The reaction centers of PSI and PSII in chloroplast thylakoids are the major generation site of reactive oxygen species (ROS). Photoreduction of oxygen to hydrogen peroxide (H2O2) in PSI was discovered over 50 years ago by Mehler (1951). Subsequently, the primary reduced product was identified to be superoxide anion (O2−), and its disproportionation produces H2O2 and O2 (Asada et al., 1974). On the other hand, in PSII, oxygen of the ground (triplet) state (3O2) is excited to singlet state (1O2) by the reaction center chlorophyll (Chl) of triplet excited state (3P680*; Telfer et al., 1994; Hideg et al., 1998).
The photoproduction of ROS is largely affected by physiological and environmental factors; the rate is enhanced under the conditions where photon intensity (P) is in excess of that required for the CO2 assimilation (A). Under the conditions of photon excess (P > A), the relaxation systems suppress the photoproduction of ROS in chloroplasts, such as photorespiration, the cyclic electron flows through either PSI or PSII, and the down-regulation of PSII quantum yield as regulated by the xanthophyll cycle and the proton gradient across thylakoid membrane. Prompt scavenging of the ROS produced in thylakoids prior to its diffusion from the generation site is indispensable to protect the target molecules in thylakoid and stroma.
Here, the production of reduced and excited species of ROS and their scavenging system in chloroplasts are overviewed. The photoproduction and subsequent scavenging of ROS not only protect chloroplasts from the direct effects of ROS, but also relax the photon (electron) excess stress, and these physiological functions of ROS production and scavenging are discussed.
PHOTOREDUCTION OF OXYGEN IN PSI TO WATER VIA O2− AND H2O2: WATER-WATER CYCLE
In thylakoids, H2O2 is photoproduced via O2− and accumulates, but in intact chloroplasts, H2O2 does not accumulate. Localization of ascorbate peroxidase (APX) and related enzymes indicates that chloroplasts reduce H2O2 with APX using the electrons derived from water in PSII as follows:
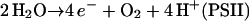 |
(1) |
 |
(2) |
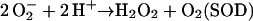 |
(3) |
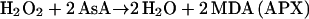 |
(4) |
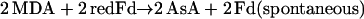 |
(5) |
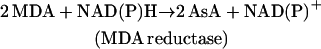 |
(6) |
 |
(7) |
 |
(8) |
The primary product of oxygen reduction, O2− (Eq. 2), is disproportionated to H2O2 and O2 catalyzed with superoxide dismutase (SOD; Eq. 3). The H2O2 generated by SOD is reduced to water by ascorbate (AsA) catalyzed with APX, and AsA is oxidized to monodehydroascorbate radical (MDA; Eq. 4). Subsequently, MDA is directly reduced to AsA by either reduced ferredoxin (redFd; Miyake and Asada, 1994; Eq. 5) or NAD(P)H catalyzed with chloroplastic MDA reductase (Sano et al., 2005; Eq. 6). If MDA fails to reduce directly to AsA, it is spontaneously disproportionated to dehydroascorbate (DHA) and AsA. DHA is then reduced to AsA by reduced glutathione (GSH) catalyzed with DHA reductase (Shimaoka et al., 2003; Eq. 7). Finally, Fd and NADP+ for the regeneration of AsA (Eqs. 5–7) are reduced in PSI (Eq. 8). Thus, in any pathways of the regeneration of AsA, the half electrons derived from water in PSII (Eq. 1) are used for the univalent reduction of oxygen (Eq. 2) and another half for the generation of reductants (Eq. 8) to reduce H2O2 (Eqs. 4–7), which has been referred to as the water-water (W-W) cycle.
PARTICIPATING ENZYMES IN THE W-W CYCLE
SOD
Several plants contain Fe-SOD in addition to CuZn-SOD, but no Mn-SOD, in chloroplasts. In anaerobic bacteria, the reduced form of iron-sulfur protein, such as rubredoxin and neelaredoxin, can reduce O2− to H2O2 (Abreu et al., 2001), but in chloroplasts no such iron-sulfur proteins are found.
