Reactive oxygen species (ROS) are emerging as important regulators of plant development. There is now abundant evidence that ROS play roles in cell growth and that spatial regulation of ROS production is an important factor controlling plant form. Here we will review evidence that supports a role for ROS in development, but first we will define what we mean by development. The body of the vascular plant sporophyte (diploid life cycle stage) is derived from meristems and much of the action of development occurs where organs are formed in and around meristems (Martienssen and Dolan, 1998). Organogenesis, the development of organs, involves an early patterning stage that roughs out boundaries where the organs will form. Within these boundaries, groups of founder cells divide and growth occurs, leading to the formation of structures containing arrays of differentiated cells. There are two major contributors to growth: First, there is cell division, which increases the number of cells in an organ; second, there is expansion of those cells. The huge increase in volume in plant cells as they transit from the founder population in and around meristems to the differentiated cells of the mature organ means that cell growth is an important component of plant development (Sugimoto-Shirasu and Roberts, 2003). Recent discoveries suggest that ROS may control development through their role in regulating cell growth.
NADPH OXIDASES GENERATE ROS INVOLVED IN DEVELOPMENT
ROS that have been shown to play a role in development are produced by NADPH oxidases (NOXs) that generate the superoxide radical (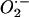 ), using NADPH as an electron donor. These NOX proteins are similar to the enzymes first identified in mammals that are responsible for the respiratory burst that occurs in activated mammalian neutrophils (Segal and Abo, 1993). Since then, homologs of this enzyme have been found in a range of organisms (Keller et al., 1998; Suh et al., 1999; Cheng et al., 2001; Torres et al., 2002; Aguirre et al., 2005). In Arabidopsis (Arabidopsis thaliana), this class of genes is referred to as Arabidopsis respiratory burst oxidase homologs (Atrboh; Keller et al., 1998; Torres et al., 1998). The activity of three members of this family has been shown to be involved in different aspects of root growth (Foreman et al., 2003; Kwak et al., 2003).
), using NADPH as an electron donor. These NOX proteins are similar to the enzymes first identified in mammals that are responsible for the respiratory burst that occurs in activated mammalian neutrophils (Segal and Abo, 1993). Since then, homologs of this enzyme have been found in a range of organisms (Keller et al., 1998; Suh et al., 1999; Cheng et al., 2001; Torres et al., 2002; Aguirre et al., 2005). In Arabidopsis (Arabidopsis thaliana), this class of genes is referred to as Arabidopsis respiratory burst oxidase homologs (Atrboh; Keller et al., 1998; Torres et al., 1998). The activity of three members of this family has been shown to be involved in different aspects of root growth (Foreman et al., 2003; Kwak et al., 2003).
The ROOT HAIR DEFECTIVE2 (RHD2)/AtrbohC protein is required for root elongation. The roots of plants homozygous for loss-of-function rhd2 mutations have decreased levels of ROS and are 20% shorter than the wild type, indicating that cell expansion is defective in these plants (Foreman et al., 2003; Renew et al., 2005). By using inhibitors such as diphenylene iodonium (DPI), it has been suggested that NOX-derived ROS control cell expansion in maize (Zea mays) roots (Liszkay et al., 2004). This indicates that ROS-mediated growth is not an Arabidopsis-specific phenomenon. Because DPI is a general inhibitor of flavin-containing enzymes, DPI treatment may affect the activities of other proteins besides NOX (Bolwell, 1999; Moulton et al., 2000). Therefore, without direct genetic evidence for the involvement of NOX in this pathway, the role of NOX remains to be confirmed in maize.
The roots of plants lacking both AtrbohD and AtrbohF (atrbohd, atrbohf double mutants) are indistinguishable from the wild type, indicating that they are not involved in growth per se under standard conditions, but the double-mutant roots are less sensitive to the inhibitory effects of abscisic acid (ABA) on root elongation (Kwak et al., 2003). Given that AtrbohD and AtrbohF are also required for the stomatal response to ABA, it suggests that ROS derived from the D and F proteins are involved in the ABA-signaling mechanism that controls plant growth responses in drought conditions. We can therefore say that there are at least two distinct ROS-requiring mechanisms that occur during root growth in Arabidopsis. There is the requirement of RHD2/AtrbohC for elongation and there is an ABA-related growth inhibition process that requires AtrbohD and AtrbohF.
