Abstract
Aluminum (Al) toxicity and phosphorus (P) deficiency often coexist in acid soils that severely limit crop growth and production, including soybean (Glycine max). Understanding the physiological mechanisms relating to plant Al and P interactions should help facilitate the development of more Al-tolerant and/or P-efficient crops. In this study, both homogeneous and heterogeneous nutrient solution experiments were conducted to study the effects of Al and P interactions on soybean root growth and root organic acid exudation. In the homogenous solution experiments with a uniform Al and P distribution in the bulk solution, P addition significantly increased Al tolerance in four soybean genotypes differing in P efficiency. The two P-efficient genotypes appeared to be more Al tolerant than the two P-inefficient genotypes under these high-P conditions. Analysis of root exudates indicated Al toxicity induced citrate exudation, P deficiency triggered oxalate exudation, and malate release was induced by both treatments. To more closely mimic low-P acid soils where P deficiency and Al toxicity are often much greater in the lower soil horizons, a divided root chamber/nutrient solution approach was employed to impose elevated P conditions in the simulated upper soil horizon, and Al toxicity/P deficiency in the lower horizon. Under these conditions, we found that the two P-efficient genotypes were more Al tolerant during the early stages of the experiment than the P-inefficient lines. Although the same three organic acids were exuded by roots in the divided chamber experiments, their exudation patterns were different from those in the homogeneous solution system. The two P-efficient genotypes secreted more malate from the taproot tip, suggesting that improved P nutrition may enhance exudation of organic acids in the root regions dealing with the greatest Al toxicity, thus enhancing Al tolerance. These findings demonstrate that P efficiency may play a role in Al tolerance in soybean. Phosphorus-efficient genotypes may be able to enhance Al tolerance not only through direct Al-P interactions but also through indirect interactions associated with stimulated exudation of different Al-chelating organic acids in specific roots and root regions.
Acid soils comprise up to 50% of the world's potentially arable land and thus are a significant limitation to crop production worldwide (von Uexküll and Mutert, 1995). On most acid soils, there are several limiting factors for plant growth, including toxic levels of aluminum (Al), manganese, and iron (Fe), as well as deficiencies of some essential elements, such as phosphorus (P), nitrogen, potassium (K), calcium (Ca), magnesium, and some micronutrients (Kochian et al., 2004). Among these constraints, Al toxicity and P deficiency are the most important due to their ubiquitous existence and overwhelming impact on plant growth (Kochian et al., 2004).
Aluminum toxicity limits plant growth mainly through its adverse effects on root growth and development. Under acidic soil conditions, active, phytotoxic forms of Al are released to the soil solution to levels that can inhibit root growth and damage roots (Foy, 1984, 1988; Delhaize et al., 1993a). The phytotoxicity of active Al species can be ameliorated by chelation with organic acids (Ma et al., 2001), and there is considerable evidence in support of root organic acid exudation as a major mechanism of Al tolerance in plants. Prior to the recent identification of the first gene that confers root organic acid exudation, ALMT1, in wheat (Triticum aestivum; Sasaki et al., 2004), most conclusions on the relationships between organic acid exudation and Al tolerance were based on the frequent observations of a positive correlation between Al tolerance and root organic acid exudation, such as for malate release in wheat (Delhaize et al., 1993b) and citrate exudation in snapbean (Phaseolus vulgaris; Miyasaka et al., 1991), maize (Zea mays; Pellet et al., 1995), Cassia tora (Ma et al., 1997), and soybean (Glycine max; Yang et al., 2001). Delhaize et al. (2004) showed that when the wheat ALMT1 gene was expressed in highly Al-sensitive transgenic barley (Hordeum vulgare) seedlings, this resulted in a significant increase in Al tolerance, both for seedlings grown on nutrient solutions and acid soils, providing direct evidence for the role of root organic acid exudation in plant Al tolerance. However, in some species, root organic acid exudation does not correlate with differential Al tolerance. For example, Al did not induce organic acid exudation in oat (Avena sativa; Zheng et al., 1998), and Al tolerance in signalgrass (Brachiaria decumbens) and maize was not necessarily correlated with organic acid exudation (Wenzl et al., 2001; Piñeros et al., 2005), implying that organic acid exudation is not the only mechanism of Al tolerance in plants.
Low-P availability is another important limiting factor to plant growth in acid soils. In most humid tropical and subtropical regions where acid soils prevail, warm and moist conditions result in weathered soil types (mostly Ustisols, Oxisols), in which free Fe and Al oxides bind native and applied P into forms unavailable to plants (Barber, 1995). Plants have evolved a number of adaptive mechanisms for growth on low-P soils, and these include the exudation of several solutes from roots, including organic acids, phosphatases, and other compounds that may mobilize P from bound P pools in the soil (especially Fe-P, Al-P compounds, and organic phosphate esters) and thus contribute to P efficiency in plants. In fact, release of organic acids in response to P deficiency has been reported in rape (Brassica napus; Hoffland et al., 1989), white lupin (Lupinus albus; Gardner et al., 1983), and purple lupin (Lupinus pilosus; Ligaba et al., 2004). However, results have also been obtained that do not support this correlation, including studies with wheat, buckwheat (Fagopyrum esculentum), and taro (Colocasia esculenta) where organic acid exudation did not seem to be related to P efficiency (Delhaize et al., 1993b; Ma et al., 1997; Ma and Miyasaka, 1998).
Although many studies have been conducted on plant Al tolerance and P efficiency on acid soils, Al toxicity and P deficiency are almost always studied separately as independent factors (Foy, 1988; Yan et al., 1995a, 1995b). In reality, however, Al toxicity and P deficiency often coexist in acid soils, and these two factors may strongly interact through chemical and biochemical reactions. In relation to this point, inconsistent results have often been obtained from different studies with regard to the relationship between plant Al tolerance or P efficiency studied in the laboratory, and the performance of the same genotypes on acid soils. For instance, the relative ranking of Al tolerance in soybean can change when different growth media are used (Villagarcia et al., 2001). In another study, soybean genotype 416937 was found to be Al tolerant when studied in hydroponic-based experiments, but was considerably less tolerant when grown on acid soils in the field (Ritchey and Carter, 1993; Ferrufino et al., 2000). In contrast, a soybean genotype that was scored as Al sensitive in hydroponic experiments was found to be relatively tolerant when grown on acid soils (Horst and Klotz, 1990; Foy et al., 1992). These discrepancies might be due, in part, to overlooking possible interactions between Al and other factors in the soil, particularly P status.