APX
APX is classified as class I peroxidase similar to Cyt c peroxidase (CcP) and is different from class III peroxidase such as horseradish (Armoracia lapathifolia) peroxidase (Raven, 2003). Even though CcP and APX are classified in the same family, compound I of CcP is stable, but that of APX is not so stable in the absence of AsA (Miyake and Asada, 1996). Compound I of chloroplastic APX is more labile as compared with that of cytosolic APX (Amako et al., 1994). In contrast to plant APX, compound I of APX from a red alga is stable (Sano et al., 2001). APX is inhibited by thiol-modifying reagents, and the Cys residue participates in the binding of AsA to the heme (Raven, 2003).
Chloroplastic APX is classified into thylakoid-bound (tAPX) and stroma-localized forms. tAPX binds in the vicinity of PSI (Miyake et al., 1993), and its anchor is the C-terminal, hydrophobic 50 residues. Except for Arabidopsis (Arabidopsis thaliana), a single gene codes tAPX and stroma-localized APX, and both isoforms are generated by alternative splicing (Yoshimura et al., 2002).
Peroxiredoxin
Prokaryotes lack AsA and cyanobacteria scavenge H2O2 with the thioredoxin-peroxiredoxin (Prx) system (Yamamoto et al., 1999). Thioredoxin and Prx are localized in plant chloroplasts, but it is not known under what conditions this system functions as an alternative W-W cycle in place of APX. Prx Q associates to PSII complexes (Lamkemeyer et al., 2006).
MDA Reductase
In addition to MDA, MDA reductase is able to reduce phenoxyl radicals, such as quercetin radicals to their parent phenols (Sakihama et al., 2000). Thus, this enzyme would participate also in the regeneration of antioxidants. In Arabidopsis, MDA reductase (Obara et al., 2001), stromal APX, and glutathione reductase gene products (Chew et al., 2003) have dual targeting into chloroplasts and mitochondria.
Catalase
In chloroplast stroma, no catalase has been found. However, PSII membranes associate a heme catalase (Sheptovitsky and Brudig, 1996). This catalase would not directly participate in the W-W cycle but protects water oxidase in the lumen if the W-W cycle does not operate properly and H2O2 diffuses to the lumen.
A-Type Flavoprotein
This protein participates in the photoreduction of O2 in Synechocystis PCC6803, catalyzing the four electron reduction of O2 to 2 H2O using 2 NAD(P)H without releasing ROS (Helman et al., 2005).
THYLAKOIDAL AND STROMAL SCAVENGING SYSTEMS OF ROS IN CHLOROPLASTS
In chloroplasts, over one-half of CuZn-SOD attaches on the stroma thylakoids where PSI is localized (Ogawa et al., 1995). PSI-attached SOD, tAPX bound to in the vicinity of PSI and Fd-dependent reduction of MDA form the thylakoidal scavenging system (Eqs. 3–5), which functions as the first defense against ROS (Fig. 1). Other scavenging enzymes would be compartmented in the stroma and play a role as the second defense (stromal scavenging system). In the thylakoidal system, local concentrations of SOD and tAPX of around 1 mm in the vicinity of PSI and respective reaction rates of the participating enzymes allow us to estimate the scavenging rates of O2− and H2O2 and their steady-state concentrations (Asada, 1999, 2000; Polle, 2001). These simulations indicate that the limiting step of the W-W cycle is the reduction of oxygen (Eq. 2), and its rate is lower at least two orders of magnitude than those of the SOD-catalyzed disproportionation of O2− (Eq. 3), the reduction of H2O2 by APX (Eq. 4), and the reduction of MDA (Eq. 5). Therefore, the total electron flux through the W-W cycle is spontaneously twice the rate of Equation 2. In the presence of methyl viologen (MV) the rate of Equation 2 is higher due to rapid autooxidation of the photoreduced MV radicals to form O2− in PSI, then few electrons are available for Equation 8 to regenerate AsA, and H2O2 cannot be reduced. Under such conditions, APX is rapidly inactivated due to decomposition of the compound I, and oxidative damages are amplified (Mano et al., 2001).
