Abstract
In this study, the function of the NAD(P)H dehydrogenase (NDH)-dependent pathway in suppressing the accumulation of reactive oxygen species in chloroplasts was investigated. Hydrogen peroxide accumulated in the leaves of tobacco (Nicotiana tabacum) defective in ndhC-ndhK-ndhJ (ΔndhCKJ) at 42°C and 4°C, and in that of wild-type leaves at 4°C. The maximum quantum efficiency of PSII decreased to a similar extent in both strains at 42°C, while it decreased more evidently in ΔndhCKJ at 4°C. The parameters linked to CO2 assimilation, such as the photochemical efficiency of PSII, the decrease of nonphotochemical quenching following the initial rise, and the photosynthetic O2 evolution, were inhibited more significantly in ΔndhCKJ than in wild type at 42°C and were seriously inhibited in both strains at 4°C. While cyclic electron flow around PSI mediated by NDH was remarkably enhanced at 42°C and suppressed at 4°C. The proton gradient across the thylakoid membranes and light-dependent ATP synthesis were higher in wild type than in ΔndhCKJ at either 25°C or 42°C, but were barely formed at 4°C. Based on these results, we suggest that cyclic photophosphorylation via the NDH pathway might play an important role in regulation of CO2 assimilation under heat-stressed condition but is less important under chilling-stressed condition, thus optimizing the photosynthetic electron transport and reducing the generation of reactive oxygen species.
Oxygen-evolving photosynthesis operates with two photosystems (PSI and PSII). Light energy absorbed by antenna pigments is transferred to the photosystem reaction centers and is converted to assimilative power (ATP and NADPH) via a series of electron transporters. Photosynthetic electron transport is comprised of noncyclic electron transport from water to NADP+, cyclic electron transport from reduced Fd or NADPH recycling to plastoquinone (PQ) or the cytochrome b6f complex, and a number of O2-consuming alternative pathways. The function of noncyclic electron transport has been well studied, but the physiological function of PSI-cyclic electron transport has only recently been clarified, although Arnon et al. (1954) first reported cyclic photophosphorylation 50 years ago. Bendall and Manasse (1995) summarized the multiple pathways of cyclic electron flow. In higher plants, cyclic electron transport is mediated by the chloroplast NAD(P)H dehydrogenase (NDH) complex, a homolog of mitochondrial complex I (Mi et al., 1995; Burrows et al., 1998; Kofer et al., 1998; Shikanai et al., 1998), and by PGR5, a proton gradient regulation protein (Munekage et al., 2002). In Arabidopsis (Arabidopsis thaliana), research using mutants in which cyclic flow pathways were impaired proved that cyclic electron transport is essential for efficient photosynthesis (Munekage et al., 2004).
It is generally accepted that the PSI-cyclic electron transport mediated by NDH functions in photoprotection. Barley (Hordeum vulgare) leaves incubated under photooxidative conditions showed a large increase in NdhA, indicating that NDH may be involved in the protection of chloroplasts against photooxidative stress (Martín et al., 1996). Endo et al. (1999) reported that the repeated application of suprasaturating light eventually resulted in more severe photoinhibition and even chlorosis in the NDH-defective mutant, while the wild type sustained less photodamage and was able to recover from it. These results suggest that NDH compensates the stromal overreduction that induces formation of reactive oxygen species (ROS) through mediation of cyclic electron transfer. A detergent-containing system able to oxidize NADH with hydrogen peroxide (H2O2) in a PQ-dependent process was constructed using purified NDH and peroxidase (Casano et al., 2000). It has been thought that NDH and PQ are involved in the chlororespiratory process that consumes ROS and might poise the reduced and oxidized forms of the intermediates of cyclic electron transport. Further work showed the possible involvement of intrachloroplastic H2O2-mediated signaling in the photooxidative induction of increased NADH dehydrogenase activity and ndhB/F transcripts (Casano et al., 2000, 2001).
Inhibition of CO2 assimilation induced by heat, chilling, or water stress could also lead to overreduction of electron transport chain. We observed increased NDH activity and NdhK in chloroplasts after intact tobacco (Nicotiana tabacum) plants were heat stressed at 50°C in the light (Yao et al., 2001). Li et al. (2004) suggested that NDH functions in providing extra protons, thereby promoting the xanthophylls cycle and mitigating overreduction of the stroma. Horváth et al. (2000) found that ndhB-deficient tobacco mutants were sensitive to humidity stress and proposed that NDH may retard the inhibition of photosynthesis by providing extra proton gradient (ΔpH).
Thus, many works have shown the increased expression and activity of NDH under stressful conditions and postulated that NDH-mediated cyclic electron transport may function in alleviating the stressors. However, no direct experimental evidence has demonstrated the mechanism involved in the mitigation of oxidative damage by NDH-dependent pathways.
In this work, we demonstrate a remarkable increase in the amount of H2O2 in leaves of tobacco ndhC-ndhK-ndhJ defective mutant (ΔndhCKJ) either at 42°C or 4°C. We compared the photosynthetic electron transport activities, CO2 assimilation, and photophosphorylation between wild type and the mutant under unstressed and stressed conditions. Based on the results, a possible mechanism leading to the increased production of H2O2 in ΔndhCKJ at 42°C or 4°C, and the role of NDH in alleviating the stress will be discussed.
RESULTS
Accumulation of H2O2 in Leaves under Temperature-Stressed Conditions
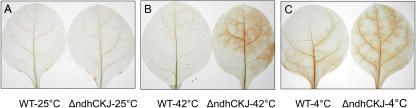
3,3-Diaminobenzidine (DAB) reacts with H2O2 in the presence of peroxidase and immediately generates brown polymers that are stable in most solutions. With DAB uptake by leaves, H2O2 can be localized in vivo and in situ or on a subcellular level (Thordal-Christensen et al., 1997). Brown marks were not detectable in either ΔndhCKJ or wild-type leaves after treatment at 25°C for 3 h (Fig. 1). At 42°C, brown traces were usually seen in ΔndhCKJ leaves within 3 h, and a larger brown area appeared the longer the leaves were exposed to high-temperature stress. Very few brown marks emerged within the wild-type leaves. At 4°C, brown traces usually began to emerge in both wild type and ΔndhCKJ after 1 h of treatment, with a more prominent appearance in the ΔndhCKJ leaves. The results indicated that temperature stress causes an accumulation of H2O2 in tobacco leaves with defective ndh genes.
Figure 1.