Relatively few studies have been done to investigate Al and P interactions in plants. Tan and Keltjens (1990) found that increasing P supply might play a role in ameliorating Al phytotoxicity, possibly through improved root growth and P uptake. Phosphorus efflux was speculated to be a potential mechanism of Al tolerance in wheat (Pellet et al., 1996). Zheng et al. (2005) found that the P content of the root apex of buckwheat was significantly correlated with the immobilization and detoxification of Al, indicating that there can be a significant P by Al interaction in roots. In a recent study from one of our laboratories, Dong et al. (2004) provided evidence for root Al and P interactions that had an influence on soybean growth and also on the root organic acid exudation patterns induced by Al toxicity and P deficiency. In that study, oxalate and malate release was induced by P deficiency, while Al activated root citrate exudation. However, in a study on purple lupin, Ligaba et al. (2004) found that citrate exudation was enhanced by P deficiency but not by Al toxicity. These previous studies were all conducted under conditions based on the exposure of root systems to uniform hydroponic media where Al and P are homogeneously distributed. However, in most acid soils, Al and P are heterogeneously distributed in the soil profile with relatively higher Al content in the subsoil and relatively higher P availability in the topsoil due to long-term soil genesis processes and agricultural practices such as fertilization and liming (Pothuluri et al., 1986; Sumner, 1995). Hence, different regions of the intact root system may be exposed to varying degrees of Al toxicity and P deficiency, depending on their depth in the soil horizon, and this may lead to the development of spatially separate and coordinated mechanisms for plant adaptation to acid soils. Therefore, studies based on the application of a uniform hydroponic medium to the entire root system may not be representative of the actual soil conditions plants have evolved in, and thus the findings from such studies may have to be considered with caution.
We have recently established an applied core collection of soybean germplasm and identified materials with varying growth performance and yield potential on the acidic low-P soils of South China (Zhao et al., 2004). In this study, we selected four soybean genotypes contrasting in P efficiency from this core collection to investigate the interactions between Al and P on soybean growth and their relationship to spatial and specific exudation of organic acids using a specially designed system to apply Al toxicity and P deficiency to root systems in a stratified manner. For comparison, we also tested organic acid exudation of soybean roots using a more conventional homogeneous hydroponic system, where Al toxicity and/or P deficiency was imposed uniformly across the entire root system. Our objectives were to elucidate possible mechanisms of Al and P interaction in soybean roots in relation to the genetic basis for P efficiency and Al tolerance.
RESULTS
Al and P Interactions on Soybean Root Growth in Homogeneous Nutrient Solution
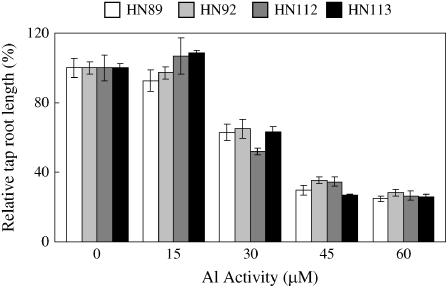
In this study, four soybean genotypes were used that we previously have shown to differ in P efficiency (the ability to tolerate P deficiency stress; Zhao et al., 2004). This previous study indicated that the soybean genotypes HN89 and HN92 performed significantly better than HN112 and HN113 both under the P-deficient conditions on acid soils, and also under field conditions of P fertilization (see Zhao et al., 2004, and also Supplemental Fig. 1). Al tolerance of these four soybean genotypes was determined using relative taproot growth as the quantitative measure of tolerance. Under homogenous low-P conditions, the taproot lengths of all the genotypes were inhibited by Al addition and the inhibition was more severe with increasing Al activities (Fig. 1). For example, the relative taproot lengths were about 60%, 40%, and 30% at 30, 45, and 60 μm Al3+ activities, respectively.
Figure 1.
Relative taproot growth at different Al3+ activities for soybean seedlings grown in homogenous nutrient solution. Relative taproot growth was calculated as the percentage of taproot growth for roots grown at different Al3+ activities relative to the taproot growth without Al addition.
Under these homogenous low-P conditions, no significant genotypic differences in Al tolerance were observed among the four genotypes that were previously shown to contrast under low-P and acidic field conditions (Fig. 1).
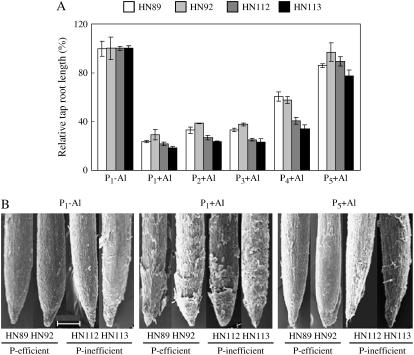
Phosphorus addition to the homogeneous hydroponic media significantly increased Al tolerance of the four soybean genotypes as indicated by relative taproot lengths, and the root growth inhibition by Al toxicity was significantly alleviated at the highest P level (320 μm P), thus demonstrating a significant Al-by-P interaction (Fig. 2A).
Figure 2.
Soybean root growth as affected by different P concentrations in the homogenous nutrient solution. A, Relative taproot growth in response to Al exposure. Five P concentrations were used, where P1, P2, P3, P4, and P5 are 0, 20, 40, 80, and 320 μm P, respectively, added as KH2PO4, and an Al3+ activity of 38 μm (as AlCl3) was used (see “Materials and Methods” for details). B, Scanning electron micrograph showing the effect of Al and P interactions on the morphology of the root apex (scale bar = 200 μm).
