Abstract
The role of mitogen-activated protein kinase (MAPK) in abscisic acid (ABA)-induced antioxidant defense was investigated in leaves of maize (Zea mays) plants. Treatments with ABA or H2O2 induced the activation of a 46-kD MAPK and enhanced the expression of the antioxidant genes CAT1, cAPX, and GR1 and the total activities of the antioxidant enzymes catalase, ascorbate peroxidase, glutathione reductase, and superoxide dismutase. Such enhancements were blocked by pretreatment with several MAPK kinase inhibitors and reactive oxygen species inhibitors or scavengers. Pretreatment with MAPK kinase inhibitors also substantially arrested the ABA-induced H2O2 production after 2 h of ABA treatment, but did not affect the levels of H2O2 within 1 h of ABA treatment. Pretreatment with several inhibitors of protein tyrosine phosphatase, which is believed to be a negative regulator of MAPK, only slightly prevented the ABA-induced H2O2 production, but did not affect the ABA-induced MAPK activation and ABA-enhanced antioxidant defense systems. These results clearly suggest that MAPK but not protein tyrosine phosphatase is involved in the ABA-induced antioxidant defense, and a cross talk between H2O2 production and MAPK activation plays a pivotal role in the ABA signaling. ABA-induced H2O2 production activates MAPK, which in turn induces the expression and the activities of antioxidant enzymes. The activation of MAPK also enhances the H2O2 production, forming a positive feedback loop.
The phytohormone abscisic acid (ABA) regulates many important aspects of plant growth and development, including the synthesis of seed storage proteins, the promotion of seed desiccation tolerance and dormancy, and the inhibition of seed germination and seedling growth (Finkelstein et al., 2002). Although ABA has broad functions in plant growth and development, its main function is to regulate plant adaptive responses to various adverse environmental conditions (Zhu, 2002).
An increasing body of evidence indicates that one mode of ABA action is associated with oxidative stress in plant cells. ABA can cause the generation of reactive oxygen species (ROS) in various plant cells or tissues (Guan et al., 2000; Pei et al., 2000; Jiang and Zhang, 2001; Lin and Kao, 2001; Kwak et al., 2003; Kuo and Kao, 2004; Laloi et al., 2004; Hu et al., 2005), induce the expression of antioxidant genes (Bueno et al., 1998; Guan and Scandalios, 1998; Guan et al., 2000; Fryer et al., 2003; Park et al., 2004), and enhance the capacity of antioxidant defense systems, including enzymatic and nonenzymatic constituents (Bueno et al., 1998; Bellaire et al., 2000; Jiang and Zhang, 2001, 2002a, 2002b, 2003; Kuo and Kao, 2004; Hu et al., 2005). ROS are important intermediate components in the ABA-induced antioxidant defense (Jiang and Zhang, 2002a, 2002b, 2003; Hu et al., 2005). However, the mechanisms that ABA-induced ROS production up-regulates antioxidant defense with have yet to be determined.
Several lines of evidence from biochemical and genetic studies of plant stress signaling indicate that reversible protein phosphorylation plays an important role in the regulation of physiological status and gene expression in response to various environmental stresses (Yuasa et al., 2001; Xiong and Yang, 2003). The mitogen-activated protein kinase (MAPK) cascade is one of the major pathways by which extracellular stimuli are transduced into intracellular responses in all eukaryotic cells (Tena et al., 2001; Zhang and Klessig, 2001; Jonak et al., 2002). MAPK and immediate upstream activators, MAPK kinase (MAPKK) and MAPKK kinase, constitute a functionally interlinked MAPK cascade. Activation of the MAPK can facilitate its translocation to the nucleus where it can phosphorylate and activate transcription factors, thereby modulating gene expression (Neill et al., 2002). It has been shown that MAPKs are involved in plant signal transduction in response to pathogens, drought, salinity, cold, wounding, O3, ROS, and hormone stimuli (Tena et al., 2001; Zhang and Klessig, 2001; Jonak et al., 2002; Lu et al., 2002; Mittler, 2002; Samuel and Ellis, 2002; Moon et al., 2003; Xiong and Yang, 2003; Mittler et al., 2004).
ABA treatment activates AtMPK3 and p46MAPK in Arabidopsis (Arabidopsis thaliana; Lu et al., 2002), OsMAPK5 in rice (Oryza sativa; Xiong and Yang, 2003), p45MAPK in pea (Pisum sativum; Burnett et al., 2000), and several MAPK isoforms between 40 and 43 kD in barley (Hordeum vulgare) aleurone protoplasts (Knetsch et al., 1996). Oxidative stress leads to the activation of AtMPK3 and AtMPK6 in Arabidopsis (Kovtun et al., 2000; Moon et al., 2003) and the salicylate-induced protein kinase in tobacco (Nicotiana tabacum; Samuel et al., 2000; Samuel and Ellis, 2002). The activation of MAPKs enhances tolerance to multiple stresses, including oxidative stress (Kovtun et al., 2000; Samuel and Ellis, 2002; Moon et al., 2003; Xiong and Yang, 2003). However, it is not clear whether a MAPK pathway is involved in ABA-enhanced antioxidant defense systems in plants. Moreover, both ABA and H2O2 can activate the same MAPK in Arabidopsis (Lu et al., 2002) and pea (Desikan et al., 2004), and the MAPK mediates both ABA- and H2O2-induced stomatal closure (Desikan et al., 2004), suggesting that ABA and H2O2 may converge on MAPK-signaling pathways regulating stomatal closure. However, the relationship between ABA, MAPK, and H2O2 production remains to be determined in ABA signaling.
In this study, an effort was made to elucidate whether the MAPK pathway is involved in ABA-enhanced antioxidant defense systems in plants and, if so, what the relationship between ABA, MAPK, and H2O2 production in ABA signaling is. First of all, the effects of ABA or H2O2 treatment on the activation of MAPKs; the expression of several antioxidant genes, such as CAT1 (encoding catalase [CAT] isozyme 1), cAPX (encoding a cytosolic isoform of ascorbate peroxidase [APX]), and GR1 (encoding a plastidial isoform of glutathione reductase [GR]); and the total activities of the antioxidant enzymes CAT, APX, GR, and superoxide dismutase (SOD) were investigated. The effects of pretreatment with PD98059 and U0126, two widely used specific inhibitors of MAPKK (Favata et al., 1998; Romeis et al., 1999; Mockaitis and Howell, 2000; Desikan et al., 2001; Lu et al., 2002; Samuel and Ellis, 2002), and phenylarsine oxide (PAO) and 3,4 dephosphatin (3,4 DP), two specific inhibitors of protein Tyr phosphatase (PTP; MacRobbie, 2002), on the ABA- or H2O2-induced activation of MAPK, H2O2 production, and the expression and the total activities of antioxidant enzymes were also examined. PTP has been suggested to serve as a primary target for oxidative stress and the activation of a MAPK cascade in Arabidopsis (Gupta and Luan, 2003). Several other ROS manipulators were then used, such as diphenylene iodonium (DPI) and imidazole, two inhibitors of NADPH oxidase (Jiang and Zhang, 2002a, 2003), and Tiron and dimethylthiourea (DMTU), the scavengers for O2 and H2O2, respectively (Jiang and Zhang, 2002b). The manipulation of ROS levels should help to assess the possible link between MAPK activation, H2O2 production, and antioxidant defense in ABA signaling.
