The shoot apical meristem (SAM) is the source of all postembryonic aerial organs in plants. Because of their central importance in plant development, SAMs have been studied for more than 100 years and have also become a major focus of molecular genetic studies. These studies indicate that signaling between cells is important in the maintenance of SAM size and structure and for the positioning and development of new organs. Two signaling molecules are currently known to regulate SAMs: the secreted protein CLV3 and the classical plant hormone auxin. Studies of SAMs have been hindered by their small size and by their location within developing buds. Innovations in confocal microscopy and the development of markers for individual cells and for cell type–specific genes now allow for in vivo visualization of SAMs. These methods provide powerful tools for examining the dynamics of gene expression patterns and the instantaneous consequences of perturbation of gene function in living SAMs. In this essay, we describe the structure of SAMs, summarize experimental and molecular data for signaling events, and describe the impact of two new studies (Heisler et al., 2005; Reddy and Meyerowitz, 2005) that increase our understanding of signaling mechanisms in SAM maintenance and in organogenesis.
MERISTEM FORM AND FUNCTION
The primary SAM is formed during embryogenesis (Figure 1) and typically produces organs such as leaves and stems. Meristems that form in the axil of a leaf (axillary meristems) have the same organ-forming potential as the SAM. When an Arabidopsis plant undergoes the transition from vegetative to reproductive growth, the SAM changes to an inflorescence meristem (IM), and initiation of flower buds begins from axillary meristems in bracts or directly from the IM. The floral meristem, which gives rise to the flower bud, is responsible for initiating the four types of floral organs, the sepals, petals, stamens, and carpels, which are produced in four concentric rings. While the SAM and IM are usually indeterminate and will produce organs throughout the life of the plant, the floral meristem is unique in that it terminates after producing the floral organs.
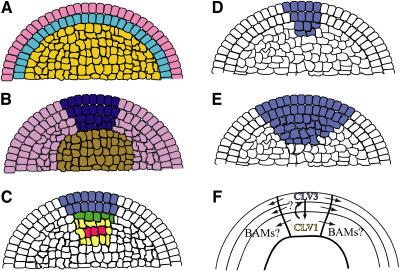
Figure 1.
Models for SAM Organization and Maintenance.
(A) Tunica corpus model for SAM structure. L1, pink; L2, light blue; L3, orange.
(B) Zonal model for SAM structure. CZ, dark blue; PZ, purple; rib zone, brown.
(C) Expression pattern of CLV pathway genes. CLV1 expression, yellow; CLV3 expression, blue; CLV3/CLV1 overlap, green; WUS expression, red.
(D) CLV3 expression domain (blue) in the wild type. Note similarity of CLV3 expression to CZ in (B).
(E) CLV3 expression in clv3 mutant background.
(F) Composite of SAM layers and zones. Arrows indicate direction of signaling between SAM regions based on experiments described in the text.
Two independent conceptual frameworks based on morphology and function are used to label SAMs (Lyndon, 1998). In the first, the SAM is divided into the tunica and corpus. The tunica contains the outer two cell layers in most dicots, known as the L1 and L2 (Figure 1A), or contains only one cell layer in monocots. The individual layers are maintained by anticlinal (within a layer) cell divisions (Satina et al., 1940). The region underlying the tunica makes up the corpus, or L3, in which cells can divide in all planes. The layers contribute differentially to organs that are initiated from the SAM, although there is some variation among species. For example, analysis of mosaics in tobacco revealed that while the L1 generated solely epidermis, both the L2 and L3 contributed to inner cell types of the leaf (Stewart and Derman, 1975). This indicates that while the L2 is restricted to a single cell layer within the SAM, the L2 and L3 contribute to several internal lineages in mature leaves.
In the second framework, SAMs are characterized into three radially distinct zones based on function (Vaughan, 1952). The central zone (CZ) contains slowly dividing stem cells and spans the tunica and corpus from the apex to the base of the dome of the SAM (Figure 1B). The peripheral zone (PZ) surrounds the CZ and contains cells that will be incorporated into primordia, the beginning stage of growth of leaves and flowers. Similarly, the rib zone lies basal to the CZ and contains cells that will be incorporated into the developing stem (Vaughan, 1952).
INTERCELLULAR SIGNALING IN THE SAM
The organization of the SAM is extremely stable, as the SAMs in long-lived trees or in saguaro cacti can be hundreds of years old. From a quick look at the structure of SAMs (Figure 1), it is obvious that cell division patterns need to be coordinated among layers both to maintain the identity of SAM layers and zones and to position organs. Cell division rates also need to be coordinated in organ primordia. This coordination would most likely involve signals.