Effects of heat (42°C) or chilling (4°C) temperature on the accumulation of H2O2 in the leaves of wild-type (WT) and ΔndhCKJ tobacco plants. Petioles were steeped in solutions containing 1 mg mL−1 DAB (pH 3.8) at 25°C in the dark for 1 h to take up the stain. Samples were then incubated at 25°C, 42°C, or 4°C, under illumination at 100 μmol photons m−2 s−1. H2O2 accumulation was detected as brown areas after 3 h (A and B) and after 1 h (C) of treatment.
Changes in PSII Photochemical Activity and CO2 Assimilation in Response to Temperature Stress
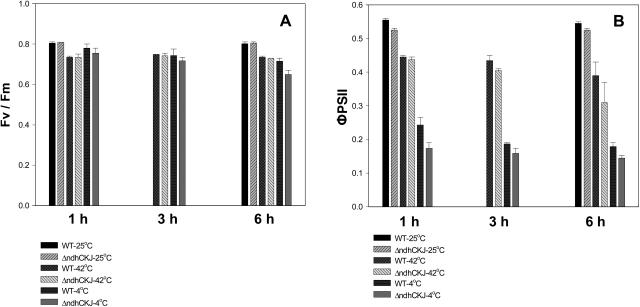
Fv/Fm is a chlorophyll (Chl) fluorescence parameter used to evaluate the maximum or potential quantum efficiency of PSII (Genty et al., 1989; Maxwell and Johnson, 2000). Fv/Fm of leaf discs did not significantly decline during incubation at 25°C for 1 or 6 h, with no obvious differences between wild type and ΔndhCKJ (Fig. 2A). During treatment at 42°C, Fv/Fm decreased to around 0.74, but there was still no noticeable difference between wild type and ΔndhCKJ. At 4°C, Fv/Fm gradually declined in both wild-type and ΔndhCKJ leaf discs, but the decline in ΔndhCKJ was more remarkable (Fig. 2A). The results indicated that there was no difference between the wild type and mutant in their light-harvesting ability during heat treatment, but that of ΔndhCKJ was more sensitive under chilling treatment.
Figure 2.
Changes of Chl fluorescence parameters during temperature treatment. Leaf discs were floated on the surface of temperature-controlled cyclic water bath with the epidermal side upward, and treated at indicated temperatures (25°C, 42°C, or 4°C) for the indicated time (1 h, 3 h, or 6 h) under illumination of about 100 μmol photons m−2 s−1. They were dark adapted at the corresponding temperatures for 10 min. Chl fluorescence was then measured at the same temperature using a PAM emitter-detector unit 101 ED as described in “Materials and Methods.” Values for Fv/Fm = (Fm − F0)/Fm (A) and ΦPSII = (Fm′ − F)/Fm′ (B) are the averages of four independent measurements. Standard errors are indicated by the vertical bars.
ΦPSII represents the effective photochemical efficiency of PSII, which can indirectly reflect linear electron transport (Genty et al., 1989; Maxwell and Johnson, 2000). Figure 2B shows that ΦPSII of ΔndhCKJ was slightly lower than that of wild type even at 25°C. During incubation at 42°C, ΦPSII continuously decreased, and the value declined even faster in ΔndhCKJ. After incubation at 42°C for 6 h, ΦPSII declined by 28.4% in wild type and by 41.0% in ΔndhCKJ. After treatment at 4°C for 1 h, ΦPSII in wild type had declined by 56.2%, and the decline was more notable in ΔndhCKJ (by 68.2%). Nevertheless, the decline in ΦPSII slackened during subsequent treatment, and the difference between wild type and ΔndhCKJ became less evident. The results indicated that the linear electron transport rate gradually slowed during 42°C stress, and the difference between wild type and ΔndhCKJ also progressively increased up to 6 h after treatment. While ΦPSII dropped rapidly at 4°C, the drop was slower in wild type than in ΔndhCKJ within 1 h of chilling stress.
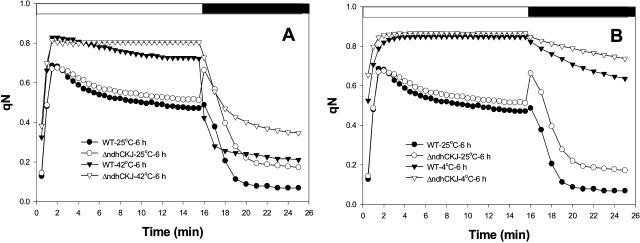
To obtain further confirmation of the differences in linear electron transport linked to CO2 assimilation, the dynamic changes in nonphotochemical quenching (qN) were compared between wild type and ΔndhCKJ. The decreasing phases of qN during illumination (light recovery) and after illumination (dark relaxation) reflect the activities of CO2 assimilation and of energy-dependent heat dissipation, respectively (Horton et al., 1994, 1996; Jones et al., 1998; Ivanov and Edwards, 2000; Maxwell and Johnson, 2000; Müller et al., 2001). Figure 3A shows that the light recovery ability of qN was similar in wild type and ΔndhCKJ at 25°C but was higher in wild type at 42°C. After treatment at 42°C for 6 h, qN stayed at an abnormally high level in ΔndhCKJ, but still showed light recovery ability in wild type, indicating that CO2 assimilation was much less inhibited in wild type than in ΔndhCKJ when suffering heat stress. On the other hand, after treatment at 4°C for 6 h, there was a great increase in qN, and light recovery was seriously inhibited in both wild type and ΔndhCKJ. The dark relaxation of qN was notably slower in ΔndhCKJ than in wild type but was significantly retarded in both strains (Fig. 3B). These results suggest a regulative role of NDH in alleviating the inhibition of photosynthetic electron transport and in the dissipation of the transthylakoid energy gradient.
Figure 3.
Effects of heat (42°C) and chilling (4°C) treatments on the kinetics of qN. Leaf discs were treated for 6 h and dark adapted as in Figure 2. qN was measured as described in “Materials and Methods.” The AL was turned on at 0 min and off at 15 min, but saturating pulses lasted for another 10 min. qN was calculated as 1 − (Fm′−F0′)/(Fm−F0).
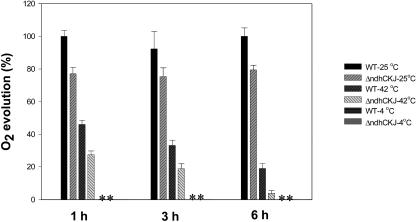
The measurement of O2 evolution using leaf fragments suspended in a solution of bicarbonate more directly reflects the activity of CO2 assimilation. Figure 4 shows that O2 evolution was inhibited in both wild type and ΔndhCKJ at 42°C, and that the inhibition was much more significant in ΔndhCKJ than in wild-type fragments after 6 h of treatment. No O2 evolution was detected at 4°C. These results confirmed that CO2 assimilation was more strongly inhibited in the NDH-defective mutant at the high temperature and was almost totally inhibited at the low temperature in both strains.
