Abstract
Glucosinolates are sulfur-rich plant secondary metabolites whose breakdown products have a wide range of biological activities in plant–herbivore and plant–pathogen interactions and anticarcinogenic properties. In Arabidopsis thaliana, hydrolysis by the enzyme, myrosinase, produces bioactive nitriles, epithionitriles, or isothiocyanates depending upon the plant's genotype and the glucosinolate's structure. A major determinant of this structural specificity is the epithiospecifier locus (ESP), whose protein causes the formation of epithionitriles and nitriles. A quantitative trait locus (QTL) on chromosome 3 epistatically affects nitrile formation in combination with ESP; this QTL has been termed EPITHIOSPECIFIER MODIFIER1 (ESM1). We identified a myrosinase-associated protein as the ESM1 QTL in Arabidopsis using map-based cloning with recombinant inbred lines, natural variation transcriptomic analysis, and metabolic profiling. In planta and in vitro analyses with natural ESM1 alleles, ESM1 knockouts, and overexpression lines show that ESM1 represses nitrile formation and favors isothiocyanate production. The glucosinolate hydrolysis profile change influenced by ESM1 is associated with the ability to deter herbivory by Trichoplusia ni. This gene could provide unique approaches toward improving human nutrition.
INTRODUCTION
Plants produce a diversity of secondary metabolites that are believed to function in mediating interactions with the environment, including antiherbivory defenses. However, it has been difficult to demonstrate the defensive roles of individual compounds under natural conditions. Modern molecular and genetic methods can help unravel complex metabolically controlled plant defensive mechanisms and help test the defensive functions of individual metabolites. The identification of the underlying genes can provide insight into the evolution of secondary metabolism and plant–herbivore interactions. A model system for metabolic plant defense systems consists of the glucosinolates, a group of low-molecular-weight, sulfur-rich thioglucosides (Halkier, 1999; Kliebenstein et al., 2005), coupled with activating myrosinase enzymes (Kliebenstein, 2004).
Glucosinolates are sulfur-rich plant secondary metabolites whose basic skeleton consists of a β-thioglucose residue, an N-hydroxyiminosulfate moiety, and a variable side chain. Generally, glucosinolates are classified as aliphatic, aromatic, and indolic, depending on whether they originate from aliphatic amino acids, aromatic amino acids, or Trp (Bones and Rossiter, 1996; Halkier and Du, 1997; Kliebenstein et al., 2001a, 2005; Wittstock and Halkier, 2002; Kliebenstein, 2004; D'Auria and Gershenzon, 2005). The Met-derived glucosinolates are the largest and most structurally diverse class. This structural variety is generated by elongation of the basic Met side chain with one to six additional methylene groups. A single genetic locus in Arabidopsis thaliana, GS-Elong, regulates the length of the aliphatic side chain. The GS-Elong locus contains the genes, MAM1, MAM2, and MAML (de Quiros et al., 2000; Kliebenstein et al., 2001b; Kroymann et al., 2001, 2003; Reichelt et al., 2002; Falk et al., 2004; Field et al., 2004). The natural structural diversity is further increased by natural variation in gene expression at biosynthetic loci responsible for side chain modification (Kliebenstein et al., 2001c, 2006).
Glucosinolates are not bioactive until they have been enzymatically hydrolyzed. This occurs when endogenous myrosinase is released during the disruption of plant cells by harvesting, processing, or mastication. Glucosinolate breakdown products play prominent roles in plant–herbivore and plant–pathogen interactions (Chew, 1988; Borek et al., 1998; Halkier, 1999; Lambrix et al., 2001) and have potential antiulcer and anticarcinogenic properties (Zhang et al., 1994; Hecht, 2000). Hydrolysis of a single glucosinolate can produce a nitrile, an isothiocyanate, or a thiocyanate, with the final structure controlling the bioactivity (Lambrix et al., 2001). For example, the isothiocyanate product of 4-methylsulfinylbutyl glucosinolate, sulforaphane (4-methylsulfinylbutyl isothiocyanate), induces cancer defenses in humans and is intensely studied for its potential benefits to human nutrition (Zhang et al., 1994; Barcelo et al., 1998; Fahey et al., 2001). By contrast, the nitrile product of 4-methylsulfinylalkyl glucosinolate appears to be less active in humans. Glucosinolate bioactivity is thus strongly dependent upon the structural specificity of the hydrolysis process. Understanding the molecular basis for the specificity could aid in developing more nutritious crops.
In Arabidopsis and Brassica, the hydrolysis products formed depend on the chemical nature of the glucosinolate side chain and the presence of several genetic loci (Mitchell-Olds and Pedersen, 1998; Bernardi et al., 2000; Foo et al., 2000; Lambrix et al., 2001; Mithen et al., 2003). Our analysis of glucosinolate hydrolysis products among 122 Arabidopsis accessions showed that the accessions can be grouped by their predominant hydrolysis products: isothiocyanates or nitriles (Figure 1). The difference in hydrolysis structure is genetically controlled by variation at two quantitative trait loci (QTL) in the Landsberg erecta (Ler) × Columbia (Col-0) recombinant inbred line (RIL) population (Lister and Dean, 1993; Lambrix et al., 2001). The major QTL on chromosome 1 affecting hydrolysis specificity is controlled by variation in transcript accumulation of the epithiospecifier protein (ESP; Figure 1). ESP promotes epithionitrile formation during alkenyl glucosinolate hydrolysis by transfer of the sulfur atom from the basic glucosinolate backbone to the terminal alkene residue of the side chain (Lambrix et al., 2001). During nonalkenyl glucosinolate hydrolysis, ESP promotes simple nitrile formation through sulfur loss (Bernardi et al., 2000; Foo et al., 2000; Lambrix et al., 2001; Kliebenstein, 2004). A QTL on chromosome 3 epistatically affects nitrile formation in combination with ESP and has been termed EPITHIOSPECIFIER MODIFIER1 (ESM1; Figure 1). This QTL controls the ratio of nitrile to isothiocyanate production during glucosinolate hydrolysis without affecting the total level of hydrolysis product (Lambrix et al., 2001). The gene and molecular polymorphism controlling the glucosinolate hydrolysis variation at ESM1 are described in this report.
Figure 1.
Model of Structural Specificity in Glucosinolate Hydrolysis.
ESP promotes nitrile and epithionitrile formation, whereas ESM1 has an unknown function. R indicates a variable side chain. n = 1 or 2.
Previous studies have suggested that glucosinolate hydrolysis is a simple system controlled by ESP, myrosinase, and potentially free iron or pH. However, identification of the additional QTL, ESM1, controlling glucosinolate hydrolysis suggests that the system is more complex. Identifying the genetic basis of ESM1 could provide insight into additional determinants of structural specificity in glucosinolate hydrolysis (Figure 1). In this study, we cloned the ESM1 QTL using map-based cloning, metabolic profiling, and transcriptomics. We used a T-DNA insertion line and transgenic complementation for rapid validation of ESM1 identity. We conducted in vitro analysis of ESM1's biochemical function in controlling the formation of nitriles and isothiocyanates. We investigated the association between the ESM1-altered nitrile production ratio and the ability to deter generalist lepidopteran herbivory by Trichoplusia ni (cabbage looper).
RESULTS
Genetic Analysis of ESM1
To genetically analyze the ESM1 QTL and to initiate a mapping population, we crossed two Ler × Col-0 RILs, CS1945 and CS1995 (Lister and Dean, 1993). These two lines contain opposite parental alleles around ESM1 on the top of chromosome 3 but are identical at the three major QTL known to affect glucosinolate content, structure, and/or hydrolysis (GS-AOP, GS-Elong, and ESP). Crossing CS1945 and CS1995 forces the previously known glucosinolate hydrolysis locus, ESP, into a homozygous nitrile- producing state (Lambrix et al., 2001). In addition, we selected these two lines because they are homozygous for 4-methylsulfinylbutyl glucosinolate production, which accumulates to the highest level within the Ler × Col-0 population. Maximizing the endogenous glucosinolate production increases the sensitivity of detection for the resulting endogenous hydrolysis products (Kliebenstein et al., 2001b, 2002). Furthermore, because Ler and Col-0 contain different intact glucosinolate structural profiles, our use of these two RILs eliminated structural variation of the precursor in the mapping population, enabling us to map ESM1 without confounding variations at unlinked loci such as ESP, GS-Elong, and GS-AOP (Kliebenstein et al., 2001a, 2001c; Lambrix et al., 2001; Kroymann et al., 2003).