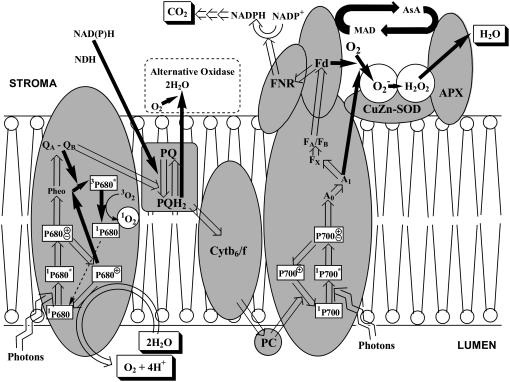
Figure 1.
Productions of 1O2 in PSII and of O2− in PSI in chloroplast thylakoids. White arrows represent the photoexcitation of reaction center Chl and the electron flow under the photon intensity where all of the electrons generated are utilized for the CO2 assimilation (P < A or P = A). Black arrows represent where photon intensity exceeds the capacity of the flux of electron transport and the flux to CO2 assimilation (P > A). To make it simple, the stromal scavenging system of ROS is omitted and the thylakoidal scavenging system only is shown. Alternative oxidase is a four-electron oxidase using plastoquinol as the electron donor (2 PQH2 + O2 → 2 PQ + 2 H2O) without releasing ROS and functions in chlororespiration in the dark (Aluru and Rodermel, 2004). NDH, NAD(P)H dehydrogenase complex; PQ, plastoquinone; PQH2, plastoquinol.
In chloroplasts, AsA is rapidly regenerated from either MDA or DHA (Eqs. 5–8). In addition to the electron donors for APX, AsA participates in the following reactions: the cofactor for violaxanthin deepoxidase in the xanthophyll cycle, acute electron donors to PSI and PSII in the lumen (Mano et al., 2004), maintenance of the cyclic electron flow as the compensatory electron donor (Ivanov et al., 2005), and regeneration of tocopherols from its oxidized radicals to suppress lipid oxidation in thylakoids (Munne-Bosch and Alegre, 2002).
INCREASED ELECTRON FLUX THROUGH W-W CYCLE UNDER P > A CONDITIONS
The electron flux through the W-W cycle was estimated from CO2 assimilation and parameters of Chl fluorescence or 18O2 uptake. The flux through the W-W cycle of dark-adapted leaves (Makino et al., 2002) and algal cells (Radmer and Kok, 1976) prior to the photoactivation of the Calvin cycle is very similar to that for the CO2 assimilation at steady state after the photoactivation. These observations indicate that the capacity of the W-W cycle is high enough to allow the electron flux at the steady state of photosynthesis, and prior to the photoactivation, the W-W cycle is the major alternative pathway. In this respect, the W-W cycle appears to be indispensable to start photosynthesis of dark-adapted leaves without photooxidative damages. In maize (Zea mays), oxygen at low concentration (half saturation; 0.15 kPa) is required to initiate CO2 assimilation (T. Ohwaki, K. Asada, unpublished data).
A similar increase in the electron flux through the W-W cycle has been observed when the CO2 assimilation (A) is suppressed by CO2-limiting conditions (Miyake and Yokota, 2000), low temperatures (Hirotsu et al., 2004; Li et al., 2005), and salt stress (Chen et al., 2004).