RESULTS
MAPK But Not PTP Is Involved in ABA-Induced Antioxidant Defense
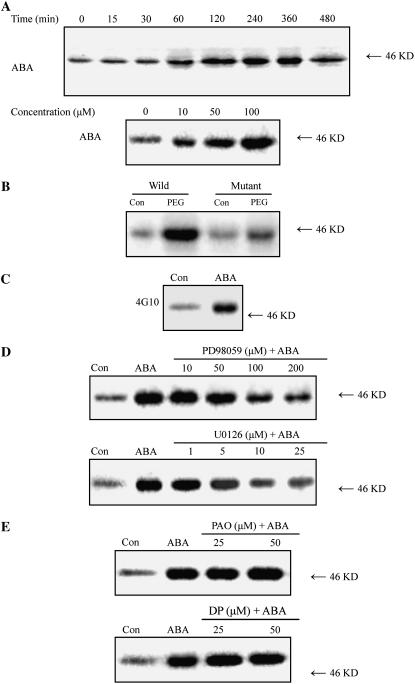
To investigate the effects of ABA on the activation of MAPKs in leaves of maize (Zea mays) plants, in-gel kinase assays were performed on protein extracts from the leaves of maize plants treated with ABA, using myelin basic protein (MBP) as a substrate. Treatment with 100 μm ABA led to a significant increase in the activity of a 46-kD kinase within 1 h, maximized at 4 h, remained high for 6 h after ABA treatment, and then decreased after 8 h of ABA treatment (Fig. 1A). The ABA-induced activation of the 46-kD MBP kinase occurred in a dose-dependent manner (Fig. 1A). To investigate whether the MBP kinase can be activated by endogenous ABA, the ABA-deficient maize vp5 mutant, which interrupts ABA biosynthesis early in the biosynthetic pathway (Guan and Scandalios, 1998; Sharp, 2002), was used. Treatment with 10% polyethylene glycol (PEG) for 2 h resulted in a significant increase in the activity of the MBP kinase in the wild type, but only a slight increase in the mutant (Fig. 1B), indicating that water stress-induced ABA accumulation can activate the MBP kinase. To demonstrate the exogenous and endogenous ABA-activated MBP kinase is a MAPK-like enzyme, immunoprecipitation was performed on protein extracts using the anti-phosphotyrosine monoclonal antibody 4G10, which has been widely used to demonstrate Tyr phosphorylation of MAPKs, an important characteristic of MAPKs (Zhang and Klessig, 1997; Zhang et al., 1998; Desikan et al., 1999; Burnett et al., 2000; Hoyos and Zhang, 2000; Ichimura et al., 2000). Protein extracts from control- or ABA-treated leaves were immunoprecipitated with 4G10 and then subjected to the in-gel kinase assay. As shown in Figure 1C, treatment with 100 μm ABA resulted in an increase in immunoprecipitated Tyr-phosphorylated MBP kinase activity when compared to the control. Moreover, pretreatment with the widely used specific MAPKK inhibitors PD98059 and U0126 inhibited the increase in the activity of the MBP kinase induced by ABA in a dose-dependent manner (Fig. 1D). PD98059 (100 μm) or U0126 (10 μm) substantially reduced the activation of the kinase induced by ABA. These results obtained from the above clearly suggest that the ABA-activated MBP kinase is a MAPK-like enzyme. However, pretreatment with PAO (25 or 50 μm) and 3,4 DP (25 or 50 μm), two specific inhibitors of PTP, which has been shown to be a negative regulator of MAPK in vitro (Gupta and Luan, 2003), did not affect the activity of MBP kinase induced by ABA in leaves of maize plants (Fig. 1E).
Figure 1.
ABA activates an MBP kinase that is Tyr phosphorylated and is inhibited by PD98059 and U0126 in leaves of maize plants. A, Time course and dose dependence for ABA-induced MBP kinase activation. The detached plants were treated with either 100 μm ABA for various times (top) or 10, 50, and 100 μm ABA for 4 h (bottom). B, Water stress-induced ABA activates the MBP kinase. The detached vp5 mutant and wild-type plants were treated with 10% PEG for 2 h. C, Tyr phosphorylation of ABA-activated MBP kinase. The detached plants were treated with 100 μm ABA for 4 h. Protein extracts from control- or ABA-treated leaves were immunoprecipitated with 4G10 and then subjected to the in-gel kinase assay. D, Effects of the MAPKK inhibitors PD98059 and U0126 on ABA-induced activation of MBP kinase. The detached plants were pretreated with different concentrations of PD98059 (top) or U0126 (bottom) for 8 h, then exposed to 100 μm ABA or distilled water (control [Con]) treatment for 4 h. E, Effects of the PTP inhibitors PAO and 3,4 DP on ABA-induced activation of MBP kinase. The detached plants were pretreated with 25 or 50 μm PAO (top) and 25 or 50 μm 3,4 DP (bottom) for 8 h, then exposed to 100 μm ABA or distilled water (control [Con]) treatment for 4 h. All experiments were repeated at least three times with similar results.
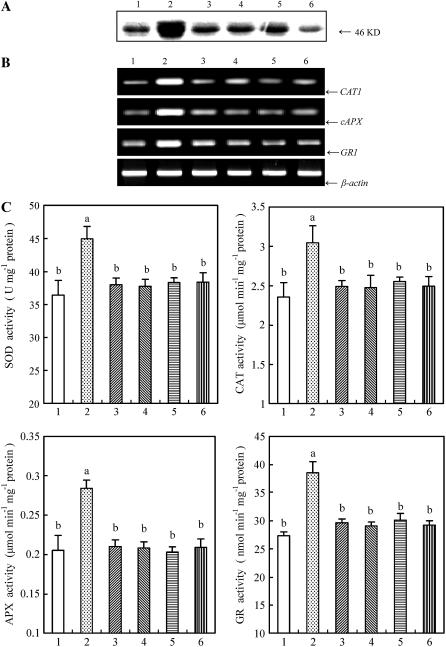
ABA treatment also resulted in the enhancement in the transcript levels of several antioxidant genes, such as CAT1, cAPX, and GR1 (Fig. 2A), and the total activities of the antioxidant enzymes CAT, APX, GR, and SOD (Fig. 2B) in leaves of maize plants. The time-course analysis of gene expression and the enzyme activities showed that the transcript levels and the total activities of antioxidant enzymes reached the maximum values after 8 and 12 h of ABA treatment, respectively (data not shown). Pretreatment with 100 μm PD98059 and 10 μm U0126 significantly blocked the increases in the transcript levels and the total activities of these antioxidant enzymes induced by ABA (Fig. 2), but pretreatment with 25 (Fig. 2) or 50 μm (data not shown) PAO and 25 (Fig. 2) or 50 μm (data not shown) 3,4 DP did not affect the ABA-induced increases in those of antioxidant enzymes in leaves of maize plants exposed to ABA treatment.
Figure 2.