Previous analysis showed that the ESM1 QTL altered the hydrolysis of aliphatic glucosinolates, but the use of a RIL population prevented the estimation of dominance. We assayed leaves of Col-0, CS1945, CS1995, and CS1995 × CS1945 F1 progeny using a gas chromatography–flame ionization detection (GC-FID) protocol for detecting endogenous hydrolysis products of 4-methylsulfinylbutyl glucosinolate, 5-methylsulfinylpentyl nitrile (5MSOP-NIT), and 4-methylsulfinylbutyl isothiocyanate (4MSOB-ITC) (Figure 2) (Kliebenstein et al., 2001b, 2002). In an attempt to use the summation of the two hydrolysis forms to estimate the quantity of intact glucosinolate that was hydrolyzed, the nitrile formation activity is described in terms of nitrile as the sum of nitrile plus isothiocyanate (5MSOP-NIT / 5MSOP-NIT + 4MSOB-ITC). The average aliphatic glucosinolate nitrile formation ratio was 0.50 for CS1945 (ESM1Col/Col), 0.67 for the F1 (ESM1Ler/Col), and 0.95 for CS1995 (ESM1Ler/Ler) (Figures 2 and 3). All three values were significantly different (P ≤ 0.05) by t test, which indicated that the ESM1 QTL is semidominant for aliphatic glucosinolate hydrolysis. We confirmed this semidominance in an analysis of 286 F2 individuals, in which 72 plants showed an ESM1Col/Col nitrile formation ratio, 141 plants showed a heterozygous phenotype, and 73 plants showed a homozygous Ler phenotype (χ2 = 0.063 < P0.05,2 = 5.991). Thus, the ESM1 QTL acts as a single semidominant locus when assayed via the hydrolysis of endogenous aliphatic glucosinolates.
Figure 2.
Glucosinolate Hydrolysis Phenotypes.
GC-FID was used to measure the hydrolysis of endogenous 4-methylsulfinylbutyl glucosinolate in Col-0, CS1945, and CS1995. Representative GC-FID traces are shown, with the positions of the nitrile (5MSOP-NIT) and isothiocyanate (4MSOB-ITC) labeled. Accessions and RILs are listed for each trace with the respective genotypes at the ESP and ESM1 loci.
Figure 3.
ESM1 Is Semidominant.
GC-FID was used to measure the hydrolysis of the endogenous 4-methylsulfinylbutyl glucosinolate into 5MSOP-NIT and 4MSOB-ITC. The structural outcome of endogenous glucosinolate hydrolysis is shown as the ratio of 5MSOP-NIT to the sum of 5MSOP-NIT and 4MSOB-ITC. GC-FID was conducted in the following three lines: CS1945, ESM1Col/Col; CS1945 × CS1995 F1, ESM1Col/Ler; and CS1995, ESM1Ler/Ler. All three lines are homozygous Ler for ESP. Four plants were separately measured per line, and the experiment was independently replicated twice. Lowercase letters above the bars show statistically significant groupings as obtained by t tests within analysis of variance (ANOVA) that combine the two replicate experiments. Error bars indicate se.
Fine-Mapping of ESM1
The single locus, semidominant nature of the ESM1 QTL allowed us to proceed with simple Mendelian fine-mapping approaches to clone the underlying gene using the CS1945 × CS1995 F2 progeny. We used sequence information from The Arabidopsis Information Resource (www.arabidopsis.org) and the Monsanto Arabidopsis Landsberg Sequence (www.ncgr.org/cgi-bin/cereon/cereon_seq_login.pl) to design 27 insertion/deletion PCR markers or cleaved-amplified polymorphism PCR markers for ESM1 fine-mapping (see Supplemental Table 1 online). Nine markers were evenly spaced at approximately one per megabase pair along the long arm of chromosome 3 (Figure 4A). We used these markers to genotype 286 random F2 individuals that were also phenotyped for their nitrile production ratio using GC-FID. Our analysis showed that the ESM1 QTL is a single locus positioned within a 0.9-Mb interval between 4.2 and 5.1 Mb (Figure 4B). We used 18 additional markers, developed to query this 0.9-Mb interval, to screen an additional 1056 F2 progeny for recombination events and to position the recombination breakpoints within all F2 progeny. The screen identified 77 F2 lines with recombination breakpoints that positioned ESM1 within a 100-kb region, flanked by markers at 4.65 and 4.75 Mb. Our final resolution was limited by the fact that no recombination event was found within this 100-kb region in 2684 tested meioses (the original 286 F2 progeny plus the later 1056 F2 progeny). The final genetic region identified as containing the ESM1 QTL encompasses parts of four BAC clones that contain 36 annotated genes within the reference Col-0 genomic sequence (Figure 4C).
Figure 4.
Map-Based Cloning of ESM1.
The genetics methodology used for map-based cloning of the ESM1 QTL is shown.
(A) Approximate map position of the glucosinolate hydrolysis ESM1 QTL on chromosome 3.
(B) Fine-scale mapping of ESM1. Relative marker positions by physical distance are shown, with the number of recombinants per total number of progeny shown below.
(C) BAC contig and genic content for the final recombinant region containing the ESM1 QTL.
(D) Shown for each gene is the maximal difference in transcript accumulation between two accessions when comparing seven Arabidopsis accessions. ANOVA showed that only the At3g14210 and At3g14070 genes had statistical evidence for natural variable transcript accumulation (Kliebenstein et al., 2006).
(E) Semiquantitative RT-PCR of the At3g14210 and At3g14070 candidate genes in Ler and Col-0. RAN and ESP are controls. At3g14225 is a homolog of At3g14210.
Differential Transcript Accumulation and RT-PCR Analysis of Candidate Genes
To filter through the 36 genes, we used a data set in which ATH1 GeneChip Affymetrix microarrays were used to test for variable transcript accumulation among seven Arabidopsis accessions (Kliebenstein et al., 2006) (elp.ucdavis.edu/parental_survey_ELP_finder.htm). We analyzed the resulting transcript accumulation data with ANOVA to test whether any of the 36 genes showed evidence of naturally variable transcript accumulation (Figure 4D). Of the 36 genes, 2 showed statistically significant variation in transcript accumulation among seven Arabidopsis accessions: At3g14070, a putative cation exchanger, and At3g14210, annotated as a myrosinase-associated protein (MyAP) containing a lipase/acylhydrolase-like motif. The tandem neighbors of At3g14210, At3g14220, and At3g14225 belong to a homologous GDSL-motif lipase/hydrolase protein family.
Because the microarray analysis of Arabidopsis accessions did not include Ler, we used semiquantitative RT-PCR to test the candidate genes for differential transcript accumulation between Col-0 and Ler. We used RAN as a loading control because it does not show differential transcript accumulation between Ler and Col-0, whereas ESP was used as a control for a transcript known to differentially accumulate between Ler and Col-0 (Lambrix et al., 2001). Our analysis showed that transcripts for the At3g14070 and At3g14225 genes do not detectably accumulate in leaves of 3-week-old Ler or Col-0 plants (Figure 4E). At3g14220 is expressed in leaf tissue but does not show a difference between Col-0 and Ler. Only At3g14210 shows a difference in transcript accumulation between Ler and Col-0. This difference is correlated with increased isothiocyanate production, such that the Col-0 allele at ESM1 has high At3g14210 transcript accumulation and the nitrile-producing Ler allele has low transcript levels. Because At3g14210 shows differential transcript accumulation at ESM1, we focused on testing whether At3g14210 mediates the phenotypic variation inherent to the ESM1 QTL.
Knockout Analysis in Planta
We obtained a T4 heterozygous SALK T-DNA insertion line in the Col-0 background (signal.SALK.edu) located in the At3g14210 open reading frame, SALK-043148, from the ABRC (www.Arabidopsis.org/abrc). The homozygous T-DNA knockout is in the middle of the At3g14210 open reading frame and abolished the accumulation of the At3g14210 transcript and, possibly, the resulting gene function (data not shown). We used GC-FID to test whether the homozygous At3g14210 knockout altered the ratio of endogenous nitrile to isothiocyanate hydrolysis products compared with the parental Col-0 genotype. The homozygous At3g14210 knockout has a significantly higher ratio of 5MSOP-NIT formation than the Col-0 parent in planta, indicating that At3g14210 from Col-0 represses nitrile formation relative to isothiocyanate production during glucosinolate hydrolysis (Figure 5A). Lines heterozygous for the At3g14210 knockout had an intermediate phenotype (data not shown). Our hypothesis that At3g14210 is the ESM1 QTL is supported by the facts that an At3g14210 knockout causes the same phenotypes as the ESM1 QTL, both polymorphisms correlate low gene transcript accumulation with increased nitrile ratio, and both lesions are semidominant. An alternative hypothesis is that SALK-043148 has other T-DNA insertions or sequence polymorphisms that generate the observed phenotype.
Figure 5.