GENETIC MODIFICATION OF W-W CYCLE ENZYMES
The key enzymes in the thylakoidal scavenging system, tAPX and chloroplastic CuZn-SOD, have genetically altered. Mutants of tAPX are thought to be lethal (Yabuta et al., 2002). Plants with reduced tAPX activity are sensitive to MV stress (Tarantino et al., 2005), and show decreases in PSII activity, CO2 assimilation, and biomass production (Danna et al., 2003). Phenotypes of knockdown mutant of chloroplastic CuZn-SOD are suppressed growth, small chloroplasts, and low photosynthetic activity (Rizhsky et al., 2003). These phenotypes confirm that the thylakoidal scavenging system of ROS is essential for photosynthesis even under mild conditions. A low scavenging capacity of ROS in the vicinity of PSI, for example, during the photoactivation of the Calvin cycle, would inactivate several enzymes for CO2 assmilation. On the contrary, plants overexpressing tAPX (Yabuta et al., 2002; Murgia et al., 2004) are tolerant to MV stress. When Escherichia coli catalase is overexpressed in chloroplasts of tobacco (Nicotiana tabacum), plants show enhanced resistance to photooxidative stress by MV (Miyagawa et al., 2000). Because chloroplastic APX is labile as compared with the cytosolic one, cytosolic APX was overexpressed in chloroplasts. Such plants show enhanced tolerance to salt and drought stresses (Badawi et al., 2004). Transformants overexpressing a stable red algal APX (Sano et al., 2001) effectively scavenge H2O2 even under conditions where CO2 assimilation is limited (Miyake et al., 2006). The knockout mutant of the cytosolic APX disturbs the chloroplastic scavenging system and enhances H2O2 accumulation in leaves, indicating that the cytosolic APX is functional in ROS signaling (Davletova et al., 2005).
PHOTOPRODUCTION OF 1O2 IN CHLOROPLASTS
PSII Reaction Center (P680)
In contrast to P700, the life time of P680+ is very short, because of rapid withdrawal of electrons from water to P680+ and the charge recombination of P680+ with the primary electron acceptors of PSII pheophytin, QA, and QB to form 3P680*, especially when the intersystem electron carriers are reduced. Such a situation is likely to occur under the conditions of P > A, where either light intensity is too high or the CO2-assimilation rate is low due to either environmental stresses or physiological conditions. In illuminated PSII reaction center, 3P680* was produced under anaerobic conditions, but on addition of 3O2 it decayed to 1P680, accompanied with the generation of 1O2, as detected by luminescence at 1,270 nm or a chemical trapping (Macpherson et al., 1993; Telfer et al., 1994): 3O2 + 3P680* → 1O2 + 1P680 (Fig. 1). In leaf tissues, photoproduction of 1O2 in chloroplasts has been demonstrated using the fluorescence and spin probe DanePy. The yield of 1O2 increases, accompanied by the photoinhibition of PSII by high light or UV (Hideg et al., 2002).
Using another fluorescence probe of 1O2, DPAX (Umezawa et al., 1999), a real-time detection system of 1O2 in illuminated intact thylakoids and chloroplasts was established. In this assay, 1O2 was generated just after the start of the actinic illumination prior to photoinhibition. Under anaerobic conditions, the production rate of 1O2 is immediately enhanced by 5- to 6-fold (E. Yamamoto, N. Umezawa, T. Nagano, Y. Urano, and K. Asada, unpublished data). The similar increase in production rate of 1O2 under anaerobic state was observed using DanePy as a fluorescence probe of 1O2 (K. Asada and E. Hideg, unpublished data).
Enhanced production of 1O2 in thylakoids under anaerobic state is different from that in isolated PSII reaction centers where under anaerobic conditions 1O2 is not photoproduced but only 3P680*. In thylakoids, oxygen functions as the electron acceptor in PSI; therefore, under anaerobic state, the electron flux through the intersystem is suppressed, and the recombination to form 3P680* in PSII is likely to occur. The source of oxygen for 1O2 generation would be the 3O2 evolved by the water oxidase in the lumen. The anaerobiosis-induced generation of 1O2, however, disappears on addition of the electron acceptor ferricyanide, which confirms that an increased production of 1O2 by 3P680* is caused by the overreduction of the intersystem carriers.
Anaerobiosis-induced photoinhibition in thylakoids (Trebst, 1962) and Euglena cells (Asada and Takahashi, 1987) has been observed. These paradoxical observations in the respect of photoinhibition by ROS can be inferred by the enhanced photoproduction of 1O2 in PSII under anaerobic state. In other words, the oxygen-dependent electron flux to PSI including the W-W cycle would suppress the generation of 1O2 in PSII and protect the photoinactivation of PSII.
Biosynthetic and Catabolic Intermediates of Chl
Biosynthetic and catabolic intermediates of Chl are photosensitizers to generate 1O2. The catabolic enzyme-deficient and pheophorbide a-accumulating mutant is sensitive to light after dark treatment (Tanaka et al., 2003; Pruzinska et al., 2005). Similarly, the biosynthetic intermediate of Chl, protochlorophillide-accumulating mutant, is also sensitive to light (Wagner et al., 2004).