Signals have also long been thought to play a role in the species-specific stereotypical phyllotaxy or arrangement of SAM-derived organs such as leaves or flowers (reviewed in Lyndon, 1998). In Arabidopsis, organs appear in a spiral pattern that approximates the mathematical properties of the Fibonacci series. This series can be explained by the newly emerging primordia inhibiting the surrounding cells from forming new organs. If the inhibition becomes less active with time, the influence of older primordia is lessened. The combination of inhibitor produced by the newest developing leaf and the older leaves results in a primordium forming at ∼137° away from the most recent primordium (Figure 2).
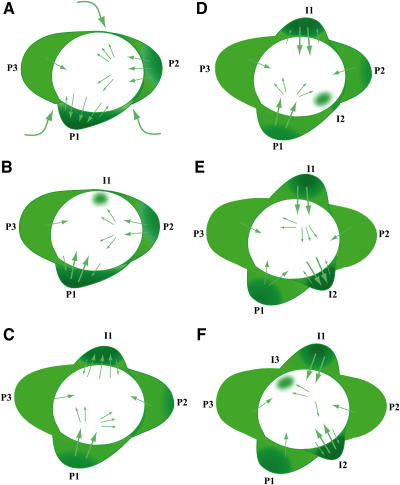
Figure 2.
Model for Auxin Dynamics in the IM.
(A) Auxin is transported from basal region in the stem through the L1 layer (indicated by curved arrows) and from the center out to the first primordium (P1).
(B) Auxin concentrates (dark green) at the tip of P2, while auxin in the base of the primordium is redirected back toward the center where it will be moved to the region of IM where I1 will emerge (green circle).
(C) Auxin is concentrated in I1.
(D) Auxin is redirected to center of IM from P2 and P1. Intersection of auxin flow from P1 and P2 marks the site of I2.
(E) Auxin concentrated in I2.
(F) Auxin is redirected to center of IM from P1, I1, and I2, resulting in positioning of new primordium at P4.
Existing leaf primordia are numbered P1, P2, and P3 in order of ascending age, and future (incipient) primordial are numbered I1, I2, and I3 in sequential order of appearance.
Experimental evidence demonstrating that intercellular signaling is required for the maintenance of SAM organization initially came from the study of colchicine-induced chimeras in Datura (Satina and Blakeslee, 1941). While most cell divisions in the L1 and L2 are anticlinal, maintaining the layer, there are occasional periclinal cell divisions in these layers that displace a cell into a new layer. These cells always adopted the fates of their new layer, demonstrating that the location of a cell is more important than lineage for determining its fate. Chimeras created from grafting experiments also indicated that there are signals between SAM layers (reviewed in Szymkowiak and Sussex, 1996). These graft experiments were performed between varieties with different leaf or floral morphologies. For example, a camellia chimera resulting from a graft between a variety with stamens and carpels was able to generate these organs from L2 and L3 from a variety with no stamens or carpels (Stewart et al., 1972). This suggested that a signal from L1 regulates the fates of L2 and L3 cells. Another series of chimeras was produced between all possible combinations of layers with two tomato strains differing in carpel number (Szymkowiak and Sussex, 1992). The genotype of the L3 in these chimeras determined carpel number, indicating that L3 can send signals to L2 and L1.
These experiments suggest that several intercellular signals originating from different layers of the meristem are involved in regulating SAM maintenance and cell fate. One pathway that signals from the L1 to L2 and L3 cells has been identified that plays a role in meristem maintenance (see the description of the CLAVATA signaling pathway below). The camellia chimeras suggest there is an additional signal from the L1 to L2 and L3 that regulates cell fates. Ligands or receptors for signaling originating from the L3 to the L2 or L1 have yet to be identified.
SIGNALING MOLECULES I: THE CLV PATHWAY
The signaling pathways in SAM communication remained elusive until the era of molecular genetic studies in Arabidopsis. Mutants in three genes with a variety of phenotypes, including extra leaves, extra floral organs with club-shaped carpels, altered phyllotaxy, and flattened stems, were identified and named clavata1 (clv1), clv2, and clv3 (Clark et al., 1993, 1995; Kayes and Clark, 1998). Molecular analysis revealed that CLV3 encodes a secreted protein (Fletcher et al., 1999), CLV1 a leucine-rich repeat (LRR) receptor-like protein kinase (RLK) (Clark et al., 1997), and CLV2 a receptor-like protein resembling CLV1 but without a kinase domain (Jeong et al., 1999).