Moderate but statistically significant genotypic differences in Al tolerance were observed among the four soybean genotypes when the P level in the hydroponic media was increased from 0 to 80 μm, in that the two P-efficient soybean genotypes exhibited higher levels of Al tolerance than did the P-inefficient varieties. This differential Al tolerance disappeared at the highest P level employed (320 μm P; Fig. 2A). The observation of differential Al tolerance was also supported by scanning electron micrographs of the root apices, which showed that roots exposed to low-P media plus Al were severely damaged, whereas exposure of roots to increasing P levels plus Al exhibited far fewer symptoms of root damage less affected by Al, particularly for the two P-efficient genotypes (Fig. 2B). It should be noted in Figure 2B that at the highest P level employed (320 μm P), a greater degree of root damage was still seen in the two P-inefficient genotypes. However, at this P level there was only a small degree of Al inhibition of root growth. Thus, it appears that symptoms of Al-induced physical damage to roots does not necessarily correlate with Al-induced root growth inhibition.
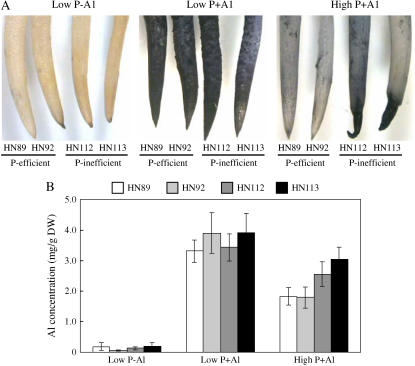
Phosphorus supplementation of the hydroponic media also significantly decreased Al accumulation in the root tips (the terminal 2 cm of the root) as indicated by both hematoxylin staining and direct quantification of root Al content using inductively coupled plasma (ICP) emission spectroscopy (Fig. 3, A and B). In accordance with the findings for relative root growth in Figure 2, the two P-efficient soybean genotypes accumulated less Al in the root apices than did the P-inefficient varieties under P supplementation (Fig. 3, A and B), indicating that Al exclusion from the root tips might be an Al tolerance mechanism that is influenced by increased plant P availability and nutrition.
Figure 3.
Effect of low P and high P in nutrient solution on the Al tolerance of soybean roots in a homogenous solution. A, Roots stained with hematoxylin to visualize root Al content. For each treatment, from left to right, the names of the soybean genotypes are HN89, HN92, HN112, and HN113, respectively. B, Root tip Al concentrations (first 2 cm of root), determined using ICP-emission spectroscopy. The Al3+ activity was 38 μm; low P was no P added and high P was 320 μm P, added as KH2PO4 (see “Materials and Methods” for details).
Root Organic Acid Exudation in Homogeneous Nutrient Solution
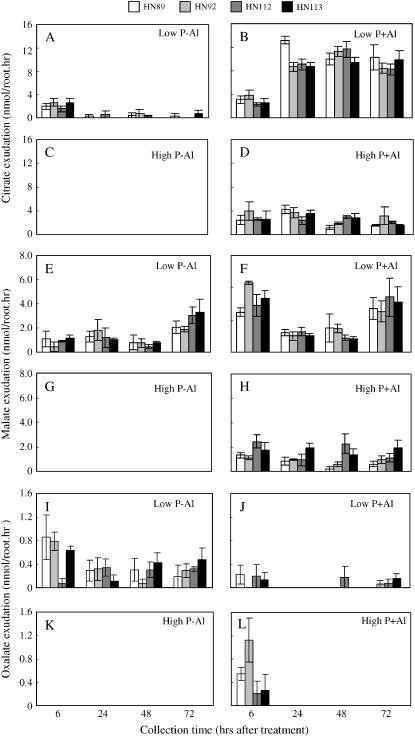
Citrate and malate were the two major organic acids induced by Al toxicity and/or low-P availability in the homogenous collection system, while oxalate appeared to be mainly induced by P deficiency (Fig. 4). Citrate exudation was strongly induced by Al toxicity, but much less so by P deficiency (Fig. 4, A–D). Al-induced citrate exudation was greatly decreased when roots were also grown on high-P levels, indicating that there is a clear Al-by-P interaction regarding root citrate exudation. It should be noted that this was not simply an effect of chelation of exogenous Al by P, as the nutrient solutions were designed to maintain the same free Al3+ activity as the level of P in the nutrient solution was increased, using the speciation program GEOCHEM-PC to calculate Al3+ activities in solution. Interestingly, the rate of Al-activated root citrate exudation increased from 6 to 24 h of Al exposure, and then maintained a relatively high constant level of exudation over the subsequent time points (48 and 72 h). On the other hand, the relatively low levels of citrate exudation induced by low-P status were only seen over the first 6 h of the exudation period. This demonstrates that the Al-activated citrate exudation is a relatively long-term response while low-P-induced citrate exudation is transient.
Figure 4.
Organic acid exudation of soybean roots in homogenous solution. There were four treatments, including two P levels (0 and 320 μm P added as KH2PO4) and two Al3+ activities (0 and 38 μm Al added as AlCl3). Organic acid exudation was measured by capillary electrophoresis (see “Materials and Methods” for details). A to D, Citrate exudation; E to H, malate exudation; I to L, oxalate exudation.
Similar to citrate exudation, malate exudation was induced by both Al and low P, but its pattern was different from that of citrate exudation. For example, the low-P treatment elicited a significant malate exudation, which completely disappeared at high P (320 μm; Fig. 4, E–H). Malate exudation was also activated by Al exposure. As was seen for citrate release, this response was greatly reduced under high-P treatment, again indicating there is a significant Al-by-P interaction on root malate exudation.
Unlike citrate and malate release, oxalate exudation was activated primarily by P deficiency, and the relative rate of P deficiency-induced oxalate exudation was much higher than the rates of citrate and malate exudation induced by low-P status (Fig. 4, I–L). Interestingly, Al-activated oxalate exudation was detectable in the high-P plants over the first 6 h of exudation, particularly for the two P-efficient genotypes, and then disappeared, indicating this Al-activated oxalate exudation is transient.