Figure 4.
Effects of heat (42°C) and chilling (4°C) treatments on photosynthetic oxygen evolution. Leaf discs were treated as in Figure 2, cut into fragments of 1 mm2, and stirred in a 1.8-mL suspension (0.11 mg Chl mL−1) containing 0.1 m NaHCO3 and 0.05 m Tris (pH 7.5) in the thermostated glass vessel of a Clark-type oxygen electrode. O2 evolution was normally detected several minutes after the start of illumination (800 μmol photons m−2 s−1) at 25°C or 42°C, but was not detectable at 4°C (indicated with asterisks [*]). Values are the averages of four independent measurements. Standard errors are indicated by the vertical bars. The control rate of O2 evolution (wild type, 25°C, 1 h) was 67.2 μmol O2 mg Chl−1 h−1.
Effects of Temperature Stress on PSI-Cyclic Electron Transport Mediated by NDH
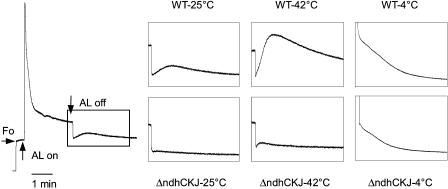
A transient postillumination increase in Chl fluorescence is considered to arise from the reduction of PQ by NAD(P)H or other reducing substances that accumulated in the light. This reaction mainly involves PSI-cyclic electron transport mediated by NDH in cyanobacteria (Mi et al., 1995) and in higher plants (Burrows et al., 1998; Kofer et al., 1998; Shikanai et al., 1998). Figure 5 shows that there is a visible transient postillumination increase in Chl fluorescence in wild-type leaves at 25°C, while it was severely impaired in ΔndhCKJ. When the leaves were treated at 42°C, either the initial rate of the increasing phase or the amplitude of the postillumination increase in Chl fluorescence was enhanced in wild type, with only a trace increase in ΔndhCKJ. In contrast, when treated at 4°C, both wild type and ΔndhCKJ showed a decreased level of fluorescence with slower kinetics and no transient peaks after removal of the white actinic light (AL).
Figure 5.
Effects of heat (42°C) and chilling (4°C) on postillumination increase of Chl fluorescence in the leaves of wild type and ΔndhCKJ. F0, Dark fluorescence level; and AL, white actinic light (200 μmol photons m−2 s−1, lasted for 2 min). Insets show transient increase in Chl fluorescence following light-to-dark transition. Leaf discs were dark adapted on a temperature-controlled plate at indicated temperatures for 10 min and then the fluorescence was measured at the same temperature.
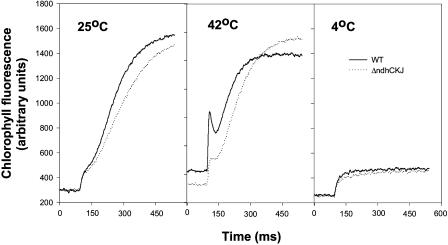
The fast kinetics of Chl fluorescence following a pulse light reflects the reduction of PQ (Joët et al., 2002b; Joliot and Joliot, 2002). The rise in fluorescence observed in the first 200-millisecond (ms) time range showed a prominent peak in wild type after treatment at 42°C but only slightly increased in ΔndhCKJ leaves (Fig. 6), indicating that PQ could be rapidly reduced and reoxidized in wild-type leaves. However, neither the wild type nor the mutant exhibited reduction of PQ at 4°C. These data further confirm that the reduction of PQ by NDH-dependent cyclic electron flow is augmented by heat but not chilling exposure.
Figure 6.
Effects of heat (42°C) and chilling (4°C) treatments on the kinetics of fluorescence induction curve. Leaf discs were treated for 6 h and dark adapted as in Figure 2. Chl fluorescence was measured and recorded as described in “Materials and Methods.”
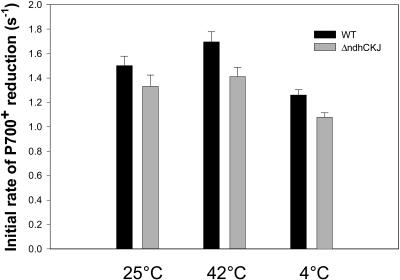
The dark rereduction of P700+ is a more direct reflection of the rate of cyclic electron transport around PSI and of the electron donation to the intersystem electron transport chain by stromal reductants (Maxwell and Biggins, 1976; Mi et al., 1992a, 1992b; Havaux, 1996). At 25°C, the initial rate of P700+ rereduction after turning off of the far-red light was slower in ΔndhCKJ than in wild type by 14.2% (Fig. 7). After 42°C stress, the initial rate was accelerated in both and more notably accelerated in wild type. It was slower in ΔndhCKJ than in wild type by 16.9%. After 4°C treatment, the initial rate measured under this temperature was slowed and was slower in ΔndhCKJ than in wild type by 14.6%. Together, these results reveal that the cyclic electron transport mediated by NDH is enhanced at the high temperature and suppressed at the low temperature.
Figure 7.
Effects of heat (42°C) and chilling (4°C) on the initial rates (0–1 s) of P700+ rereduction following far-red light in wild type and ΔndhCKJ. Leaf discs were treated as in Figure 5. Dark reduction of P700+ was measured by turning off of the far-red light (>705 nm, 5.2 μmol photons m−2 s−1) after a 30-s illumination that allowed the oxidation of P700 to a steady state. Each experiment was repeated four times. Standard errors are indicated by the vertical bars.