Effects of pretreatment with MAPKK inhibitors and PTP inhibitors on the gene expression and total activities of antioxidant enzymes in leaves of maize plants exposed to ABA treatment. A, The transcript levels of the antioxidant genes CAT1, cAPX, and GR1 analyzed by reverse transcription (RT)-PCR. B, The total activities of the antioxidant enzymes SOD, CAT, APX, and GR. The detached plants were treated as follows: 1, distilled water (control); 2, 0.1% DMSO; 3, 100 μm ABA; 4, 100 μm PD98059 + 100 μm ABA; 5, 10 μm U0126 + 100 μm ABA; 6, 25 μm PAO + 100 μm ABA; and 7, 25 μm 3,4 DP + 100 μm ABA. The detached plants were pretreated with various inhibitors or DMSO for 8 h, and then exposed to ABA or distilled water treatment for 8 h (A) or 12 h (B). In A, experiments were repeated at least five times with similar results. In B, the values are the means ± se (n = 6) of three different experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
ROS Are Required for the Activation of MAPK and the Induction of Antioxidant Defense in ABA Signaling
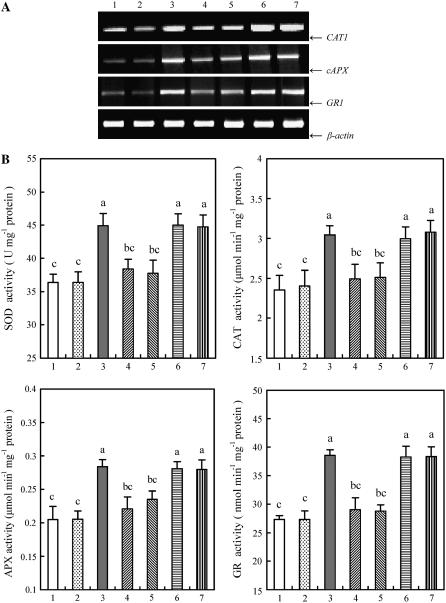
Previous studies have shown that both ABA and H2O2 can activate the same MAPK (Lu et al., 2002; Desikan et al., 2004), and ROS are required for the ABA-induced antioxidant defense (Jiang and Zhang, 2002a, 2002b; Hu et al., 2005). To establish a link between ROS, MAPK, and antioxidant defense in ABA signaling, the detached plants were pretreated with several ROS manipulators, such as DPI and imidazole, two inhibitors of NADPH oxidase; Tiron and DMTU, the scavengers for O2 and H2O2, respectively; and then exposed to ABA treatment. Experimental results showed that pretreatment with these ROS inhibitors or scavengers nearly fully arrested the ABA-induced activation of MBP kinase (Fig. 3A), and also blocked the enhancement in the transcript levels (Fig. 3B) and the total activities of antioxidant enzymes induced by ABA (Fig. 3C), indicating that ROS are required for the activation of MAPK and the up-regulation in the expression and the activities of antioxidant enzymes in ABA signal transduction.
Figure 3.
Effects of pretreatment with ROS scavengers or inhibitors on the activation of MBP kinase, and the expression and total activities of antioxidant enzymes in leaves of maize plants exposed to ABA treatment. A, MBP in-gel kinase activity. B, The transcript levels of the antioxidant genes CAT1, cAPX, and GR1 analyzed by RT-PCR. C, The total activities of the antioxidant enzymes SOD, CAT, APX, and GR. The detached plants were treated as follows: 1, distilled water (control); 2, 100 μm ABA; 3, 5 mm DMTU + 100 μm ABA; 4, 100 μm DPI + 100 μm ABA; 5, 20 mm imidazole + 100 μm ABA; and 6, 10 mm Tiron + 100 μm ABA. The detached plants were pretreated with ROS scavengers or inhibitors for 8 h, and then exposed to ABA treatment for 4 h (A) or 8 h (B) or 12 h (C). In A and B, experiments were repeated at least three (A) or five (B) times with similar results. In C, the values are the means ± se (n = 6) of three different experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
MAPK But Not PTP Is Involved in Exogenous H2O2-Induced Antioxidant Defense
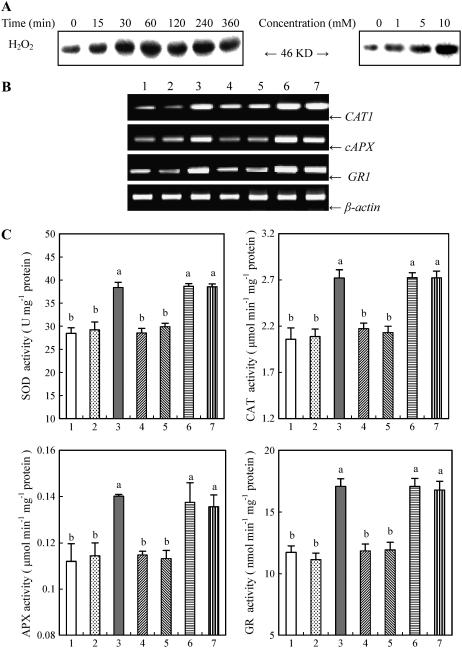
To further determine whether it is ROS that activate MAPK, which in turn leads to the induction of the antioxidant defense system, the effects of exogenously applied H2O2 on the activation of MBP kinase, the transcript levels and the total activities of antioxidant enzymes, and pretreatment with the inhibitors of MAPKK and PTP on the H2O2-induced antioxidant defense were examined. Treatment with different concentrations of H2O2 resulted in the activation of the 46-kD MBP kinase in a dose-dependent manner in leaves of maize plants (Fig. 4A). Time-course analysis showed that a significant increase in the activity of the kinase occurred within 30 min and maximized at 60 min, and then remained high for 4 h after 10 mm H2O2 treatment. Treatment with 10 mm H2O2 also led to significant increases in the transcript levels of CAT1, cAPX, and GR1 (Fig. 4B), and the total activities of CAT, APX, GR, and SOD in leaves of maize plants (Fig. 4C). Pretreatment with 100 μm PD98059 and 10 μm U0126 substantially blocked the H2O2-induced increases in the transcript levels and the total activities of these antioxidant enzymes, but pretreatment with 25 μm PAO and 25 μm 3,4 DP did not affect those of antioxidant enzymes induced by H2O2 in leaves of maize plants exposed to H2O2 treatment (Fig. 4, B and C).
Figure 4.