Whereas there is clearly a role for ROS in root elongation, there is also evidence that NOX-derived ROS are required during the growth of other organs. During leaf expansion, a wave of ROS-dependent cell growth sweeps through the leaf (Rodriguez et al., 2002). This local expansion zone is the site of the accumulation of ROS, and inhibition of ROS formation by treatment with DPI inhibits leaf growth. This indicates that not only are ROS involved in growth, but also that a flavin-containing oxidase such as a NOX is required for its production. Furthermore, the accelerated elongation that occurs upon auxin treatment is accompanied by the formation of higher levels of ROS than in coleoptiles grown without auxin treatment (Rodriguez et al., 2002; Schopfer et al., 2002). This suggests that the rate of cell growth may be proportional to the amount of ROS produced in growing organs.
Whereas the above evidence suggests that NOXs control cell growth, there is some evidence to suggest that these enzymes control other aspects of development, such as apical dominance and leaf shape (Sagi et al., 2004). Transgenic plants harboring NOX-antisense constructs not only had both reduced NOX activity and ROS levels, but also exhibited a number of morphological defects, including reduced apical dominance, leading to an increase in branching, reduced leaf lobing, and curled leaflets. These phenotypes suggest that ROS control more processes than cell expansion. The mechanism by which ROS act on these different facets of development remains mysterious, but unraveling them will provide important insights into the mechanism by which ROS controls development.
AN UNIDENTIFIED CALCIUM CHANNEL AND THE OXIDATIVE BURST INDUCIBLE1 SER-THR KINASE ARE DOWNSTREAM OF ROS DURING ROOT HAIR DEVELOPMENT
Whereas there is a plethora of cellular activities that may be regulated by ROS during growth, characterization of the mechanism by which RHD2/AtrbohC controls growth has uncovered at least one process. RHD2/AtrbohC activity produces ROS that accumulate at the sites of root hair growth, the tip (Fig. 1). Relatively high cytoplasmic Ca2+ concentrations are found at the tip, leading to the formation of a so-called tip-high calcium gradient, and this gradient is absent in the rhd2 mutant (Wymer et al., 1997). This suggests that RHD2/AtrbohC activity and, by extension, ROS are required for the formation of the Ca2+ gradient. This is supported by the finding that the hyperpolarization-activated calcium channel (HACC) that is required for the formation of the gradient is activated by ROS, and that ROS treatment results in an elevation of cytoplasmic Ca2+ levels in rhd2 mutant root hairs (Very and Davies, 2000; Foreman et al., 2003). Therefore, an as yet unidentified HACC is likely to be a target of RHD2-/AtrbohC-derived ROS regulation. Molecular identification of the HACC will allow this hypothesis to be tested.
Figure 1.
Nitroblue tetrazolium stains the growing tips of wild-type root hairs soon after initiation (A) and during subsequent rapid tip growth (B). This indicates that superoxide accumulates at the site of growth. C, Plants that are homozygous for the rhd2 mutation do not stain with nitroblue tetrazolium, indicating that accumulation of superoxide at the root hair tip requires the activity of the RHD2/AtrbohC NOX (Figure 1 is courtesy of Dr. Seiji Takeda, John Innes Centre).
Another target of ROS in growing root hairs is the OXIDATIVE BURST INDUCIBLE1 (OXI1) kinase (Rentel et al., 2004). OXI1 was identified in a screen for mRNAs that increase in abundance upon ROS treatment in Arabidopsis. OXI1 is a Ser-Thr kinase that is also required for root hair elongation; oxi1 mutant root hairs are slightly shorter than the wild type. Therefore, it is likely that RHD2-/AtrbohC-derived ROS leads to activation of a mitogen-activated protein kinase cascade via OXI1. Whereas it is formally possible that activation of OXI1 is dependent on the HACC channel, it is more likely that ROS activate OXI1 independently of the HACC.
CELL WALL LOOSENING AND TIGHTENING ARE MEDIATED BY ROS
The cell wall plays an important role in cell expansion; loosening the wall allows cells to expand, whereas wall cross-linking can inhibit expansion. There is evidence that ROS are involved in both processes. They have been implicated in the loosening of cell walls in growing tissues (Fry, 1998; Potikha et al., 1999; Liszkay et al., 2004) and in making cell walls stiff as growth ceases and cells differentiate (Hohl et al., 1995; Ros-Barcelo et al., 2002).
