Abstract
Glutathione (GSH) has been implicated in maintaining the cell cycle within plant meristems and protecting proteins during seed dehydration. To assess the role of GSH during development of Arabidopsis (Arabidopsis thaliana [L.] Heynh.) embryos, we characterized T-DNA insertion mutants of GSH1, encoding the first enzyme of GSH biosynthesis, γ-glutamyl-cysteine synthetase. These gsh1 mutants confer a recessive embryo-lethal phenotype, in contrast to the previously described GSH1 mutant, root meristemless 1(rml1), which is able to germinate, but is deficient in postembryonic root development. Homozygous mutant embryos show normal morphogenesis until the seed maturation stage. The only visible phenotype in comparison to wild type was progressive bleaching of the mutant embryos from the torpedo stage onward. Confocal imaging of GSH in isolated mutant and wild-type embryos after fluorescent labeling with monochlorobimane detected residual amounts of GSH in rml1 embryos. In contrast, gsh1 T-DNA insertion mutant embryos could not be labeled with monochlorobimane from the torpedo stage onward, indicating the absence of GSH. By using high-performance liquid chromatography, however, GSH was detected in extracts of mutant ovules and imaging of intact ovules revealed a high concentration of GSH in the funiculus, within the phloem unloading zone, and in the outer integument. The observation of high GSH in the funiculus is consistent with a high GSH1-promoter∷β-glucuronidase reporter activity in this tissue. Development of mutant embryos could be partially rescued by exogenous GSH in vitro. These data show that at least a small amount of GSH synthesized autonomously within the developing embryo is essential for embryo development and proper seed maturation.
Embryo development is a crucial part of the life cycle of plants during which the body plan of the daughter plant is established, storage products required for germination are accumulated, and desiccation tolerance develops. Development of desiccation tolerance enables both seed and embryo to overcome prolonged times with unfavorable conditions. Each of these phases of seed development requires specific and overlapping genetic programs involving cell division, cell differentiation, and general housekeeping functions (Goldberg et al., 1989).
Oxidation-reduction status is an important regulator of various metabolic functions in all eukaryotic cells. Perturbation of the finely balanced cellular redox system by biotic and abiotic stresses results in molecular responses ultimately leading to alterations in cell function and adaptation. The glutathione system (reduced, GSH; oxidized, GSSG) acts as a homoeostatic redox buffer that contributes to maintenance of the cellular redox balance (Schafer and Buettner, 2001; Meyer and Hell, 2005) and it may also act as a redox sensor of environmental cues (May et al., 1998). Thus, glutathione may form part of a complex regulatory network underlying adaptation processes and coordinating gene expression and cell division. Beyond stress perception and signaling processes, glutathione is involved in a wide range of different metabolic functions, ranging from detoxification of heavy metals (Cobbett and Goldsbrough, 2002) and conjugation of electrophilic xenobiotics (Marrs, 1996) to reductive processes, such as scavenging of reactive oxygen species (ROS; Noctor and Foyer, 1998). Furthermore, GSH is required as a cofactor for several other metabolic processes, including the detoxification of methylglyoxal, a cytotoxic compound that is formed as a by-product of glycolysis under stress conditions (Zang et al., 2001; Singla-Pareek et al., 2003) and the reduction of adenosine 5′-phosphosulfate as the first reductive step of sulfate assimilation (Bick et al., 1998; Prior et al., 1999; Weber et al., 2000).
Glutathione biosynthesis takes place in two consecutive steps catalyzed by γ-glutamyl-cysteine synthetase (γ-ECS or GSH1) and glutathione synthetase (GSH2), respectively. In Arabidopsis (Arabidopsis thaliana [L.] Heynh.), both enzymes are encoded by single-copy genes (May et al., 1998). GSH1 is located in plastids, whereas GSH2 is targeted to both cytosol and plastids (Wachter et al., 2005). Several mutants of GSH1 have been described, including the Cd2+-sensitive cad2-1 (Howden et al., 1995) and regulator of ascorbate peroxidase1-1 (rax1-1; Ball et al., 2004), which both have only about 30% wild-type levels of GSH and display distinct defense gene expression profiles. More severe is the root meristemless1 (rml1) mutant of GSH1, which lacks postembryonic cell division due to a severe deficiency of GSH (Cheng et al., 1995; Vernoux et al., 2000). Antisense suppression of GSH1 resulted, in the most extreme case, in smaller plants with significantly increased sensitivity to environmental stresses (Xiang et al., 2001).
In nonstressed cells, GSH is present in millimolar concentrations with the highest concentrations of 2 to 4 mm in meristematic cells of the Arabidopsis root tip and cells from the logarithmic phase of a suspension culture (Fricker et al., 2000; Meyer et al., 2001). The increased concentrations of glutathione in dividing cells support the hypothesis of specific functions of GSH during meristematic growth (Sánchez-Fernández et al., 1997; Vernoux et al., 2000). A significant function for GSH is in scavenging ROS and as a redox buffer (Noctor and Foyer, 1998; Meyer and Hell, 2005). Several different ROS are generally produced as by-products during normal aerobic metabolism (Apel and Hirt, 2004). Detoxification of ROS is mainly achieved through the ascorbate-glutathione cycle (Noctor and Foyer, 1998; Foyer and Noctor, 2000) and also through the glutathione peroxide cycle by which hydrogen peroxide is reduced to water by glutathione peroxidase (Roxas et al., 1997; Mullineaux et al., 1998).
Developing seeds are an important sink for sulfur in either oxidized or reduced form. Thus, sulfur nutrition of maternal plants may directly affect the delivery and metabolism of sulfur in, for example, the developing endosperms of wheat (Triticum aestivum; Fitzgerald et al., 2001). The demand of developing seeds for sulfur can potentially be satisfied either through supply of sulfate or, alternatively, in the form of reduced phloem-mobile sulfur compounds. Potential transport metabolites for reduced sulfur are GSH (Rennenberg, 1982; Herschbach et al., 2000) and S-methylmethionine (Bourgis et al., 1999). It has been proposed that glutathione translocated from shoot to root via the phloem acts as a signal responsible for repression of sulfate uptake in the roots (Lappartient and Touraine, 1997; Lappartient et al., 1999). Artificial inhibition of GSH biosynthesis in maize (Zea mays) by l-buthionine-(S,R)-sulfoximine, an inhibitor of GSH1, however, provided evidence for Cys rather than GSH as repressor signal for expression of sulfur status-responsive genes (Bolchi et al., 1999). Whereas sulfate is the major form of soluble sulfur in the endosperm of high-sulfur wheat plants, low-sulfur plants contained predominantly GSH (Fitzgerald et al., 2001). The interpretation that seeds can be supplied with reduced sulfur in the form of GSH is supported by the observation of high GSH concentrations in rice (Oryza sativa) phloem (Kuzuhara et al., 2000). Awazuhara et al. (2002) showed that feeding of GSH, but not the precursor Cys, to immature cotyledons affected storage protein accumulation. Thus, these results suggest a key role of GSH as a transport metabolite for reduced sulfur to the seed. In wheat endosperm, GSH is metabolized to the single amino acids required for synthesis of storage proteins (Anderson and Fitzgerald, 2001; Fitzgerald et al., 2001). Whereas seeds of legumes and cereals contain vascular tissue, the only vascular bundle in Arabidopsis seed is the bundle that terminates at the end of the funiculus. Using a noninvasive approach based on phloem-mobile green fluorescent protein and other fluorescent tracers, it was recently shown that within Arabidopsis seed several isolated symplastic domains with three apoplastic borders between the phloem and the embryo can be distinguished (Stadler et al., 2005). Transport of GSH into the developing embryo would thus require efficient transport systems, and it has been suggested that members of the oligopeptide transporter family might be involved in GSH transport (Cagnac et al., 2004).