Root Growth and Organic Acid Exudation in Response to Localized Application of Al and P in Nutrient Solution
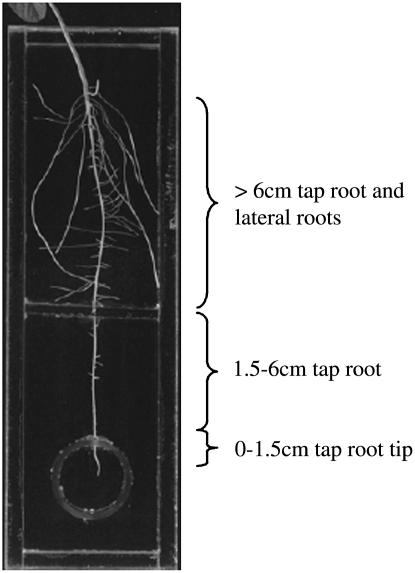
The specially designed Plexiglas chamber depicted in Figure 5 was used to isolate the solution bathing the intact root system into two compartments, upper and lower, where the upper chamber housed the first approximate 6 cm of the taproot and associated lateral roots, and the lower chamber housed the rest of the taproot. Furthermore, root exudation of organic acids was collected specifically from the terminal approximately 1.5 cm of the taproot, using a Plexiglas ring that straddled the root and isolated the root apex from the rest of the root system. This system was used to mimic the soil conditions for a low-P acid soil, such that ±P treatments were applied only to the upper chamber, and Al exposure under −P conditions was imposed in the lower chamber.
Figure 5.
Representative photograph of one of the divided root chambers. The soybean root system was separated into three compartments, including the 0 to 1.5 cm taproot tip, the 1.5 to 6 cm taproot, and the taproot region >6 cm from the root tip that included lateral roots. Each compartment received its own P and Al treatments. Organic acid exudation was collected from the first 1.5 cm of the taproot tip and measured by capillary electrophoresis (see “Materials and Methods” for details).
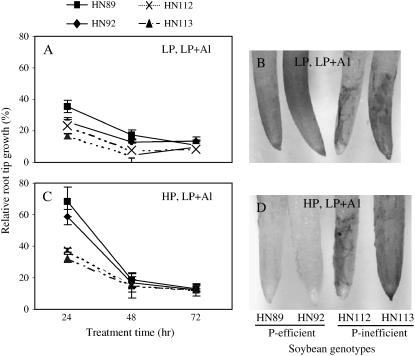
In this heterogeneous nutrient solution system, soybean taproot growth for all four soybean genotypes was significantly inhibited by Al toxicity under −P conditions in both chambers (Fig. 6, A and C). When the solution P concentration was increased in the upper compartment, the Al tolerance measured as relative taproot growth was greatly improved specifically for the two P-efficient genotypes over the first 24 h of the experiment. This finding was further confirmed by hematoxylin staining, which showed that the two P-efficient genotypes accumulated less Al than the two P-inefficient genotypes when high P was applied to the upper compartment (Fig. 6, B and D). At the later time points (48 and 72 h), this improved Al tolerance disappeared, indicating that, under the conditions imposed by these experiments, localized P supply could only transiently increase Al tolerance in the P-efficient genotypes.
Figure 6.
Effect of localized P and Al on Al tolerance of soybean roots in the divided chamber experiment. The Al3+ activity was 38 μm; low P was no P added and high P was 320 μm P, added as KH2PO4. A and C, Relative taproot growth for the four soybean genotypes in the divided chamber experiment. Relative root tip growth (%) was calculated as the percentage of taproot growth within the Plexiglas ring with Al addition relative to that without Al addition at a given P level; B and D, roots stained with hematoxylin to visualize root Al content at 24 h after treatments.
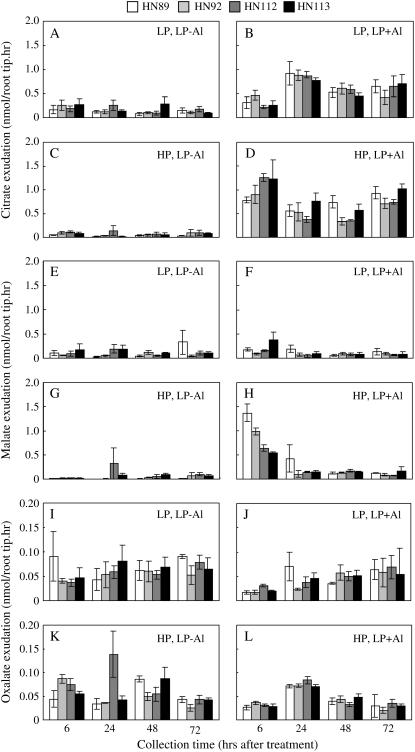
As was done for roots homogeneously exposed to Al and/or different P regimes, organic-acid exudation specifically from the taproot apex was studied under the stratified conditions of P and Al exposure. As seen in Figure 7 (A–D), citrate exudation from the root tip was strongly activated by Al exposure in the lower chamber, when the roots in the upper chamber were grown under both ±P conditions. The major difference between the low- versus high-P treatment in the upper chamber was the rate of Al-activated citrate exudation at the earliest time point (6 h). Here, the high-P treatment imposed on the upper portion of the root system resulted in a much larger Al-activated citrate release from the taproot apex residing in the lower chamber. No significant genotypic differences were observed in Al-activated citrate exudation from root tips among the four soybean genotypes, suggesting that, under these conditions that more closely mimic the real-world situation, root citrate exudation might not be a major mechanism of soybean Al tolerance, at least for the taproot.
Figure 7.
Organic acid exudation of soybean taproot tips in the divided root chamber experiment. There were four treatments, combining two P levels (0 or 320 μm P as KH2PO4) in the upper compartment and two Al activities (0 or 38 μm Al3+ as AlCl3) in the lower compartment. Organic acid exudation was performed with the terminal 1.5 cm of the taproot and measured by capillary electrophoresis (see “Materials and Methods” for details). A to D, Citrate exudation; E to H, malate exudation; I to L, oxalate exudation.