ESM1 Knockout Analysis for Nitrile Production.
GC-FID was used to measure the hydrolysis of the endogenous 4-methylsulfinylbutyl glucosinolate into 5MSOP-NIT and 4MSOB-ITC. The structural outcome of endogenous glucosinolate hydrolysis is shown as the ratio of 5MSOP-NIT to the sum of 5MSOP-NIT and 4MSOB-ITC.
(A) GC-FID was conducted in the following lines: CS1945, ESPLer/LerESM1Col/Col; CS1945 × SALK-043148 F1 (the ESM1 knockout), ESPLer/ColESM1Col/Δ; CS1945 × Col-0 F1, ESPLer/ColESM1Col/Col; SALK-043148 (the ESM1 knockout), ESPCol/ColESM1Δ/Δ; and Col-0, ESPCol/ColESM1Col/Col. SALK-043148, the ESM1 knockout, is indicated by ESM1 KO for clarity. Four plants were separately measured per line per experiment, and the experiment was conducted twice. Lowercase letters above the bars show lines with statistically different nitrile production ratios as determined by t tests within ANOVA that combine the three experiments. Error bars indicate se.
(B) Endogenous glucosinolate hydrolysis in 90 F2 progeny, 10 for each genotypic class, of the CS1945 × SALK-043148 cross was measured by GC-FID. The genotypes at the ESP and ESM1 loci are shown on the x and y axes, respectively. KO represents the ESM1 knockout allele obtained from SALK-043148. Lowercase letters at the top of each bar show genetic groupings that are statistically different as determined by t tests within ANOVA. se values for the nine genotypic classes range from 0.01 to 0.02.
At3g14210/ESM1 Epistatically Influences ESP Activity
The ESM1 QTL in the Ler × Col-0 RIL population epistatically interacts with the ESP locus. This type of epistasis meets the quantitative genetics definition of epistasis, which involves nonadditivity of effects between loci (Lynch and Walsh, 1998; Lambrix et al., 2001; Mackay, 2001). To test whether the At3g14210 knockout epistatically influences ESP, we created crosses of CS1945 (ESPLer/LerESM1Col/Col) × SALK-043148 (ESPCol/ColESM1Δ/Δ) and CS1945 (ESPLer/LerESM1Col/Col) × Col-0 (ESPCol/ColESM1Col/Col) and assayed eight F1 individuals of each cross for nitrile formation using GC-FID. Both ESP and the ESM1 loci have been shown to be semidominant, permitting analysis in the F1. Our hypothesis is that if At3g14210 functions epistatically to ESP, F1 of CS1945 × SALK-043148 (ESPLer/ColESM1Col/Δ) should have higher nitrile production than F1 of CS1945 × Col-0 (ESPLer/ColESM1Col/Col), because the F1s differ in their ESM1 dosage. Furthermore, this higher nitrile production should not be attributable only to the additive effects of the ESP and ESM1 loci but instead should have a significant nonadditive component. GC-FID analysis of the F1 showed that the CS1945 × SALK-043148 F1 (nitrile ratio = 0.54) has statistically significant (P ≤ 0.05) higher nitrile formation than the CS1945 × Col-0 F1 (nitrile ratio = 0.36) (Figure 5A). Furthermore, ANOVA indicated that there is a significant nonadditive effect of the interaction between ESP and ESM1 when comparing the two different F1s with the Col-0 and SALK-043148 parents (P = 0.002 for the interaction term, P < 0.0001 for the ESP and ESM1 terms separately).
To further investigate the quantitative epistasis between ESP and ESM1, we genotyped the ESP and ESM1 loci from 200 CS1945 × SALK-043148 (ESM1 knockout) F2 individuals. We then selected 10 lines for each of the 9 different genotypic classes with respect to ESP and ESM1 and measured their endogenous glucosinolate hydrolysis via GC-FID (Figure 5B). ANOVA showed a quantitative epistatic interaction between ESP and ESM1 (P < 0.0001 for the interaction term, P < 0.0001 for the ESP and ESM1 terms separately). The epistasis is predominantly attributable to the nonfunctional ESP allele multiplicatively diminishing the phenotypic impact of allele substitution at the ESM1 locus in a nonadditive manner (Figure 5B). Thus, ESM1 (At3g14210) epistatically diminishes the ESP-mediated formation of nitrile glucosinolate hydrolysis products in planta. The cosegregation of the T-DNA insertion and altered nitrile formation in this cross supports the hypothesis that At3g14210 controls glucosinolate hydrolysis and minimizes the possibility that unlinked mutations in SALK-043148 generate the altered glucosinolate hydrolysis phenotype.
Complementation Test
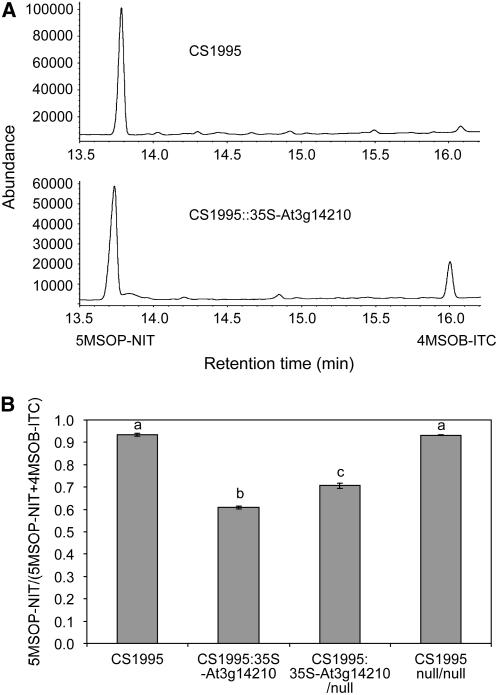
ESM1 knockout analysis in planta suggested that the ESM1 Col-0 allele in CS1945 is fully functional and leads to lower nitrile formation. To confirm this hypothesis, we performed a transgenic complementation test using the 35S cauliflower mosaic virus promoter to drive ectopic expression of At3g14210 from Col-0 in the CS1995 background containing the ESM1 Ler/knockdown allele (Clough and Bent, 1998; Bent, 2000; Hellens et al., 2000). We confirmed the presence of the transgene in the T1 and T2 with PCR and tested the endogenous nitrile conversion ratio via GC-FID. Both the T1 and T2 generations of CS1995∷35S-At3g14210 have a significant decrease (P ≤ 0.05) in nitrile formation and a correspondingly higher level of isothiocyanate formation compared with CS1995 (Figure 6A). Four independent T1 plants of CS1995∷35S-At3g14210 have an average aliphatic nitrile formation ratio of 0.81. T2 plants from two independent T1 plants segregated as three groups: T2 CS1995∷35S-At3g14210 plants homozygous for the transgene have a nitrile formation ratio of 0.61; T2 CS1995∷35S-At3g14210 plants hemizygous for the transgene have a nitrile formation ratio of 0.71; and T2 plants not containing the transgene have a nitrile formation ratio of 0.93, the same as CS1995 (Figure 6B). These differences were significant at P ≤ 0.05 by t test.
Figure 6.
Complementation Tests Show That ESM1 Represses Nitrile Formation and Increases Isothiocyanate Production.
GC-FID was used to measure the hydrolysis of the endogenous 4-methylsulfinylbutyl glucosinolate into 5MSOP-NIT and 4MSOB-ITC.
(A) Representative GC-FID traces of CS1995 and CS1995∷35S-At3g14210 (ESM1). This CS1995∷35-At3g14210 is a homozygous T3 line generated by transforming CS1995 with a construct overexpressing At3g14210, the hypothesized ESM1, driven by a 35S promoter. CS1995 has the functional Ler allele of ESP.
(B) Quantification of the structural outcome of endogenous glucosinolate hydrolysis as the ratio of 5MSOP-NIT to the sum of 5MSOP-NIT and 4MSOB-ITC. Nitrile ratios were quantified in eight CS1995 and 72 T2 plants obtained from two independent T1 progeny obtained by transforming CS1995 with 35S-At3g14210. These T2 plants were genotyped as homozygous (CS1995∷35S-At3g14210), hemizygous (CS1995∷35S-At3g14210/null), or null (CS1995∷null/null). Both T1 lines had a single insertion event leading to a 1:2:1 segregation ratio. The T3 genotypes were used to group the T2 phenotypic data. Lowercase letters above the bars show statistically significant phenotypic values as determined by t test. Error bars indicate se.