QUENCHERS AND SCAVENGERS OF 1O2, AND CYCLIC ELECTRON FLOW THROUGH PSII
Since 1O2 is rapidly quenched by water, its life time and diffusion distance from the generation site are very short: 3.1 to 3.9 μs and 190 nm, respectively. In chloroplast thylakoids, the diffusion distance is further shortened because of a higher viscosity and is estimated to be 5.5 nm (Krasnovsky, 1998). Thus, the diffusion distance of 1O2 is the shortest among ROS. For estimation of its biological effects, then, the distances among the generation site of 1O2, quenchers, and target are a critical factor.
In PSII reaction center and antenna subunit complex 11 molecules of β-carotene have been assigned. In the reaction center core, two molecules of β-carotene participate in the quenching of 1O2 generated via 3P680*. Distance between β-carotene and P680 in PSII (Loll et al., 2005) does not allow the quenching of 3P680* by the β-carotene (for review, see Telfer, 2002). Oxidation of the β-carotene in D2 to its cation radical has been demonstrated, indicating this β-carotene participates in the cyclic electron flow with Cyt b-559 in PSII (Miyake et al., 2002; Telfer et al., 2003). This cyclic electron transfer through PSII would suppress the generation of 3P680* and then 1O2 under the conditions of P > A.
Tocopherols can quench 1O2, but its rate (3 × 108 m−1 s−1) is two orders of magnitude lower than that with β-carotene (Krasnovsky, 1998). α-Tocopherol is oxidized by 1O2 to α-tocopherol quinone via 8-hydroxyperoxy-tocopherol (for review, see Trebst, 2003), but it is not known by what way α-tocopherol is regenerated. Tocopherols also play a role as an antioxidant to suppress the lipid peroxidation in thylakoids by trapping of lipid radicals (Munne-Bosch and Alegre, 2002).
CONCLUDING REMARKS
Photoproduction of reduced and excited species of ROS in PSI and PSII reaction centers, respectively, is enhanced under the conditions where P is in excess of A. Even under the conditions of P > A, where P is too high or A is low by either environmental stress or physiological state such as prior to the photoactivation of the Calvin cycle, rapid scavenging of ROS by the W-W cycle protects chloroplasts from the direct action of ROS. Further, as long as the W-W cycle operates properly and ROS is promptly scavenged prior to its diffusion to stromal targets, the W-W cycle works as an alternative electron flux and can down-regulate PSII quantum yield by the generation of the proton gradient across the thylakoid membrane. Thus, the W-W cycle functions also as a relaxation system to suppress the photoproduction of 1O2 in PSII.
This work was supported by a Grants-in-Aids for Scientific Research from the Ministry of Education, Science and Culture, Japan (no. 15370026).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Kozi Asada (asada@bt.fubt.fukuyama-u.ac.jp).