The major difference in root malate exudation between this experiment and the one depicted in Figure 4 for the homogenous treatments of roots was that high-P status in the upper chamber dramatically stimulated Al-activated malate release from the taproot, at least for the early stages of the experiment (6 and 24 h; Fig. 7, E–H). Furthermore, the two P-efficient soybean genotypes had significantly higher malate exudation from the root tips over the first 6 h (Fig. 7, E–H), indicating that the increased root tip growth in the first 24 h might be attributed to the higher rates of taproot apical malate exudation that was stimulated by providing more P to the upper portions of the root system. In comparing the data for taproot tip Al-activated malate exudation in Figure 7, G and H, with whole root Al-activated malate exudation in Figure 4, G and H, it is not clear if the Al-activated malate exudation from the whole root system under high-P nutrition in Figure 4 is primarily due to the taproot tip malate exudation depicted in Figure 7. However, as shown in Supplemental Figure 2 where Al-activated root malate exudation was measured from all three compartments (upper compartment, lower compartment, and taproot tip) under conditions where the upper compartment received high P, it is clear that high-P status in the upper chamber dramatically stimulated malate release from the whole root system for the first 6 h of the experiment, especially in the two P-efficient genotypes. Furthermore, almost all of the malate was secreted from the taproot tips in response to Al.
With regard to oxalate exudation from the taproot apex, this release was only induced by low-P status, as was also seen for exudation from the entire root system. As seen in Figure 7, I to L, high rates of root tip oxalate exudation were found in all four treatments, indicating that the low-P status of the chamber where the taproot tip grew was the primary factor triggering root tip oxalate exudation.
DISCUSSION
Soybean Al Tolerance as Influenced by Changes in P Status
In the literature, plant Al tolerance is commonly determined by measuring the relative root growth in a simple salt solution, such as CaCl2 or CaSO4, in the absence or presence of different Al concentrations (Kinraide et al., 1985). Soybean has a taproot system and therefore relative taproot growth is a good indicator for Al tolerance. To avoid chemical precipitation of P with Al, most studies have used P-free or low-P solutions to screen for Al tolerance (Wenzl et al., 2001; Piñeros et al., 2005). However, since plants need at least 17 essential nutrients for normal growth (Marschner, 1995) under some conditions, the study of Al tolerance using simple salt solutions may generate results that do not necessarily reflect the real-world situation, particularly for longer-term Al treatments where other nutrients may interact to various degrees with Al toxicity. There have been a number of studies in the literature where scientists employed dilute complete nutrient solutions to quantify relative root growth associated with Al tolerance. For example, Dong et al. (2004) used Al treatments in one-fifth-strength Hoagland nutrient solution to screen soybean for Al tolerance. This previous study dealt with both Al tolerance and P efficiency instead of focusing on Al tolerance alone, but because of the design of the experiments employing a homogenous nutrient solution, it was difficult to distinguish effects associated with P nutrition from those involving Al toxicity and tolerance. In this study we again used a dilute nutrient solution (one-half-strength Hoagland nutrient solution) varying in P concentration with or without Al. Our results confirmed that Al tolerance in soybean is influenced by varying the P concentrations in the nutrient solution, under conditions where the solution Al3+ activity was held constant (Fig. 2).
Because of the complexities of Al solution chemistry, particularly with regards to its interactions with phosphate in solution, it is critical to model the solution chemistry with a computer speciation program, such as the GEOCHEM-PC used here (Parker et al., 1995), to calculate the Al concentrations needed to maintain a specific-free Al3+ activity over a range of solution P concentrations.
The Role of P Efficiency in Soybean Al Tolerance
Al toxicity and P deficiency often coexist in acid soils, and therefore the relative ranking of Al tolerance in plants may be affected by P and Al interactions. This was the case in our study. The four soybean genotypes differing in P efficiency did not significantly differ in Al tolerance when no or very low levels of P were included in the homogeneous nutrient solution (Fig. 2). However, when higher levels of P were included in the nutrient solution, the Al tolerance of all the four soybean genotypes was significantly increased, with basically no Al toxicity symptoms seen at the highest solution P concentration employed (320 μm; Fig. 2). Under these conditions, evidence for some differential Al tolerance was seen, as the two P-efficient genotypes appeared to be more Al tolerant than the P-inefficient genotypes. This agrees with some previous findings suggesting that P had positive effects with regard to ameliorating Al tolerance (Gaume et al., 2001; Zheng et al., 2005).
It could be assumed that plants that perform well on low-P acid soils should possess root systems that are both Al tolerant and P efficient. But this is not necessarily the case in many soils that are heterogeneous with regard to distribution of Al and P in the soil horizon, in which case different root type may respond differently to Al toxicity and low P. In this study, we used a divided root chamber system that allowed us to maintain a localized supply of P and/or Al to simulate the actual growth conditions in low-P acid soils. We found that soybean taproot growth of the two P-efficient genotypes was less affected by Al toxicity (more Al tolerant) than the two P-inefficient genotypes in experiments where high P was supplied to the roots in the upper horizon, and Al toxicity and P deficiency was imposed in more deeply situated roots. This result is consistent with what we found in field studies with soybean, where P-efficient soybean genotypes had greater growth of shallow roots in the upper soil horizons where soil pH and P levels are higher, while the taproot grew more effectively in more acidic, low-P subsoil, compared with P-inefficient genotypes (Zhao et al., 2004).
One obvious possible explanation for the greater taproot Al tolerance of the P-efficient genotypes in a soil with a heterogeneous distribution of Al and P is that these genotypes may more effectively acquire P from the upper soil horizons and thus maintain a more optimal P nutrition, which could result in more robust root function. It was recently suggested that P could help ameliorate Al tolerance not only through Al complexation and possible precipitation of Al in the rhizosphere, but also through Al-P interactions in the root apoplast or even at the root-cell plasma membrane (Zheng et al., 2005). The P-efficient soybean genotypes in this study usually have more developed basal roots, which are located in the more shallow soil layers with higher P availability and less Al toxicity (Zhao et al., 2004). With improved P nutrition, the P-efficient genotypes may transport more P to the other regions of the entire root system, including the taproot tip, and thus may enhance taproot Al tolerance. Another possibility is that improved overall plant P nutrition may stimulate exudation of organic acids in the root parts exposed to Al, and thus may better serve to protect the roots from Al toxicity. This possibility will be discussed in more detail below.