At least part of the stimulatory effect of ROS on expansion is through the ability of the hydroxyl (⋅OH) radical to promote cell growth. ⋅OH radicals are present in the walls of cells in elongating organs in vivo (Liszkay et al., 2004; Renew et al., 2005). One way in which ⋅OH radicals regulate growth is through the scission of xyloglucan polymers allowing walls to stretch (Fry, 1998). If, as is likely to be the case, the ⋅OH radical has the same effect in vivo, then it is likely that it contributes to growth by decreasing the resistance of the wall to the pressure from the expanding protoplast through xyloglucan scission. A likely route for the production of these radicals is through the Fenton reaction from hydrogen peroxide (H2O2), which is catalyzed by a metal ion such as Cu2+. It is likely that this reaction occurs in vivo because all of the reagents required are present in the plant cell wall (Fry, 1998). However, there is also evidence for enzymatic production of ⋅OH in plasma membrane extracts that is stimulated by NADH and insensitive to oxygen, indicating a mechanism independent of superoxide (Mojovic et al., 2004).
H2O2 production is correlated with the stiffening of cell walls as growth ceases in a number of cell types. For example, H2O2 is produced at the same time as lignification in the walls of Zinnia elegans xylem tracheary elements and required for the formation of the complex cross bridges found in the walls of this cell type (Ros-Barcelo et al., 2002). Similar H2O2-induced stiffening of the cell wall by increasing cross-linking of polymers occurs as growth ceases at the onset of differentiation in many cell types (Brisson et al., 1994; Hohl et al., 1995; Schopfer, 1996). A variation on this theme is the oxidative H2O2-mediated cross-linking of cell wall proteins, which reinforces the cell wall, thereby blocking the pathogen spread that occurs upon infection (Brisson et al., 1994).
H2O2 has also been shown to be involved in differentiation of the cellulose-rich cell wall in cotton fibers. An increase in H2O2 production coincides with deposition of the secondary walls of cotton (Gossypium hirsutum) fibers (Potikha et al., 1999). Furthermore, incubation of developing cotton fibers with H2O2 scavengers blocks differentiation, whereas application of H2O2 stimulates secondary wall formation. Its precise role in the wall differentiation process is unknown, but it may act by stimulating the activity of cellulose synthases that are active during the growth of the cotton fiber.
SMALL GTPASES SPATIALLY CONTROL ROS PRODUCTION AND GROWTH
Whereas ROS are clearly required for growth, spatial regulation of their production is important in determining organ shape and form (morphogenesis). Local accumulation of ROS at the tips of growing root hairs suggests that spatial regulation of ROS production is an important determinant of cell shape (Foreman et al., 2003; Carol et al., 2005). This conclusion is supported by the finding that application of ROS (in the form of ⋅OH) results in isotropic growth instead of tip growth (Foreman et al., 2003). Genetic evidence suggests that GTPases of the Rho class (called ROPs in plants) are involved in spatial regulation of ROS production and, by extension, spatial control of growth (Carol et al., 2005). The activities of Rho-GTPases are negatively controlled by a group of proteins called Rho-GDP dissociation inhibitors (RhoGDI). Loss of function of one member of this family, called SUPERCENTIPEDE1 (SCN1)/AtRhoGDI1, results in both spatially deregulated ROS accumulation and hair outgrowth. Furthermore, the ectopic sites of ROS accumulation in the scn1/atrhogdi1 mutant require the activity of RHD2/AtrbohC, indicating that spatial regulation of RHD2/AtrbohC involves SCN1/AtRhoGDI1. If SCN1/AtRhoGDI1 were active in the spatial control of growth, then it might be expected that its regulatory targets, the ROP GTPases, should also be involved. The finding that dominant negative versions of ROPs cause root hair defects and, in extreme cases, lead to complete loss of spatially controlled growth lends weight to this model (Molendijk et al., 2001; Jones et al., 2002). Similarly, a constitutively active form of a cotton small GTPase that is highly expressed during cotton fiber development has been shown to induce ROS production in cultured Arabidopsis and soybean (Glycine max) cells (Potikha et al., 1999). This suggests that this GTPase activates a NOX protein during the rapid elongation of cotton fibers. Therefore, data from a number of systems indicate that small GTPases regulate the activity of NOX enzymes during development.