References
- Abreu IA, Saraiva LM, Soares CM, Teixeira M, Cabelli DE (2001) The mechanism of superoxide scavenging by Archaeoglobus fulgidus neelarredoxin. J Biol Chem 276: 38995–39001 [DOI] [PubMed] [Google Scholar]
- Aluru MR, Rodermel SR (2004) Control of chloroplast redox by the IMMUTANS terminal oxidase. Physiol Plant 120: 4–11 [DOI] [PubMed] [Google Scholar]
- Amako K, Chen G-X, Asada K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35: 497–504 [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Asada K (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond B Biol Sci 355: 1419–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K, Kiso K, Yoshikawa K (1974) Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J Biol Chem 249: 2175–2181 [PubMed] [Google Scholar]
- Asada K, Takahashi M (1987) Production and scavenging of active oxygen in chloroplasts. In DJ Kyle, CB Osmond, CJ Arntzen, eds, Photoinhibition. Elsevier, Amsterdam, pp 227–287
- Badawi GH, Kawano N, Yamauchi Y, Shimada E, Sasaki R, Kubo A, Tanaka K (2004) Overexpression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol Plant 121: 231–238 [DOI] [PubMed] [Google Scholar]
- Chen H-X, Gao H-Y, An S-Z, Li W-J (2004) Dissipation of excess energy in Mehler-peroxidase reaction in Rumex leaves during salt shock. Photosynthetica 42: 117–122 [Google Scholar]
- Chew O, Whelan J, Millar AH (2003) Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria dual targeting of antioxidant defenses in plants. J Biol Chem 278: 46869–46877 [DOI] [PubMed] [Google Scholar]
- Danna CH, Bartoli CG, Sacco F, Ingala LR, Santa-Maria GE, Guiamet JJ, Ugalde RA (2003) Thylakoid-bound ascorbate peroxidase mutant exhibits impaired electron transport and photosynthetic activity. Plant Physiol 132: 2116–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman Y, Barkan E, Eisenstadt D, Luz B, Kaplan A (2005) Fractionation of the three stable isotopes by oxygen-producing and oxygen-consuming reactions in photosynthetic organisms. Plant Physiol 138: 2292–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideg E, Barta C, Kalai T, Vass M, Hideg K, Asada K (2002) Detection of singlet oxygen and superoxide with fluorescence sensors in leaves under stress by photoinhibition or UV radiation. Plant Cell Physiol 43: 1154–1164 [DOI] [PubMed] [Google Scholar]
- Hideg E, Kalai T, Hideg K, Vass I (1998) Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. Biochemistry 237: 11405–11411 [DOI] [PubMed] [Google Scholar]
- Hirotsu N, Makino A, Ushio A, Mae T (2004) Changes in the thermal dissipation and the electron flow in the water-water cycle in rice grown under conditions of physiologically low temperature. Plant Cell Physiol 45: 635–644 [DOI] [PubMed] [Google Scholar]
- Ivanov B, Asada K, Kramer DM, Edwards G (2005) Characterization of photosynthetic electron transport in bundle sheath cells of maize. I. Ascorbate effectively stimulates cyclic electron flow around PSI. Planta 220: 572–581 [DOI] [PubMed] [Google Scholar]
- Krasnovsky AA Jr (1998) Singlet molecular oxygen in photobiochemical systems: IR phosphorescence studies. Membr Cell Biol 12: 665–690 [PubMed] [Google Scholar]
- Lamkemeyer P, Laxa M, Collin V, Li W, Finkemeier I, Schottler MA, Holtkamp V, Tognetti VB, Issakidis-Bourguet E, Kandlbinder A, et al (2006) Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. Plant J 45: 968–981 [DOI] [PubMed] [Google Scholar]
- Li X-G, Bi Y-P, Zhao S-J, Meng Q-W, Zou Q, He Q-W (2005) Cooperation of xanthophyll cycle with water-water cycle in the protection of photosystems 1 and 2 against inactivation during chilling stress under low irradiance. Photosynthetica 43: 261–266 [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J (2005) Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 438: 1040–1044 [DOI] [PubMed] [Google Scholar]
- Macpherson AN, Telfer A, Barber J, Truscott TG (1993) Direct detection of singlet oxygen from isolated photosystem II reaction centers. Biochim Biophys Acta 1143: 301–309 [Google Scholar]