Is Exudation of Specific Organic Acids Localized to Different Root Regions an Important Component of Al Tolerance in Soybean?
Organic acid exudation is considered as a major mechanism of Al tolerance and P efficiency in certain plant species (Ma et al., 2001; Ligaba et al., 2004). In this study, we investigated organic acid exudation in soybean using both homogeneous solutions where the entire root system was exposed to uniform Al toxicity and/or P deficiency, and a divided root chamber system where localized stresses and responses were investigated. Our results demonstrated that there are strong effects of Al-by-P interaction on the type of organic acid released as well as the exudation patterns, and the responses were different in the two experimental systems. In the homogeneous solution system, citrate was the major organic acid whose exudation was triggered by Al toxicity, as previously reported by Yang et al. (2001). Malate release was induced by both Al toxicity and P deficiency, but the total amount of malate exuded was much less than that of citrate. Meanwhile, oxalate was induced only by P deficiency, which agrees with our previous findings (Dong et al., 2004). However, no significant genotypic difference was found in the exudation of the above three organic acids in the homogeneous solution system, indicating that organic acid exudation in a homogenous bulk solution may not reflect the plant's ability to detoxify exogenous Al.
On the other hand, organic acid exudation patterns were quite different in the divided root chamber system that employed a stratified distribution of Al and P in the root bathing solution. Although the same three organic acids (i.e. citrate, malate, and oxalate) were detected in root exudates in the various treatments applied in this system, their exudation patterns were very different from those in the homogeneous solution system. For example, Al-activated citrate exudation from the taproot apex was significantly increased by localized supply of P to the upper portion of the root system over the first 6 h, indicating that alterations in P nutrition may play a role in soybean Al tolerance mediated by citrate exudation (Fig. 7, A–D).
It is quite likely that the protection of roots from Al during their early stages of development is important to their long-term growth and survival. Therefore, the increased organic acid exudation observed during the early time points of root exposure to Al could contribute to Al tolerance of soybean. In our divided root system experiments, genotypic differences in organic acid exudation in response to localized P and Al supply were observed that were not seen when the entire root system was exposed to the same Al and P treatments. In comparing Figures 4 and 7, it is seen that the two P-efficient genotypes secreted approximately the same amount of citrate but much more malate from the taproot apex when the lateral roots in the upper portion of the root system were supplied with higher levels of P and Al toxicity was imposed to the terminal 6 cm of the taproot. These findings suggest that improved plant P nutrition, which is linked to genotypic differences in P efficiency, may play a role in enhanced Al toxicity exhibited by more deeply penetrating taproots.
In conclusion, our results demonstrate that P efficiency may play a role in Al tolerance in soybean. Phosphorus-efficient genotypes may be able to enhance Al tolerance not only through direct Al-P interactions but also through indirect interactions associated with stimulated exudation of different Al-chelating organic acids in specific roots and root regions, which in turn enhances plant tolerance to Al toxicity.
MATERIALS AND METHODS
Plant Materials
Four soybean (Glycine max L. Merr.) genotypes contrasting in their adaptability to low-P soil conditions and acidic soils were selected from an applied core collection of soybean germplasm (Zhao et al., 2004). Previous studies indicated that HN89 and HN92 performed significantly better than HN112 and HN113 under P-deficient, Al-toxic acid soil conditions (Zhao et al., 2004).
Characterization of Al Tolerance of Different Genotypes in a Homogeneous Nutrient Solution
Surface-sterilized seeds of the four soybean genotypes were germinated on paper towels moistened with one-half-strength modified Hoagland nutrient solution for 3 d at 25°C. The nutrient solution contained the following macronutrients (in mm): Ca, 2.0; K, 3.0; magnesium, 0.5; NO3−, 7.0; and SO42−, 0.5, and micronutrients (in μm): Fe-EDTA, 12.5; manganese, 1.0; Zn, 1.0; Cu, 1.0; NH4+, 0.25; MoO4−, 0.25; H3BO3, 12.5; and Cl−, 25. Aluminum treatments were imposed after this 3-d period by replacing the nutrient solution with the same solution containing Al3+ as AlCl3. Five Al3+ activity levels were employed, ranging from 0, 15, 30, 45, or 60 μm Al3+. The desired Al3+ activities were estimated using the GEOCHEM-PC speciation software (Parker et al., 1995). Seedlings were grown in a growth chamber at 26°C/24°C (light/dark, 12/12 h) with a light intensity of 550 μmol photons m−2 s−1. The solution was well aerated and the pH was maintained at 4.2 with daily additions of dilute HCl. After 3 d of Al treatment, plants were harvested and the taproot length was measured with a ruler as the indicator of Al tolerance.
Determination of the Effects of P Concentrations on Al Tolerance in Homogeneous Nutrient Solution
Sterilized seeds of the four soybean genotypes were germinated as described above with one-half-strength modified Hoagland nutrient solution with five different P treatments, P1, P2, P3, P4, and P5, which contained 0, 20, 40, 80, or 320 μm P as KH2PO4, respectively, for 3 d at 25°C. After 3 d of germination, seedlings were transplanted into 8-L polyethylene containers with the specific Al and P treatments. There were six treatments in total, including P1 + Al, P2 + Al, P3 + Al, P4 + Al, and P5 + Al, in which 0, 20, 40, 80, and 320 μm P were added as KH2PO4, respectively, together with 38 μm Al3+ activity based on the results from the above initial Al tolerance characterization experiment. An additional treatment without P and Al addition (P1-Al) was used as a control. The solution was well aerated and the pH was maintained at 4.2 with daily additions of dilute HCl. After 3 d of Al treatment, the morphology of the root apex from the taproot under P1-Al, P1 +Al, and P5 +Al treatments was documented using scanning electron microscopy, then plants were harvested and the taproot length was measured with a ruler as the indicator of Al tolerance.