The observation that homozygous rml1 seedlings develop normally through seed maturation leaves open the question of whether the rml1 embryo produces sufficient residual amounts of GSH or whether GSH required during embryogenesis might be supplied by maternal tissues. Approaches to analyze the glutathione status in seeds so far have used conventional tissue extraction and subsequent HPLC analysis (Fitzgerald et al., 2001; Hagan et al., 2003). This approach, however, has the disadvantage that uneven distribution of GSH between different seed tissues is difficult to analyze, especially in small seeds such as in Arabidopsis. GSH levels in different adjacent cells can be visualized and quantified by GSH-specific fluorescent labeling with monochlorobimane (MCB) and confocal laser-scanning microscopy (CLSM; Fricker et al., 2000; Gutiérrez-Alcalá et al., 2000; Meyer and Fricker, 2000; Meyer et al., 2001; Hartmann et al., 2003). To investigate whether developing Arabidopsis embryos are fully autonomous in their GSH metabolism or whether the embryos can be supplied with GSH from maternal tissues during their development, we have isolated Arabidopsis loss-of-function alleles of GSH1 from T-DNA mutant collections and studied the development of homozygous embryos in these mutants in comparison to homozygous rml1 embryos and wild-type embryos. The presence of GSH in the different embryos and the distribution of GSH within developing ovules were observed in situ by CLSM after MCB labeling.
RESULTS
The rml1 Mutant Has Residual GSH Detectable in Situ Using MCB
The rml1 mutation results in a single amino acid substitution in GSH1, which renders the homozygous seedling almost entirely GSH deficient. The lack of GSH prevents normal meristematic growth of mutant seedlings and normal root hair formation (Fig. 1, A, B, and E). Vernoux et al. (2000) reported that, using an HPLC assay, a residual level of GSH (2.7% of wild type) could be detected in rml1. We have used MCB in combination with CLSM to detect GSH in rml1 mutants in situ and imaging confirmed a small amount of MCB-dependent fluorescence. In different experiments, rml1 seedlings were shown to contain about 2% to 10% of GSH compared with wild-type seedlings (Fig. 1, C–G). Simultaneous labeling of cell walls with 50 μm propidium iodide (PI) indicated that cells of both wild-type and mutant seedlings were intact and viable. When rml1 seedlings were germinated in the presence of 1 mm l-buthionine-(S,R)-sulfoximine, an inhibitor of GSH biosynthesis, no MCB-dependent fluorescence was detected (data not shown), suggesting that the observed GSH in the mutant seedlings is due to autonomous GSH biosynthesis from residual GSH1 activity. Because embryonic development and seed maturation appear to occur normally in the rml1 mutant, we wished to determine whether this was dependent on a level of endogenous GSH biosynthesis. To test this, we have characterized T-DNA insertion alleles of GSH1.
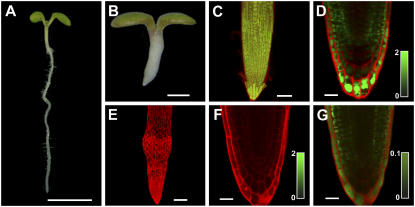
Figure 1.
In situ imaging of GSH using the MCB reagent in the rml1 GSH-deficient mutant. A, Wild-type seedling (scale bar = 5 mm). B, Homozygous rml1 seedling (scale bar = 1 mm) 10 d after germination. C to G, In situ labeling of root tips of wild-type and mutant seedlings with 100 μm MCB (green) and 50 μm PI (red) for 30 min. C, Maximal projection of an intact wild-type root tip (scale bar = 50 μm). D, Median optical section of a labeled wild-type root (scale bar = 20 μm). E, Maximal projection of an intact rml1 root tip (scale bar = 20 μm). F, Median optical section of a labeled rml1 root imaged under the same conditions used for the wild-type root tip shown in D (scale bar = 20 μm). G, The same median optical section of a labeled rml1 root as in F, imaged with increased detection sensitivity to visualize residual amounts of GSH (scale bar = 20 μm). Intensity bars for estimation of GSB concentrations in D, F, and G give GSB concentrations in millimolars.
Genetic Characterization of GSH1 T-DNA Insertion Mutants
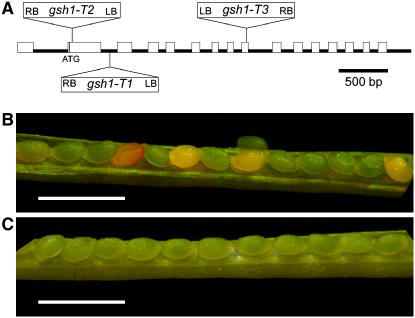
Three different lines with T-DNA insertions in GSH1 were obtained from the SIGnAL collection available through the Arabidopsis stock center. These mutants are referred to here as gsh1-T1, -T2, and -T3 (Table I; Fig. 2A). The sequence of DNA spanning the T-DNA left border in each mutant was determined to confirm the position of insertion (Fig. 2A). Among the seed of self-fertilized heterozygous individuals for each mutant, approximately 25% failed to germinate and, of those that did, no homozygous gsh1 mutant individuals were detected. Ratios of 2:1 heterozygous-homozygous wild-type individuals were observed (Table I). This suggested that gsh1 insertion mutations conferred an embryo-lethal phenotype. Siliques of heterozygous individuals contained approximately 25% developing embryos that were white in color rather than green (Fig. 2B). For gsh1-T1, the green-to-white ratio observed was 1,858:591 (χ2 = 0.98; P > 0.3).
Table I.
Genetic characterization of gsh1 T-DNA insertion mutants
| Cross | Seedling Genotypea T/T:T/+:+/+ |
|---|---|
| gsh1-T1 self (SALK 011665) | 0:90:56 (χ2 = 1.7; P > 0.1) |
| gsh1-T2 self (SALK 081530) | 0:41:18 (χ2 = 0.21; P > 0.6) |
| gsh1-T3 self (SALK 102540) | 0:38:11 (χ2 = 2.6; P > 0.1) |
| gsh1-T1 × gsh1-T2 F1 | 0:63:33 (χ2 = 0.05; P > 0.8) |
| gsh1-T1 × gsh1-T3 F1 | 0:56:27 (χ2 = 0.02; P > 0.8) |
| gsh1-T2 × gsh1-T3 F1 | 0:57:20 (χ2 = 1.88; P > 0.1) |
Heterozygous plants were allowed to self fertilize (self) or were cross pollinated by hand (F1). Progeny were germinated on agar nutrient medium and genotyped by PCR. T/T and T/+ individuals are homozygous and heterozygous, respectively, for T-DNA insertion alleles; χ2 values are calculated on an expected ratio of 2 T/+:1 +/+.