We also transformed CS1945 with the 35S-At3g14210 transgene and tested 10 homozygous T2 transgenic plants. These tests showed that even in the higher ESM1-expressing CS1945 background (Figure 4), the 35S construct led to a slight decrease in nitrile production (CS1945, 0.52 ± 0.02, versus CS1945∷35S-At3g14210, 0.45 ± 0.03; P < 0.05 by t test). As expected from the original QTL analysis (Lambrix et al., 2001), the absolute level of total glucosinolate hydrolysis product (5MSOP-NIT + 4MSOB-ITC) was unchanged in any of the transgenic plants, suggesting that At3g14210/ESM1 does not alter the rate of glucosinolate hydrolysis or glucosinolate accumulation but instead changes the ratio of the resulting products (data not shown). Our results indicate that At3g14210/ESM1 decreases 5MSOP-NIT formation and increases 4MSOB-ITC production (sulforaphane, the anticancer agent) during glucosinolate hydrolysis in planta.
In Vitro Analysis of ESM1 Functions
To test whether ESM1 regulates the hydrolysis of different glucosinolates, we conducted an in vitro analysis using exogenous allyl glucosinolate (sinigrin) and benzyl glucosinolate (glucotropaeolin) as substrates. When using exogenous benzyl glucosinolate, the ESM1 knockout (SALK-043148) shifts the hydrolysis toward the benzyl nitrile and away from the benzyl isothiocyanate compared with wild-type Col-0 (ESM1) (Figure 7). To account for the potential complication of second-site polymorphisms in SALK-043148, we also tested benzyl glucosinolate hydrolysis in ESM1 overexpression lines. Transgenic plants of CS1995∷35S-ESM1 showed repression of benzyl nitrile formation compared with CS1995, confirming that ESM1 can affect structural specificity during aromatic glucosinolate hydrolysis (Figure 7). By contrast, SALK-043148 (ESM1 knockout) and Col-0 produced identical epithionitrile, allyl nitrile, and allyl isothiocyanate levels when exogenous allyl glucosinolate (sinigrin) was used as a substrate (see Supplemental Figure 1 online).
Figure 7.
In Vitro Analysis with Benzyl Glucosinolate.
Exogenous benzyl glucosinolate was added to the glucosinolate hydrolysis assays. The resulting levels of nitrile (Benzyl-NIT) and isothiocyanate (Benzyl-ITC) products were assayed by GC-FID.
(A) Representative GC-FID traces of Benzyl-NIT and Benzyl-ITC formation after hydrolysis of exogenous benzyl glucosinolate using commercial myrosinase, Ler leaf extract (functional [fxnl] ESP, low ESM1 transcript), SALK-043148 leaf extract, the ESM1 knockout (nonfunctional ESP, nonfunctional ESM1), and Col-0 leaf extract (nonfunctional ESP, high ESM1 transcript).
(B) Quantification of the structural outcome of endogenous glucosinolate hydrolysis. Four independent plants from each line, Col-0 and SALK-043148 (ESM1 knockout [KO]), were measured in two independent experiments. Lowercase letters above the bars show statistically significant differences as determined by t tests within ANOVA that combine the data from both experiments. Error bars indicate se.
Based on ESM1 knockout analysis, ESM1 overexpression analysis, and complementation tests in planta and in vitro analysis, we conclude that the QTL ESM1 functions in the hydrolysis of aliphatic glucosinolate and aromatic glucosinolate but not alkenyl glucosinolates. The difference in ESM1 function between benzyl and allyl glucosinolate is not likely caused by their exogenous nature, because both glucosinolates were exogenously applied. In general, ESM1 alters the structural specificity during glucosinolate hydrolysis away from nitrile formation and toward isothiocyanates.
Sequence Analysis
We sequenced the Ler allele of At3g14210/ESM1 in an attempt to identify the putative causative polymorphisms leading to the differential transcript accumulation of the Ler and Col-0 alleles. We identified a number of sequence polymorphisms between the two accessions, several of which could lead to altered gene expression (Figure 8, Table 1). Most of these polymorphisms, 13 of 21, clustered within the promoter. In addition to the promoter polymorphisms, there is a G-C conversion immediately preceding the poly(A) signal that may alter polyadenylation. In contrast with potential transcript expression or processing polymorphisms, there are only four polymorphisms in the coding region. Two of these four polymorphisms are silent, whereas the other two differences in the coding region lead to conservative amino acid changes (V to A and S to G in Col-0 and Ler, respectively).
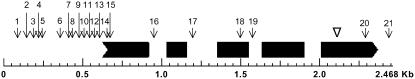
Figure 8.
GENSCAN-Predicted Gene Structure of ESM1 in Ler and Polymorphisms between Col-0 and Ler in the Region of ESM1.
The black ribbon at left indicates the initial exon, the black rectangles at center indicate internal exons, and the thick black arrow at right indicates the terminal exon to show the organization of the ESM1 gene. The spaces between exons represent introns. The Col-0 and Ler sequences at ESM1 were aligned to identify sequence polymorphisms. The numbers 1 to 21, and the corresponding vertical arrows, show the locations of sequence polymorphisms for ESM1 between Col-0 and Ler. The arrowhead shows the position of the T-DNA insertion in SALK-043148.
Table 1.
Sequence Polymorphisms along ESM1 between Col-0 and Ler
| Series Number of Polymorphism | Polymorphisms between Col-0 and Ler | Position with Regard to Ler Sequence (bp) |
|---|---|---|
| 1 | −(AT)12 | 75 to 98 |
| 2 | T→G | 132 |
| 3 | T→G | 160 |
| 4 | A→T | 182 |
| 5 | T→C | 187 |
| 6 | C→A | 397 |
| 7 | A→C | 448 |
| 8 | A→C | 468 |
| 9 | A→G | 507 |
| 10 | T→A | 525 |
| 11 | −A | 564 |
| 12 | T→G | 647 |
| 13 | T→C | 650 |
| 14 | T→C | 679 |
| 15 | A→G | 681 |
| 16 | T→C | 922 |
| 17 | A→G | 1223 |
| 18 | T→A | 1496 |
| 19 | C→A | 1554 |
| 20 | G→T | 2319 |
| 21 | G→C | 2454 |
Series numbers refer to positions in Figure 8. In the polymorphisms column, the first base is the nucleotide in Col-0 and the second base is the corresponding nucleotide in Ler. If no base is given, it is absent in that accession.
Investigation of ESM1 Effects on Insect Herbivory
Variation in glucosinolate hydrolysis structure has been correlated with plant resistance against herbivores (Chew, 1988; Borek et al., 1998; Halkier, 1999; Jander et al., 2001; Lambrix et al., 2001). The ESP and ESM1 loci were first identified as QTL for nitrile formation, resistance to T. ni (cabbage looper), and taste preference of T. ni (Jander et al., 2001; Lambrix et al., 2001). To functionally test the link between glucosinolate hydrolysis and plant–insect interactions, we conducted a preference test for T. ni using SALK-043148, the ESM1 knockout, and wild type Col-0, the accession with high levels of ESM1 transcript. The presumption is that if the nitrile increase and isothiocyanate decrease in the ESM1 knockout do not influence insect preference, then the damage by T. ni feeding on both ESM1 knockout and wild-type Col-0 should be equal. However, the insect test results demonstrated that out of 40 pairs of leaves, 12 pairs showed that Col-0 had slightly more damage than SALK-043148 (the ESM1 knockout) and 28 pairs showed that SALK-043148 was more damaged than Col-0. The comparison indicated a T. ni feeding choice difference between the ESM1 knockout and wild-type Col-0 that was statistically significant (χ2 = 15.7, df = 1, P < 0.0001). This result could be biased by the presence of second-site polymorphisms within the SALK-043148 line.
To further test this taste preference and provide experimental support for At3g14210 as the causative gene, we compared CS1995 with CS1995∷35S-At3g14210 in a choice experiment with T. ni (Figure 6). T. ni greatly preferred the high-nitrile CS1995 line compared with the higher-isothiocyanate CS1995∷35S-At3g14210 line (42 insects chose CS1995, 8 chose CS1995∷35S-At3g14210; χ2 = 23.1, df = 1, P < 0.0001). We included two independent CS1995∷35S-At3g14210 lines in our analysis; there was no significant difference between them, suggesting that this feeding preference is caused by the transgene (P > 0.2).