Determination of Organic Acids in Root Exudates from Bulk Nutrient Solution
Sterilized seeds of the four soybean genotypes were germinated as described above. Seedlings were transplanted into 8-L polyethylene containers with the specific Al and P treatments 3 d after germination. There were four treatments in total, including two P levels (0 and 320 μm P added as KH2PO4) and two Al3+ activities (0 and 38 μm Al3+ added as AlCl3). At 0, 24, 48, and 72 h after treatment, two plants for each treatment were then transferred into 50-mL sterilized plastic tubes filled with 45 mL of exudation media to collect organic acids exuded from the roots for 6 h. The exudation media consisted of 4.3 mm CaCl2, plus the Al and P treatments as described above. The pH of exudation media was adjusted to 4.5 with HCl or KOH. After collection, plants were carefully taken out of the tubes and put back into the polyethylene containers for further growth. After the last collection of root exudates, the first 2 cm of the taproot from half of the tested plants was stained by hematoxylin solution for 1 h as described by Delhaize et al. (1993b), then the other half of plants was harvested and the first 2 cm of the root tips was collected and dried in an oven at 55°C overnight for Al content analysis. Dry weight of the root tips was determined using a microgram balance (MT2; Mettler). Dry samples were digested with ultrapure concentrated HNO3 at 135°C for 35 min, and Al concentrations in solutions were quantified by ICP-emission spectroscopy (Sciex).
All the root exudate samples collected were passed through a silver cartridge (OnGuardII Ag; Dionex) to remove excess Cl−, then mixed with a cation-exchange resin (100:1, v/w) to remove cations. The mixtures were centrifuged at 1,000g for 3 min at 4°C. The supernatants were used for organic acid determination with a capillary electrophoresis system (P/ACE 5510; Beckman Instruments) controlled by a Pentium II computer interfaced via PACE 1.2.1 software (Beckman Instruments). The background electrolyte cleaning and organic acid separation method were as described by Piñeros et al. (2002).
Determination of Organic Acids in Root Exudates from a Divided Root Chamber System
Following germination in paper towels for 3 d, plants were transplanted into the specially designed divided root chambers (Fig. 5). One plant was transplanted into each chamber, and the terminal 6 cm of the taproot was isolated from the rest of the root system by a Plexiglas block that straddled the taproot via a notch cut into the Plexiglas. For collection of root exudates, an additional Plexiglas ring with a notch cut into its base was used to isolate the terminal 1.5 cm of the taproot for exudate collection. The treatment solutions within the three compartments were isolated from each other using silicon grease to make a watertight seal of each divider with the chamber base. The root tip compartment was filled with 10 mL of collection solution for root exudates. After root exudates were collected, each compartment was refilled with the same volume of treatment solutions. There were four treatments: 1, LP, LP − Al, which was −P solution without Al in both the upper and lower chambers as well as the root exudate chamber; 2, LP, LP + Al, which was −P media without Al in the upper chamber, and −P media plus 38 μm Al3+ (activity) in the lower and root exudate chambers; 3, HP, LP − Al, which was 320 μm P without Al in the upper chamber, and −P media without Al in the lower and root exudates chambers; and 4, HP, LP + Al, which was 320 μm P without Al in the upper chamber, and −P media plus 38 μm Al3+ in the lower and root exudate chambers. Relative root tip growth (%) was determined as the percentage of taproot growth within the Plexiglas ring at low P and high P with Al addition relative to that without Al addition at a given P level. Root exudate solutions were collected over a 6-h time period at 0, 24, 48, and 72 h after transplanting. The organic acids in the root exudate solutions were determined as described above. Taproot growth was measured at 0, 24, 48, and 72 h after treatment and before root exudate collection.
Another short-term experiment was carried using the same divided root growth chamber above for Al staining with hematoxylin. After 24 h of Al treatment, the first 2 cm of the taproot was stained by hematoxylin solution for 1 h as described by Delhaize et al. (1993b).
Statistical Analyses
All of the data were analyzed statistically using Microsoft Excel 2000 (Microsoft) for calculating mean and se and SAS (SAS Institute) for ANOVA.
Supplementary Material
Acknowledgments
We thank Eric Craft and Randy Clark for technical help and Drs. Vera M. Carvalho Alves, Jonathan Lynch, Rebecca Nelson, and Hong Shen for valuable discussions.
This work was supported by grants from National Key Basic Research Special Funds of China (grant no. 2005CB120902), the McKnight Foundation Collaborative Crop Research Program and the National Natural Science Foundation of China (grants to X.Y. and H.L.), and by grants from the McKnight Foundation Collaborative Crop Research Program and U.S. Department of Agriculture National Research Initiative (grant no. 2001–35301–10647 to L.K.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Leon V. Kochian (lvk1@cornell.edu).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.076497.