GTPases also spatially control nongrowth-related processes associated with differentiation. During the development of Z. elegans xylem tracheary elements, NOX-derived ROS are required for formation of the highly lignified banded thickenings in the cell wall (Ros-Barcelo et al., 2002). The ROS are produced in adjacent xylem parenchyma cells and not in the differentiating tracheary elements themselves (Nakanomyo et al., 2002; Ros Barcelo, 2005). In this neighboring cell, mRNA of a Rac small GTPase accumulates in the cytoplasm on the face of the parenchyma cell next to the developing tracheary element, but does not accumulate in other regions of these cells (Ros Barcelo, 2005). It is hypothesized that local accumulation of this mRNA results in preferential localization of the small GTPase in the region of the parenchyma cell next to the differentiating tracheary element (Nakanomyo et al., 2002). This GTPase may then activate a NOX enzyme that produces the ROS required for wall lignification in the adjacent cell.
Given that RHD2/AtrbohC controls both tip and intercalary growth and that there is evidence for a role for ROPs in both mechanisms, it is possible that NOX-derived ROS are required during the growth of all plant cells. If this were the case, then perhaps ROPs are responsible for the spatial regulation of NOX activity not only in tip-growing cells, but also in cells undergoing intercalary growth.
NOXS CONTROL DEVELOPMENT IN THE THREE DOMAINS OF EUKARYOTIC LIFE
Control of development by NOX proteins is not a plant-specific phenomenon; NOX-derived ROS control development in fungi and animals. For example, NADPH oxidase A (NoxA) regulates sexual development in Aspergillus nidulans (Lara-Ortiz et al., 2003). NoxA gene transcription is induced during sexual development, suggesting that it may be involved in the formation of the fruiting body (cleistothecia) and, indeed, ROS are produced during fruiting-body development. Both formation of ROS and fruiting-body development are defective in noxA deletion mutants, which lack NoxA activity.
NOX genes are also required for development of the fruiting body (perithecium) in Podospora anserina (Malagnac et al., 2004). Mutants that lack the two NOX-encoding genes undergo defective fruiting-body development. Furthermore, germination of the ascospore is defective in these mutants. This suggests that ROS may not only control fruiting-body development in Podospora as they do in the other ascomycete Aspergillus, but they may also control the formation of tip-growing cells as they do in the root hairs of flowering plants.
NOX genes are also involved in animal development. Cell migration is a process that distinguishes plant from animal development; cell migration is an integral part of metazoan organogenesis, but cells do not crawl past each other in plants. The migration of mammalian endothelial cells has been shown to be regulated by NOX-derived ROS (Wu et al., 2005). Furthermore, this NOX activity is also controlled by the activity of a Rho GTPase as may be the case in plants. Whereas the role of NOX-derived ROS in cell migration has no parallel in plants, the role for ROS in promoting animal cell growth is remarkably similar to its role in plants; overexpression of a mouse NOX gene results in elevated superoxide production and increased cell growth in fibroblasts (Suh et al., 1999). Therefore, ROS control development in all three eukaryotic domains (plants, animals, and fungi). Their role in controlling growth may be ancestral because the cells of the common ancestors of the plants, animals, and fungi must have grown!
PERSPECTIVES
Most of the developmental processes involving ROS that we have reviewed involve the growth of cells and organs. We are beginning to identify the pieces of the complex machine that integrates ROS and growth. The startling phenotypes of plants reported by Sagi et al. (2004) suggests that ROS may be doing something more complex than simply regulating cell growth during development; they may be involved in controlling organ number and initiation. Getting to the root of ROS in these processes will be important in understanding the regulation of multicellular development.
This work was supported by a grant in aid to the John Innes Centre from the Biotechnology and Biological Science Research Council, John Innes Foundation, and a grant from the Biotechnology and Biological Science Research Council.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Liam Dolan (liam.dolan@bbsrc.ac.uk).