The Transthylakoid Proton Gradient and Photophosphorylation
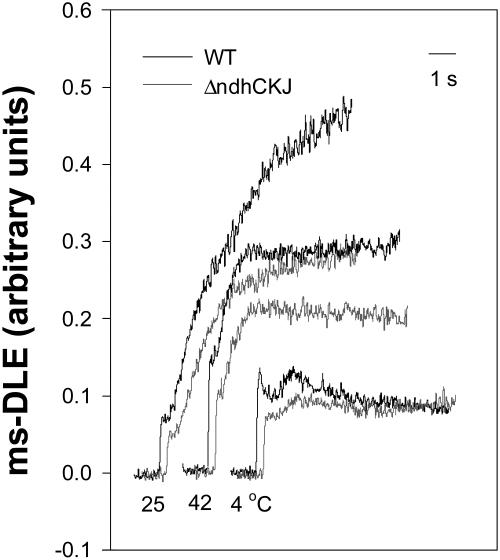
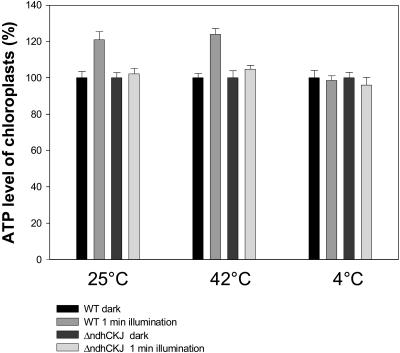
The ms-delayed light emission (ms-DLE) of Chl fluorescence was used to monitor the transthylakoid proton gradient (ΔpH; Wraight and Crofts, 1971; Li and Shen, 1994). The ms-DLE from wild-type leaf discs was higher than that from ΔndhCKJ both before and after temperature treatment (Fig. 8), suggesting the involvement of the NDH pathway in the formation of ΔpH. To investigate whether the NDH-dependent transthylakoidal ΔpH contributes to ATP synthesis, light-induced ATP synthesis in intact chloroplasts was analyzed (Fig. 9). After illumination of the dark-adapted chloroplasts for 1 min at 25°C, there was ATP synthesis in wild-type chloroplasts but very little in those from the ΔndhCKJ mutant. This finding is indicative of the operation of photophosphorylation through rapidly activated cyclic electron transport mediated by NDH under CO2 assimilation retarded conditions. ATP synthesis was also observed in both wild type and ΔndhCKJ at 42°C (much more in wild type), while no photophosphorylation occurred at 4°C. The results indicate that cyclic photophosphorylation via NDH functions when CO2 assimilation was inhibited under heat-stressed conditions.
Figure 8.
Effects of heat (42°C) and chilling (4°C) treatments on ms-DLE. Leaf discs were treated for 6 h and dark adapted as in Figure 2. The ms-DLE was measured as described in “Materials and Methods.”
Figure 9.
Light-induced ATP synthesis of chloroplasts. The 1-mL reaction mixture contained 0.4 m Suc, 50 mm Tris-HCl (pH 7.6), 10 mm NaCl, 5 mm MgCl2, 2 mm ADP, 10 mm Na2HPO4, and intact chloroplasts with 30 μg of Chl. After illumination (800 μmol photons m−2 s−1) for 1 min at 25°C, 42°C, or 4°C, ATP contents of the illuminated and dark-controlled sample were analyzed using the Luciferin-luciferase method. Values are the averages of six independent measurements. Standard errors are indicated by the vertical bars. The control value of ATP contents (100%) was about 31.4 nmol ATP mg Chl−1.
DISCUSSION
Temperature Stress Induced H2O2 Production in ΔndhCKJ Strain
ROS such as superoxide anion radical and H2O2 are inevitably photoproduced in chloroplasts (Asada and Kiso, 1973; Asada et al., 1974) and are rapidly scavenged through water-water cycle (Asada, 1999). However, under heat- or chilling-stressed conditions, Calvin cycle enzymes (especially Rubisco activase) are depressed, leading to suppression of CO2 assimilation (Weis, 1981; Kingston-Smith et al., 1997; Allen and Ort, 2001; Salvucci and Crafts-Brandner, 2004a, 2004b) and overreduction of stromal components. Inhibition of CO2 assimilation enhances the electron flow to O2, generating more·superoxide anion radical (Asada, 1992). Compared with the accumulation of H2O2 (Fig. 1), decrease in CO2 assimilation (Fig. 4) occurred much earlier during temperature stress. Thus, accumulation of H2O2 in the leaves of ΔndhCKJ (under either heat or chilling stress) and wild-type (under chilling stress) leaves (Fig. 1) should be the result of the inhibition of linear electron transport (Fig. 2B). It has been suggested that NDH and PQ are involved in the chlororespiratory process that consumes ROS, and that they might poise the reduced and oxidized forms of the intermediates of cyclic electron transport with a constructed in vitro system (Casano et al., 2000). However, there was no direct evidence for involvement of the NDH pathway in ROS scavenging. This work provides direct evidence for the involvement of the NDH-dependent pathway in the suppression of ROS generation in chloroplasts.
CO2 Assimilation Was Greatly Inhibited in the ΔndhCKJ Mutant
At the high temperature, the light energy harvested and transferred by PSII was not obviously reduced, and it maintained a similar level between wild type and ΔndhCKJ (Fig. 2A). Nevertheless, linear electron transport (Fig. 2B), as well as CO2 assimilation (Fig. 4), was increasingly limited. The ΔndhCKJ mutant exhibited a more severe inhibition, especially after 6 h of stress treatment (Figs. 2B, 3, and 4). These results implied that the NDH pathway was involved in regulation of CO2 assimilation, thus reducing the generation of ROS under heat stress. Differently, chilling stress induced a continuous decrease of Fv/Fm and a large drop in ΦPSII (Fig. 2), indicating that the light energy harvested and transferred by PSII was reduced, leading to a slowdown of apparent electron transport. As a result, the protective role of the NDH pathway became less important (Fig. 1).
A typical qN induction curve measured under optimal physiological conditions is composed of an initial rise to a high level, which reflects the rapid buildup of ΔpH, and the gradual decrease to a steady-state level in the course of activation of the Calvin cycle, consuming ATP and thus relaxing ΔpH (Jones et al., 1998). After 6 h of heat stress, the qN induction curve in the ΔndhCKJ mutant lost the decreasing phase and stayed at a high level, while wild type retained the typical induction curve due to less inhibition of CO2 assimilation (Fig. 3A). Under chilling stress, qN induction curves achieved even higher levels in both strains, while the dark relaxation of qN was more retarded in ΔndhCKJ than in wild type (Fig. 3B). The results further supported the regulative role of NDH in alleviating the inhibition of CO2 assimilation and also its possible involvement in the transthylakoid energy gradient. The much more significant inhibition of photosynthetic O2 evolution in ΔndhCKJ than in wild type after 6-h heat treatment (Fig. 4) again strengthened our suggestions.
PSI-Cyclic Electron Transport Mediated by NDH Functions in Acclimating to Temperature-Stressed Conditions
Previous work has shown that wild-type, but not Ndh-deficient tobaccos, exhibited a postillumination increase in fluorescence after photooxidative treatment that paralleled with higher Ndh complex level, activity, and an increase in thylakoid peroxidase (Martín et al., 2004). Consistent with this result, enhancement of the postillumination increase in Chl fluorescence and the more notable acceleration of P700+ rereduction in wild type under heat stress (Figs. 5 and 7) indicated that the NDH-dependent cyclic pathway was stimulated under oxidative conditions caused by heat stress. Similarly, the fast kinetics of Chl fluorescence indicated that PQ could be rapidly reduced and reoxidized in wild type under heat treatment (Fig. 6), further demonstrating the effective activation of the NDH-related pathway in response to heat stress.