Figure 2.
Characterization of T-DNA insertion alleles of Arabidopsis GSH1. A, GSH1 gene structure and location of three independent T-DNA insertions in GSH1. B, Opened silique from self-fertilized gsh1-T1/+ heterozygote showing 25% embryos with lethal phenotype. C, Wild-type control. Scale bars in B and C = 1 mm.
To confirm that the lethal phenotypes resulted from the insertions in the GSH1 gene in each mutant, a gsh1-T1 heterozygote was crossed with either a gsh1-T2 or gsh1-T3 heterozygote. All F1 progeny carried a wild-type GSH1 allele, demonstrating that the lethal phenotypes were not complemented (Table I). In addition, a gsh1 mutant should not complement either cad2-1 or rml1. A gsh1-T1 heterozygote was crossed with a rml1 heterozygote and the progeny were examined. Four possible genotypic classes were expected in equal ratios. The phenotype of the gsh1-T1/rml1 individuals was expected to exhibit the embryo-lethal or rootless phenotypes of either homozygous parent (or possibly some intermediate phenotype). Twenty-five of 105 F1 progeny exhibited the rml1 rootless phenotype. The remaining 80 phenotypically wild-type individuals were grown to maturity and their progeny were examined to determine their genotype. Of these, 25 segregated white embryos consistent with a gsh1-T1/+ genotype, 25 segregated rml1-like seedlings consistent with a rml1/+ genotype, and 30 gave only wild-type progeny. Overall, the numbers were consistent with the expected 1:1:1:1 ratio of the four classes (χ2 = 0.71; P > 0.7). The phenotype of the gsh1-T1/rml1 individuals was indistinguishable from rml1 homozygotes, indicating that only one copy of the partially functional rml1 allele is sufficient for embryo survival. Heterozygous gsh1-T1/+ plants were also crossed to homozygous cad2-1 individuals and the F1 were tested for the cad2-1 Cd-sensitive phenotype. Approximately 50% of the F1 individuals were Cd sensitive (59 wild type/49 Cd sensitive; χ2 = 0.93; P > 0.3), indicating that the gsh1-T1 mutant is unable to complement the Cd sensitivity of the cad2-1 mutant. These experiments confirm at a genetic level that all these mutations are allelic and demonstrate the embryo-lethal phenotype of a gsh1 T-DNA insertion mutant.
GSH Partially Rescues the Phenotype of Mutant Embryos
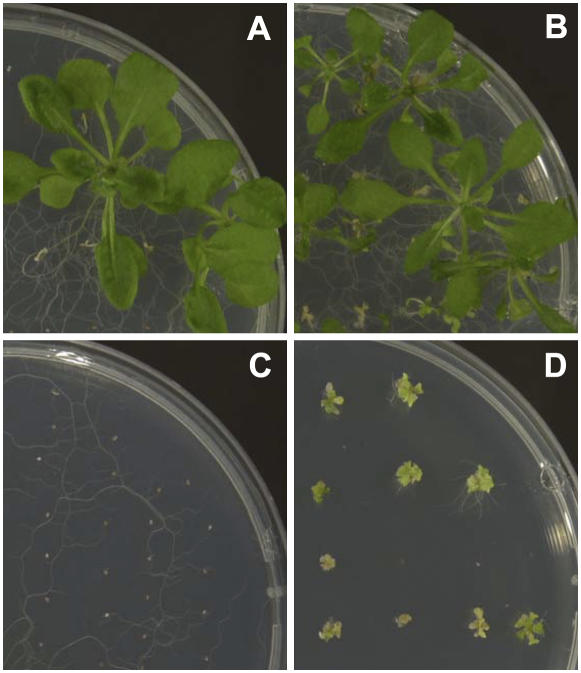
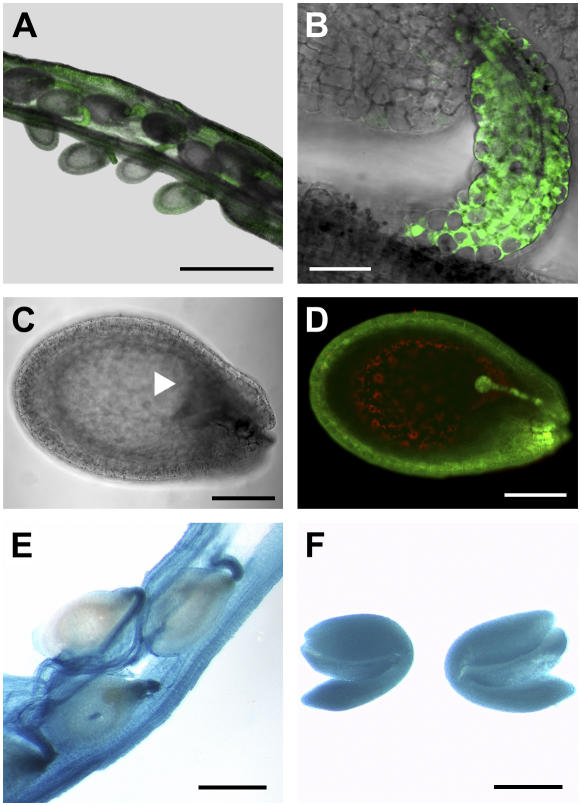
Root growth of rml1 mutants can be rescued by germinating seeds on agar medium containing GSH (Vernoux et al., 2000). To test whether gsh1-T1 mutants could be similarly rescued, white and green embryos were removed from siliques 8 to 10 d postfertilization and placed on agar medium with or without 1 mm GSH. In different experiments, 20% to 50% of green embryos developed into seedlings on both media. Similarly, 20% to 50% of white embryos developed on media containing GSH, but none developed in the absence of GSH (Fig. 3). Those that developed in the presence of GSH were confirmed by PCR to be homozygous mutants and, although they grew roots and leaves in the presence of exogenous GSH, they became progressively chlorotic and died (Fig. 3D).
Figure 3.
Partial rescue of gsh1-T1 homozygous plants by GSH. Green (A and B) and white (C and D) embryos collected from siliques of a heterozygous gsh1-T1/+ plant were plated onto either nutrient medium (A and C) or nutrient medium containing 1 mm GSH (B and D) and allowed to grow for a further 24 d.
To confirm that gsh1-T1 is a null allele, RNA was extracted from green and white embryos and expression of GSH1 mRNA was measured by using reverse transcription-PCR and primers that flanked the insertion point of the T-DNA in gsh1-T1. No GSH1-specific PCR product was detected using RNA from white embryos (data not shown), consistent with this being a null mutation.