References
- Aguirre J, Rios-Momberg M, Hewitt D, Hansberg W (2005) Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol 13: 111–118 [DOI] [PubMed] [Google Scholar]
- Bolwell GP (1999) Role of active oxygen species and NO in plant defence responses. Curr Opin Plant Biol 2: 287–294 [DOI] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C (1994) Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 6: 1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L (2005) A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438: 1013–1016 [DOI] [PubMed] [Google Scholar]
- Cheng G, Cao Z, Xu X, Meir EGV, Lambeth JD (2001) Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Fry SC (1998) Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem J 332: 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl M, Greiner H, Schopfer P (1995) The cryptic growth response of maize coleoptiles and its relationship to H2O2 dependent cell wall stiffening. Physiol Plant 94: 491–498 [Google Scholar]
- Jones MA, Shen JJ, Fu Y, Li H, Yang Z, Grierson CS (2002) The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C (1998) A plant homolog of the neutrophil NADPH oxidase gp91(phox) subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10: 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Ortiz T, Riveros-Rosas H, Aguirre J (2003) Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol Microbiol 50: 1241–1255 [DOI] [PubMed] [Google Scholar]
-
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates
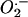 , H2O2, and OH by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136: 3114–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
, H2O2, and OH by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136: 3114–3123 [DOI] [PMC free article] [PubMed] [Google Scholar] - Malagnac F, Lalucque H, Lepere G, Silar P (2004) Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet Biol 41: 982–997 [DOI] [PubMed] [Google Scholar]
- Martienssen R, Dolan L (1998) Patterns in vegetative development. In M Anderson, J Roberts, eds, Arabidopsis. Sheffield Academic Press, Sheffield, UK, pp 262–297
- Mojovic M, Vuletic M, Baic GG, Vuinic Z (2004) Oxygen radicals produced by plant plasma membranes: an EPR spin-trap study. J Exp Bot 55: 2523–2531 [DOI] [PubMed] [Google Scholar]
- Molendijk AJ, Bischoff F, Rajendrakumar CS, Friml J, Braun M, Gilroy S, Palme K (2001) Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J 20: 2779–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton P, Martin H, Ainger A, Cross A, Hoare C, Doel J, Harrison R, Eisenthal R, Hancock J (2000) The inhibition of flavoproteins by phenoxaiodonium, a new iodonium analogue. Eur J Pharmacol 401: 115–120 [DOI] [PubMed] [Google Scholar]
- Nakanomyo I, Kost B, Chua NH, Fukuda H (2002) Preferential and asymmetrical accumulation of a Rac small GTPase mRNA in differentiating xylem cells of Zinnia elegans. Plant Cell Physiol 43: 1484–1492 [DOI] [PubMed] [Google Scholar]
- Potikha TS, Collins CC, Johnson DI, Delmer DP, Levine A (1999) The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol 119: 849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renew S, Heyno E, Schopfer P, Liszkay A (2005) Sensitive detection and localization of hydroxyl radical production in cucumber roots and Arabidopsis seedlings by spin trapping electron paramagnetic resonance spectroscopy. Plant J 44: 342–347 [DOI] [PubMed] [Google Scholar]
- Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, et al (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427: 858–861 [DOI] [PubMed] [Google Scholar]
- Rodriguez AA, Grunberg KA, Taleisnik EL (2002) Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol 129: 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros Barcelo A (2005) Xylem parenchyma cells deliver the H2O2 necessary for lignification in differentiating xylem vessels. Planta 220: 747–756 [DOI] [PubMed] [Google Scholar]
- Ros-Barcelo A, Pomar F, Lopez-Serrano M, Martinez P, Pedreno MA (2002) Developmental regulation of the H2O2-producing system and of a basic peroxidase isoenzyme in the Zinnia elegans lignifying xylem. Plant Physiol Biochem 40: 325–332 [Google Scholar]
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R (2004) Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 16: 616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P (1996) Hydrogen peroxide-mediated cell-wall stiffening in vitro in maize coleoptiles. Planta 199: 43–49 [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A (2002) Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214: 821–828 [DOI] [PubMed] [Google Scholar]
- Segal AW, Abo A (1993) The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci 18: 43–47 [DOI] [PubMed] [Google Scholar]
- Sugimoto-Shirasu K, Roberts K (2003) “Big it up”: endoreduplication and cell-size control in plants. Curr Opin Plant Biol 6: 544–553 [DOI] [PubMed] [Google Scholar]
- Suh YA, Arnold RS, Lassegue B, Shi J, Xu XX, Sorescu D, Chung AB, Griendling KK, Lambeth JD (1999) Cell transformation by the superoxide-generating oxidase Mox1. Nature 401: 79–82 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91(phox) homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defence response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JD (1998) Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J 14: 365–370 [DOI] [PubMed] [Google Scholar]
- Very AA, Davies JM (2000) Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc Natl Acad Sci USA 97: 9801–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA Jr, Terada LS (2005) Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol 171: 893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymer CL, Bibikova TN, Gilroy S (1997) Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J 12: 427–439 [DOI] [PubMed] [Google Scholar]



