH (2005) Salicylic acid and H2O2 function by independent pathways in the induction of freezing tolerance in potato. Physiol Plant 125: 430–440 [Google Scholar]
- Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22: 867–880 [Google Scholar]
- Nanasato Y, Akashi K, Yokota A (2005) Co-expression of cytochrome b561 and ascorbate oxidase in leaves of wild watermelon under drought and high light conditions. Plant Cell Physiol 46: 1515–1524 [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279 [DOI] [PubMed] [Google Scholar]
- Noctor G, Veljovic-Jovanovic SD, Driscoll S, Novitskaya L, Foyer CH (2002) Drought and oxidative load in wheat leaves: a predominant role for photorespiration? Ann Bot (Lond) 89: 841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östin A, Kowalycz M, Bhalerao RP, Sandberg G (1998) Metabolism of indole-3-acetic acid in Arabidopsis. Plant Physiol 118: 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15: 939–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, Alvarez ME, Foyer CH (2005) Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis thaliana. Plant Physiol 139: 1291–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RW, Lane PW, Digby PGN, Harding SA, Leech PK, Morgan GW, Todd AD, Thompson R, Tunnicliffe WG, Welham SJ, et al (1993) Genstat 5, Release 3, Reference Manual. Clarendon Press, Oxford
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: 2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignocchi C, Fletcher JM, Wilkinson JE, Barnes JD, Foyer CH (2003) The function of ascorbate oxidase in tobacco. Plant Physiol 132: 1631–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignocchi C, Foyer CH (2003) Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Curr Opin Plant Biol 6: 379–389 [DOI] [PubMed] [Google Scholar]
- Polidoros AN, Scandalios JG (1998) Circadian expression of the maize catalase cat3 gene is highly conserved among diverse maize genotypes with structurally different promoters. Genetics 149: 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preger V, Scagliarini S, Pupillo P, Trost P (2005) Identification of an ascorbate-dependent cytochrome b of the tonoplast membrane sharing biochemical features with members of the cytochrome b561 family. Planta 220: 365–375 [DOI] [PubMed] [Google Scholar]
- Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, et al (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427: 858–861 [DOI] [PubMed] [Google Scholar]
- Sanmartin M, Drogoudi PD, Lyons T, Pateraki I, Barnes J, Kanellis AK (2003) Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta 216: 918–928 [DOI] [PubMed] [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A (2002) Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214: 821–828 [DOI] [PubMed] [Google Scholar]
- Schopfer P, Plachy C, Frahry G (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol 125: 1591–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signora L, De Smet I, Foyer CH, Zhang H (2001) ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. Plant J 28: 655–662 [DOI] [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multi-faceted molecule. Curr Opin Plant Biol 3: 229–235 [PubMed] [Google Scholar]
- Steffens B, Wang J, Sauter B (2005) Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 14: 1–9 [DOI] [PubMed] [Google Scholar]
- Sun TP, Gubler F (2004) Molecular mechanisms of gibberellin signalling in plants. Annu Rev Plant Biol 55: 197–223 [DOI] [PubMed] [Google Scholar]
- Takahama U (1994) Changes induced by abscisic acid and light in the redox state of ascorbate in the apoplast of epicotyls of Vigna angularis. Plant Cell Physiol 35: 975–978 [Google Scholar]
- Takahama U (1998) Ascorbic acid-dependent regulation of redox levels of chlorogenic acid and its isomers in the apoplast of leaves of Nicotiana tabacum L. Plant Cell Physiol 39: 681–689 [Google Scholar]
- Takahama U, Oniki T (1994) The association of ascorbate and ascorbate oxidase in the apoplast with IAA-enhanced elongation of epicotyls from Vigna angularis. Plant Cell Physiol 35: 257–266 [Google Scholar]
- Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8: 397–403 [DOI] [PubMed] [Google Scholar]
- Yamamizo C, Kuchimura K, Kobayashi A, Katou S, Kawakita K, Jones JDG, Doke N, Yoshioka H (2006) Rewiring mitogen-activated protein kinase cascade by positive feedback confers potato blight resistance. Plant Physiol 140: 681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Bhuiyan NH, Waditee R, Tanaka Y, Esaka M, Oba K, Jagendorf AT, Takabe T (2005) Suppressed expression of the apoplastic ascorbate oxidase gene increases salt tolerance in tobacco and Arabidopsis plants. J Exp Bot 56: 1785–1796 [DOI] [PubMed] [Google Scholar]
- Zhong HH, Young JC, Pease EA, Hangarter RP, McClung CR (1994) Interactions between light and the circadian clock in the regulation of CAT2 expression in Arabidopsis. Plant Physiol 104: 889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.