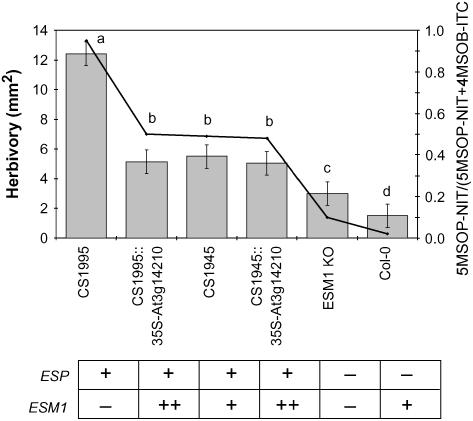
To further support the experiments showing that ESM1 influences insect herbivory choices, we measured the ability to deter T. ni herbivory in six lines that differ for both their ESP and ESM1 genes (Figure 9). Herbivory was measured as the amount of tissue eaten by the larvae over a 72-h period in a no-choice experiment. Our results showed that ESM1 deters insect herbivory in both the presence and absence of ESP. Diminished nitrile ratios were linked with a better ability to deter herbivory (Figure 9). Furthermore, the observations that knocking out ESM1 leads to increased herbivory (ESM1 knockout versus Col-0) and overexpressing ESM1 in CS1995 (the RIL with low ESM1 transcript levels) leads to decreased herbivory indicate that ESM1 alters resistance to T. ni (Figure 9). Overexpression of ESM1 affected insect herbivory only in the CS1995 parent that had low ESM1 transcript levels and not in the CS1945 line; this may be attributable to lower heritability of the transgene's effect in CS1995 compared with CS1945 (Figure 9). A larger experiment with hundreds of replicated plants might be able to identify the transgene's effects in CS1945. Overall, our results demonstrate that ESM1 has a functional role in both insect herbivory and glucosinolate hydrolysis.
Figure 9.
Glucosinolate Hydrolysis Regulates T. ni Herbivory.
Six lines, CS1995, CS1995∷35S-At3g14210, CS1945, CS1945∷35S-At3g14210, SALK-043148 (ESM1 knockout [KO]), and Col-0, were tested for their ability to deter herbivory by first instar T. ni. Below the graph, the functionality of the ESP and ESM1 loci is shown for the different lines (+, functional; −, nonfunctional/low function; ++, 35S overexpression). Twenty-five independent plants were tested per line per experiment, with two independent experiments. All data from both experiments were combined in ANOVA, and t tests were used to distinguish statistically different groups, as shown by the lowercase letters above the bars. Error bars indicate se. There was no significant line–experiment interaction, allowing the experiments to be combined. Four additional plants per line were measured by GC-FID for the structural outcome of endogenous glucosinolate hydrolysis. These results are shown by the line and are presented as the ratio of 5MSOP-NIT to the sum of 5MSOP-NIT and 4MSOB-ITC.
DISCUSSION
ESM1 QTL Cloning
We identified and cloned the gene for the ESM1 QTL using RILs, a positional cloning strategy, Affymetrix microarray data, RT-PCR, and metabolic profiling. ESM1 variation is likely attributable to naturally variable transcript accumulation in At3g14210 between the Ler and Col-0 accessions (Figures 4 to 6). Knockout and overexpression analysis confirmed that ESM1Col/Col affects the ability of Arabidopsis to hydrolyze glucosinolates into nitriles and isothiocyanates (Figures 5 to 7). Furthermore, an ESM1 knockout showed the same epistatic interaction with ESP-mediated glucosinolate hydrolysis as the ESM1 QTL. Additionally, the ESM1 T-DNA knockout and overexpressing lines altered plant–insect interactions in a similar manner to the ESM1 QTL (Figure 9) (Lambrix et al., 2001). These multiple lines of evidence strongly support the hypothesis that differential transcript accumulation caused by a cis-acting polymorphism within ESM1 is the main determinant underlying the ESM1 QTL. The actual nucleic acid change responsible for this phenotypic polymorphism remains to be identified. Because there appears to be strong recombination suppression within this region (Figure 4), the actual causal quantitative trait nucleotide polymorphism for this locus (Mackay, 2001) will be very difficult to determine. Identification of the gene, however, allows for detailed mechanistic analysis of QTL biology in terms of the biochemical and insect resistance functions.
ESM1 Identity
ESM1 is annotated as a MyAP. MyAPs were identified via copurification with myrosinase and are members of a large gene family that is typically wound– and methyl jasmonate–inducible (Taipalensuu et al., 1996). MyAPs lack sequence homology with another group of proteins that bind myrosinase, the myrosinase binding proteins (Lenman et al., 1990; Falk et al., 1995). The original proposed function of MyAP is in liberating acetylated glucosinolates from their acyl group, thereby making them available for hydrolysis by the myrosinase enzymes (Taipalensuu et al., 1997). This is likely not the case in Arabidopsis, because there are no known acylated glucosinolates and the transgenically confirmed alterations in nitrile production were with the nonacylated 4-methylsulfinylbutyl and benzyl glucosinolates. Our work identifies a novel function for MyAPs in determining structural specificity in glucosinolate hydrolysis.
ESM1 Biochemical Function
Our analysis of knockout, transgenic, and natural variation alleles showed that functional ESM1 leads to a decreased capacity to produce nitriles and an increased capacity to produce isothiocyanates from a given amount of intact glucosinolate (Figures 5 to 7 and 9). Thus, ESM1 alters the structural specificity during glucosinolate hydrolysis. The alteration occurred in both the presence and absence of significant levels of the nitrile-specifying ESP protein (Figure 5). The alteration could occur either from a direct biochemical repression of nitrile formation or a direct stimulation of isothiocyanate formation. The data presented here do not allow direct testing of these two hypotheses.
ESM1 homology and sequence analysis suggests that it is a membrane-attached carboxy-esterase/lipase that resides in the endoplasmic reticulum. Previous work has shown that a functional endoplasmic reticulum is necessary for the proper functioning of a myrosinase homolog during tissue disruption (Nagano et al., 2005). The homologous protein's function and ESM1's putative endoplasmic reticulum localization suggest a possible role for the endoplasmic reticulum in controlling glucosinolate hydrolysis and/or insect herbivory resistance.
Myrosinase Protein Complex
Myrosinase has previously been shown to be surrounded by a complex of proteins with unknown function. ESM1's identification as a MyAP, and previous suggestions that ESP is also associated with myrosinase, suggest that the protein complex may function to alter the relative production of isothiocyanates and nitriles. The semidominant nature of both loci suggests that this antagonistic influence occurs at least in part from the quantitative ratio of the two proteins. One possibility is that ESM1 may function via a direct interaction with either ESP and/or myrosinase. The nonadditive nature of the genetic interaction suggests that ESM1 somehow nonenzymatically modifies the predominantly ESP-determined ratio of isothiocyanates to nitriles. This model, in which the protein membership within the myrosinase protein complex can determine the structural specificity, establishes a system wherein the outcome of glucosinolate hydrolysis can be finely tuned. This model remains to be proven by identification and functional validation of other MyAPs.
Plant/Insect Coevolution
The observation that ESM1 functions in the absence of ESP suggests that it may alter nitrile production by means of a variety of proteins or treatments (Figure 5). ESM1 may also affect enzymatic insect defenses against glucosinolate hydrolysis products. The specialist lepidopteran, Pieris rapae, uses a nitrile-specifying protein (NSP) to force glucosinolate hydrolysis away from the toxic isothiocyanate to the less toxic nitriles (Wittstock et al., 2004). Because ESM1 has the ability to direct glucosinolate hydrolysis toward isothiocyanate production in the presence of the ESP protein, it may have the same function in the presence of NSP, thereby increasing resistance to specialist lepidopterans. This resistance could result in a coevolutionary complex of competing proteins from the insect and plant, all of which try to alter the structure of glucosinolate hydrolysis. It remains to be tested whether ESM1 can inhibit nitrile produced via NSP.
Future Avenues
In addition to the potential for enhancing resistance to insects by altering glucosinolate hydrolysis, ESM1 enables more precisely directed breeding to improve nutritional and flavor content of a variety of crucifers. As a nitrile-enhancing myrosinase cofactor, ESP activity has been found in seeds of Crambe abyssinica, turnip (Brassica rapa), brussels sprout (Brassica oleracea gemmifera), white cabbage (Brassica capitata), broccoli (Brassica oleracea botrytis), rapeseed (Brassica napus), and Lepidium sativum (Petroski and Tookey, 1982; MacLeod and Rossiter, 1985; Bernardi et al., 2000; Foo et al., 2000; Mithen et al., 2003; Matusheski et al., 2006). Furthermore, variation in the presence and absence of both ESP and ESM1 in these species could lead to altered nutritional and flavor qualities, because isothiocyanates are typically more active in anticancer assays as well as more bitter than the corresponding nitriles. Variation in the presence and absence of ESP and ESM1 could potentially lead to unique approaches to simultaneously increase the agronomic value and nutritional content of cruciferous crops. Beyond agricultural applications, the ESP/ESM1 system allows the study of how proteins that function in a complex can generate quantitative epistatic variation. Understanding the molecular mechanisms underlying quantitative epistatic variation is fundamental to both population and quantitative genetics.
