Abstract
Dimethylallyl diphosphate (DMADP) and geranyl diphosphate (GDP) are the last precursors of isoprene and monoterpenes emitted by leaves, respectively. DMADP and GDP pools were measured in leaves of plants emitting isoprene (Populus alba), monoterpenes (Quercus ilex and Mentha piperita), or nonemitting isoprenoids (Prunus persica). Detectable pools were found in all plant species, but P. persica showed the lowest pool size, which indicates a limitation of the whole pathway leading to isoprenoid biosynthesis in nonemitting species. The pools of DMADP and GDP of nonemitting, isoprene-emitting, and monoterpene-emitting species were partially labeled (generally 40%–60% of total carbon-incorporated 13C) within the same time by which volatile isoprenoids are fully labeled (15 min). This indicates the coexistence of two pools for both precursors, the rapidly labeled pool presumably occurring in chloroplasts and thereby synthesized by the methylerythritol phosphate pathway and the nonlabeled pool presumably located in the cytosol and synthesized by the mevalonic pathway. In M. piperita storing monoterpenes in specialized leaf structures, the GDP pool remained totally unlabeled, indicating either that monoterpenes are totally formed by the mevalonic pathway or that labeling occurs slowly in comparison to the large pool of stored monoterpenes in this plant. The pools of DMADP and GDP increased during the season (from May to July) but decreased when the leaf was darkened or exposed to very high temperature. In the dark, the pool of DMADP of the isoprene-emitting species decreased faster than the pool of GDP. However, after 6 h of darkness, both pools were depleted to about 10% of the pool size in illuminated leaves. This indicates that both the chloroplastic and the cytosolic pools of precursors are depleted in the dark. When comparing measurements over the season and at different temperatures, an inverse correlation was observed between isoprene emission by P. alba and the DMADP pool size and between monoterpene emission by Q. ilex and the GDP pool size. This suggests that the pool size does not limit the emission of isoprenoids. Rather, it indicates that the flux of volatile isoprenoids effectively controls the size of their pools of precursors.
A wide array of volatile organic compounds (VOCs) is emitted into the atmosphere by the leaves of many plant species. Among the biogenic VOCs studied to date, isoprene and monoterpenes are quantitatively the most important (Guenther et al., 1995), exerting profound effects on atmospheric chemistry (Chameides et al., 1988; Di Carlo et al., 2004) and on plant protection against abiotic and biotic stresses (Sharkey and Singsaas, 1995; Holopainen, 2004).
The biosynthesis of isoprene and monoterpenes has been clarified only recently. The emission of isoprene is light dependent (Loreto and Sharkey, 1990) and is rapidly labeled by 13C (Delwiche and Sharkey, 1993; Loreto et al., 1996b). The emission of monoterpenes from plants lacking specialized structures where these compounds accumulate (glands and ducts) also exhibits light dependence (Loreto et al., 1996a) and rapid 13C labeling (Loreto et al., 1996b). These observations have led to the proposal that volatile isoprenoids are formed from carbon directly shunting from the photosynthetic carbon fixation cycle. It has then been shown that isoprenoids can be synthesized in plastids from a biochemical pathway different from the cytosolic (mevalonic) pathway. This novel chloroplastic pathway, named from the intermediate methylerythritol phosphate (MEP), effectively uses photosynthetic carbon to generate volatile isoprenoids (Lichtenthaler et al., 1997).
The mevalonate and the MEP pathways generate the same precursors. Isoprene derives from the five-carbon precursor dimethylallyl diphosphate (DMADP), which may be formed in the cytosol by the mevalonic pathway and in the chloroplasts by the MEP pathway. DMADP may also condense with its isomer, isopentenyl diphosphate (IDP), generating geranyl diphosphate (GDP), the precursor of monoterpenes. Further condensation with IDP units generates the wide family of compounds characterizing sesquiterpenes and nonvolatile isoprenoids such as carotenoids. Previously, we have determined the size of chloroplastic and cytosolic pools of DMADP. We have shown that in isoprene-emitting leaves the pool of DMADP is incompletely labeled when isoprene labeling is complete (Loreto et al., 2004). This suggested that DMADP is partitioned in both chloroplasts and the cytosol and that the slightly less abundant chloroplastic pool generates isoprene. Other studies have addressed this problem with different techniques and there is general consensus about the partial labeling of DMADP. However, contrary to our findings, Rosenstiel et al. (2002) found DMADP mostly in cottonwood (Populus deltoides) chloroplasts. Wolfertz et al. (2003) noted that chloroplastic DMADP may be variable and inversely dependent on the emission rate of isoprene in kudzu (Pueraria lobata) leaves. The size of the DMADP pools in species that do not emit isoprene and in monoterpene-emitting species is not known.