To prevent overreduction of stromal components and formation of ROS, excess electrons must be efficiently consumed, either by the Calvin cycle or by other electron valves. When CO2 assimilation was inhibited under heat stress (Fig. 4), alternative electron valves such as Mehler reaction and photorespiration as well as cyclic electron flow (Figs. 5 and 7), might become evident. Since plastid terminal oxidase (PTOX) is able to transfer electrons from PQ to oxygen without generating ROS (Cournac et al., 2000; Josse et al., 2003), NDH and PTOX involved in chlororespiration were suggested to function to provide and remove electrons, respectively, thus to balance the redox state of transporters (Niyogi, 2000; Martín et al., 2004; Streb et al., 2005). Changes in the redox state of intersystem electron carriers by chlororespiration have been indicated to tightly control the rate of PSI-driven cyclic electron flow in vivo (Joët et al., 2002a). Since the potential electron consumption by chlororespiration is currently thought to be very low (Ort and Baker, 2002) and the content of PTOX is low in many plant species (Streb et al., 2005), its function remains to be clarified under the stressed conditions.
Coincident with a previous report (Savitch et al., 2001), in contrast to heat, chilling stress inhibited the cyclic electron flow (Figs. 5, 6, and 7). This inhibition might be the result of the slowing down of the apparent electron transport (Fig. 2) and inactivation of enzymes involved in the operation of photosynthesis, including NDH. Nevertheless, some studies have shown that PSI-cyclic electron transport is accelerated by chilling stress (Kim et al., 2001; Barth and Krause, 2002; Bukhov et al., 2004). The discrepancy may be attributed to different measurement conditions. The former were carried out at chilling temperature while the latter were at room temperature, which might reflect the recovery process. Actually, we found that the complete inhibition of photosynthetic O2 evolution could be completely recovered once shifted to 25°C (data not shown). The accumulation of H2O2 in wild-type leaves at the low temperature (Fig. 1) might be attributed to the suppression of NDH-dependent cyclic electron flow. Thus, the role of NDH in photoprotection at low temperature is less important compared with that at high temperature.
The NDH Pathway Probably Provides Extra ATP for Regulation of CO2 Assimilation
It has been proposed that NDH-dependent cyclic electron transport plays a role in supplying extra ATP for optimal photosynthesis, particularly under conditions when CO2 is limiting (Peltier and Cournac, 2002). However, there was no direct proof of supplementation of extra ATP by NDH-mediated cyclic electron transport. On the basis of the slower rising phase of the nonphotochemical qN induction curve in the NDH-defective mutant under water-stressed conditions, it has been suggested that PSI-cyclic electron transport mediated by NDH was responsible for enhanced proton pumping and was involved in energy dissipation when CO2 availability reduced (Burrows et al., 1998). In this work, the higher ms-DLE in wild type than in ΔndhCKJ (Fig. 8) denoted the formation of ΔpH through NDH-mediated cyclic electron transport under either stressed or nonstressed conditions. A 9-aminoacridine fluorescence quenching analysis showed a similar trend (data not shown). Moreover, when CO2 assimilation did not operate in the intact chloroplasts in the absence of acceptor at either 25°C or 42°C, light-dependent ATP synthesis via NDH pathway occurred (Fig. 9).
One of the roles of transthylakoid ΔpH is to function in photoprotection, necessary for the thermal dissipation of excess absorbed light energy (Niyogi, 1999). Li et al. (2004) suggested that NDH might function by providing extra protons, thus to promote the xanthophylls cycle and mitigate the stromal overreduction. In addition to the xanthophylls cycle (Demming-Adams, 1990), Mehler reaction (Schreiber and Neubauer, 1990; Osmond and Grace, 1995) was suggested to function in radiationless energy dissipation, and water-water cycle would be enhanced by limitation of photosynthesis (Miyake and Yokota, 2000). However, Mehler reaction alone is not sufficient for effective energy dissipation (Clarke and Johnson, 2001). There are reports that strong light also promotes radiationless energy dissipation in npq1-2 mutant, which cannot synthesize zeaxanthin (Niyogi et al., 1998), as in zeaxanthin-containing leaves (Bukhov et al., 2001). Based on this evidence, Heber et al. (2001) suggested that the combination of zeaxanthin and a low intrathylakoid pH were sufficient in some hydrated mosses and green lichens, but not in higher plants, to dissipate energy even when PSII reaction centers are open. Therefore, cyclic electron transport might be responsible for producing the extra thylakoid acidification that leads to the effective dissipation of excess excitant energy as heat. The observed involvement of NDH in protection of the tobacco plant from photooxidative damage (Fig. 1) under temperature-stressed conditions also supports this view.
On the other hand, CO2 assimilation is usually limited by its key enzyme, Rubisco, which is activated by its molecular chaperone, Rubisco activase, through an ATP-dependent reaction. A series of studies have indicated that the operation of Rubisco activase is sensitive to high and low temperature (Kingston-Smith et al., 1997; Salvucci and Crafts-Brandner, 2004a, 2004b). It is plausible that the suppression of linear electron transport (Fig. 2B) and photosynthetic O2 evolution (Fig. 4) under heat-stressed conditions might be the result of inhibition of Rubisco activation. The lower level of suppression in wild type might be attributed to the function of NDH-dependent PSI-cyclic electron transport (Figs. 5, 6, and 7). Cyclic photophosphorylation via the NDH pathway (Figs. 8 and 9) might provide extra ATP for activation and stabilization of Rubisco activase under heat stress. It has been suggested that Rubisco activase was activated only by electron transport through PSI, and not by that from PSII (Campbell and Ogren, 1990). Whether the cyclic electron transport mediated by NDH can activate or stabilize Rubisco activase is worth further study. These results suggest that photophosphorylation via NDH pathway might optimize CO2 assimilation under heat stress, leading to a reduction in the generation of ROS, which would inactivate CO2 assimilation enzymes (Ishida et al., 1998, 1999) and scavenging enzymes such as ascorbate peroxidases (Mano et al., 2001), thus cause further oxidative damage in leaves.
In conclusion, this work indicates that when the Calvin cycle is inhibited under temperature-stressed conditions, especially under heat stress, PSI-cyclic electron transport mediated by NDH might play an important role in optimization of the photosynthetic apparatus. This function is probably carried out by providing extra ΔpH and ATP to regulate CO2 assimilation, and poising the redox level of electron transporters through chlororespiration, thus reducing the generation of ROS.