Analysis of cell type–specific expression of CLV1 and CLV3 suggests that the CLV3 signal initiates from the L1 and L2 in the CZ, while CLV1 is expressed in L3 cells of the CZ (Figure1C). As a result of CLV1 activation by CLV3, the expression of WUSCHEL (WUS), which encodes a transcription factor required for stem cell maintenance, is restricted to four to eight cells in the L3 (Mayer et al., 1998). The CLV pathway might be analogous to the one responsible for the signaling events from L1 to L2 and L3 in camellia and tomato. There is evidence that WUS or a downstream target of WUS then acts in a feedback loop to upregulate CLV3 (Brand et al., 2000; Schoof et al., 2000). This signaling pathway could be analogous to the signaling from L3 to L1 and L2 observed in floral meristems in tomatoes, but these signals and receptors have not yet been identified in any plant.
SIGNALING MOLECULES II: AUXIN
Auxin plays two general roles in the meristem. First, auxin has long been thought to be the signal or one of the signals that regulates the initiation of primordia in a defined pattern, or phyllotaxy, in the PZ of the SAM. A second role for auxin is in the differentiation of organ primordia once they are initiated.
A number of synthetic auxins and auxin transport inhibitors affect phyllotaxy (reviewed in Lyndon, 1998). Inhibition of auxin transport results in the failure to form floral primordia (Okada et al., 1991). A recent experiment demonstrated that application of auxin locally to meristems in which organ initiation has been inhibited by mutation can restore initiation at the site of auxin application (Reinhardt et al., 2000). Therefore, auxin is not only important for establishing the placement of primordia in the PZ but plays a third role in initiation of the growth of primordia.
Information from molecular and genetic studies has led to the development of tools and assays that are used to probe the specific role of auxin in a variety of processes. One tool is a synthetic auxin-responsive promoter called DR5 (Ulmasov et al., 1997) that is fused to a reporter gene, such as glucuronidase or a fluorescent protein. The DR5 reporter reflects the ability of the cell to respond to auxin and activate transcription from this artificial promoter (reviewed in Woodward and Bartel, 2005). Two different assays for the presence of PIN1, a member of the putative auxin efflux carrier gene family in Arabidopsis, are used to track the movement of auxin between cells. Detection of PIN1 signal to a polar location on a cell membrane is taken as evidence that auxin movement out of the cell will take place at this site, thus indicating the direction of auxin flow (Galweiler et al., 1998). While some studies use specific antibodies to PIN1, others use a PIN1 promoter directing the expression of a PIN1 translational fusion to green fluorescent protein (pPIN1:PIN1-GFP).
The results of recent studies that examined DR5 and/or PIN1 expression in fixed tissue in SAMs suggested that auxin is first transported from more basal epidermal cells apically toward a new primordium (Figure 2A) (Benkova et al., 2003; Reinhardt et al., 2003). The new primordium then functions as a sink, pulling auxin from nearby cells. Localization experiments using a specific antibody to PIN1 suggest that the auxin in the primordium then moves into the vasculature and is transported basally out of the L1 of the SAM (Reinhardt et al., 2003). Since the primordium has locally depleted auxin from surrounding cells, a new primordium is most likely to initiate growth at a region of high auxin concentration as far away from the old primordium as possible. High levels of auxin accumulation would therefore initiate primordia growth, and differences in auxin concentration could account for the patterns of phyllotaxis.
DECIPHERING THE ROLE OF AUXIN IN SAMS
From the experiments described, it is clear that auxin plays a role in the initiation of organs in SAMs and in phyllotaxy. Could auxin be the inducer for primordia initiation? All of the PZ of the SAM appears able to form primordia—are concentrations of auxin above a certain threshold the trigger for initiation? Once an individual primordium is initiated, does auxin play a role in determining the axes of this organ?
If auxin is a key regulator of the differentiation of primordia, the localization and signaling potential of auxin is predicted to overlap with other components essential for differentiation. A number of transcription factors that play important roles in SAM development or primordial outgrowth and differentiation have been identified. These are expressed in spatially restricted regions of SAMs or primordia and can be used as markers to detect patterning. For example, SHOOT MERISTEMLESS (STM), a member of the Knotted class of homeodomain proteins, is required for SAM establishment and maintenance (Barton and Poethig, 1993; Long et al., 1996). STM is required for keeping cells in an undifferentiated state and is downregulated in leaf primordia. By contrast, the transcription factors LEAFY (LFY) and AINTEGUMENTA (ANT) are not expressed in the SAM but are expressed in primordia (Weigel et al., 1992; Elliott et al., 1996). The CUP SHAPED COTYLEDON (CUC) genes restrict tissue outgrowth, and CUC1 and CUC2 are expressed at a boundary between the SAM and primordia (Aida et al., 1999; Takada et al., 2001). Once the primordium starts to increase in size, upper and lower surfaces of the leaf begin to form. Additional transcription factors that play a role in development of the adaxial and abaxial surfaces of leaves have been identified and include REVOLUTA (REV) and FILAMENTOUS FLOWER (FIL), respectively (Chen et al., 1999; Sawa et al., 1999; McConnell et al., 2001; Otsuga et al., 2001). Previous analysis of CUC2, LFY, and ANT in the pin1 mutant, which does not transport auxin, indicated that auxin might play an active role in regulating the boundary between the meristem and primordia (Vernoux et al., 2000).