To our knowledge, nothing is also known about the partitioning of the GDP pool between the chloroplasts and the cytosol. As in the case of isoprene, monoterpenes are now believed to be formed by the MEP pathway in chloroplasts, but, contrary to isoprene, several species store large amounts of monoterpenes outside chloroplasts. The possibility therefore exists that monoterpene biosynthesis occurs in both compartments from GDP available in situ or translocated from its place of biosynthesis. Labeling of monoterpenes stored in specialized structures is very slow compared to labeling of chloroplastic monoterpenes (Schurmann et al., 1993). Labeling of the GDP pool can indicate the partitioning of GDP between the putatively chloroplastic pool, rapidly labeled and generating the monoterpenes emitted without being stored, and the putatively cytosolic pool, slowly labeled and contributing to the formation of monoterpenes stored in the specialized structures.
A series of experiments involving the determination and labeling of DMADP and GDP pools was therefore carried out to answer to the following specific questions. (1) What is the size of chloroplastic and cytosolic DMADP pools of nonisoprenoid emitters and monoterpene emitters, as compared to isoprene emitters? (2) Are there chloroplastic and cytosolic pools of GDP, and what are their sizes? (3) Are these pools different during the season? (4) How do the DMADP and GDP pools respond to environmental factors, and are these responses consistent with the light and temperature dependence of isoprene and monoterpene emission? (5) Is there any relationship between the GDP pools and the presence of stored monoterpenes? (6) Is there any general relationship between the pool size of DMADP and GDP and the emission of isoprene and monoterpenes?
RESULTS
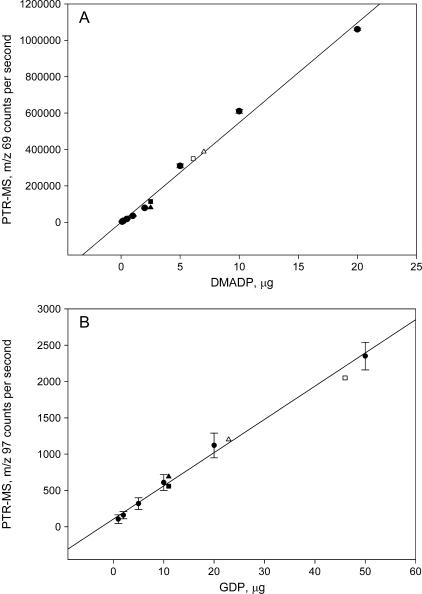
The proton transfer reaction (PTR)-mass spectrometry (MS) technique was successfully used to detect pools of isoprenoid precursors upon acid hydrolysis, as shown by the excellent correlation of the instrumental reading with different concentrations of DMADP and GDP (Fig. 1). The yield of DMADP and GDP was not influenced by leaf characteristics. Experiments mixing an aliquot of standards to extracts of Quercus ilex and Populus alba leaves with different specific leaf weight yielded DMADP and GDP amounts fitting the relationship obtained in the absence of leaf material (Fig. 1).
Figure 1.
Correlation between the concentration of DMADP (A) and GDP (B) standards subjected to acid hydrolysis and the evolved isoprene and linalool, respectively. Isoprenoid concentration was measured by PTR-MS, and the counts per second for the protonated mass of isoprene (m/z 69) and linalool most abundant fragment (m/z 97) are shown as mean ± se (black circles, n = 4). When not visible, ses are lower than symbol size. Triangles and squares represent two experiments in which 2.5 μg of DMADP and 11 μg of GDP were added to leaf extract of Q. ilex and P. alba, respectively, to determine whether the leaf characteristics could influence the yields of these compounds. White symbols show results with illuminated leaves whose endogenous pools of DMADP and GDP were previously determined and subtracted from the amount of DMADP and GDP exogenously supplied. Black symbols show results with darkened leaves whose endogenous pools of DMADP and GDP were depleted.
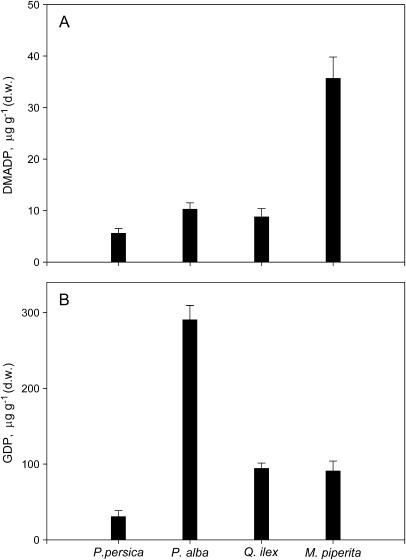
The total (chloroplastic and cytosolic) DMADP pool of nonemitting Prunus persica leaves was low in comparison to that measured in isoprene-emitting leaves of P. alba, in the species that emits monoterpenes without storing them (Q. ilex), and in the monoterpene producer that stores terpenes (Mentha piperita; Fig. 2A). The GDP pool was larger (up to 10 times in isoprene- and monoterpene-emitting leaves) than the DMADP pool in all species (Fig. 2B). As for DMADP, the nonemitter P. persica was characterized by the smallest pool of GDP.
Figure 2.