H2O2-induced MBP kinase activation, the expression and the total activities of antioxidant enzymes, and the effects of pretreatment with MAPKK inhibitors and PTP inhibitors on these changes induced by H2O2 in leaves of maize plants. A, Time course and dose dependence for H2O2-induced MBP kinase activation. The detached plants were treated with either 10 mm H2O2 for various times (left) or 1, 5, and 10 mm H2O2 for 1 h (right). B, Effects of pretreatment with MAPKK inhibitors and PTP inhibitors on H2O2-induced gene expression of antioxidant enzymes analyzed by RT-PCR. C, Effects of pretreatment with MAPKK inhibitors and PTP inhibitors on H2O2-induced total activities of antioxidant enzymes. In B and C, the detached plants were treated as follows: 1, distilled water (control); 2, 0.1% DMSO; 3, 10 mm H2O2; 4, 100 μm PD98059 + 10 mm H2O2; 5, 10 μm U0126 + 10 mm H2O2; 6, 25 μm PAO + 10 mm H2O2; and 7, 25 μm 3,4 DP + 10 mm H2O2. The detached plants were pretreated with various inhibitors or DMSO for 8 h, and then exposed to H2O2 or distilled water treatment for 8 h (B) or 12 h (C). In A and B, experiments were repeated at least three (A) or five (B) times with similar results. In C, the values are the means ± se (n = 6) of three different experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
Inhibition of MAPK Signaling Decreases ABA-Induced H2O2 Production
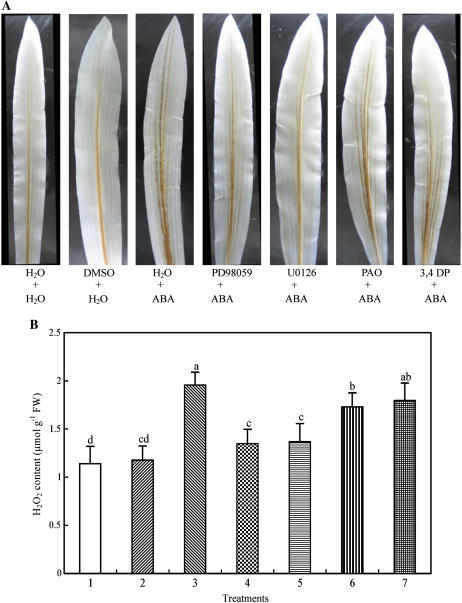
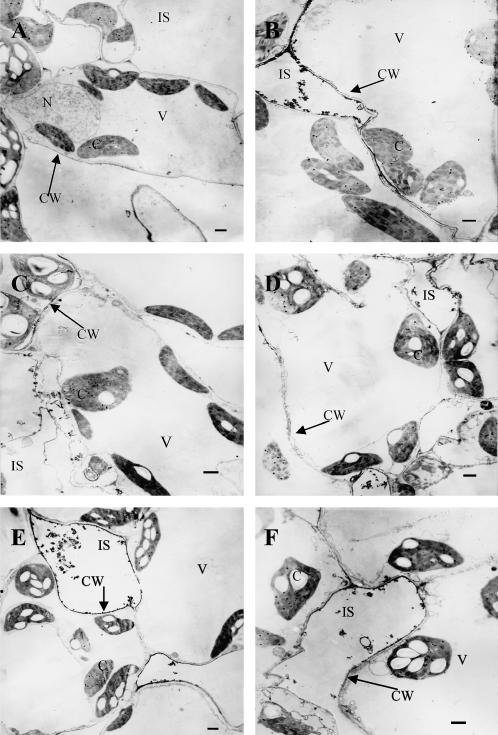
To investigate whether ABA-induced H2O2 production is regulated by MAPK or PTP, the effects of pretreatment with inhibitors of MAPKK or PTP on ABA-induced H2O2 production were examined using the methods of histochemistry with 3,3-diaminobenzidine (DAB) staining, spectrophotometry in leaf extracts, and cytochemistry with CeCl3 staining and transmission electron microscopy, respectively. Experimental results from DAB staining, in which DAB reacts with H2O2 in the presence of peroxidases to produce a brown polymerization product (Thordal-Christensen et al., 1997; Fryer et al., 2002), showed that treatment with 100 μm ABA for 2 h led to H2O2 accumulation, and the color mainly appeared in the major veins of the leaves (Fig. 5A). Pretreatment with the MAPKK inhibitors PD98059 (100 μm) and U0126 (10 μm) markedly blocked the accumulation of H2O2 induced by ABA, but pretreatment with the PTP inhibitors PAO (25 μm) and 3,4 DP (25 μm) only slightly prevented the accumulation of H2O2. The solvent dimethyl sulfoxide (DMSO) of various inhibitors did not affect the color, compared with the control. To quantify the content of H2O2 in leaves of maize plants, a spectrophotometric method was used. Treatment with ABA for 2 h increased the content of H2O2 by 72%, compared to the control value (Fig. 5B). Pretreatment with PD98059 and U0126 inhibited the increase by 75% and 72%, respectively, but pretreatment with PAO and 3,4 DP only inhibited the increase by 25% and 19%, respectively. To further confirm the effects of inhibitors on ABA-induced H2O2 production, a more sensitive detection method, staining H2O2 with CeCl3, which reacts to produce electron-dense deposits of cerium perhydroxides (Bestwick et al., 1997; Pellinen et al., 1999), was used. Treatment with ABA for 2 h led to H2O2 accumulation in apoplast of the mesophyll cells, and the greatest accumulation of H2O2 was observed in the cell walls facing intercellular spaces (Fig. 6B), as has been reported recently (Hu et al., 2005). Pretreatment with PD98059 and U0126 abolished the majority of H2O2 accumulation detectable with the CeCl3 staining (Fig. 6, C and D). Pretreatment with PAO and 3,4 DP only slightly inhibited the accumulation of H2O2 in apoplast of the mesophyll cells (Fig. 6, E and F). These results obtained with different methods and inhibitors clearly suggest that MAPK signaling mediates the ABA-induced H2O2 production, but PTP only has a minor contribution in this process.
Figure 5.
ABA-induced H2O2 accumulation and the effects of pretreatment with MAPKK inhibitors and PTP inhibitors on the production of H2O2 in leaves of detached maize plants exposed to ABA treatment. A, Histochemical detection of H2O2 production with DAB staining. B, Leaves were homogenized and H2O2 was assayed by spectrophotometry as described in “Materials and Methods.” The detached plants were treated as follows: 1, distilled water (control); 2, 0.1% DMSO; 3, 100 μm ABA; 4, 100 μm PD98059 + 100 μm ABA; 5, 10 μm U0126 + 100 μm ABA; 6, 25 μm PAO + 100 μm ABA; and 7, 25 μm 3,4 DP + 100 μm ABA. The detached plants were pretreated with various inhibitors or DMSO for 8 h, and then exposed to ABA or distilled water treatment for 2 h. In A, experiments were repeated at least five times with similar results. In B, the values are the means ± se (n = 6) of three different experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
Figure 6.
Cytochemical localization of ABA-induced H2O2 accumulation in mesophyll cells of maize leaves with CeCl3 staining and transmission electron microscopy, and the effects of pretreatment with MAPKK inhibitors and PTP inhibitors on the production of H2O2. A, Distilled water + distilled water (control). B, Distilled water + 100 μm ABA. C, 100 μm PD98059 + 100 μm ABA. D, 10 μm U0126 + 100 μm ABA. E, 25 μm PAO + 100 μm ABA. F, 25 μm 3,4 DP + 100 μm ABA. The detached plants were pretreated with various inhibitors or distilled water for 8 h, and then exposed to ABA or distilled water treatment for 2 h. Abbreviations: C, chloroplast; CW, cell wall; N, nucleus; V, vacuole; IS, intercellular space. Bar = 1 μm. Experiments were repeated at least three times with similar results.
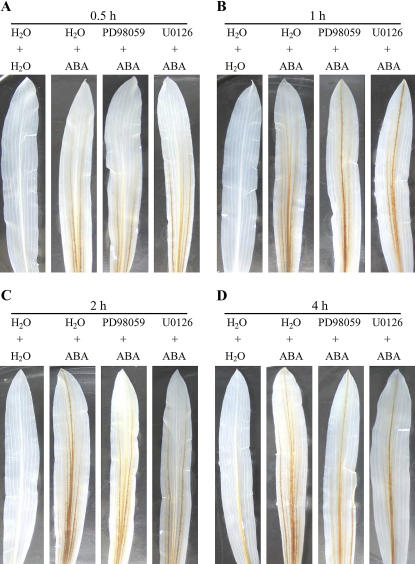
To further investigate the effects of the ABA-activated MAPK on the ABA-induced H2O2 production, the kinetics of inhibition by the MAPKK inhibitors in the ABA-induced H2O2 production was examined. Pretreatment with PD98059 and U0126 hardly affected the ABA-induced H2O2 production, detected by DAB staining, within 1 h of ABA treatment (Fig. 7, A and B), but substantially blocked the accumulation of H2O2 induced by ABA after 2 h of ABA treatment (Fig. 7, C and D). These results suggest that the initial H2O2 burst induced by ABA is not dependent on the activation of MAPK in leaves of maize plants exposed to ABA treatment.