GSH1 T-DNA Insertion Alleles Are Lethal at a Late Stage in Embryo Development
Knockout embryos started their normal developmental program and became green at the same time as wild-type embryos during the heart stage approximately 4 d after pollination. Because the initiation of the greening process is restricted to the outermost cells of the heart-stage embryos, greening was best observed by the chlorophyll autofluorescence of isolated embryos. From the torpedo stage onward, all embryos could be identified as green without isolation from the ovules. Shortly after the torpedo stage, the mutant embryos started bleaching (Fig. 2; Table II). Growth of the mutant embryos continued at the same rate compared to wild-type embryos from the same siliques (Table II).
Table II.
Development of segregating embryos from a heterozygous gsh1-T1 plant
The embryonic stages are abbreviated as follows: Q, quadrant; G, globular; H, heart; T, torpedo; U, U-turn; M, mature; and D, desiccation. n.d., Not detected.
| Days After Pollination (Embryonic Stage)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (Q) | 2 (G) | 3 (G/H) | 4 (H) | 5 (H/T) | 6 (T) | 7 (U) | 8 (U) | 10 (M) | 12 (D) | |
| Green embryosa | 0 | 0 | 0 | 32 | 215 | 173 | 168 | 176 | 159 | 167 |
| White embryosa | 229 | 225 | 241 | 208 | 28 | 75 | 62 | 63 | 58 | 59 |
| Segregation ratiob | 1 | 1 | 1 | 0.87 | 0.12 | 0.30 | 0.27 | 0.26 | 0.27 | 0.26 |
| GSH staining of embryos in % (n)c | n.d. | n.d. | 78.2 (23) | 72.7 (33) | 78.6 (28) | 76.9 (78) | 76.1 (84) | 74.3 (140) | 74.0 (46) | n.d. |
Four to six siliques from different plants were opened per day.
The ratio gives the proportion of white embryos in siliques harvested from a heterozygous plant. In wild-type controls grown under the same conditions, only green embryos were observed from the torpedo stage onward.
Observations of labeling of globular and heart-stage embryos were made on ovules incubated with MCB. More advanced stages of embryo development were dissected from the ovule prior to labeling.
GSH Is Transported into the Seed But Is Absent from Homozygous gsh1-T Embryos
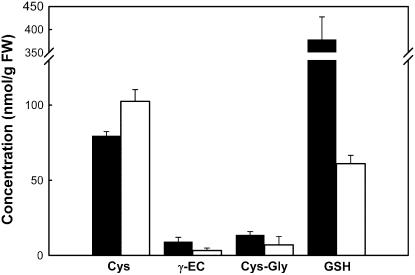
Low-Mr thiols were assayed in extracts of green wild-type and white homozygous gsh1-T1 ovules identified after the mutant phenotype was clearly visible. The mutant ovules contained about 60 nmol g−1 fresh weight (FW) GSH compared with 380 nmol g−1 FW in wild-type ovules collected at the same stage (Fig. 4). The level of Cys was higher in mutant ovules: 105 nmol g−1 FW compared to 80 nmol g−1 FW in the wild type (Fig. 4). Two other possible intermediates of GSH degradation, Cys-Gly and γ-EC, which is also the immediate product of the enzyme GSH1 deleted in the mutant embryos, were slightly decreased in the mutant.
Figure 4.
Low-Mr thiols in developing wild-type and gsh1-T1 mutant seeds. Ovules were harvested 9 to 13 d after self pollination when homozygous gsh1-T1 mutants could easily be identified by their white color. Black bars = ecotype Columbia; white bars = homozygous gsh1-T1. Values are mean ± sd; n = 3.
To study in more detail the GSH content of ovules and isolated embryos and the distribution of GSH within the ovules, we used in situ labeling with MCB and CLSM. In wild-type plants, embryos at the globular stage could be labeled within intact ovules (Fig. 5A), whereas in approximately one-fourth of the ovules collected from a heterozygous rml1 mutant, no labeling of the embryos was observed (Fig. 5B). Similarly, for about 25% of the early stage embryos from a heterozygous gsh1-T1 mutant, no labeling with MCB was found (Table II). These observations suggest that the nonlabeled embryos were homozygous for rml1 or gsh1-T1, respectively.
Figure 5.
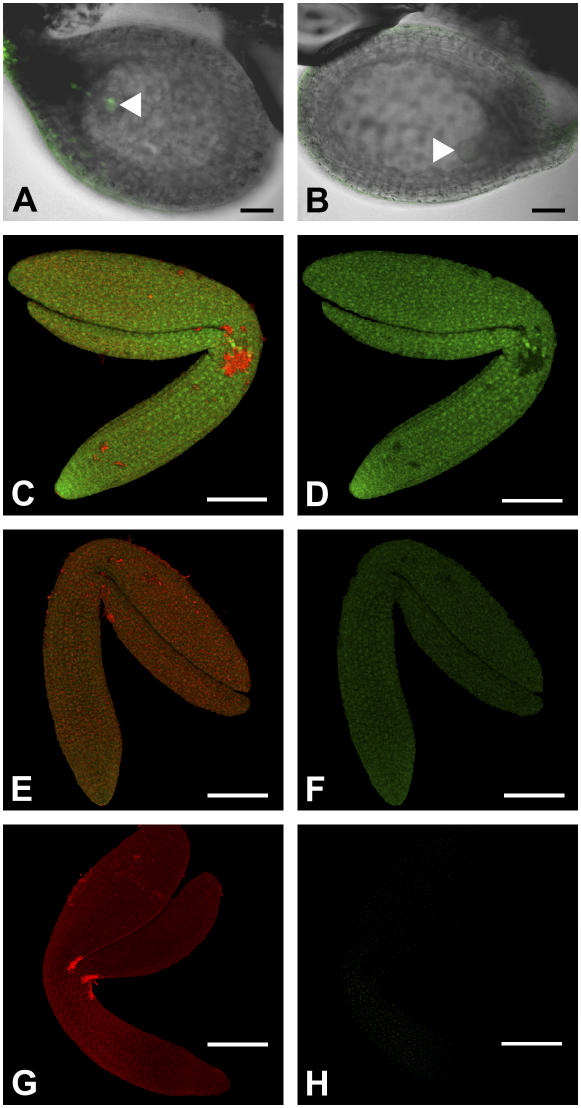
GSH imaging in intact ovules and isolated embryos. Ovules and isolated embryos were incubated in 100 μm MCB for 30 min prior to imaging. A, Superimposed transmission image (gray) and fluorescence image (green) of an ovule of a wild-type plant. The globular-stage embryo is fluorescently labeled (arrowhead). B, Ovule from a heterozygous rml1 plant after self pollination. The superimposed transmission image (gray) and fluorescence image (green) indicate the absence of GSH labeling in the globular-stage embryo (arrowhead). The presented image is representative for about 25% of the ovules in siliques of a rml1/+ heterozygote plant. C to H, Isolated embryos of wild type (C and D), rml1 (E and F), and gsh1-T1 (G and H) labeled with 100 μm MCB for GSH (green) and 50 μm PI for cell walls and viability of the cells (red). Images C, E, and G show merged green and red signals, including red autofluorescence from chloroplasts. Images D, F, and H show only the green signal for GSH labeled with MCB. The labeling pattern depicted for the rml1 and gsh1-T1 embryos is representative for 25% of the embryos of the respective self-pollinated heterozygous lines. All other embryos of these lines label like wild-type embryos. Scale bars in A and B = 50 μm; scale bars in C to H = 100 μm.