DMADP (A) and GDP (B) pools of a nonisoprenoid-emitting plant species (P. persica), an isoprene-emitting species (P. alba), a monoterpene-emitting species without specialized structures for monoterpene storage (Q. ilex), and a monoterpene-emitting species with specialized structures for monoterpene storage (M. piperita). Measurements were carried out in May. Means (n = 4) ± se are shown.
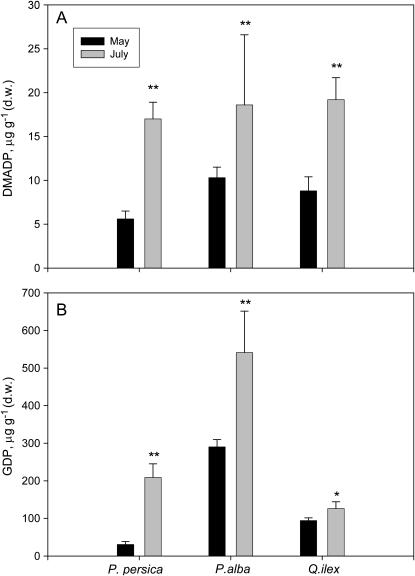
The pools were sampled in May, when leaves start to emit isoprenoids, and again in July, when isoprenoid emission is at the seasonal peak (Ciccioli et al., 2003) in a nonemitting, an isoprene-emitting, and a monoterpene-emitting species. In all cases, both DMADP and GDP pool sizes increased during the season (Fig. 3, A and B), although isoprenoid emission did not increase in July compared to May. In fact, as an average, we noted a reduction of isoprenoid emission in July, as shown later in Figure 6, perhaps due to a coincident reduction of photosynthesis, in turn caused by diurnal stomatal closure under very high vapor pressure difference between leaf and air (data not shown).
Figure 3.
Seasonal variation of DMADP (A) and GDP (B) pools of a nonisoprenoid-emitting plant species (P. persica), an isoprene-emitting species (P. alba), and a monoterpene-emitting species without specialized structures for monoterpene storage (Q. ilex). Means (n = 4) ± se are shown. Means were separated statistically between sampling period with a Tukey's HSD mean-separation test (** indicates P = 0.05; * indicates P = 0.10).
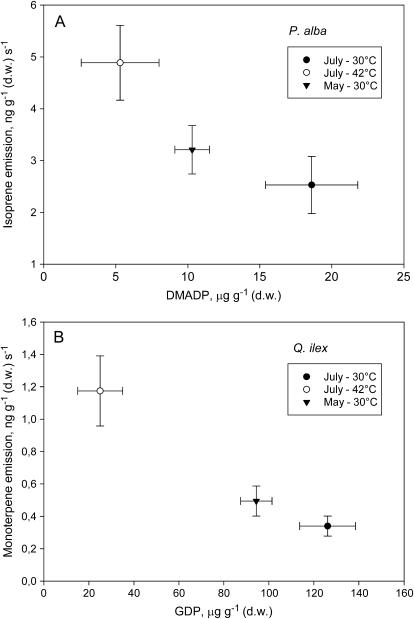
Figure 6.
Relationship between isoprene emission and DMADP pool size in P. alba (A) and between monoterpene emission and GDP pool size in Q. ilex (B). Emissions and pool sizes recorded at the two sampling periods (May and July) or at different temperatures (30°C and 42°C) are reported. For each data point, means (n = 4) ± se are shown.
The DMADP and GDP pools were incompletely labeled by 13C in nonemitting and isoprenoid-emitting leaves. About 40% of unlabeled DMADP was found in P. persica, P. alba, and Q. ilex leaves (Table I). The amount of unlabeled GDP was comparable to the unlabeled fraction of DMADP in P. alba and Q. ilex, and slightly higher in P. persica. The labeling patterns of the DMADP and GDP pools of nonemitting leaves and isoprenoid-emitting leaves with no storage organs for monoterpenes were compared with those of M. piperita, a monoterpene-emitting plant storing monoterpenes in glands. In M. piperita, the DMADP pool was again about 50% unlabeled, but the GDP pool was completely unlabeled (Table I).
Table I.
Fractions of unlabeled (12C) and labeled (13C) carbon in the DMADP and GDP pools of a nonisoprenoid-emitting plant species (P. persica), an isoprene-emitting species (P. alba), a monoterpene-emitting species without storage organs for monoterpenes (Q. ilex), and a monoterpene-emitting species with specialized structure for monoterpene storage (M. piperita)
Experiments were replicated on five leaves per plant species. Means of the two fractions are shown together with the se expressed as a percent of the labeled fraction.