Figure 7.
Time-course analysis of H2O2 production and the effects of MAPKK inhibitors on the H2O2 production in leaves of maize plants exposed to ABA treatment. The detached plants were pretreated with 100 μm PD98059 or 10 μm U0126 or distilled water for 8 h, and then exposed to ABA or distilled water treatment for 0.5 h (A), 1 h (B), 2 h (C), and 4 h (D), respectively. H2O2 production in leaves was detected by DAB staining. Experiments were repeated at least five times with similar results.
DISCUSSION
It has been well documented that ABA can cause the increased generation of ROS, induce the expression of antioxidant genes, and enhance the capacity of antioxidant defense systems in plants (Jiang and Zhang, 2004). ABA-induced ROS production plays an important role in the ABA signal transduction pathway leading to the induction of antioxidant defense systems (Jiang and Zhang, 2002a, 2002b, 2004; Hu et al., 2005). However, the detailed mechanisms about how ABA-induced ROS production is transduced into the antioxidant defense response remain to be determined. It has been shown that the MAPK cascade is one of the major pathways by which extracellular stimuli are transduced into intracellular responses in plant cells (Tena et al., 2001; Zhang and Klessig, 2001; Jonak et al., 2002). Both ABA and H2O2 can activate MAPKs, and the activation of MAPKs plays an important role in plant response to multiple stresses, including oxidative stress, drought, and salinity (Kovtun et al., 2000; Mittler, 2002; Samuel and Ellis, 2002; Moon et al., 2003; Xiong and Yang, 2003; Mittler et al. 2004). However, it is not clear whether a MAPK pathway is involved in ABA-enhanced antioxidant defense in plants. In this study, our results showed that ABA treatment induced the activation of a 46-kD MBP kinase in a dose-dependent manner in leaves of maize plants (Fig. 1A), and water stress-induced ABA accumulation also activated the MBP kinase (Fig. 1B). The MBP kinase activation was associated with Tyr phosphorylation (Fig. 1C), and was inhibited by pretreatment with the specific MAPKK inhibitors PD98059 and U0126 in a dose-dependent pattern (Fig. 1D). These results clearly suggest that the ABA-activated MBP kinase is a MAPK-like enzyme. Pretreatment with the ROS inhibitors or scavengers, such as DPI, imidazole, Tiron, and DMTU, also significantly reduced the activation of the ABA-induced MBP kinase (Fig. 3A). Meanwhile, ABA treatment induced the increases in the transcript levels of antioxidant genes such as CAT1, cAPX, and GR1; the total activities of the antioxidant enzymes CAT, APX, GR, and SOD in leaves of maize plants; and the increases were substantially blocked by pretreatment with the MAPKK inhibitors and the ROS inhibitors or scavengers (Figs. 2 and 3). Although the absolute specificity of each inhibitor used in this study can always be questioned, the similar results obtained with the different inhibitors, together with the increased production of H2O2 induced by ABA (Fig. 5) and the activation of the MBP kinase induced by H2O2 (Fig. 4A), clearly suggest that MAPK pathway is involved in the ABA-induced antioxidant defense systems and ROS are required for the activation of MAPK in the ABA signal transduction in plants.
The question of the relationship between MAPK activation and H2O2 production in plants exposed to various stresses or stimuli appears to be particularly interesting. H2O2 has been shown to activate MAPKs in plants (Desikan et al., 1999; Kovtun et al., 2000; Samuel et al., 2000; Yuasa et al., 2001; Samuel and Ellis, 2002; Moon et al., 2003), and the prolonged activation of MAPKs by the expression of a constitutively active mutant of MAPKK, MEKDD, leads to an H2O2 burst (Ren et al., 2002; Yoshioka et al., 2003). These results suggest that there exists a cross-talk mechanism between the oxidative burst and the MAPK cascade. The elicitor-induced H2O2 production might act upstream of MAPK activation in the induction of cell death (Yoshioka et al., 2003). Using DPI and PD98059, however, it was shown that fungal-elicitor-induced MAPK activation was not dependent on the H2O2 burst, and the H2O2 burst did not require MAPK activation as well (Romeis et al., 1999). Similarly, treatments with DPI or CAT also did not inhibit harpin- or hypoosmotic stress-induced activation of MAPKs (Cazalé et al., 1999; Desikan et al., 2001). It has been proposed that the pathways leading to the H2O2 burst and MAPK activation may separate early after the perception of pathogens or other stresses, and that the generation of H2O2 can feed into the MAPK pathway, forming a positive feedback loop (Zhang and Klessig, 2001).
Both ABA and H2O2 activate the same MAPK (Lu et al., 2002; Desikan et al., 2004), and the MAPK mediates both ABA- and H2O2-induced stomatal closure (Desikan et al., 2004), suggesting that ABA and H2O2 may converge on MAPK-signaling pathways that are involved in regulating stomatal closure. In a recent study, it was shown that the ABA-induced H2O2 production was blocked by PD98059, and the ABA- or H2O2-induced stomatal closure was also reversed in epidermal strips of broad bean (Vicia faba; Jiang et al., 2003). These authors concluded that MAPK cascade might function upstream of H2O2 production in the ABA-induced stomatal closure. However, these authors did not check the changes of endogenous MAPKs. Whether or not this occurs in vivo is not yet known. In this study, four lines of evidence indicate that ROS acts upstream of the MAPK cascade in the ABA-induced antioxidant defense. First, the time-course analysis of ROS production and MAPK activation showed that the accumulation of H2O2 preceded the activation of MAPK in the ABA signaling (Figs. 1 and 7). Second, the ABA-induced activation of MAPK was nearly fully arrested by the pretreatment with ROS inhibitors or scavengers (Fig. 3A), suggesting the activation of MAPK might be a result of H2O2 production. Third, exogenous H2O2 treatment also led to the activation of MAPK (Fig. 4A), and the time course of the H2O2-induced MAPK activity (within 30 min) was in line with ROS being upstream of MAPK cascade in the ABA signaling. Fourth, pretreatment with MAPKK inhibitors did not affect the ABA-induced H2O2 production within 1 h of ABA treatment (Fig. 7, A and B), suggesting that the initial H2O2 production induced by ABA does not require MAPK activation. Taken together, our data clearly suggest that the ABA-induced H2O2 production activates MAPK, which in turn leads to the up-regulation of antioxidant defense systems in plants. On the other hand, our results showed that pretreatment with MAPKK inhibitors abolished the majority of ABA-induced H2O2 accumulation detectable with the histochemical, cytochemical, and biochemical methods in leaves or mesophyll cells after 2 h of ABA treatment (Figs. 5–7), indicating that the MAPK cascade is also involved in the ABA-induced H2O2 production in plants. Our data suggest that the MAPK cascade-dependent increase in ABA-induced H2O2 production could be an amplification loop in ABA signaling. The possible existence of positive amplification loops in ROS signaling has recently been reported in plants in response to elicitor (Yoshioka et al., 2003) and oxidative stress (Rizhsky et al., 2004). It has been proposed that the activation of MAPKs can amplify ROS signals by regulating NADPH oxidase activity directly or activating transcription factors to enhance the expression of NADPH oxidase genes in ROS signal transduction (Mittler et al., 2004). It has been shown that ABA can enhance the gene expression (Kwak et al., 2003) and the activity (Jiang and Zhang, 2002a) of NADPH oxidase. Therefore, the ABA-activated MAPK might enhance ROS signals via the activity of NADPH oxidase.