METHODS
Plant Growth
Arabidopsis thaliana plants were grown in a Conviron growth chamber at 20°C and 60% RH using a photoperiod of 16 h of light and 8 h of dark with white fluorescent lights at 100 μE·m−2·s−1 PAR. Flats with 104 cells were used for planting, with a single plant per cell, generating a density of 1040 plants/m2. General-purpose growing medium (Premier Pro-Mix soil) with slow-release Osmocote fertilizer was used.
GC-FID Analysis of 4-Methylsulfinylbutyl Glucosinolate Hydrolysis Products
At 3 weeks after germination, leaves were collected for glucosinolate hydrolysis product analysis (Lambrix et al., 2001). Six mature leaves were harvested into a 6-mL, 12-mm-diameter glass vial, and the leaves were ground in 1.2 mL of water with a glass rod. This is meant to mimic the in planta conversion processes that occur during tissue disruption by either human or insect herbivores. The vial was covered with a screw-cap septum lid and incubated for 5 min at room temperature to allow endogenous proteins to hydrolyze the glucosinolates. The reaction was stopped, and hydrolysis products were extracted by adding 4 mL of dichloromethane. Samples were vortexed for 3 s and centrifuged twice at 1100g for 15 min. The organic phase was removed with a glass Pasteur pipette and dried by passing over a column containing 1 g of anhydrous sodium sulfate. The organic phase was then evaporated to 150 μL, transferred into an Agilent 100-μL pulled-point glass insert with polymer feet in an Agilent 1.5-mL screw-top vial, and covered with a septum cap for GC-FID analysis.
An HP 5890 series II gas chromatograph was used to assay the extracted glucosinolate hydrolysis products using the following program (Lambrix et al., 2001): inlet temperature of 200°C, and detector temperature of 300°C. The oven temperature was initially set at 35°C, then after 3 min it was increased to 96°C at a rate of 12°C/min. The oven was then taken to the final temperature of 240°C at a rate of 18°C/min. The temperature was then returned to 35°C and held for 6 min before the next injection. Five microliters of condensed extract was injected, and the hydrolysis products were detected with a flame ionization detector. The peak areas were manually integrated using ChemStation software. An Agilent high-resolution HP-5MS gas chromatography column (length, 30 m; i.d., 0.25 mm; film, 0.25 μm) was used for separation with 99.995% helium as the carrier gas. The hydrolysis products 4-methylsulfinylbutyl glucosinolate (glucoraphanin, a predominant glucosinolate in Arabidopsis Col-0 leaves), 5MSOP-NIT, and 4MSOB-ITC (sulforaphane, an anticancer agent) formed unique peaks with retention times of 13.7 and 16.0 min as determined by analysis with purified standards. The identities were confirmed with gas chromatography/electron impact mass spectrometry (Tierens et al., 2001).
DNA Extraction
The first pair of true leaves from 2-week-old plants was harvested into a single well of a 96-well deep-well microtiter plate for DNA extraction. Two 2.3-mm ball bearings from V&P Scientific were added to each tube, and the samples were ground to a fine powder in a paint shaker. Seven hundred microliters of extraction buffer was added to each tube and incubated at 65°C for 1 h (extraction buffer: 200 mM Tris, pH 8.0, 250 mM NaCl, 25 mM EDTA, and 0.5% SDS). The tubes were removed and allowed to cool for 10 min at room temperature. Two hundred microliters of 5 M potassium acetate, pH 7.5, was added, and the samples were centrifuged for 15 min at 3200g to precipitate the protein and insoluble materials. Seven hundred microliters of the supernatant was transferred to a 96-well deep-well microtiter plate containing 650 μL of ice-cold isopropanol per well, and the DNA was pelleted by centrifugation at 3200g for 15 min. The supernatant was removed by tipping the plate upside down, and the DNA was washed with 200 μL of 70% ethanol and centrifuged for 5 min at 3200g. The supernatant was removed, the DNA pellets were dried overnight at room temperature, and 100 μL of water was added to each well to solubilize the DNA pellet (Kliebenstein et al., 2001c).
Fine-Scale ESM1 QTL Mapping
A mapping population was generated by crossing two RILs, CS1945 and CS1995, obtained from the Ler × Col-0 RIL population (Lister and Dean, 1993). Both CS1945 and CS1995 contain a four-carbon GS-Elong locus (Col-0 allele), a nitrile-producing ESP locus (Ler allele), and a nonfunctional GS-AOP locus (Col-0 allele). However, they contain opposite parental alleles around ESM1 on the long arm of chromosome 3. This allowed mapping of the ESM1 locus without interfering variation at unlinked epistatic or confounding loci, such as ESP, GS-Elong, and GS-AOP (Kliebenstein et al., 2001c; Kroymann et al., 2001; Lambrix et al., 2001). The F1 progeny were tested via HPLC and DNA analysis to verify the allele status at the ESM1, ESP, GS-Elong, and GS-AOP loci. A single F1 individual was allowed to self to generate F2 seeds for mapping analysis.
Nine single-nucleotide polymorphism and insertion/deletion markers, equally spaced approximately every megabase pair (1, 1.9, 2.7, 4.2, 5.1, 6.0, 7.3, 8.4, and 9.6 Mb), were developed along the long arm of chromosome 3 (see Supplemental Table 1 online). PCR products and cleaved-amplified polymorphism fragments were separated on 4% agarose gels in 1× TAE buffer (40 mM Tris-acetate and 2 mM Na2EDTA). After treatment with ethidium bromide staining buffer, marker patterns were detected and recorded with AlphaImager 3400 (Alpha Innotech). Marker genotyping and GC-FID phenotyping of 286 F2 individuals localized the ESM1 locus between 4.2 and 5.1 Mb. Forty-five recombination breakpoints were identified in this collection, and the genotype-to-phenotype relationship was confirmed via analysis of eight individuals from each of the segregating 45 F3 families. The 4.2- and 5.1-Mb markers were used to identify further recombinant individuals in a population of 1056 F2 progeny. These recombinants were then phenotyped by GC-FID in the F2 and F3 generations and used to further refine the map position. Eighteen single-nucleotide polymorphism and insertion/deletion markers were developed to query the interval between 4.2 and 5.1 Mb (4.35, 4.45, 4.5, 4.59, 4.61, 4.63, 4.65, 4.69, 4.72, 4.75, 4.77, 4.79, 4.83, 4.86, 4.90, 4.93, 5.0, and 5.05 Mb). ESM1 was eventually mapped between 4.63 and 4.77 Mb, based on analysis of 1344 F2 plants. No recombination events were found between 4.65 and 4.75 Mb in the 2684 meioses tested.
RNA Extraction, Reverse Transcripts, and RT-PCR
At 3 weeks after germination, two fully expanded true leaves were harvested and total RNA was extracted with Trizol using the manufacturer's protocol (Invitrogen). Omniscript reverse transcriptase (Qiagen) was used for first-strand cDNA synthesis, and RT-PCR was conducted as described previously (Kliebenstein et al., 2001c). Primers for the ESM1 cDNA were At3g14210-2F (5′-AAGATCTTCCACAAACCTATTG-3′) and At3g14210-2R (5′-TTTGTATTCTTGTCTCACGATC-3′), with a cDNA product of 503 bp and a genomic DNA product of 895 bp. The thermocycler program for RT-PCR was as follows: 94°C for 2 min; 35 cycles of 94°C for 30 s, 55°C for 35 s, and 72°C for 45 s; and a final extension at 72°C for 10 min.
T-DNA Homozygous Line Identification
A T4 heterozygous T-DNA insertion line, SALK-043148, was obtained from the ABRC at Ohio State University (www.arabidopsis.org/abrc). Primers were designed using the SALK T-DNA verification primer design program (signal.SALK.edu/tdnaprimers): LP, 5′-TTCCATTAACTAAATTCGAGTGATACG-3′; RP, 5′-CCAGTGATCATGTAATACGTCCA-3′; and LBa1, 5′-TGGTTCACGTAGTGGGCCATCG-3′. Leaves were harvested from individual T-DNA progeny, and DNA was extracted as described above. Lines homozygous for the T-DNA insertion were identified and allowed to self as well as crossed to CS1945 and Col-0.