| Plant Species | Emission Type | 12C/13C Fractions in DMADP (% se) | 12C/13C Fractions in GDP (% se) |
|---|---|---|---|
| P. persica | Nonemitter | 34/66 (6) | 68/32 (7) |
| P. alba | Isoprene | 38/62 (6) | 40/60 (7) |
| Q. ilex | Monoterpene (no storage) | 44/56 (5) | 52/48 (5) |
| M. piperita | Monoterpene (storage) | 48/52 (6) | 100/0 (0) |
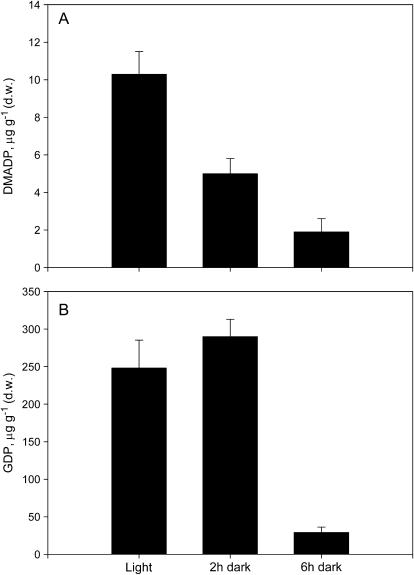
When leaves of the isoprene-emitting species P. alba were darkened, both the DMADP and the GDP pools were depleted (Fig. 4, A and B). However, the DMADP pool was already halved after 2 h of darkness (Fig. 4A), while at the same sampling time the GDP pool was comparable to that of illuminated leaves (Fig. 4B).
Figure 4.
Changes in the pool size of DMADP (A) and GDP (B) following darkening of isoprene-emitting P. alba leaves. Means (n = 4) ± se are shown.
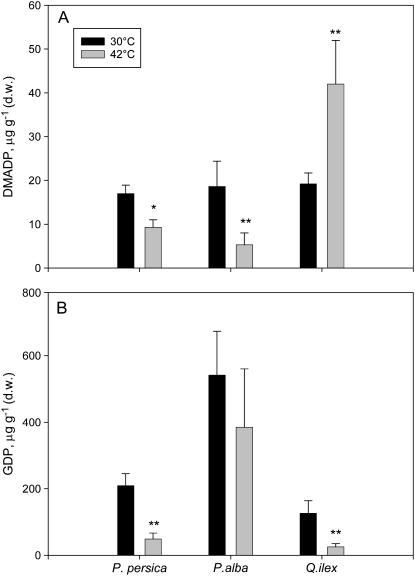
When leaves were exposed to very high temperature, the pool of DMADP decreased in nonemitting and isoprene-emitting leaves, but in the monoterpene emitter Q. ilex the DMADP pool increased in comparison to that measured at 30°C (Fig. 5A). Similarly, the GDP pool decreased at high temperature in the nonemitter P. persica and, although not significantly, in the isoprene emitter P. alba. The GDP pool also decreased in the monoterpene emitter Q. ilex exposed to high temperature (Fig. 5B).
Figure 5.
Changes in the pool size of DMADP (A) and GDP (B) following exposure to high temperature (42°C for 1 h) in a nonisoprenoid-emitting plant species (P. persica), an isoprene-emitting species (P. alba), and a monoterpene-emitting species without specialized structures for monoterpene storage (Q. ilex). Means (n = 4) ± se are shown. Means were separated statistically between temperature treatments with a Tukey's HSD mean-separation test (** indicates P = 0.05; * indicates P = 0.10).
The emission of isoprenoids was compared to the total pool size of the precursor in the two sampling dates and at the two different temperatures (Fig. 6). An inverse relationship was found between the emission of isoprene and the DMADP pool in P. alba (Fig. 6A) and between monoterpene emission and the GDP pool in Q. ilex (Fig. 6B).
DISCUSSION
There are detectable pools of DMADP and GDP in the leaves of all examined plants. The DMADP pool detected in May was generally low but comparable with the pool previously detected with a similar method on young leaves (Loreto et al., 2004). The GDP pool was in all cases much higher than the DMADP pool. This accumulation of the immediate precursor of monoterpenes may indicate a strong enzymatic control of the flux of carbon driving monoterpene biosynthesis and the availability of a large quantity of intermediates for the biosynthesis of more complex terpenes both through the MEP and the mevalonate pathways. Monoterpene synthases are reported to have a higher affinity for GDP than isoprene synthase for DMADP (Wolfertz et al., 2004). Thus, the large pool of GDP may indicate that the rate of GDP synthesis is controlled by the activity of GDP synthase but not by substrate availability. The pool of GDP was comparable in the two monoterpene-emitting species characterized by the presence (M. piperita) or the absence (Q. ilex) of large pools of monoterpene stored in specialized structures. This brings further confirmation that monoterpenes do not contribute to the amount of linalool generated by GDP upon acid hydrolysis. It remains to be tested whether monoterpenes are transformed in compounds different from linalool by acid hydrolysis or whether linalool formed by monoterpenes remains bound to leaf structures even after this treatment. Interestingly, the nonisoprenoid emitter P. persica showed the lowest pools of both DMADP and GDP among the selected plant species. This suggests that, in nonemitting plant species, early steps control the biosynthesis of these compounds. It also suggests that the lack of emission is related to low activation of both the cytosolic and the chloroplastic metabolic pathways driving DMADP and GDP formation, although, as noted later, the total absence of emission in nonemitting plants may be better explained by the low catalytic properties of the enzymes.