A detailed comparison of wild-type, homozygous rml1, and white homozygous gsh1-T1 embryos shortly before maturation showed strong MCB labeling in wild-type embryos, indicating the presence of high amounts of GSH (Fig. 5, C and D). About one-fourth of the embryos isolated from a heterozygous rml1 plant showed little labeling with MCB consistent with the low level of staining observed for rml1 seedlings (Fig. 5, E and F). Due to the weak MCB labeling, the merged image (Fig. 5E) is dominated by the red autofluorescence from chloroplasts, which is stronger than the red signal from PI bound to the cell walls. White embryos selected from a heterozygous gsh1-T1 plant lacked chloroplast autofluorescence, allowing for more sensitive imaging of the PI signal, which was restricted to the cell walls, confirming the structural integrity of the tissues (Fig. 5G). The white gsh1-T1 embryos were not labeled with MCB to a detectable level, supporting the expectation that these mutants were unable to synthesize GSH endogenously (Fig. 5H; Table II).
The apparent contradiction between the HPLC data showing the presence of GSH in white gsh1-T ovules and the lack of MCB labeling in the mutant embryos is consistent with the import of GSH into the growing seed from maternal tissues. Labeling of intact ovules still attached to the false septum of the opened siliques resulted in intense labeling of the funiculi (Fig. 6, A and B). Within the intact ovules, the chalazal point at which the funiculus is attached gave a particularly strong MCB-dependent fluorescent signal with concentrations of glutathione-bimane (GSB) conjugate up to 5 mm (Fig. 6, C and D). Strong fluorescence was also observed in all cells of the outer integument, but this was always less than that observed at the chalazal point. Only weak labeling with MCB was observed in cells of the inner integument, and very little or no labeling in the endosperm, whereas the developing embryo was again clearly labeled (Fig. 6D). Histochemical localization of GSH1 promoter activity in wild-type plants transformed with a GSH1 promoter∷β-glucuronidase (GUS) construct indicated strong activity in the embryo (Fig. 6F) and a particularly high activity in the funiculus (Fig. 6E), consistent with the levels of GSH observed.
Figure 6.
In situ labeling of GSH in intact ovules. Siliques were opened without detaching the ovules from the false septum of the ovary and then placed in 100 μm MCB for 30 min. A, Superimposed transmission image (gray) and fluorescence image (green) of an opened silique with several attached ovules, all of which show strong fluorescence (scale bar = 500 μm). B, Detail of a single ovule (scale bar = 20 μm). C, Transmission image of an ovule with a globular-stage embryo (arrowhead; scale bar = 100 μm). D, Optical section through the ovule shown in C after labeling with 100 μm MCB and 50 μm PI (scale bar = 100 μm). E and F, Histochemical assay for detection of GUS activity in siliques (E) and embryos (F) of Arabidopsis plants expressing an AtGSH1-promoter∷GUS fusion (scale bars = 250 μm).
DISCUSSION
In plants, biochemical functions of GSH may be essential for certain developmental steps such as gametogenesis, seed development, or postembryonic growth and development. It has been proposed that a complete loss of a critical chloroplast function in Arabidopsis is likely to result in embryo lethality, whereas partial loss of the function would result in defects after germination (Tzafrir et al., 2004). This proposal is supported by the different mutant alleles of GSH1. GSH1 is a nuclear gene that codes for plastid-localized γ-glutamyl-cysteine synthetase (Wachter et al., 2005). The isolation of rml1, a mutant allele of GSH1 conferring a single amino acid substitution (Vernoux et al., 2000), initially suggested that GSH deficiency results in loss of postembryonic growth but not embryo development. Here we show through the characterization of T-DNA insertion alleles that activity of GSH1 is indispensable for seed maturation. Three independent T-DNA insertion mutants exhibited the same embryo-lethal phenotype. Gametogenesis, however, is not affected because the frequency of homozygous mutant embryos was always close to the expected 25%, and crosses using heterozygotes as either the male or female parent indicated no effect on transmission of the mutant gametes. Nonrandom distribution of mutant embryos within the silique that could indicate a reduced viability of pollen tubes of the male gametophyte (Meinke, 1994) was not observed.
The rml1 mutant exhibits its characteristic phenotype only after germination (Vernoux et al., 2000), and comparison with the embryo-lethal phenotypes of the gsh1 T-DNA insertion lines is consistent with the rml1 mutant having some residual GSH1 activity. High-resolution imaging showed the presence of residual amounts of GSH in rml1 embryos and in the primary root of rml1 seedlings. MCB has been reported to nonspecifically label thiols in situ in the absence of a specific glutathione S-transferase capable of catalyzing the conjugation reaction with GSH (van der Ven et al., 1994). In this study, however, the absence of a detectable MCB-dependent fluorescence signal in homozygous gsh1-T embryos demonstrates that almost all the MCB-dependent fluorescence signal arises from conjugation with GSH, with no other low-Mr thiols or protein thiols labeled to a detectable level under the conditions used. This is consistent with earlier observations that MCB can be used for long-term incubation of suspension culture cells without apparent toxic effects on normal metabolism that would be caused by nonspecific labeling of protein thiols or low-Mr thiols other than GSH (Meyer and Fricker, 2002).
The embryo-lethal phenotype indicates that the embryo is not supplied with GSH from maternal tissues sufficiently to support full seed maturation. Developing seeds require a large amount of reduced sulfur for synthesis of structural and metabolic proteins. Sulfur assimilation from inorganic sulfate predominantly takes place within chloroplasts utilizing the reducing power generated through photosynthesis (Leustek et al., 2000). Grain tissues have the capacity to assimilate sulfate (Fitzgerald et al., 2001) and, from activities of enzymes involved in sulfate assimilation, Tabe and Droux (2001) calculated that sulfur assimilation within developing lupin cotyledons could contribute significantly to the accumulation of organic sulfur reserves in the seed. High activity of ATP sulfurylase in developing soybean (Glycine max) seeds indicates that the seeds, rather than the pods, are the dominant site of sulfate reduction (Sexton and Shibles, 1999). Similarly, high expression of adenosine 5′-phosphosulfate reductase was found in developing siliques (A.J. Meyer and S. Kopriva, unpublished data).