- Makino A, Miyake C, Yokota A (2002) Physiological functions of the water-water cycle (Mehler reaction) and the cyclic electron flow around PSI in rice leaves. Plant Cell Physiol 43: 1017–1026 [DOI] [PubMed] [Google Scholar]
- Mano J, Hideg E, Asada K (2004) Ascorbate in thylakoid lumen functions as an alternative electron donor to photosystem II and photosystem I. Arch Biochem Biophys 429: 71–80 [DOI] [PubMed] [Google Scholar]
- Mano J, Ohno C, Domae Y, Asada K (2001) Chloroplastic ascorbate peroxidase is the primary target of methylviologen-induced photooxidative stress in spinach leaves: its relevance to monodehydroascorbate radical detected with in vivo ESR. Biochim Biophys Acta 1504: 275–287 [DOI] [PubMed] [Google Scholar]
- Mehler AH (1951) Studies on reactivities of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys 33: 65–77 [DOI] [PubMed] [Google Scholar]
- Miyagawa Y, Tamoi M, Shigeoka S (2000) Evaluation of the defense system in chloroplasts to photooxidative stress caused by paraquat using transgenic tobacco plants expressing catalase from Escherichia coli. Plant Cell Physiol 41: 311–320 [DOI] [PubMed] [Google Scholar]
- Miyake C, Asada K (1994) Ferredoxin-dependent photoreduction of monodehydroascorbate radicals in spinach thylakoids. Plant Cell Physiol 35: 539–549 [Google Scholar]
- Miyake C, Asada K (1996) Inactivation of mechanism of ascorbate peroxidase at low concentrations of ascorbate: hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol 37: 423–430 [Google Scholar]
- Miyake C, Cao WH, Asada K (1993) Purification and molecular properties of thylakoid-bound ascorbate peroxidase from spinach chloroplasts. Plant Cell Physiol 343: 881–889 [Google Scholar]
- Miyake C, Shinzaki Y, Nishioka M, Horiguchi S, Tomizawa K (2006) Photoinactivation of ascorbate peroxidase in isolated tobacco chloroplasts: Galdieria partita APX maintains the electron flux through the water-water cycle in transplantomic plants. Plant Cell Physiol 47: 200–210 [DOI] [PubMed] [Google Scholar]
- Miyake C, Yokota A (2000) Determination of the rate of photoreduction of O2 in the water-water cycle in watermelon leaves and enhancement of the rate by limitation of photosynthesis. Plant Cell Physiol 41: 335–343 [DOI] [PubMed] [Google Scholar]
- Miyake C, Yonekura K, Kobayashi Y, Yokota A (2002) Cyclic flow of electron within PSII functions in intact chloroplasts from spinach leaves. Plant Cell Physiol 43: 951–957 [DOI] [PubMed] [Google Scholar]
- Munne-Bosch S, Alegre L (2002) The function of tocopherols and tocitrienols in plants. CRC Crit Rev Plant Sci 21: 31–57 [Google Scholar]
- Murgia I, Tarantino D, Vannini C, Bracale M, Carrabvieri S, Soave C (2004) Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show resistance to paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J 38: 940–995 [DOI] [PubMed] [Google Scholar]
- Obara K, Sumi K, Fukuda H (2001) The use of multiple transcription starts causes the dual targeting of Arabidopsis putative monodehydroascorbate reductase to both mitochondria and chloroplasts. Plant Cell Physiol 43: 697–705 [DOI] [PubMed] [Google Scholar]
- Ogawa K, Kenematsu K, Takabe K, Asada K (1995) Attachment of CuZn-superoxide dismutase to thylakoid membranes at the superoxide generation (PSI) in spinach chloroplasts: detection by immuno-gold labeling after rapid freezing and substitution method. Plant Cell Physiol 36: 565–573 [Google Scholar]
- Polle A (2001) Dissecting the superoxide dismutase-ascorbate-glutathione pathway in chloroplasts by metabolic modeling: computer simulation as a step towards flux analysis. Plant Physiol 126: 445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzinska A, Tanner G, Aubry S, Anders I, Moser S, Muller T, Ongania K-H, Krautler B, Youn J-Y, Liljegren SL, et al (2005) Chlorophyll breakdown in senescent Arabidopsis leaves: characterization of chlorophyll catabolites and of chlorophyll catabolic enzymes involved in the degreening reaction. Plant Physiol 139: 52–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer RJ, Kok B (1976) Photoreduction of O2 primes and replaces CO2 assimilation. Plant Physiol 58: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven EL (2003) Understanding functional diversity and substrate specificity in haem peroxidases: what can we learn from ascorbate peroxidase? Nat Prod Rep 20: 367–381 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Mittler R (2003) The water-water cycle is essential for chloroplast protection in the absence of stress. J Biol Chem 278: 38921–38925 [DOI] [PubMed] [Google Scholar]