In all plant species, the pool sizes of DMADP and GDP increased significantly during the season, indicating a strong environmental, and perhaps developmental, control on DMADP and GDP formation. However, as discussed below, the increase in the pool sizes of precursors does not seem to support a larger emission of the volatile products.
Under conditions generating high rates of isoprenoid emission (30°C and exposure to high light intensity), we have detected a 50% to 60% incorporation of 13C in the pools of both DMADP and GDP in isoprene-emitting species. Because DMADP incomplete labeling occurs when isoprene emission labeling is quasicomplete and does not proceed further (Delwiche and Sharkey, 1993), it may indicate, as previously suggested (Loreto et al., 2004), that about one-half of the total pool of DMADP is made from the MEP pathway in chloroplasts, whereas the other half is extrachloroplastic and probably formed through the mevalonic pathway. The data collected in this experiment confirm the labeling patterns found in our previous experiment for two other strong isoprene-emitting species (Phragmites australis and Populus nigra; Loreto et al., 2004). Perhaps, more interestingly, the labeling experiment also suggests a similar contribution of the MEP pathway to the formation of GDP in isoprene-emitting species.
Again, a similar degree of labeling was found in the nonemitting species as in the isoprenoid-emitting species. This points out that chloroplastic pools of DMADP and GDP are also present in nonemitters and that the emission trait and amount are not controlled by the substrate availability in these species, whereas it may be restricted by the low presence or catalytic properties of the enzymes driving isoprene and monoterpene formation from the immediate precursors (see Lehning et al., 1999; Fischbach et al., 2000).
The pool of DMADP was partially labeled in the monoterpene emitters Q. ilex and M. piperita, indicating a ubiquitous and constant distribution of the chloroplast versus cytosolic pool of DMADP in leaves. However, the GDP pool of M. piperita remained completely unlabeled, whereas in Q. ilex the labeling was partial, as for isoprene-emitting and nonemitting leaves. It is now believed that volatile isoprenoids (isoprene and monoterpenes) are made by the same MEP pathway in chloroplasts (Lichtenthaler, 1999). This should also be true for monoterpenes accumulating in the glands of M. piperita leaves (Lange et al., 2001). It has been demonstrated that the enzyme limonene synthase is localized in the plastids of peppermint (Turner et al., 1999).
Our experiment might suggest that the pool of GDP supplying stored monoterpenes is predominantly cytosolic in M. piperita leaves. Perhaps GDP is made in the cytosol and then transported to the chloroplast, where monoterpene synthesis is carried out. However, in snapdragon (Antirrhinum majus) flowers, it has been shown that the cross-talk between pathways of isoprenoid biosynthesis is unidirectional from plastids to cytosol (Dudareva et al., 2005). Schurmann et al. (1993) observed the incorporation of labeled compound in the monoterpenes of pine needle resin ducts within hours. Thus, another explanation is that an extremely slow labeling of a preexisting pool, possibly already located in the specialized structures, occurs in all plant species characterized by storage pools of isoprenoids.
The MEP pathway generating volatile isoprenoids is light regulated (Loreto and Sharkey, 1990; Loreto et al., 1996a) because carbon assimilation stops in the dark and isoprene synthase is concurrently inhibited (Lehning et al., 1999). We expected to see a fast depletion of DMADP and GDP pools in an isoprene-emitting plant after dark adaptation. However, the DMADP pool dropped faster than the GDP pool in darkened leaves. This may, in part, be explained by the different sizes of the pools, the GDP pool being larger by far than the DMADP pool. It may also indicate a slow turnover of the GDP pool, perhaps again due to some unknown factors restricting monoterpene formation. After 6 h of darkness, however, both the DMADP and GDP pools were largely depleted. The remaining pools were about 10% of the pools present in illuminated leaves, a percentage much lower than the labeled fraction probably formed by the MEP pathway. This suggests that not only the chloroplastic pools but also the cytosolic pools of both isoprene and monoterpene precursors are light dependent and, although with a different time course, may be depleted in the dark.
Temperature is the other relevant factor affecting isoprene and monoterpene emission (Loreto and Sharkey, 1990; Loreto et al., 1996a), probably reflecting the temperature dependence of isoprene synthase (Monson et al., 1992) and monoterpene synthases (Fischbach et al., 2000). However, when sampled at a temperature comparable to the optimal temperature for the enzymes catalyzing isoprene and monoterpene synthesis, the pool of DMADP was reduced in the isoprene-emitting species P. alba and the pool of GDP was reduced in the monoterpene-emitting species Q. ilex. Assuming a fixed contribution of the chloroplastic pool to the total pool of DMADP and GDP (about 50%, as indicated by the labeling experiment), and a constant cytosolic pool size, this indicates an inverse relationship between the pool sizes and the emission of isoprene and monoterpenes. In fact, an inverse relationship can be observed between the emission rates of isoprene in P. alba and of monoterpenes in Q. ilex and their respective precursors, measured at different temperature or during the season. The chloroplastic DMADP pool was found to be inversely associated with isoprene emission by Wolfertz et al. (2003), whereas other reports suggest that the pool of DMADP may be directly associated with the emission (Rosenstiel et al., 2002; Brüggeman and Schnitzler, 2002; Wolfertz et al., 2004). Our finding supports the idea that the pools of precursors do not control the emission of volatile isoprenoids. It rather indicates a strong flux control over the pools because the size of DMADP and GDP pools gets smaller when isoprene and monoterpene are more released in the atmosphere. Interestingly, such an inverse relationship was not found between emissions and pool sizes of intermediates other than the direct precursors of the emitted compounds (i.e. isoprene and GDP or monoterpenes and DMADP; data not shown; e.g. compare Figs. 5 and 6). This suggests that the emission flux only sets the pool sizes of the most immediate precursors rather than controlling the flux of carbon over the entire pathway of isoprenoid formation.