MATERIALS AND METHODS
Tobacco Strains, Growth Conditions, and Treatment
Homoplasmic ΔndhCKJ tobacco (Nicotiana tabacum cv Xanthi) mutants in which the chloroplastic ndhC, ndhK, and ndhJ genes were inactivated (Takabayashi et al., 2002) were cultivated along with wild-type plants in a phytotron (about 200–300 μmol photons m−2 s−1, 12-h light at 25°C and 12-h dark at 20°C). Four- to 6-week-old plants were used for experiments. To perform heat and chilling treatments, leaf discs were floated on the surface of a temperature-controlled cyclic water bath of 25°C, 42°C, or 4°C, with the epidermal side turned up and an illumination of about 100 μmol photons m−2 s−1.
Detection of H2O2 in the Leaves
Young leaves of similar size were cut from 4-week-old plants. The leafstalks were immediately dipped into water containing 1 mg mL−1 DAB (pH = 3.8; Thordal-Christensen et al., 1997) and kept at 25°C in the dark for 1 h to take up the stain. The samples were then placed in illuminating incubators at 25°C, 42°C, or 4°C (about 100 μmol photons m−2 s−1) for the indicated time, keeping the petioles immersed in the DAB solutions. The Chl was bleached by boiling the leaves in 95% ethanol before taking pictures.
Chloroplast Preparation
Intact chloroplasts were isolated at 4°C according to a modification of the method described by Mills and Joy (1980). Dark-adapted leaves were homogenized in cold medium containing 0.4 m Suc, 50 mm Tris-HCl (pH 7.6), 10 mm NaCl, 2 mm MgCl2, and 2 mm EDTA. After filtration through two layers of nylon cloth and centrifugation (1,000g for 3 min at 4°C), the pellet was resuspended in the medium and layered onto a 40% and 60% Percoll step gradient. Intact chloroplasts were recovered from the 40%/60% Percoll interface after centrifugation at 3,500g for 10 min at 4°C. After washing, the chloroplasts were pelleted at 2,000g for 3 min at 4°C, suspended in the homogenization medium, and stored on ice in the dark. The intactness of the Percoll-purified chloroplasts from both wild type and ΔndhCKJ was above 90%, which was estimated by ferricyanide-dependent O2 evolution before and after osmotic shock (Heber and Santarius, 1970).
ATP Synthesis Measurements
Light-induced ATP synthesis of chloroplasts was measured by comparing the ATP level in the dark and 1 min after illumination. One-milliliter reaction mixture contained 0.4 m Suc, 50 mm Tris-HCl (pH 7.6), 10 mm NaCl, 5 mm MgCl2, 2 mm ADP, 10 mm Na2HPO4, and intact chloroplasts with 30 μg of Chl. After illumination (800 μmol photons m−2 s−1) for 1 min at 25°C, 42°C, or 4°C, 10% TCA was immediately added to the illuminated and dark-controlled samples and neutralized with 3 m Na2CO3. ATP content was then analyzed by the Luciferin-luciferase method using a luminometer (RS 9901 luminometer) and ATP bioluminescence assay kit (Shanghai Institute of Plant Physiology, Chinese Academy of Sciences).
Measurements and Analysis of Chl Fluorescence Parameters and the Redox Change of P700
Chl fluorescence was measured according to Schreiber et al. (1986, 1988) using a pulse-amplitude modulated fluorimeter (PAM 101, Walz). After treatment, the leaf discs were dark adapted at the indicated temperatures and the measurement was carried out at the same temperatures. The modulated nonactinic measuring beam (1.6 kHz) was switched on to obtain the initial fluorescence (F0). Maximal fluorescence (Fm) was measured by illumination with a 0.8-s pulse of white saturating light. Maximum quantum efficiency of PSII was determined by Fv/Fm. The kinetics of the fluorescence induction curve was recorded with a PDA-100 data acquisition system. Using a previously described method with slight modifications (Shikanai et al., 1998), a transient postillumination increase in Chl fluorescence was recorded after termination of the 2-min illumination by AL (200 μmol photons m−2 s−1). qN was calculated as 1 − (Fm′−F0′)/(Fm−F0) based on the Fm′ measured every 30 s with saturating pulses, from turning on the AL until steady-state photosynthesis was reached (15 min of induction). ΦPSII, the photochemical efficiency of PSII, was also calculated at steady state as (Fm′ − F)/Fm′. The redox change of P700 was monitored by absorbance at 810 − 830 nm, using an ED-P700DW-E unit of the PAM fluorometer, and the initial rate of P700+ rereduction following far-red light (>705 nm, 5.2 μmol m−2 s−1) was calculated (Klughammer and Schreiber, 1998).
Photosynthetic Oxygen Evolution Measurements on Leaf Fragments
After the leaf discs were treated at the indicated temperatures for the indicated time, they were cut into fragments of 1 mm2 and stirred into a 1.8-mL suspension (0.11 mg Chl mL−1) containing 0.1 m NaHCO3 and 0.05 m Tris (pH 7.5), in the thermostated glass vessel of a Clark-type oxygen electrode. The leaf discs were vacuum infiltrated with 0.1 m NaHCO3 after temperature treatment, and the suspension medium was stabilized to the corresponding temperatures for the measurement. O2 evolution was normally detected several minutes after the beginning of the illumination (800 μmol photons m−2 s−1).
Measurements of ms-DLE
Measurements of ms-DLE were carried out using a phosphoroscope according to Wang et al. (2003) with modifications. The sample was irradiated with light passing through holes arranged on two rotating wheels, so that the measuring process might be divided into consecutive cycles of 1-ms excitation by light followed by 4.6 ms darkness. The DLE between 2.8 and 3.8 ms after every flash was measured with a photomultiplier, and the signal was recorded continuously by a computer through an analog-digital converter. The leaf discs were treated and dark adapted at the same temperature and were immediately inserted into the sample cell to measure ms-DLE.
Acknowledgments
We appreciate Dr. D.S. Bendall, Department of Biochemistry, University of Cambridge, and Professor T. Ogawa, a visiting professor of this institute; Professor J.M. Wei, Y.K. Shen, Professor Z.H. He, Dr. M.X. Jin, and Dr. G.Y. Chen in the institute for reviewing the manuscript and for fruitful discussion.
This work was supported by the National Natural Science Foundation of China (grant nos. 30270123 and 2003CCA01100).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Hualing Mi (mihl@iris.sipp.ac.cn).
Open Access articles can be viewed online without a subscription.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.070490.