Sequencing of At3g14210
Three overlapping fragments spanning the At3g14210 genomic region from 668 bp before the start codon to 245 bp after the stop codon were amplified from both Col-0 and Ler genomic DNA. Primers used are as follows: At3g14210-1F, 5′-CAGTTGCCAATAGAACCGATTC-3′; At3g14210-1R, 5′-TCAAAAGCATACCGATGAAGTC-3′; At3g14210-2F, 5′-AAGATCTTCCACAAACCTATTG-3′; At3g14210-2R, 5′-TTTGTATTCTTGTCTCACGATC-3′; At3g14210-3F, 5′-GCACTTTAATTATGTTCAAATGAC-3′; At3g14210-3R, 5′-TATTAGTCCACGTAGCAATGAG-3′. PCR conditions were 94°C for 2 min; 35 cycles of 94°C for 30 s, 55°C for 35 s, and 72°C for 1 min; and a final extension at 72°C for 10 min. Amplified products were purified with the Qiagen PCR purification kit and sequenced on an ABI 3730 capillary electrophoresis genetic analyzer, and the sequences were analyzed with Vector NTI 9.0 from Invitrogen.
Complementation Test
The full-length At3g14210 cDNA was cloned into binary vector pBIN m-gfp5-ER (Haseloff and Siemering, 1998) by replacing the GFP reporter gene on the vector with a BamHI–SacI fragment of At3g14210. This places the At3g14210 cDNA under the control of the 35S cauliflower mosaic virus promoter (Kay et al., 1987; Kuhlemeier et al., 1987). The construct was verified and confirmed by sequencing. The plasmid containing At3g14210 was transformed into Agrobacterium tumefaciens strain C58C1 via electroporation at 1800 V for 5 ms using a presterilized 1-mm gap cuvette (Molecular BioProducts) and Eppendorf electroporator 2510. Transformants were selected on yeast extract peptone (YEP) plates with tetracycline (5 mg/L) and kanamycin (50 mg/L). Positive colonies were validated using the RT-PCR primers described above to test for the presence of the At3g14210 cDNA. A single positive colony was selected and cultured in liquid YEP. The transformed agrobacteria were collected by centrifugation at 2000g for 10 min and washed with 5% sucrose solution containing 20 μL of Silwet L-77 and 1 mL of 0.5 M MES per 100 mL. Agrobacteria at OD600 = 1 were sprayed evenly over inflorescences of CS1995 and covered overnight. Seeds were harvested from transformed CS1995 and sterilized. The sterilized seeds were suspended with 0.1% phytoagar, and transformants were selected on Murashige and Skoog plates containing kanamycin (50 mg/mL). Transformants were transferred into soil. Primers were designed to amplify the 35S promoter region and used for validation of transgenic plants (35S-F, 5′-ATTCAGGACTAACTGCATCAAG-3′; 35S-R, 5′-CAGTGGAGATATCACATCAATC-3′). RT-PCR was conducted as described above to test for cDNA expression in transformants containing the 35S∷At3g14210 cDNA construct. Individuals of CS1995 were planted side by side together with individuals of the T2 generation of transgenic CS1995∷35S-At3g14210. Verified T1 and T2 transformants were tested for nitrile and isothiocyanate formation as described above. The same experimental design was used to introduce the 35S-At3g14210 transgene into CS1945.
In Vitro Analysis
Allyl glucosinolate (sinigrin; Sigma-Aldrich) and benzyl glucosinolate (glucotropaeolin; Calbiochem) dissolved in 100 mM MES, pH 6.0, at 0.4 μmol/mL were used as substrates for in vitro analysis. As a control, 1 mg of Sinapis alba thioglucosidase (myrosinase) (Sigma-Aldrich) in 100 mM MES, pH 6.0, was added to 1.2 mL of glucosinolate solutions to catalyze the formation of allyl isothiocyanate from allyl glucosinolate and benzyl isothiocyanate from benzyl glucosinolate. Six leaves from plants at 3 weeks after germination were harvested into a 6-mL, 12-mm-diameter glass vial, and the leaves were ground in 1.2 mL of glucosinolate solution with a glass rod. Control leaves from Ler were used as an endogenous ESP source to catalyze the formation of epithionitrile from allyl glucosinolate and benzyl nitrile from benzyl glucosinolate. Glucosinolate hydrolysis product extraction and GC-FID analysis were as described above. The retention times were 9.4 and 11.5 min for allyl isothiocyanate and epithionitrile and 13.1 and 15.6 min for benzyl nitrile and benzyl isothiocyanate, respectively.
Insect Resistance Analysis
Cabbage looper (Trichoplusia ni) eggs were obtained from Bio-Serv. The eggs were hatched at room temperature, and neonates were allowed to grow into first instars on lepidoptera diet from Bio-Serv. For the choice experiments, plants were grown in a randomized block design. At 4 weeks after germination, one leaf of each genotype to be tested for the choice experiment was removed and placed side by side on a wet paper towel in 3 × 6-cm Petri dishes (Fisher). One larva was placed in each Petri dish between the leaves. Herbivory was digitally recorded every 24 h. The percentage of the leaves removed was measured with freehand line selection in ImageJ (version 1.32j). After 72 h, the leaves were scored with regard to which leaf was eaten the most, but only if that leaf had 60% or more of the total herbivory observed on the plate.
T. ni herbivory-resistance experiments were conducted as described previously (Lambrix et al., 2001). Twenty-five plants of each of six genotypes were planted in a randomized design. At 4 weeks after germination, a single larva was added to each plant and allowed to eat the plant for 72 h. No migration was observed, as had been noted previously (Lambrix et al., 2001). At 72 h, the leaves were digitally imaged and the absolute amount of leaf material per plant was measured with the freehand line selection tool in ImageJ. This experiment was conducted twice. The data from the two experiments were combined and tested for significance using ANOVA.
Accession Number
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number DQ286725 (for At3g14210/ESM1 in Ler).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table 1. Markers Used for ESM1 Fine-Scale Mapping.
Supplemental Figure 1. Exogenous Allyl Glucosinolate Hydrolysis.
Supplementary Material
Acknowledgments
This research was supported by the National Science Foundation (Grant MCB-0323759 to D.J.K.). We thank Andrew Waterhouse for the use of his GC-FID device and Clark Dodsworth, Heather Rowe, Dina St. Clair, and several anonymous reviewers for help in improving the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Daniel J. Kliebenstein (kliebenstein@ucdavis.edu).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.039602.
References
- Barcelo, S., Mace, K., Pfeifer, A.M.A., and Chipman, J.K. (1998). N-Nitrosodimethylamine and 2-amino-3-methylimidazo[4,5-f]quinoline in THLE cells expressing human CYP isoenzymes and inhibition by sulforophane. Mutat. Res. 402 111–120. [DOI] [PubMed] [Google Scholar]
- Bent, A.F. (2000). Arabidopsis in planta transformation. Uses, mechanisms, and prospects for transformation of other species. Plant Physiol. 124 1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi, R., Negri, A., Ronchi, S., and Palmieri, S. (2000). Isolation of the epithiospecifier protein from oil-rape (Brassica napus ssp. oleifera) seed and its characterization. FEBS Lett. 467 296–298. [DOI] [PubMed] [Google Scholar]
- Bones, A.M., and Rossiter, J.T. (1996). The myrosinase–glucosinolate system, its organisation and biochemistry. Physiol. Plant. 97 194–208. [Google Scholar]
- Borek, V., Elberson, L.R., McCaffrey, J.P., and Morra, M.J. (1998). Toxicity of isothiocyanates produced by glucosinolates in Brassicaceae species to black vine weevil egglucosinolate. J. Agric. Food Chem. 46 5318–5323. [Google Scholar]
- Chew, F.S. (1988). Searching for defensive chemistry in the Cruciferae, or, Do glucosinolates always control interactions of Cruciferae with their potential herbivores and symbionts? In Chemical Mediation of Coevolution, K.A. Spencer, ed (New York: Academic Press), pp. 81–111.
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- D'Auria, J.C., and Gershenzon, J. (2005). The secondary metabolism of Arabidopsis thaliana: Growing like a weed. Curr. Opin. Plant Biol. 8 308–316. [DOI] [PubMed] [Google Scholar]
- de Quiros, H.C., Magrath, R., McCallum, D., Kroymann, J., Schnabelrauch, D., Mitchell-Olds, T., and Mithen, R. (2000). α-Keto acid elongation and glucosinolate biosynthesis in Arabidopsis thaliana. Theor. Appl. Genet. 101 429–437. [Google Scholar]
- Fahey, J.W., Zalcmann, A.T., and Talalay, P. (2001). The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56 5–51. [DOI] [PubMed] [Google Scholar]
- Falk, A., Taipalensuu, J., Ek, B., Lenman, M., and Rask, L. (1995). Characterization of rapeseed myrosinase-binding protein. Planta 195 387–395. [DOI] [PubMed] [Google Scholar]
- Falk, K.L., Vogel, C., Textor, S., Bartram, S., Hick, A., Pickett, J.A., and Gershenzon, J. (2004). Glucosinolate biosynthesis: Demonstration and characterization of the condensing enzyme of the chain elongation cycle in Eruca sativa. Phytochemistry 65 1073–1084. [DOI] [PubMed] [Google Scholar]
- Field, B., Cardon, G., Traka, M., Botterman, J., Vancanneyt, G., and Mithen, R. (2004). Glucosinolate and amino acid biosynthesis in Arabidopsis. Plant Physiol. 135 828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo, H.L., Gronning, L.M., Goodenough, L., Bones, A.M., Danielsen, B.-E., Whiting, D.A., and Rossiter, J.A. (2000). Purification and characterisation of epithiospecifier protein from Brassica napus: Enzymic intramolecular sulphur addition within alkenyl thiohydroximates derived from alkenyl glucosinolate hydrolysis. FEBS Lett. 468 243–246. [DOI] [PubMed] [Google Scholar]
- Halkier, B.A. (1999). Glucosinolates. In Naturally Occurring Glycosides: Chemistry, Distribution and Biological Properties, R. Ikan, ed (New York: John Wiley), pp. 193–223.