In conclusion, our experiments provided the following answers to the questions we have asked in the introduction. (1) All plant species do have pools of DMADP and GDP even if they do not emit isoprenoids. (2) These pools of precursors may be roughly equally distributed in the cytosol and in the chloroplasts, indicating that the chloroplastic biosynthesis of isoprenoids is also active in nonisoprenoid-emitting species. (3) The pools of DMADP and GDP increase during the vegetative season (at least from early summer to full summer). (4) The pools decrease at increasing temperatures and in the dark. (5) Pool sizes are not related to the presence of stored monoterpenes. (6) Pool sizes are inversely related to the emissions of isoprene (DMADP) and monoterpenes (GDP), indicating that emission fluxes strongly control the pool sizes of the immediate precursors of the emitted compounds.
MATERIALS AND METHODS
Plant Materials, Protocols, and Statistics
Plants of a tree species that does not emit isoprene or monoterpenes (Prunus persica), a tree emitting isoprene (Populus alba), a monoterpene-emitting tree that does not store monoterpenes in specialized structures (Quercus ilex), and a perennial herb storing monoterpenes in glands (Mentha piperita) were grown in 50-L pots under optimal water and nutrient conditions. Plants were grown in the experimental field of our Institute, near Rome. Experiments were carried out on leaves of 3-year-old (tree species) and 1-year-old plants of Mentha. Two samplings were carried out: in May, only on fully expanded leaves, and in July, on mature leaves. The selected samples were exposed to the south and unshaded by other leaves of the same plants or by neighboring trees. Plants sampled in May were grown at average day temperature of 20°C with peaks of 30°C during that month, while July samples endured a 2-month growth under hot summer conditions, with a mean day temperature of 27°C and maximal temperatures often exceeding 35°C.
Experiments were carried out during the morning hours (9 am–12 pm) and were repeated on at least four different leaves of different plants. Data are presented as means ± se. Statistical significance of mean differences was assessed, when needed, by Tukey's honestly significant difference (HSD) mean-separation test, and the statistically significant differences are shown with P = 0.05 (**) or P = 0.10 (*).
Gas-Exchange and Labeling Experiments
A variable area of single leaves was clamped in a 0.5-L gas-exchange plastic cuvette entirely coated with Teflon, as previously explained (Loreto et al., 1996a, 2004). All experiments were done on leaves still attached to the plants. Leaves were exposed to a flux of synthetic air, free of contaminants and pollutants, such as ozone or exogenous isoprenoids, and composed of N2, O2, and CO2 in atmospheric concentrations (80%, 20%, and 370 μL L−1, respectively). All experiments were done exposing leaves at a light intensity of 1,000 μmol photons m−2 s−1, except when assaying the impact of a light-to-dark transition on the pools of precursors. Experiments were done maintaining the leaf at a temperature of 30°C, except when examining the impact of elevated temperatures on the pools of precursors. In the latter case, the leaf temperature was raised to 42°C with thermoelectric Peltier modules. The leaf temperature was controlled with an array of three thermocouples appressed to the center and two margins of the abaxial leaf side. Experiments were carried out when all physiological parameters (photosynthesis, transpiration, and isoprenoid emission) were steady, unless otherwise noted. The high temperature measurements were carried out after a 1-h exposure to 42°C. Photosynthesis and transpiration were measured comparing the CO2 and water content in the flux of air out of the cuvette and in a reference line bypassing the cuvette with a CO2/water differential infrared gas analyzer (Li-6262; LI-COR). The emission of isoprenoids (isoprene and monoterpenes) was measured online with a PTR-MS (Ionicon) diverting to the instrument a small flux of the same air flowing out of the cuvette. The instrument was calibrated daily by comparing isoprene and monoterpene standards with the reading of a gas chromatograph (GC855; Synthec Spectras), which was also used to discriminate and measure the emission of single monoterpenes with the same Mr. Further details about the gas-exchange setup and physiological measurements are reported by Loreto et al. (1996a). Details on the theory and practice of the PTR-MS technique are reported by Lindinger et al. (1998).