Transport of GSH has been reported for grains of wheat grown under conditions of low sulfur nutrition. Within the seeds, the delivered GSH is rapidly catabolized to provide free Cys required for synthesis of storage proteins (Fitzgerald et al., 2001). Rapid catabolism of supplied GSH could thus explain the lack of MCB labeling of the endosperm even in wild-type seeds. Degradation of GSH involves γ-glutamyl transpeptidases, which cleave the amino-terminal bond between Glu and Cys (Martin, 2003). Similar to extracellular degradation of GSH in animal tissues, an Arabidopsis γ-glutamyl transpeptidase, which is catalytically active on the apoplastic side of the plasma membrane, has been identified (Storozhenko et al., 2002). Degradation of GSH within the endosperm cavity has been reported for wheat (Fitzgerald et al., 2001). Alternatively, the absence of bimane labeling in the endosperm might be due to lack of a suitable glutathione S-transferase capable of catalyzing the conjugation with GSH. In Arabidopsis, the endosperm develops first as a syncytium and then cellularizes after the growing embryo reaches the heart stage (Berger, 2003). During the syncytical phase, the endosperm is lacking vacuoles and thus it may be that the GSH-dependent detoxification pathway, which ultimately leads to vacuolar sequestration of the GSH conjugates, is not highly expressed in the early endosperm. However, absence of intense bimane labeling during later cellular phases of the endosperm would favor the hypothesis that GSH is catabolized.
The importance of GSH for embryo development is further corroborated by the observation that T-DNA insertion mutations of the plastidic (and mitochondrial) glutathione reductase (At3g54660) are also embryo lethal (A.J. Meyer, unpublished data; Tzafrir et al., 2004). That these mutations cause termination of development at the earlier globular stage, emphasizes the importance of glutathione metabolism and redox homeostasis for embryo development. The early lethal effect of a plastidic glutathione reductase mutation may suggest that gsh1-T mutant embryos receive sufficient GSH from maternal tissues in the earlier stages of development to provide catalytic amounts of GSH within the plastids or mitochondria. This would then also imply an efficient uptake of GSH into the chloroplasts at extremely low cytosolic concentrations of GSH. So far, high-affinity transport of GSH into plastids has only been shown for isolated wheat plastids with a Km of 30 to 50 μm (Noctor et al., 2002). The general possibility of GSH transport into the embryo and further transport into organelles is supported by the fact that mutant GSH-deficient embryos could be rescued with an external supply of 1 mm GSH. However, uptake of GSH from external medium is still by no means sufficient to enable knockout mutants to complete their life cycle.
The direct cause of embryo lethality arising from GSH deficiency is not apparent. The GSH-deficient embryos begin to bleach shortly after chlorophyll biosynthesis begins, suggesting either a block in further chlorophyll synthesis or artificial destruction of chlorophyll. Destruction of chlorophyll can occur under high light intensity due to photooxidative stress. The loss of chlorophyll during embryogenesis, however, cannot account for the lethal effect. Cell divisions proceed apparently without any significant aberrations and the mutant embryos reach their full size. This phenotype is different from mutants with aberrant plastid development like schlepperless, which shows retarded growth (Apuya et al., 2001). In contrast, albino mutants, which lack the ability to produce pigments, remain morphologically normal and are able to germinate (Budziszewski et al., 2001). Labeling of mutant embryos with PI confirmed that shortly before onset of desiccation the cells of the embryo were still viable. Thus, the embryo-lethal effect of GSH deficiency became manifested at a very late stage in development, most likely the desiccation stage.
During seed development, increased levels of ROS occur and are normally controlled by increased levels of antioxidant compounds and activity of ROS scavenging enzymes (Bailly, 2004). Uncontrolled ROS production due to tocopherol deficiency can lead to oxidative stress and cellular damage, resulting in decreased seed longevity (Sattler et al., 2004). A typical result of increased ROS formation is protein carbonylation, leading to alteration of activity and increased likelihood of proteolytic attack (Johansson et al., 2004). Protein carbonylation can occur during germination of Arabidopsis seed and might be required for counteracting and utilizing the production of ROS resulting from the recovery of metabolic activity in germinating seeds (Job et al., 2005). GSH is directly involved in the ability of resurrection plants to dehydrate and recover after rewatering (Kranner et al., 2002). Glutathione has also been suggested to be directly involved in seed desiccation not only as a ROS scavenger, but also as a protective agent for protein thiols through protein glutathionylation (Kranner et al., 2002). The molecular basis for the essential role of GSH during seed maturation is not understood yet and is the subject of future work.
In conclusion, we have demonstrated the essential need for autonomous biosynthesis of glutathione within the embryo for normal development of the embryo and especially maturation of the seed. Glutathione is apparently transported to seeds, but does not reach the embryo in sufficient quantities to enable normal embryo maturation and formation of viable seeds capable of germination. It is not yet clear whether the GSH is metabolized within the endosperm or after delivery to the seed and whether the resulting amino acids can be taken up by the embryo.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana [L.] Heynh.) ecotype Columbia was used as wild type. Three different T-DNA insertion lines for AtGSH1 (SALK_011665 = gsh1-T1; SALK_081530 = gsh1-T2; SALK_102540 = gsh1-T3) were provided by the Salk Institute (Alonso et al., 2003) and obtained from the Arabidopsis Biological Resource Center. Plants were grown in soil in a controlled growth room with a 16-h light/8-h dark cycle, 150 μmol m−2 s−1 photons, 21°C, and 50% humidity. For growth of seedlings, seeds were surface sterilized with 70% (v/v) ethanol for 5 min, washed twice with sterile water, and placed on full nutrient medium solidified with 1% phytagel (Meyer and Fricker, 2000). Seedlings were grown under short-day conditions (8.5-h light, 22°C/15.5-h dark, 18°C) and 120 μmol photons m−2 s−1. Developing embryos were isolated from ovules by microdissection of the ovules under a stereomicroscope.
Screening for T-DNA Knockout Lines and Identification of Insertion Sites
To determine the position of each T-DNA insert, a DNA fragment was amplified by PCR using primers specific for the T-DNA left border and a flanking GSH1 sequence and the nucleotide sequence of the resulting products was determined. To determine the genotype of plants for a T-DNA insertion mutation, PCR using GSH1-specific primers that flanked the insertion point was used to identify the presence of a wild-type allele, whereas a GSH1-specific primer in combination with a T-DNA left-border primer was used to identify the mutant allele.
HPLC Analysis of Low-Mr Thiols
Siliques from wild-type and heterozygous gsh1-T1 plants were harvested 9 to 13 d after self fertilization and opened under a dissecting microscope. From gsh1-T1 plants, only white ovules were collected for further analysis. Three to 4 mg of fresh material were extracted in 0.1 n HCl, fully reduced with dithiothreitol for 1 h, and derivatized with 30 mm monobromobimane (THIOLYTE; Calbiochem) for 15 min in the dark. Derivatization was stopped by acidification with 5% (v/v) acetic acid. Samples were analyzed by reverse-phase HPLC (Waters 600E multisolvent delivery system, Autosampler 717plus; Waters) on a C-18 column (Nova-Pak 4.6 × 250 mm; pore size 4 μm). Separated fluorescent thiol-bimane conjugates were detected with 380-nm excitation at an emission wavelength of 480 nm on an attached fluorescence detector (Fluorometer RF-551; Shimadzu). Samples were separated with a mixture of 91% 100 mm potassium acetate, pH 5.5, and 9% methanol for 12.5 min and a flow rate of 1.3 mL min−1. Data acquisition and processing were performed by Millenium32 software (Waters).