Protein dephosphorylation has also been shown to play an important role in ABA or H2O2 signal transduction. In plants, several protein phosphatases have been characterized to be able to inactivate MAPKs, at least in vitro. These enzymes include members of the protein phosphatase 2C, PTP, and dual-specificity PTP (Tena et al., 2001; Gupta and Luan, 2003). In barley aleurone protoplasts, it was reported that the specific PTP inhibitor PAO blocked the activation of MAPK by ABA (Knetsch et al., 1996), suggesting PTP is a positive regulator of MAPK activation. However, in vitro experiments have shown that the Arabidopsis AtPTP1 is reversibly inactivated by H2O2, and the inactivation of AtPTP1 is strongly associated with the activation of AtMPK6 (Gupta and Luan, 2003). These data suggest that AtPTP1 may serve as a primary target for oxidative stress and the activation of a MAPK cascade. Using several specific PTP inhibitors, which include PAO and 3,4 DP, it has been suggested that PTP activity is essential for stomatal closure induced by four different factors, such as ABA, H2O2, Ca2+, and darkness (MacRobbie, 2002). Recent studies have also shown that pretreatment with PAO can inhibit the ABA-induced H2O2 production and reverse the ABA-induced stomatal closure (Shi et al., 2004), and enhance the sensitivity to the inhibitory effect of ABA on seed germination (Reyes et al., 2006). However, the effects of PAO and 3,4 DP on the activation of MAPKs were not examined in these studies. Whether PTP is a negative regulator of MAPK activation in vivo is still unknown. Furthermore, there is no information about whether PTP is involved in the ABA-induced antioxidant defense in plants. In this study, our results showed that pretreatment with 25 μm PAO and 25 μm 3,4 DP, a concentration applied to inhibit the activity of PTP (Knetsch et al., 1996; MacRobbie, 2002; Shi et al., 2004) or affect MAPK activation (Knetsch et al., 1996), only slightly prevented the increased generation of H2O2 induced by ABA (Figs. 5 and 6), but did not affect the ABA-activated MAPK and the ABA- or H2O2-induced increases in the gene expression and the total activities of antioxidant enzymes (Figs. 1, 2, and 4). A higher concentration (50 μm) of inhibitors also did not affect the activation of MAPK (Fig. 1E), or the expression and the total activities of antioxidant enzymes induced by ABA (data not shown). These data clearly suggest that PTPs are not involved in the ABA- or H2O2-induced up-regulation of antioxidant defense systems in plants. However, we cannot exclude the possible involvement of other protein phosphatases, such as protein phosphatase 2C, which also has been shown to be involved in the ABA-induced stomatal closure (Murata et al., 2001) in the ABA-induced antioxidant defense.
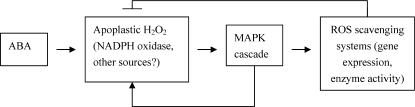
In conclusion, our results clearly suggest that MAPK but not PTP is involved in ABA- and H2O2-induced antioxidant defense, and a cross talk between H2O2 production and MAPK activation plays a pivotal role in the ABA signaling. ABA-induced H2O2 production, which mainly originates from apoplast (Hu et al., 2005), activates MAPK, which in turn induces the expression of antioxidant genes and up-regulates the activities of antioxidant enzymes. The activation of MAPK also enhances H2O2 production, forming a positive amplification loop. The up-regulation of antioxidant defense systems conversely controls ROS levels, resulting in the suppression of ROS (Fig. 8). Further studies are needed to identify the nature of MAPK, and elucidate the molecular mechanisms of interaction between H2O2 production and MAPK activation and how MAPK up-regulates the antioxidant defense system in ABA signaling.
Figure 8.
Model summarizing the interaction of H2O2, MAPK, and antioxidant defense systems in ABA signaling in maize plants.
MATERIALS AND METHODS
Plant Materials and Treatments
Seeds of maize (Zea mays L. cv Nongda 108; from Nanjing Agricultural University, China) were sown in trays of sand in a light chamber at a temperature of 22°C to 28°C, photosynthetic active radiation of 200 μmol m−2 s−1 and a photoperiod of 14/10 h (day/night), and watered daily. Seeds of the vp5 mutant and wild-type maize were obtained by selfing plants grown from heterozygous seed (Maize Genetics Stock Center, Urbana, IL). Selfed ears with kernels segregating for the mutation were chosen; mutant kernels were identified by the lack of carotenoid pigmentation. Mutant and wild-type seedlings were grown as described above. When the second leaves were fully expanded, they were collected and used for all investigations.
The plants were excised at the base of the stem and placed in the distilled water for 1 h to eliminate wound stress. After treatment, the cut ends of the stems were placed in the beakers wrapped with aluminum foil containing 100 μm ABA or 10 mm H2O2 solution for 12 h at 25°C, with a continuous light intensity of 200 μmol m−2 s−1. To investigate the role of endogenous ABA, the detached mutant and wild-type plants were treated with 10% PEG (PEG 6000) for 2 h under the same conditions as described above. To study the effects of various inhibitors or scavengers, the detached plants were pretreated with 100 μm PD98059, 10 μm U0126, 25 or 50 μm PAO, 25 or 50 μm 3,4 DP, 100 μm DPI, 20 mm imidazole, 10 mm Tiron, and 5 mm DMTU for 8 h, then exposed to 100 μm ABA or 10 mm H2O2 treatment for 12 h under the same conditions as described above. Detached plants were treated with distilled water under the same conditions for the whole period and served as controls for the above. After treatments of detached maize plants, the second leaves were sampled and immediately frozen under liquid N2 for further analysis.
Protein Extraction and In-Gel Kinase Activity Assay
Protein was extracted from leaves with an extraction buffer (100 mm HEPES, pH 7.5, 5 mm EDTA, 5 mm EGTA, 10 mm dithiothreitol [DTT], 10 mm Na3VO4, 10 mm NaF, 1 mm PMSF, 5 μg mL−1 leupeptin, 5 μg mL−1 aprotinin, 5% glycerol, 50 mm β-glycerophosphate) using the method of Zhang and Klessig (1997) with minor modifications. After centrifugation at 15,000g for 30 min at 4°C, the supernatants were transferred into clean tubes, immediately frozen with liquid N2, and stored at −80°C. Protein content was determined according to the method of Bradford (1976) with bovine serum albumin as standard.