To label isoprenoids, the 12CO2 source was replaced instantaneously with a 13CO2 source as described by Loreto et al. (1996b), maintaining the concentration of CO2 at ambient levels (370 μL L−1). Labeling of isoprene emission and of the monoterpenes emitted and not stored in specialized structures is almost complete (80%−100% of the emitted compounds incorporate 13C) after 15 min (Delwiche and Sharkey, 1993; Loreto et al., 1996b). We also carried out the labeling for 15 min before determining 13CO2 incorporation into DMADP and GDP.
DMADP and GDP Measurements
When physiological parameters were steady, leaf discs (4 cm2) were instantaneously freeze clamped in two metal drums prechilled in liquid nitrogen. Ultrafast freeze clamping is needed when measuring metabolites turning over very rapidly (Loreto and Sharkey, 1993). This might have been the case for DMADP and GDP, and, in the absence of evidence about the turnover time of these compounds, the freeze-clamping technique was also used in this experiment.
In independent measurements, we determined the specific leaf weight of these samples, which was variable between species but constant during the season. In P. alba and Q. ilex, a specific leaf weight of 32 ± 2 and 44 ± 5 mg (dry weight) cm−2 was measured, respectively, and these values were used to express measurements of emission rates on a mass basis when comparing emissions and pool sizes (Fig. 6). The leaf disc was then rapidly pulverized in a chilled mortar and the powder was treated as described by Loreto et al. (2004). In brief, the leaf extract was subjected to acid hydrolysis, maintaining it under a H2SO4 1 m solution at 37°C for 30 min. We performed the assay in a T-shaped glass tube. When the reaction ended, pure N2 was flushed at a flux of 100 mL min−1 into the tube still maintained at high temperature, and the volatile reaction products were concentrated in another T-shaped glass tube maintained in liquid N2. Finally, the second tube was rapidly brought to room temperature (25°C), and the collected samples were driven by a second flux of N2 into the PTR-MS for analysis. The amount of DMADP was derived by measuring the evolved isoprene (protonated m/z 69) as indicated by Fisher et al. (2001). The yield of DMADP was linear with isoprene emission and with the amount of DMADP standard added as an internal control (Fig. 1), but, as explained by Fisher et al. (2001), only about 5% of the total DMADP could be converted to isoprene with this chemical extraction and in the absence of appropriate proton acceptors from the intermediate carbocation. The limit for DMADP standard detection was 0.2 μg DMADP.
Also, the amount of GDP was derived by measuring the evolution of a volatile compound formed by acid hydrolysis. The blend of volatile compounds generated after hydrolyzing an internal standard of GDP included linalool and several furans. Furan yield can be reduced by decreasing the acidity of the solution, and this is the reason why the acidity used in this experiment was lower than previously adopted (Loreto et al., 2004). Linalool was positively recognized as a product of this reaction by comparison with MS spectra and by comparison with a linalool standard also subjected to acid hydrolysis. Because we have not checked for linalool glucosides that can be hydrolyzed by acid catalysis, we have measured the sum of free and conjugated linalool. Under our experimental conditions, the GDP yield of linalool was similar to that reached as isoprene for the DMADP assay (5%; see also Loreto et al., 2004) and linear with increasing amount of GDP used as internal standard (Fig. 1). The limit for GDP standard detection was 1 μg GDP.
Care was taken to maintain the acidity of the solution high enough to avoid reduction of isoprene yield (Brüggeman and Schnitzler, 2002). Probably acidity similarly affects linalool yield, but this was not tested as all experiments were carried out under the same pH (1.5). Linalool produced by GDP (protonated m/z 155) fragments in the PTR-MS was the most abundant fragment collected, with m/z 97. This fragment represents 2.25% of the total yield of linalool. Acid hydrolysis of liquid monoterpene standards (α-pinene, β-pinene, sabinene, and myrcene) in concentrations comparable to those present in monoterpene-emitting leaves (Loreto et al., 1998) did not yield detectable amounts of linalool, which suggests no interference of endogenous monoterpenes with the detection of linalool derived by the GDP pool. Also, acid hydrolysis of geraniol, sesquiterpenes (β-cariophyllene), and xanthophylls did not yield detectable amounts of linalool.
The labeled amount of DMADP and GDP was detected by freeze clamping the leaves after 13CO2 labeling for 15 min, as explained above. In these leaves, we detected by PTR-MS the m/z shift from unlabeled (m/z 69 for DMADP and m/z 97 for GDP) to partial or fully labeled DMADP (m/z 70–74) or GDP (m/z 98–104), following the procedure outlined by Loreto et al. (2004).
Acknowledgments
We thank Paolo Ciccioli and Enzo Brancaleoni for useful discussions on the chemistry of DMADP and GDP labeling and determination.
This work was supported by the European Commission (contract MC–RTN–CT-2003–504720; “ISONET”) and by the European Science Foundation scientific program VOCBAS.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Francesco Loreto (francesco.loreto@ibaf.cnr.it).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.073213.