Fluorescent Dyes
Stock solutions of 100 mm MCB were prepared with dimethyl sulfoxide and PI was prepared as a 5 mm aqueous stock. All stock solutions were stored as 100-μL aliquots at −20°C. Aliquots were thawed immediately prior to use and diluted with basal nutrient medium to final concentrations of 100 μm MCB and 50 μm PI. All dyes were obtained from Molecular Probes.
GSH Imaging
Seedlings and isolated embryos were transferred to a drop of dye solution on a slide separated from a coverslip by using 120-μm-thick plastic tape as a spacer. Serial optical sections were obtained by a Zeiss LSM 510 META attached to an inverted microscope stand (Axiovert 200 M; Zeiss) using either a Zeiss 10× Plan-Neofluar, a Zeiss 25× Plan-Neofluar 0.8 NA with water immersion, or a Zeiss 63× C-Apochromat 1.2 NA water immersion lens, as appropriate. Fluorescence was excited by a 405-nm diode laser for GSB and by a 543-nm HeNe laser for PI in a single track using a HFT 405/488/543 as the main dichroic beamsplitter and a NFT 545 as the secondary dichroic beam splitter. Bimane-dependent fluorescence was recorded on channel 2, with a selected bandwidth of 475 to 525 nm. The PI signal was monitored together with red autofluorescence from chloroplasts on channel 3 with a 560-nm long pass emission filter. Scanning was conducted in line mode and images were displayed as the mean of four subsequent scans. Besides the confocal images, a transmission image was normally recorded simultaneously.
Image analysis and processing was done in LSM3.2 (Zeiss) and Image J (version 1.33g; Wayne Rasband, National Institutes of Health). Final processing and compiling of images was done in Adobe Photoshop 7.0.
Analysis of AtGSH1 Promoter Activity
For analysis of AtGSH1 promoter activity, a 1,606-bp fragment in front of the start codon was amplified by PCR with primers 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTATCGATATGTAACACAATAAT-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTGGTATATATAGCTCCTGCA-3′. By use of a Gateway recombination system, the promoter sequence was cloned into vector pKGWFS7 in front of the reporter genes EGFP and uidA. Arabidopsis plants were transformed by the floral-dip method (Clough and Bent, 1998) and 10 independent transformant lines were used for further analysis. For detection of GUS activity, tissue samples were treated with GUS staining buffer (100 mm Na2HPO4/NaH2PO4, pH 7.0, 10 mm Na2EDTA, 0.5 mm K3[Fe(CN)6], 0.5 mm K4[Fe(CN)6], and 0.08% [w/v] X-GlucA [Duchefa]) for 16 h at 37°C. Green tissues were bleached with ethanol before examination.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (grant no. ME 1567/3–2 to A.J.M.), the University of Heidelberg (to A.J.M.), and the University of Melbourne (to C.S.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Andreas J. Meyer (ameyer@hip.uni-hd.de).
Some figures in this article are displayed in color online but in black and white in the print edition.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.077982.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Anderson JW, Fitzgerald MA (2001) Physiological and metabolic origin of sulphur for the synthesis of seed storage proteins. J Plant Physiol 158: 447–456 [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Apuya NR, Yadegari R, Fischer RL, Harada JJ, Zimmerman JL, Goldberg RB (2001) The Arabidopsis embryo mutant schlepperless has a defect in the chaperonin-60α gene. Plant Physiol 126: 717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awazuhara M, Kim H, Hayashi H, Chino M, Kim S-G, Fujiwara T (2002) Composition of seed storage proteins changed by glutathione treatment of soybeans. Biosci Biotechnol Biochem 66: 1751–1754 [DOI] [PubMed] [Google Scholar]
- Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14: 93–107 [Google Scholar]
- Ball L, Accotto GP, Bechtold U, Creissen G, Funck D, Jimenez A, Kular B, Leyland N, Mejia-Carranza J, Reynolds H, et al (2004) Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 16: 2448–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F (2003) Endosperm: the crossroad of seed development. Curr Opin Plant Biol 6: 42–50 [DOI] [PubMed] [Google Scholar]
- Bick JA, Aslund F, Chen Y, Leustek T (1998) Glutaredoxin function for the carboxyl-terminal domain of the plant-type 5′-adenylylsulfate reductase. Proc Natl Acad Sci USA 95: 8404–8409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolchi A, Petrucco S, Tenca PL, Foroni C, Ottonello S (1999) Coordinate modulation of maize sulfate permease and ATP sulfurylase mRNAs in response to variations in sulfur nutritional status: stereospecific down-regulation by l-cysteine. Plant Mol Biol 39: 527–537 [DOI] [PubMed] [Google Scholar]
- Bourgis F, Roje S, Nuccio ML, Fisher DB, Tarczynski MC, Li C, Herschbach C, Rennenberg H, Pimenta MJ, Shen TL, et al (1999) S-methylmethionine plays a major role in phloem sulfur transport and is synthesized by a novel type of methyltransferase. Plant Cell 11: 1485–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budziszewski GJ, Lewis SP, Glover LW, Reineke J, Jones G, Ziemnik LS, Lonowski J, Nyfeler B, Aux G, Zhou Q, et al (2001) Arabidopsis genes essential for seedling viability: isolation of insertional mutants and molecular cloning. Genetics 159: 1765–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnac O, Bourbouloux A, Chakrabarty D, Zhang M-Y, Delrot S (2004) AtOPT6 transports glutathione derivatives and is induced by primisulfuron. Plant Physiol 135: 1378–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JC, Seeley KA, Sung ZR (1995) RML1 and RML2, Arabidopsis genes required for cell proliferation at the root tip. Plant Physiol 107: 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S, Bent A (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Fitzgerald MA, Ugalde TD, Anderson JW (2001) Sulphur nutrition affects delivery and metabolism of S in developing endosperms of wheat. J Exp Bot 52: 1519–1526 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signalling. New Phytol 146: 359–388 [Google Scholar]
- Fricker MD, May M, Meyer AJ, Sheard N, White NS (2000) Measurement of glutathione levels in intact roots of Arabidopsis. J Microsc (Oxf) 198: 162–173 [DOI] [PubMed]
- Goldberg R, Barker S, Perez-Grau L (1989) Regulation of gene expression during plant embryogenesis. Cell 56: 149–160 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Alcalá G, Gotor C, Meyer AJ, Fricker M, Vega JM, Romero LC (2000) Glutathione biosynthesis in Arabidopsis trichome cells. Proc Natl Acad Sci USA 97: 11108–11113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan N, Upadhyaya N, Tabe L, Higgins T (2003) The redistribution of protein sulfur in transgenic rice expressing a gene for a foreign, sulfur-rich protein. Plant J 34: 1–11 [DOI] [PubMed] [Google Scholar]
- Hartmann TN, Fricker MD, Rennenberg H, Meyer AJ (2003) Cell-specific measurement of cytosolic glutathione in poplar leaves. Plant Cell Environ 26: 965–975 [DOI] [PubMed] [Google Scholar]