References
- Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 6: 36–42 [DOI] [PubMed] [Google Scholar]
- Arnon DI, Allen MB, Whatley FR (1954) Photosynthesis by isolated chloroplasts. Nature 174: 394–396 [DOI] [PubMed] [Google Scholar]
- Asada K (1992) Ascorbate peroxidase: a hydrogen peroxide scavenging enzyme in plants. Physiol Plant 85: 235–241 [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Asada K, Kiso K (1973) Initiation of aerobic oxidation of sulfite by illuminated spinach chloroplasts. Eur J Biochem 33: 253–257 [DOI] [PubMed] [Google Scholar]
- Asada K, Kiso K, Yoshikawa K (1974) Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J Biol Chem 249: 2175–2181 [PubMed] [Google Scholar]
- Barth C, Krause GH (2002) Study of tobacco transformants to assess the role of chloroplastic NAD(P)H dehydrogenase in photoprotection of photosystems I and II. Planta 216: 273–279 [DOI] [PubMed] [Google Scholar]
- Bendall DS, Manasse RS (1995) Cyclic phosphorylation and electron transport. Biochim Biophys Acta 1229: 23–38 [Google Scholar]
- Bukhov NG, Govindachary S, Rajagopal S, Joly D, Carpentier R (2004) Enhanced rates of P700+ dark-reduction in leaves of Cucumis sativus L. photoinhibited at chilling temperature. Planta 218: 852–861 [DOI] [PubMed] [Google Scholar]
- Bukhov NG, Heber U, Wiese C, Shuvalov VA (2001) Energy dissipation in photosynthesis: Does the quenching of chlorophyll fluorescence originate from the antenna complexes of photosystem II or from the reaction center? Planta 212: 749–758 [DOI] [PubMed] [Google Scholar]
- Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ (1998) Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J 17: 868–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WJ, Ogren WL (1990) Electron transport through photosystem I stimulates light activation of ribulose bisphosphate carboxylase/oxygenase (Rubisco) by Rubisco activase. Plant Physiol 94: 479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casano LM, Martín M, Sabater B (2001) Hydrogen peroxide mediates the induction of chloroplastic Ndh complex under photooxidative stress in barley. Plant Physiol 25: 1450–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casano LM, Zapata JM, Martín M, Sabater B (2000) Chlororespiration and poising of cyclic electron transport. J Biol Chem 275: 942–948 [DOI] [PubMed] [Google Scholar]
- Clarke JE, Johnson GN (2001) In vivo temperature dependence of cyclic and pseudocyclic electron transport in barley. Planta 212: 808–816 [DOI] [PubMed] [Google Scholar]
- Cournac L, Redding K, Ravenel J, Rumeau D, Josse EM, Kuntz M, Peltier G (2000) Electron flow between photosystem II and oxygen in chloroplasts of photosystem I-deficient algae is mediated by a quinol oxidase involved in chlororespiration. J Biol Chem 275: 17256–17262 [DOI] [PubMed] [Google Scholar]
- Demming-Adams B (1990) Carotenoids and photoprotection of plants: a role for the xanthophylls zeaxathin. Biochim Biophys Acta 1020: 1–24 [Google Scholar]
- Endo T, Schikanai T, Takabayashi A, Asada K, Sato F (1999) The role of chloroplastic NAD(P)H dehydrogenase in photoprotection. FEBS Lett 457: 5–8 [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Havaux M (1996) Short-term responses of photosystem I to heat stress. Photosynth Res 47: 85–97 [DOI] [PubMed] [Google Scholar]
- Heber U, Bukhov NG, Shuvalov VA, Kobayashi Y, Lange OL (2001) Protection of the photosynthetic apparatus against damage by excessive illumination in homoiohydric leaves and poikilohydric mosses and lichens. J Exp Bot 52: 1999–2006 [DOI] [PubMed] [Google Scholar]
- Heber U, Santarius KA (1970) Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B 25: 718–728 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG (1994) Regulation of light harvesting in green plants (indication by nonphotochemical quenching of chlorophyll fluorescence). Plant Physiol 106: 415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47: 655–684 [DOI] [PubMed] [Google Scholar]
- Horváth EM, Peter SO, Joët T, Rumeau D, Cournac L, Horváth GV, Kavanagh TA, Schäfer C, Peltier G, Medgyesy P (2000) Targeted inactivation of the plastid ndhB gene in tobacco results in an enhanced sensitivity of photosynthesis to moderate stomatal closure. Plant Physiol 123: 1337–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H, Makino A, Mae T (1999) Fragmentation of the large subunit of ribulose-1,5-bisphosphate carboxylase by reactive oxygen species occurs near Gly-329. J Biol Chem 274: 5222–5226 [DOI] [PubMed] [Google Scholar]
- Ishida H, Shimizu S, Makino A, Mae T (1998) Light-dependent fragmentation of the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase in chloroplasts isolated from wheat leaves. Planta 204: 305–309 [DOI] [PubMed] [Google Scholar]
- Ivanov B, Edwards G (2000) Influence of ascorbate and the Mehler peroxidase reaction on non-photochemical quenching of chlorophyll fluorescence in maize mesophyll chloroplasts. Planta 210: 765–774 [DOI] [PubMed] [Google Scholar]
- Joët T, Cournac L, Peltier G, Havaux M (2002. a) Cyclic electron flow around photosystem I in C(3) plants: in vivo control by the redox state of chloroplasts and involvement of the NADH-dehydrogenase complex. Plant Physiol 128: 760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joët T, Genty B, Josse EM, Kuntz M, Cournac L, Peltier G (2002. b) Involvement of a plastid terminal oxidase in plastoquinone oxidation as evidenced by expression of the Arabidopsis thaliana enzyme in tobacco. J Biol Chem 277: 31623–31630 [DOI] [PubMed] [Google Scholar]
- Joliot P, Joliot A (2002) Cyclic electron transfer in plant leaf. Proc Natl Acad Sci USA 99: 10209–10214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U (1998) Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ 21: 1219–1230 [Google Scholar]
- Josse EM, Alcaraz JP, Labouré AM, Kuntz M (2003) In vitro characterization of a plastid terminal oxidase (PTOX). Eur J Biochem 270: 3787–3794 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee CH, Hope AB, Chow WS (2001) Inhibition of photosystems I and II and enhanced back flow of photosystem I electrons in cucumber leaf discs chilled in the light. Plant Cell Physiol 42: 842–848 [DOI] [PubMed] [Google Scholar]