References
- Brüggeman N, Schnitzler JP (2002) Diurnal variation of dimethylallyl diphosphate concentrations in oak (Quercus robur leaves). Physiol Plant 115: 190–196 [DOI] [PubMed] [Google Scholar]
- Chameides WL, Lindsay RW, Richardson J, Kiang CS (1988) The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science 241: 1473–1475 [DOI] [PubMed] [Google Scholar]
- Ciccioli P, Brancaleoni E, Frattoni M, Marta S, Brachetti A, Vitullo M, Tirone G, Valentini R (2003) Relaxed eddy accumulation, a new technique for measuring emission and deposition fluxes of volatile organic compounds by capillary gas chromatography and mass spectrometry. J Chromatogr A 985: 283–296 [DOI] [PubMed] [Google Scholar]
- Delwiche CD, Sharkey TD (1993) Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ 16: 587–591 [Google Scholar]
- Di Carlo P, Brune WH, Martinez M, Harder H, Lesher R, Ren X, Thornberry T, Carroll MA, Young V, Shepson PB, et al (2004) Missing OH reactivity in a forest: evidence for unknown reactive biogenic VOCs. Science 304: 722–725 [DOI] [PubMed] [Google Scholar]
- Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gershenzon J (2005) The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci USA 102: 933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach RJ, Zimmer I, Steinbrecher R, Pfichner A, Schnitzler JP (2000) Monoterpene synthase activities in leaves of Picea abies (L.) Karst. and Quercus ilex L. Phytochemistry 54: 257–265 [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Rosenstiel TN, Shirk MC, Fall R (2001) Nonradioactive assay for cellular dimethylallyl diphosphate. Anal Biochem 292: 272–279 [DOI] [PubMed] [Google Scholar]
- Guenther A, Hewitt CN, Erickso D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, McKay WA, et al (1995) A global model of natural volatile organic compound emissions. J Geophys Res 100: 8873–8892 [Google Scholar]
- Holopainen JK (2004) Multiple functions of inducible plant volatiles. Trends Plant Sci 9: 529–533 [DOI] [PubMed] [Google Scholar]
- Lange BM, Ketchum REB, Croteau RB (2001) Isoprenoid biosynthesis. Metabolite profiling of peppermint oil gland secretory cells and application to herbicide target analysis. Plant Physiol 127: 305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehning A, Zimmer I, Steinbrecher R, Bruggemann N, Schnitzler JP (1999) Isoprene synthase activity and its relation to isoprene emission in Quercus robur L. leaves. Plant Cell Environ 22: 495–504 [Google Scholar]
- Lichtenthaler HK (1999) The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50: 47–65 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Schwendler J, Disch A, Rohmer M (1997) Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett 400: 271–274 [DOI] [PubMed] [Google Scholar]
- Lindinger W, Hansel A, Jordan A (1998) On-line monitoring of volatile organic compounds at ppt levels by means of proton transfer reaction-mass spectrometry (PTR-MS). Medical applications, food control and environmental research. Int J Mass Spect Ion Proc 173: 191–241 [Google Scholar]
- Loreto F, Ciccioli P, Brancaleoni E, Cucinato A, Frattoni M (1998) Measurement of isoprenoid content in leaves of Mediterranean Quercus spp. by a novel and sensitive method and estimation of the isoprenoid partition between liquid and gas phase inside the leaves. Plant Sci 136: 25–30 [Google Scholar]
- Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Tricoli D (1996. a) Influence of environmental factors and air composition on the emission of α-pinene from Quercus ilex leaves. Plant Physiol 110: 267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Tricoli D (1996. b) Evidence of the photosynthetic origin of monoterpenes emitted by Quercus ilex leaves by 13C labeling. Plant Physiol 110: 1317–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Pinelli P, Brancaleoni E, Ciccioli P (2004) 13C labeling reveals chloroplastic and extra-chloroplastic pools of dimethylallyl pyrophosphate and their contribution to isoprene formation. Plant Physiol 135: 1903–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD (1990) A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta 182: 523–531 [DOI] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD (1993) On the relationship between isoprene emission and photosynthetic metabolites under different environmental conditions. Planta 189: 420–424 [DOI] [PubMed] [Google Scholar]
- Monson RK, Jaeger CH, Adams WW, Driggers EM, Silver GM, Fall R (1992) Relationship among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiol 98: 1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel TN, Fisher AJ, Fall R, Monson RK (2002) Differential accumulation of dimethylallyl diphosphate in leaves and needles of isoprene- and methylbutenol-emitting and nonemitting species. Plant Physiol 129: 1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurmann W, Ziegler H, Kotzias D, Schonwitz R, Steinbrecher R (1993) Emission of biosynthesized monoterpenes from needles of Norway spruce. Naturwissenschaften 80: 276–278 [Google Scholar]
- Sharkey TD, Singsaas EL (1995) Why plants emit isoprene. Nature 374: 769 [Google Scholar]
- Turner G, Gershenzon J, Nielson EE, Froehlich JE, Croteau R (1999) Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol 120: 879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kuenhemann F, Yeh S, Weise SE (2003) Biochemical regulation of isoprene emission. Plant Cell Environ 26: 1357–1364 [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kunhnemann F (2004) Rapid regulation of the methylerythritol 4-phosphate pathway during isoprene synthesis. Plant Physiol 135: 1939–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]