In-gel kinase activity assays were performed using the method as described by Zhang and Klessig (1997). Extracts containing 20 μg of protein were electrophoresed on 10% SDS-polyacrylamide gels embedded with 0.25 mg mL−1 MBP in the separating gel as a kinase substrate. After electrophoresis, SDS was removed by washing the gel with a washing buffer (25 mm Tris, pH 7.5, 0.5 mm DTT, 0.1 mm Na3VO4, 5 mm NaF, 0.5 mg mL−1 bovine serum albumin, and 0.1% Triton X-100) three times for 30 min each at room temperature. The kinases were allowed to renature in 25 mm Tris, pH 7.5, 1 mm DTT, 0.1 mm Na3VO4, and 5 mm NaF at 4°C overnight with three changes of buffer. The gel was then incubated at room temperature in 30 mL of reaction buffer (25 mm Tris, pH 7.5, 2 mm EGTA, 12 mm MgCl2, 1 mm DTT, and 0.1 mm Na3VO4) with 200 nm ATP plus 50 μCi [γ-32P]ATP (3,000 Ci mm−1) for 60 min. The reaction was stopped by transferring the gel into 5% trichloroacetic acid (w/v)/1% sodium pyrophosphate (w/v). The unincorporated [γ-32P]ATP was removed by washing with the same solution for at least 6 h with five changes. The gel was dried onto Whatman 3 MM paper and exposed to Kodak XAR-5 film. Prestained size markers (Bio-Rad) were used to calculate the size of the kinases.
Immunoprecipitation Kinase Activity Assay
Protein extract (50 μg) was incubated with anti-phosphotyrosine monoclonal antibody 4G10 (2 μg; Upstate Biotechnology) in immunoprecipitation buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 2 mm EGTA, 1 mm Na3VO4, 1 mm NaF, 10 mm β-glycerophosphate, 2 μg mL−1 antipain, 2 μg mL−1 aprotinin, 2 μg mL−1 leupeptin, 0.5% [v/v] Triton X-100, and 0.5% [v/v] Nonidet P-40) at 4°C for 4 h on a rocker. About 20 μL packed volume of protein G agarose was added, and the incubation was continued for another 2 h. Agarose bead-protein complexes were pelleted by brief centrifugation. After washing with immunoprecipitation buffer three times, 1× SDS sample buffer was added and boiled for 3 min. After centrifugation, the supernatant fraction was electrophoresed on 10% SDS-polyacrylamide gels, and the in-gel kinase assay was performed.
Isolation of Total RNA and Reverse Transcription-PCR
Total RNA was isolated from leaves by using RNeasy mini kit (Qiagen) according to the instruction supplied by the manufacturer. Approximately 3 μg of total RNA were reverse transcribed using oligo(dT) primer and SuperScript II reverse transcriptase (Invitrogen). cDNA was amplified by PCR using the following primers: CAT1, forward CCAAGGGTTTCTTTGAGGT and reverse AGGGTCGAAGGAACGATAT; cAPX, forward TCGGCACCATGAAGAACCC and reverse TCCTCGTCCGCTGCGTATT; GR1, forward GAAGGTCGTGGAAAGATA and reverse TTGGCAACGAAGACATCA; and β-actin, forward AAA TGA CGC AGA TTA TGT TTG A and reverse GCT CGT AGT GAG GGA GTA CC. To standardize the results, the relative abundance of β-actin was also determined and used as the internal standard.
The cycle number of the PCR reactions was adjusted for each gene to obtain barely visible bands in agarose gels. Aliquots of the PCR reactions were loaded on agarose gels and stained with ethidium bromide.
Enzyme Assays
Frozen leaf segments (0.5 g) were homogenized in 10 mL of 50 mm potassium phosphate buffer, pH 7.0, containing 1 mm EDTA and 1% polyvinylpyrrolidone, with the addition of 1 mm ascorbate in the case of APX assay. The homogenate was centrifuged at 15,000g for 20 min at 4°C and the supernatant was immediately used for the following antioxidant enzyme assays.
The total activities of antioxidant enzymes were determined as described previously (Jiang and Zhang, 2001). Total SOD activity was assayed by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium. One unit of SOD activity was defined as the amount of enzyme that was required to cause 50% inhibition of the reduction of nitro blue tetrazolium as monitored at 560 nm. Total CAT activity was assayed by measuring the rate of decomposition of H2O2 at 240 nm. Total APX activity was measured by monitoring the decrease in absorbance at 290 nm as ascorbate was oxidized. Total GR activity was measured by following the change in A340 as oxidized glutathione-dependent oxidation of NADPH.
Histochemical Detection of H2O2
H2O2 was visually detected in the leaves of plants by using DAB as substrate (Orozco-Cárdenas and Ryan, 1999). Briefly, plants were excised at the base of stems with a razor blade and supplied through the cut stems with a 1 mg mL−1 solution of DAB, pH 3.8, for 8 h under light at 25°C, and then exposed to various treatments. After these treatments, the second leaves were decolorized by immersion of leaves in boiling ethanol (96%) for 10 min. This treatment decolorized the leaves except for the deep brown polymerization product produced by the reaction of DAB with H2O2. After cooling, the leaves were extracted at room temperature with fresh ethanol and photographed.
Cytochemical Detection of H2O2
H2O2 was visualized at the subcellular level using CeCl3 for localization (Bestwick et al., 1997). Electron-dense CeCl3 deposits are formed in the presence of H2O2 and are visible by transmission electron microscopy. Tissue pieces (approximately 1 to 2 mm2) were excised from the treated and untreated leaves and incubated in freshly prepared 5 mm CeCl3 in 50 mm MOPS at pH 7.2 for 1 h. The leaf sections were then fixed in 1.25% (v/v) glutaraldehyde and 1.25% (v/v) paraformaldehyde in 50 mm sodium cacodylate buffer, pH 7.2, for 1 h. After fixation, tissues were washed twice for 10 min in the same buffer and postfixed for 45 min in 1% (v/v) osmium tetroxide, and then dehydrated in a graded ethanol series (30%–100%; v/v) and embedded in Eponaraldite (Agar Aids). After 12 h in pure resin, followed by a change of fresh resin for 4 h, the samples were polymerized at 60°C for 48 h. Blocks were sectioned (70–90 nm) on a Reichert-Ultracut E microtome and mounted on uncoated copper grids (300 mesh). Sections were examined using a transmission electron microscope at an accelerating voltage of 75 kV.
Determination of H2O2 Content in Leaf Extracts
The content of H2O2 was measured by monitoring the A415 of the titanium-peroxide complex following the method described by Jiang and Zhang (2001). Absorbance values were calibrated to a standard curve generated with known concentrations of H2O2. Recovery was checked by adding various amounts of H2O2 to the leaf extracts as internal standard.
This work was supported by the Major State Basic Research Program of China (grant no. 2003CB114302 to M.J.), the National Natural Science Foundation of China (grant no. 30471048 to M.J.), the Key Project of Chinese Ministry of Education (grant no. 104100 to M.J.), the Science Foundation of Doctoral Subject Point of Chinese Ministry of Education (grant no. 20040307011 to M.J.), and the Hong Kong Research Grants Council (HKBU 2149/04M to J.Z.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Mingyi Jiang (myjiang@njau.edu.cn).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.075416.