- Sakihama Y, Mano J, Sano S, Asada K, Yamasaki H (2000) Reduction of phenoxyl radicals mediated by monodehydroascorbate reductase. Biochem Biophys Res Commun 279: 949–954 [DOI] [PubMed] [Google Scholar]
- Sano S, Tao S, Endo Y, Inaba T, Hossain MA, Miyake C, Matsuo M, Aoki H, Asada K, Saito K (2005) Purification and cDNA cloning of chloroplastic monodehydroascorbate reductase from spinach. Biosci Biotechnol Biochem 69: 762–772 [DOI] [PubMed] [Google Scholar]
- Sano S, Ueda M, Kitajima S, Takeda T, Shigeoka S, Kurano N, Miyachi S, Miyake C, Yokota A (2001) Characterization of ascorbate peroxidase from unicellular red alga Galdiera partita. Plant Cell Physiol 42: 433–440 [DOI] [PubMed] [Google Scholar]
- Sheptovitsky YG, Brudig GW (1996) Isolation and characterization of spinach photosystem II membrane-associated catalase and polyphenol oxidase. Biochemistry 35: 16255–16263 [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Miyake C, Yokota A (2003) Mechanism of the reaction catalyzed by dehydroascorbate reductase from spinach chloroplasts. Eur J Biochem 270: 921–928 [DOI] [PubMed] [Google Scholar]
- Tanaka R, Hirashima M, Satoh S, Tanaka A (2003) The Arabidopsis-accelerated cell death gene ACD1 is involved in oxygenation of pheophorbide a: inhibition of the pheophorbide a oxygenase activity does not lead to the “stay-green” phenotype in Arabidopsis. Plant Cell Physiol 44: 1266–1274 [DOI] [PubMed] [Google Scholar]
- Tarantino D, Vannini C, Bracale M, Campa M, Soave C, Murgia I (2005) Antisense reduction of thylakoidal ascorbate peroxidase in Arabidopsis enhances paraquat-induced photooxidative stress and nitric oxide-induced cell death. Planta 221: 757–765 [DOI] [PubMed] [Google Scholar]
- Telfer A (2002) What is β-carotene doing in the photosystem II reaction centre? Philos Trans R Soc Lond B Biol Sci 357: 1431–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer A, Bishop SM, Phillips D, Barber J (1994) Isolated photosynthetic reaction center of photosystem II as a sensitizer for the formation of singlet oxygen. J Biol Chem 269: 13244–13253 [PubMed] [Google Scholar]
- Telfer A, Frolov D, Barber J, Robert B, Pascal A (2003) Oxidation of the two β-carotene molecules in the photosystem II reaction center. Biochemistry 42: 1008–1015 [DOI] [PubMed] [Google Scholar]
- Trebst A (1962) Lichtinaktivierung der O2-entwickling in der photosynthese. Naturwissenschaften B 17: 660–663 [Google Scholar]
- Trebst A (2003) Function of β-carotene and tocopherol in photosystem II. Z Naturforsch 58c: 609–620 [DOI] [PubMed] [Google Scholar]
- Umezawa N, Tanaka K, Urano Y, Kikuchi K, Higuchi T, Nagano T (1999) Novel fluorescence probes for singlet oxygen. Angew Chem Int Ed Engl 38: 2899–2901 [DOI] [PubMed] [Google Scholar]
- Wagner DE, Przybyla D, op den Camp RG, Kim C, Landgraf F, Lee KP, Wursch M, Laloi C, Nater M, Hideg E, et al (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306: 1183–1185 [DOI] [PubMed] [Google Scholar]
- Yabuta Y, Motoki T, Yoshimura K, Takada T, Ishikawa T, Shigeoka S (2002) Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J 32: 912–925 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Miyake C, Dietz K-J, Tomizawa K, Murata N, Yokota A (1999) Thioredoxin peroxidase in the cyanobacterium Synechocystis sp. PCC6803. FEBS Lett 447: 269–273 [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S (2002) Identification of a cis-element for tissue-specific alternative splicing of chloroplast ascorbate peroxidase pre-mRNA in higher plants. J Biol Chem 277: 40623–40632 [DOI] [PubMed] [Google Scholar]