- Herschbach C, van Der Zalm E, Schneider A, Jouanin L, De Kok LJ, Rennenberg H (2000) Regulation of sulfur nutrition in wild-type and transgenic poplar over-expressing gamma-glutamylcysteine synthetase in the cytosol as affected by atmospheric H2S. Plant Physiol 124: 461–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Andersen CR, Goldsbrough PB, Cobbett CS (1995) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 107: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job C, Rajjou L, Lovigny Y, Belghazi M, Job D (2005) Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol 138: 790–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E, Olsson O, Nystrom T (2004) Progression and specificity of protein oxidation in the life cycle of Arabidopsis thaliana. J Biol Chem 279: 22204–22208 [DOI] [PubMed] [Google Scholar]
- Kranner I, Beckett RP, Wornik S, Zorn M, Pfeifhofer HW (2002) Revival of a resurrection plant correlates with its antioxidant status. Plant J 31: 13–24 [DOI] [PubMed] [Google Scholar]
- Kuzuhara Y, Isobe A, Awazuhara M, Fujiwara T, Hayashi H (2000) Glutathione levels in phloem sap of rice plants under sulfur deficient conditions. Soil Sci Plant Nutr 46: 265–270 [Google Scholar]
- Lappartient AG, Touraine B (1997) Glutathione-mediated regulation of ATP sulfurylase activity, SO42− uptake, and oxidative stress response in intact canola roots. Plant Physiol 114: 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappartient AG, Vidmar JJ, Leustek T, Glass AD, Touraine B (1999) Inter-organ signaling in plants: regulation of ATP sulfurylase and sulfate transporter genes expression in roots mediated by phloem-translocated compound. Plant J 18: 89–95 [DOI] [PubMed] [Google Scholar]
- Leustek T, Martin MN, Bick J-A, Davies JP (2000) Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol 51: 141–165 [DOI] [PubMed] [Google Scholar]
- Marrs KA (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 127–158 [DOI] [PubMed] [Google Scholar]
- Martin MN (2003) Biosynthesis and metabolism of glutathione in plants. In JK Setlow, ed, Genetic Engineering, Vol 25. Kluwer Academic/Plenum Publishers, New York, pp 163–188 [DOI] [PubMed]
- May M, Vernoux T, Leaver C, Van Montagu M, Inze D (1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49: 649–667 [Google Scholar]
- Meinke DW (1994) Seed development in Arabidopsis thaliana. In EM Meyerowitz, CR Somerville, eds, Arabidopsis, Vol 27. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 253–295
- Meyer A, Hell R (2005) Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth Res 86: 435–457 [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Fricker MD (2000) Direct measurement of glutathione in epidermal cells of intact Arabidopsis roots by two-photon laser scanning microscopy. J Microsc (Oxf) 198: 174–181 [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Fricker MD (2002) Control of demand-driven biosynthesis of glutathione in green Arabidopsis suspension culture cells. Plant Physiol 130: 1927–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ, May MJ, Fricker M (2001) Quantitative in vivo measurement of glutathione in Arabidopsis cells. Plant J 27: 67–78 [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Karpinski S, Jimenez A, Cleary SP, Robinson C, Creissen GP (1998) Identification of cDNAs encoding plastid-targeted glutathione peroxidase. Plant J 13: 375–379 [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279 [DOI] [PubMed] [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53: 1283–1304 [DOI] [PubMed] [Google Scholar]
- Prior A, Uhrig J, Heins L, Wiesmann A, Lillig C, Stoltze C, Soll J, Schwenn J (1999) Structural and kinetic properties of adenylyl sulfate reductase from Catharanthus roseus cell cultures. Biochim Biophys Acta 1430: 25–38 [DOI] [PubMed] [Google Scholar]
- Rennenberg H (1982) Glutathione metabolism and possible biological roles in higher plants. Phytochemistry 21: 2771–2781 [Google Scholar]
- Roxas V, Smith R, Allen E, Allen R (1997) Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat Biotechnol 15: 988–991 [DOI] [PubMed] [Google Scholar]
- Sánchez-Fernández R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inze D, May MJ (1997) Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc Natl Acad Sci USA 94: 2745–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16: 1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30: 1191–1212 [DOI] [PubMed] [Google Scholar]
- Sexton P, Shibles R (1999) Activity of ATP sulfurylase in reproductive soybean. Crop Sci 39: 131–135 [Google Scholar]
- Singla-Pareek SL, Reddy MK, Sopory SK (2003) Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Natl Acad Sci USA 100: 14672–14677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Lauterbach C, Sauer N (2005) Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol 139: 701–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storozhenko S, Belles-Boix E, Babiychuk E, Herouart D, Davey MW, Slooten L, Van Montagu M, Inze D, Kushnir S (2002) Gamma-glutamyl transpeptidase in transgenic tobacco plants: cellular localization, processing, and biochemical properties. Plant Physiol 128: 1109–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabe LM, Droux M (2001) Sulfur assimilation in developing lupin cotyledons could contribute significantly to the accumulation of organic sulfur reserves in the seed. Plant Physiol 126: 176–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzafrir I, Pena-Muralla R, Dickerman A, Berg M, Rogers R, Hutchens S, Sweeney TC, McElver J, Aux G, Patton D, et al (2004) Identification of genes required for embryo development in Arabidopsis. Plant Physiol 135: 1206–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ven A, Mier P, Peters W, Dolstra H, van Erp P, Koopmans P, van der Meer J (1994) Monochlorobimane does not selectively label glutathione in peripheral blood mononuclear cells. Anal Biochem 217: 41–47 [DOI] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inze D, et al (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter A, Wolf S, Steininger H, Bogs J, Rausch T (2005) Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J 41: 15–30 [DOI] [PubMed] [Google Scholar]
- Weber M, Suter M, Brunold C, Kopriva S (2000) Sulfate assimilation in higher plants characterization of a stable intermediate in the adenosine 5′-phosphosulfate reductase reaction. Eur J Biochem 267: 3647–3653 [DOI] [PubMed] [Google Scholar]
- Xiang C, Werner BL, Christensen EM, Oliver DJ (2001) The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol 126: 564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang TM, Hollman DA, Crawford PA, Crowder MW, Makaroff CA (2001) Arabidopsis glyoxalase II contains a zinc/iron binuclear metal center that is essential for substrate binding and catalysis. J Biol Chem 276: 4788–4795 [DOI] [PubMed] [Google Scholar]