- Kingston-Smith AH, Harbinson J, Williams J, Foyer CH (1997) Effect of chilling on carbon assimilation, enzyme activation, and photosynthetic electron transport in the absence of photoinhibition in maize leaves. Plant Physiol 114: 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U (1998) Measuring P700 absorbance changes in the near infrared spectral region with a dual wavelength pulse modulation system. In G Grab, ed, Photosynthesis: Mechanism and Effects, Vol 5. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 4357–4360
- Kofer W, Koop HU, Wanner G, Steinmuller K (1998) Mutagenesis of the genes encoding subunits A, C, H, I, J and K of the plastid NAD(P)H-plastoquinone-oxidoreductase in tobacco by polyethylene glycol-mediated plastome transformation. Mol Gen Genet 258: 166–173 [DOI] [PubMed] [Google Scholar]
- Li DY, Shen YK (1994) The relation between components of proton motive force and photosynthesis. Chin Sci Bull 39: 1712–1715 [Google Scholar]
- Li XG, Duan W, Meng QW, Zou Q, Zhao SJ (2004) The function of chloroplastic NAD(P)H dehydrogenase in tobacco during chilling stress under low irradiance. Plant Cell Physiol 45: 103–108 [DOI] [PubMed] [Google Scholar]
- Mano J, Ohno C, Domae Y, Asada K (2001) Chloroplastic ascorbate peroxidase is the primary target of methylviologen-induced photooxidative stress in spinach leaves: its relevance to monodehydroascorbate radical detected with in vivo ESR. Biochim Biophys Acta 1504: 275–287 [DOI] [PubMed] [Google Scholar]
- Martín M, Casano LM, Sabater B (1996) Identification of the product of ndhA gene as a thylakoid protein synthesized in response to photooxidative treatment. Plant Cell Physiol 37: 293–298 [DOI] [PubMed] [Google Scholar]
- Martín M, Casano LM, Zapata JM, Guéra A, del Campo EM, Schmitz-Linneweber C, Maier RM, Sabater B (2004) Role of thylakoid Ndh complex and peroxidase in the protection against photo-oxidative stress: fluorescence and enzyme activities in wild-type and ndhF-deficient tobacco. Physiol Plant 122: 1–10 [Google Scholar]
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence: a practical guide. J Exp Bot 51: 659–668 [DOI] [PubMed] [Google Scholar]
- Maxwell PC, Biggins J (1976) Role of cyclic electron transport in photosynthesis as measured by the photoinduced turnover of P700+ in vivo. Biochemistry 15: 3975–3981 [DOI] [PubMed] [Google Scholar]
- Mi H, Endo T, Asada K (1992. a) Donation of electrons to the intersystem chain in the cyanobacterium Synechococcus sp. PCC7002 as determined by the reduction of P700+. Plant Cell Physiol 33: 1099–1105 [Google Scholar]
- Mi H, Endo T, Ogawa T, Asada K (1995) Thylakoid membrane-bound pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacteria Synechocystis PCC6803. Plant Cell Physiol 36: 661–668 [Google Scholar]
- Mi H, Endo T, Schreiber U, Ogawa T, Asada K (1992. b) Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis PCC6803. Plant Cell Physiol 33: 1233–1237 [Google Scholar]
- Mills WR, Joy KW (1980) A rapid method for isolation of purified physiologically active chloroplasts, used to study the intracellular distribution of amino acids in pea leaves. Planta 148: 75–83 [DOI] [PubMed] [Google Scholar]
- Miyake C, Yokota A (2000) Determination of the rate of photoreduction of O2 in the water-water cycle in watermelon leaves and enhancement of the rate by limitation of photosynthesis. Plant Cell Physiol 41: 335–343 [DOI] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429: 579–582 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110: 361–371 [DOI] [PubMed] [Google Scholar]
- Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3: 455–460 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophylls cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Baker NR (2002) A photoprotective role of O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5: 193–198 [DOI] [PubMed] [Google Scholar]
- Osmond CB, Grace CE (1995) Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? J Exp Bot 46: 1351–1362 [Google Scholar]
- Peltier G, Cournac L (2002) Chlororespiration. Annu Rev Plant Biol 53: 523–550 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. a) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120: 179–186 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. b) Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol 134: 1460–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitch LV, Barker-Åstrom J, Ivanov AG, Hurry V, Öquist G, Huner NP, Gardeström P (2001) Cold acclimation of Arabidopsis thaliana results in incomplete recovery of photosynthetic capacity, associated with an increased reduction of the chloroplast stroma. Planta 214: 295–303 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Klughammer C, Neubauer C (1988) Measuring P700 absorbance changes around 830 nm with a new type of pulse modulation system. Z Naturforsch 43c: 686–698 [Google Scholar]
- Schreiber U, Neubauer C (1990) O2-dependent electron flow, membrane energization and the mechanisms of nonphotochemical quenching of chlorophyll fluorescence. Photosynth Res 25: 279–293 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10: 51–62 [DOI] [PubMed] [Google Scholar]
- Shikanai T, Endo T, Hashimoto T, Yamada Y, Asada K, Yokota A (1998) Directed disruption of the tobacco ndhB gene impaired cyclic electron flow around photosystem I. Proc Natl Acad Sci USA 95: 9705–9709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb P, Josse EM, Gallouët E, Baptist F, Kuntz M, Cornic G (2005) Evidence for alternative electron sinks to photosynthetic carbon assimilation in the high mountain plant species Ranunculus glacialis. Plant Cell Environ 28: 1123–1135 [Google Scholar]
- Takabayashi A, Endo T, Shikanai T, Sato F (2002) Post-illumination reduction of the plastoquinone pool in chloroplast transformants in which chloroplastic NAD(P)H dehydrogenase was inactivated. Biosci Biotechnol Biochem 66: 2107–2111 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Wang HW, Mi H, Ye JY, Deng Y, Shen YK (2003) Low concentrations of NaHSO3 increase cyclic photophosphorylation and photosynthesis in cyanobacterium Synechocystis PCC6803. Photosynth Res 75: 151–159 [DOI] [PubMed] [Google Scholar]
- Weis E (1981) Reversible heat-inactivation of the Calvin Cycle: a possible mechanism of the temperature regulation of photosynthesis. Planta 151: 33–39 [DOI] [PubMed] [Google Scholar]
- Wraight CA, Crofts AR (1971) Delayed light emission and high energy state of chloroplasts. Eur J Biochem 19: 386–397 [DOI] [PubMed] [Google Scholar]
- Yao ZJ, Ye JY, Mi H (2001) The role of chloroplast Ndh complex in resisting heat stress in tobacco strain. In PS2001 Proceedings, 12th International Congress on Photosynthesis. CSIRO Publishing, Collingwood, Australia