References
- Bellaire BA, Carmody J, Braud J, Gossett DR, Banks SW, Lucas MC, Fowler TE (2000) Involvement of abscisic acid-dependent and -independent pathways in the up-regulation of antioxidant enzyme activity during NaCl stress in cotton callus tissue. Free Radic Res 33: 531–545 [DOI] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennett MH, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Bueno P, Piqueras A, Kurepa J, Savouré A, Verbruggen N, Van Montagu M, Inzé D (1998) Expression of antioxidant enzymes in response to abscisic acid and high osmoticum in tobacco BY-2 cell cultures. Plant Sci 138: 27–34 [Google Scholar]
- Burnett EC, Desikan R, Moser RC, Neill SJ (2000) ABA activation of an MBP kinase in Pisum sativum epidermal peels correlates with stomatal responses to ABA. J Exp Bot 51: 197–205 [DOI] [PubMed] [Google Scholar]
- Cazalé A-C, Droillard M-J, Wilson C, Heberle-Bors E, Barbier-Brygoo H, Lauriére C (1999) MAP kinase activation by hypoosmotic stress of tobacco cell suspensions: towards the oxidative burst response? Plant J 19: 297–307 [DOI] [PubMed] [Google Scholar]
- Desikan R, Cheung M-K, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signaling in stomatal guard cells. J Exp Bot 55: 205–212 [DOI] [PubMed] [Google Scholar]
- Desikan R, Clarke A, Hancock JT, Neill SJ (1999) H2O2 activates a MAP kinase-like enzyme in Arabidopsis thaliana suspension cultures. J Exp Bot 50: 1863–1866 [Google Scholar]
- Desikan R, Hancock JT, Ichimura K, Shinozaki K, Neill SJ (2001) Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol 126: 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuch KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, et al (1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273: 18623–18632 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR (2003) Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organization of Arabidopsis leaves. Plant J 33: 691–705 [DOI] [PubMed] [Google Scholar]
- Fryer MJ, Oxborough K, Mullineaux PM, Baker NR (2002) Imaging of photo-oxidative stress responses in leaves. J Exp Bot 53: 1249–1254 [PubMed] [Google Scholar]
- Guan L, Scandalios JG (1998) Two structurally similar maize cytosolic superoxide dismutase genes, Sod4 and Sod4A, respond differentially to abscisic acid and high osmoticum. Plant Physiol 117: 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan L, Zhao J, Scandalios JG (2000) Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signalling molecule for the response. Plant J 22: 87–95 [DOI] [PubMed] [Google Scholar]
- Gupta R, Luan S (2003) Redox control of protein tyrosine phosphatases and mitogen-activated protein kinases in plants. Plant Physiol 132: 1149–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos ME, Zhang S (2000) Calcium-independent activation of salicylic acid-induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant Physiol 122: 1355–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Jiang M, Zhang A, Lu J (2005) Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta 223: 57–68 [DOI] [PubMed] [Google Scholar]
- Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K (2000) Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J 24: 655–665 [DOI] [PubMed] [Google Scholar]
- Jiang J, An G, Wang P, Wang P, Han J, Jia Y, Song C-P (2003) MAP kinase specifically mediates the ABA-induced H2O2 generation in guard cells of Vicia faba L. Chin Sci Bull 48: 1919–1926 [Google Scholar]
- Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42: 1265–1273 [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J (2002. a) Involvement of plasma membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215: 1022–1030 [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J (2002. b) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53: 2401–2410 [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J (2003) Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defense in leaves of maize seedlings. Plant Cell Environ 26: 929–939 [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J (2004) Abscisic acid and antioxidant defense in plant cells. Acta Bot Sin 46: 1–9 [Google Scholar]
- Jonak C, Ökrész L, Bögre L, Hirt H (2002) Complexity, cross talk and integration of plant MAP kinase signaling. Curr Opin Plant Biol 5: 415–424 [DOI] [PubMed] [Google Scholar]
- Knetsch MLW, Wang M, Snaar-Jagalska BE, Heimovaara-Dijkstra S (1996) Abscisic acid induces mitogen-activated protein kinase activation in barley aleurone protoplasts. Plant Cell 8: 1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu W-L, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TH, Kao CH (2004) Hydrogen peroxide is necessary for abscisic acid-induced senescence of rice leaves. J Plant Physiol 161: 1347–1357 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei Z-M, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi C, Mestres-Ortega D, Marco Y, Meyer Y, Reichheld J-P (2004) The Arabidopsis cytosolic h5 gene induction by oxidative stress and its w-box-mediated response to pathogen elicitor. Plant Physiol 134: 1006–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Kao CH (2001) Abscisic acid induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Sci 160: 323–329 [DOI] [PubMed] [Google Scholar]
- Lu C, Han MH, Guevara-Garcia A, Fedoroff NV (2002) Mitogen-activated protein kinase signaling in postgermination arrest of development by abscisic acid. Proc Natl Acad Sci USA 99: 15812–15817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EAC (2002) Evidence for a role for protein tyrosine phosphatase in the control of ion release from the guard cell vacuole in stomatal closure. Proc Natl Acad Sci USA 99: 11963–11968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Mockaitis K, Howell SH (2000) Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J 24: 785–796 [DOI] [PubMed] [Google Scholar]
- Moon H, Lee B, Choi G, Shin D, Prasad DT, Lee O, Kwak S-S, Kim DH, Nam J, Bahk J, et al (2003) NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc Natl Acad Sci USA 100: 358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder JI (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signaling molecules in plants. J Exp Bot 53: 1237–1247 [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Ryan CA (1999) Hydrogen peroxide is generated systematically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96: 6553–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Ryu SH, Jang IC, Kwon SY, Kim JG, Kwak SS (2004) Molecular cloning of a cytosolic ascorbate peroxidase cDNA from cell cultures of sweetpotato and its expression in response to stress. Mol Genet Genomics 271: 339–346 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Pellinen R, Palva T, Kangasjärvi J (1999) Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J 20: 349–356 [DOI] [PubMed] [Google Scholar]
- Ren D, Yang H, Zhang S (2002) Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem 277: 559–565 [DOI] [PubMed] [Google Scholar]
- Reyes D, Rodríguez D, Nicolás G, Nicolás C (2006) Evidence of a role for tyrosine dephosphorylation in the control of postgermination arrest of development by abscisic acid in Arabidopsis thaliana L. Planta 223: 381–385 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Davletova S, Liang H, Mittler R (2004) The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J Biol Chem 279: 11736–11743 [DOI] [PubMed] [Google Scholar]
- Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JDG (1999) Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11: 273–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Ellis BE (2002) Double jeopardy: Both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. Plant Cell 14: 2059–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Miles GP, Ellis BE (2000) Ozone treatment rapidly activates MAP kinase signaling in plants. Plant J 22: 367–376 [DOI] [PubMed] [Google Scholar]
- Sharp RE (2002) Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ 25: 211–222 [DOI] [PubMed] [Google Scholar]
- Shi W, Jia W, Liu X, Zhang S (2004) Protein tyrosine phosphatases involved in signaling of the ABA-induced H2O2 generation in guard cells of Vicia faba L. Chin Sci Bull 49: 1841–1846 [Google Scholar]
- Tena G, Asai T, Chiu WL, Sheen J (2001) Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol 4: 392–400 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Xiong L, Yang Y (2003) Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell 15: 745–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa T, Ichimura K, Mizoguchi T, Shinozaki K (2001) Oxidative stress activates AtMPK6, an Arabidopsis homologue of MAP kinase. Plant Cell Physiol 42: 1012–1016 [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N (2003) Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15: 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Du H, Klessig DF (1998) Activation of the tobacco SIP kinase by both a cell wall-derived carbohydrate elicitor and purified proteinaceous elicitins from Phytophthora spp. Plant Cell 10: 435–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF (1997) Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell 9: 809–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF (2001) MAPK cascades in plant defense signaling. Trends Plant Sci 6: 520–527 [DOI] [PubMed] [Google Scholar]
- Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]