- Halkier, B.A., and Du, L. (1997). The biosynthesis of glucosinolates. Trends Plant Sci. 2 425–431. [DOI] [PubMed] [Google Scholar]
- Haseloff, J., and Siemering, K.R. (1998). The uses of green fluorescent protein in plants. In Green Fluorescent Protein: Properties, Applications, and Protocols, M. Chalfie and S. Kain, eds (New York: Wiley-Liss), pp. 191–220.
- Hecht, S.S. (2000). Inhibition of carcinogenesis by isothiocyanates. Drug Metab. Rev. 32 395–411. [DOI] [PubMed] [Google Scholar]
- Hellens, R., Mullineaux, P., and Klee, H. (2000). A guide to Agrobacterium binary Ti vectors. Trends Plant Sci. 5 446–451. [DOI] [PubMed] [Google Scholar]
- Jander, G., Cui, J., Nhan, B., Pierce, N.E., and Ausubel, F.M. (2001). The TASTY locus on chromosome 1 of Arabidopsis affects feeding of the insect herbivore Trichoplusia ni. Plant Physiol. 126 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, R., Chan, A., Daly, M., and McPherson, J. (1987). Duplication of CaMV35S promoter sequences creates a strong enhancer for plant genes. Science 236 1299–1302. [DOI] [PubMed] [Google Scholar]
- Kliebenstein, D.J. (2004). Secondary metabolites and plant/environment interactions: A view through Arabidopsis thaliana tinged glasses. Plant Cell Environ. 27 675–684. [Google Scholar]
- Kliebenstein, D.J., Gershenzon, J., and Mitchell-Olds, T. (2001. a). Comparative quantitative trait loci mapping of aliphatic, indolic and benzylic glucosinolate production in Arabidopsis thaliana leaves and seeds. Genetics 159 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein, D.J., Kroymann, J., Brown, P., Figuth, A., Pedersen, D., Gershenzon, J., and Mitchell-Olds, T. (2001. b). Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 126 811–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein, D.J., Kroymann, J., and Mitchell-Olds, T. (2005). The glucosinolate–myrosinase system in an ecological and evolutionary context. Curr. Opin. Plant Biol. 8 264–271. [DOI] [PubMed] [Google Scholar]
- Kliebenstein, D.J., Lambrix, V.M., Reichelt, M., Gershenzon, J., and Mitchell-Olds, T. (2001. c). Gene duplication and the diversification of secondary metabolism: side chain modification of glucosinolates in Arabidopsis thaliana. Plant Cell 13 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein, D.J., Pedersen, D., Barker, B., and Mitchell-Olds, T. (2002). Comparative analysis of quantitative trait loci controlling glucosinolates, myrosinase and insect resistance in Arabidopsis thaliana. Genetics 161 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein, D.J., West, M.A.L., Van Leeuwen, H., Kyunga, K., Doerge, R.W., Michelmore, R.W., and St. Clair, D.A. (2006). Genomic survey of gene expression diversity in Arabidopsis thaliana. Genetics 172 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroymann, J., Donnerhacke, S., Schnabelrauch, D., and Mitchell-Olds, T. (2003). Evolutionary dynamics of an Arabidopsis insect resistance quantitative trait locus. Proc. Natl. Acad. Sci. USA 100 14587–14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroymann, J., Textor, S., Tokukisa, J.G., Falk, K.L., Bartram, S., Gershenzon, J., and Mitchell-Olds, T. (2001). A gene controlling variation in Arabidopsis glucosinolate composition is part of the methionine chain elongation pathway. Plant Physiol. 127 1077–1088. [PMC free article] [PubMed] [Google Scholar]
- Kuhlemeier, C., Green, P., and Chua, N.-H. (1987). Regulation of gene expression in higher plants. Annu. Rev. Plant Physiol. 38 221–257. [Google Scholar]
- Lambrix, V.M., Reichelt, M., Mitchell-Olds, T., Kliebenstein, D.J., and Gershenzon, J. (2001). The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell 13 2793–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenman, M., Rödin, M., Josefsson, L.-G., and Rask, L. (1990). Immunological characterization of rapeseed myrosinase. Eur. J. Biochem. 194 747–753. [DOI] [PubMed] [Google Scholar]
- Lister, C., and Dean, D. (1993). Recombinant inbred lines for mapping RFLP and phenotypic markers in Arabidopsis thaliana. Plant J. 4 745–750. [DOI] [PubMed] [Google Scholar]
- Lynch, M., and Walsh, B. (1998). Genetics and Analysis of Quantitative Traits. (Sunderland, MA: Sinauer Associates).
- Mackay, T.F.C. (2001). The genetic architecture of quantitative traits. Annu. Rev. Genet. 35 303–339. [DOI] [PubMed] [Google Scholar]
- MacLeod, A.J., and Rossiter, J.T. (1985). The occurrence and activity of epithiospecifier protein in some Cruciferae seeds. Phytochemistry 24 1895–1898. [Google Scholar]
- Matusheski, N.V., Swarup, R., Juvik, J.A., Mithen, R., Bennett, M., and Jeffery, E.H. (2006). Epithiospecifier protein from broccoli (Brassica oleracea L. ssp italica) inhibits formation of the anticancer agent sulforaphane. J. Agric. Food Chem. 54 2069–2076. [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds, T., and Pedersen, D. (1998). The molecular basis of quantitative genetic variation in central and secondary metabolism in Arabidopsis. Genetics 149 739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithen, R., Faulkner, K., Magrath, R., Rose, P., Williamson, G., and Marquez, J. (2003). Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theor. Appl. Genet. 106 727–734. [DOI] [PubMed] [Google Scholar]
- Nagano, A.J., Matsushima, R., and Hara-Nishimura, I. (2005). Activation of an ER-body-localized beta-glucosidase via a cytosolic binding partner in damaged tissues of Arabidopsis thaliana. Plant Cell Physiol. 46 1140–1148. [DOI] [PubMed] [Google Scholar]
- Petroski, R.J., and Tookey, H.L. (1982). Interactions of thioglucoside glucohydrolase and epithiospecifier protein of cruciferous plants to form 1-cyanoepithioalkanes. Phytochemistry 21 1903–1905. [Google Scholar]
- Reichelt, M., Brown, P.D., Schneider, B., Oldham, N.J., Stauber, E., Tokuhisa, J., Kliebenstein, D.J., Mitchell-Olds, T., and Gershenzon, J. (2002). Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana. Phytochemistry 59 663–671. [DOI] [PubMed] [Google Scholar]
- Taipalensuu, J., Andreasson, S., Eriksson, S., and Rask, L. (1997). Regulation of the wound-induced myrosinase-associated protein transcript in Brassica napus plants. Eur. J. Biochem. 247 963–971. [DOI] [PubMed] [Google Scholar]
- Taipalensuu, J., Falk, A., and Rask, L. (1996). A wound- and methyl jasmonate-inducible transcript coding for a myrosinase-associated protein with similarities to an early nodulin. Plant Physiol. 110 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierens, K.F.M.-J., Thomma, B.P.H.J., Brouwer, M., Schmidt, J., Kistner, K., Porzel, A., Mauch-Mani, B., Cammue, B.P.A., and Broekaert, W.F. (2001). Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant Physiol. 125 1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock, U., Agerbirk, N., Stauber, E.J., Olsen, C.E., Hippler, M., Mitchell-Olds, T., Gershenzon, J., and Vogel, H. (2004). Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc. Natl. Acad. Sci. USA 101 4859–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock, U., and Halkier, B.A. (2002). Glucosinolate research in the Arabidopsis era. Trends Plant Sci. 7 263–270. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Kensler, T.W., Cho, C.G., Posner, G.H., and Talalay, P. (1994). Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. USA 94 10367–10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.