References
- Barber SA (1995) Soil Nutrient Bioavailability: A Mechanistic Approach. John Wiley and Sons, New York, pp 202–230
- Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ (1993. a) Aluminum tolerance in wheat (Triticum aestivum L.): I. Uptake and distribution of aluminum in root apices. Plant Physiol 103: 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H (2004) Engineering high-level aluminum tolerance in barley with the ALMT1gene. Proc Natl Acad Sci USA 101: 15249–15254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ (1993. b) Aluminum tolerance in wheat (Triticum aestivum L.): II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol 103: 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Peng X, Yan X (2004) Organic acid exudation induced by phosphorus deficiency and/or aluminum toxicity in two contrasting soybean genotypes. Physiol Plant 122: 190–199 [Google Scholar]
- Ferrufino A, Smyth TJ, Israel DW, Carter TE Jr (2000) Root elongation of soybean genotypes in response to acidity constraints in a subsurface solution compartment. Crop Sci 40: 413–421 [Google Scholar]
- Foy CD (1984) Physiological effects of hydrogen, aluminium, and manganese toxicities in acid soil. In F Adams, ed, Soil Acidity and Liming. American Society of Agronomy, Madison, WI, pp 57–97
- Foy CD (1988) Plant adaptation to acid, aluminium-toxic soils. Commun Soil Sci Plant Anal 19: 959–987 [Google Scholar]
- Foy CD, Duke JA, Devine TE (1992) Tolerance of soybean germplasm to an acid Tatum subsoil. J Plant Nutr 15: 527–547 [Google Scholar]
- Gardner WK, Barber DA, Parbery DG (1983) The acquisition of phosphorus by Lupinus albus L. III. The probable mechanism by which phosphorus movement in the soil root interface is enhanced. Plant Soil 70: 107–124 [Google Scholar]
- Gaume A, Mächler F, Frossard E (2001) Aluminum resistance in two cultivars of Zea may L.: root exudation of organic acids and influence of phosphorus nutrition. Plant Soil 234: 73–81 [Google Scholar]
- Hoffland E, Findenegg GR, Nelemans JA (1989) Utilization of rock phosphate by rape. Plant Soil 113: 155–160 [Google Scholar]
- Horst WJ, Klotz F (1990) Screening soybean for aluminum tolerance and adaptation to acid soils. In NE Bassam, ed, Genetic Aspects of Plant Mineral Nutrition. Kluwer Academic Publisher, Dordrecht, The Netherlands, pp 355–360
- Kinraide TB, Arnold RC, Baligar VC (1985) A rapid assay for aluminum phytotoxicity at submicromolar concentrations. Physiol Plant 65: 245–250 [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55: 459–493 [DOI] [PubMed] [Google Scholar]
- Ligaba A, Yamaguchi M, Shen H, Sasaki T, Yamamoto Y, Matsumoto H (2004) Phosphorus deficiency enhances plasma membrane H+-ATPase activity and citrate exudation in greater purple lupin (Lupinus pilosus). Funct Plant Bio 31: 1075–1083 [DOI] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6: 273–278 [DOI] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H (1997) Specific secretion of citric acid induced by Al stress in Cassia tora L. Plant Cell Physiol 38: 1019–1025 [Google Scholar]
- Ma Z, Miyasaka SC (1998) Oxalate exudation as a mechanism of aluminum tolerance in taro. Plant Physiol 118: 861–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants. Academic Press, Harcourt Brace & Company Publishers, London, pp 3–5
- Miyasaka SC, Buta JG, Howell RK, Foy CD (1991) Mechanism of aluminum tolerance in snapbean, root exudation of citric acid. Plant Physiol 96: 737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DR, Norvell WA, Chaney RL (1995) GEOCHEM-PC: a chemical speciation program for IBM and compatible computers. In RH Loeppert, AP Schwab, S Goldberg, eds, Chemical Equilibrium and Reaction Models. Soil Science Society of America, Madison, WI, pp 253–269
- Pellet DM, Grunes DL, Kochian LV (1995) Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.). Planta 196: 788–795 [Google Scholar]
- Pellet DM, Papernik LA, Kochian LV (1996) Multiple aluminum resistance mechanisms in wheat: roles of root apical phosphate and malate exudation. Plant Physiol 112: 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros MA, Magalhaes J, Carvalho AVM, Kochian L (2002) The physiology and biophysics of an aluminum tolerance mechanism based on root citrate exudation in maize. Plant Physiol 137: 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros MA, Shaff JE, Manslank HS, Carvalho AVM, Kochian L (2005) Aluminum resistant in maize cannot be solely explained by root organic acid exudation. A comparative physiological study. Plant Physiol 137: 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothuluri JV, Kissel DE, Whitney DA, Thien SJ (1986) Phosphorus uptake from soil layers having different soil test phosphorus levels. Agron J 78: 991–994 [Google Scholar]
- Ritchey KD, Carter TE Jr (1993) Emergence and growth of two non-nodulated soybean genotypes in response to soil acidity. Plant Soil 151: 175–183 [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki E, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37: 645–653 [DOI] [PubMed] [Google Scholar]
- Sumner ME (1995) Amelioration of subsoil acidity with minimum disturbance. In NS Jayawardane, BA Stewart, eds, Subsoil Management Techniques. Advances in Soil Science. Lewis Publishers, London, pp 147–185
- Tan K, Keltjens WG (1990) Interaction between aluminium and phosphorus in sorghum plants. I. Studies with the aluminium sensitive sorghum genotypes TAM428. Plant Soil 124: 15–23 [Google Scholar]
- Villagarcia MR, Carter TE Jr, Rufty TW, Niewoehner AS, Jennette MW, Arrellano C (2001) Genotypic rankings for aluminum tolerance of soybean roots grown in hydroponics and sand culture. Crop Sci 41: 1499–1507 [Google Scholar]
- von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. In RA Date, NJ Grundon, GE Raymet, ME Probert, eds, Plant-Soil Interactions at Low pH: Principles and Management. Kluwer Academic Publisher, Dordrecht, The Netherlands, pp 5–19
- Wenzl P, Patiño GM, Chaves AL, Mayer JE, Rao IM (2001) The high level of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiol 125: 1473–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Lynch JP, Beebe SE (1995. a) Genetic variation for phosphorus efficiency of common bean in contrasting soil types: I. Vegetative response. Crop Sci 35: 1086–1093 [Google Scholar]
- Yan X, Lynch JP, Beebe SE (1995. b) Genetic variation for phosphorus efficiency of common bean in contrasting soil types: II. Yield response. Crop Sci 35: 1094–1099 [Google Scholar]
- Yang ZM, Sivaguru M, Horts WJ, Matsumoto H (2001) Aluminum tolerance is achieved by exudation of citric acid from roots of soybean (Glycine max). Physiol Plant 110: 72–74 [Google Scholar]
- Zhao J, Fu J, Liao H, He Y, Nian H, Hu Y, Qiu L, Dong Y, Yan X (2004) Characterization of root architecture in an applied core collection for phosphorus efficiency of soybean germplasm. Chin Sci Bull 49: 1611–1620 [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H (1998) Continuous secretion of organic acids is related to aluminum resistance during relatively long-term exposure to aluminum stress. Physiol Plant 103: 209–214 [Google Scholar]
- Zheng SJ, Yang JL, He YF, Yu XH, Zhang L, You JF, Shen RF, Matsumoto H (2005) Immobilization of aluminum with phosphorus in roots is associated with high aluminum resistance in buckwheat. Plant Physiol 138: 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.