Heisler et al. (2005) looked at the dynamics of in vivo auxin movement in IM cells using pPIN1:PIN1-GFP to monitor the direction of auxin movement and a DR5 promoter fused to a rapid folding variant of yellow fluorescent protein (YFP) to record auxin responsiveness. They visualized flower primordia from plants grown on agar in clear plastic boxes using methods that had been previously developed (Grandjean et al., 2004; Reddy et al., 2004). The plants were submerged in water and visualized for 30 s to 1 min using confocal microscopy. After imaging, the water was removed from the plants that were then allowed to continue their growth on agar. This technique is particularly powerful in that it not only allows access to meristems that are often obscured by developing primordia but also allows real-time analysis of in vivo gene expression patterns.
Imaging of PIN1 and DR5 expression identified a stereotypical pattern in which PIN1 is localized in the lateral membranes of cells in older primordia, directing flow of auxin toward an incipient primordium. As the primordium begins to grow, some PIN1-GFP reverses cellular polarity, directing auxin back to the IM center and to newer primordia (Figure 2B). Imaging of DR5:YFP is also consistent with the predicted auxin movement. There is an additional reversal of auxin at the tip of the primordium from adaxial cells to abaxial cells. The movies are visually impressive!
The clear prediction of these results is that accumulation and depletion of auxin are important regulators of primordia initiation and phyllotaxis in IM epidermal cells and that auxin is not simply moved out of the way to the vasculature (Reinhardt et al., 2003). Therefore, auxin could be acting as a signaling molecule in that differences in concentration lead to different developmental outcomes. But what is its specific role in differentiation relative to the known factors that regulate primordial growth and differentiation of young leaves? Heisler et al. (2005) looked at several markers to correlate auxin movement with expression of the transcription factors mentioned above that regulate meristem maintenance and primordia differentiation.
The results of these experiments are perhaps best described based on auxin dynamics. High levels of auxin response are detectable in the cells that will generate the next primordium, and this is correlated with downregulation of both STM and CUC2. After the PIN1-GFP polarity reversal directs auxin away from the new primordium, STM and CUC2 are upregulated at the boundary between the leaf primordia and the CZ. By contrast, LFY shows the opposite pattern, and its expression is coincident with high levels of PIN1 and DR5. A comparison of the expression of REV, FIL, and PIN1 also suggests that auxin movement influences the domains of expression of REV and FIL. Thus, auxin may play a role in positioning the boundary between abaxial and adaxial cell fates. These experiments point to a pivotal role for auxin in establishing boundaries and cell fates and in combination with the genetic approach described below can generate an extraordinary amount of information about the components of phyllotaxis and organ initiation.
APPLYING A NEW STANDARD IN PHENOTYPIC ANALYSIS TO THE CLV SIGNALING PATHWAY
In the past 20 years, mutants and their phenotypes have been the main tools used to study signaling pathways. While this approach has generated a number of well-established signaling pathways in plants, a major problem arises in that it becomes difficult to discern between the direct effects of the loss of a single gene and the indirect effects of the loss of that gene on other genes in the genome (Cutler and McCourt, 2005). Indirect effects can be caused by the feedback regulation that is often found in signaling pathways.
Reddy and Meyerowitz (2005) addressed this problem by combining genetics and live imaging. The goal of these authors was to determine the function of CLV3 in IM organization and growth. First, a double-stranded RNA interference (RNAi) construct was used to knock down CLV3 expression. The RNAi construct was placed under the control of a two-component transcriptional activation system that is induced upon the application of the hormone dexamethasone, thus enabling the authors to regulate the timing of CLV3 silencing. Once silencing was induced, its effects were monitored using live-image confocal microscopy. Transgenic GFP markers for nuclei and plasma membranes (Reddy et al., 2004) allowed for visualization of growth dynamics, while markers for specific genes were used to follow changes in expression patterns. Using this approach, the authors were able to determine the role of CLV3 in the regulation of IM size and in maintenance of the CZ.
Previous experiments have shown that the increase in IM size is due to increases in cell number in both the CZ and rib zone (Clark et al., 1995). Reddy and Meyerowitz (2005) described three mechanisms that may explain how clv3 mutants produce larger SAMs. First, mutations in clv3 simply result in an increase in cell division, resulting in an increased number of cells. Second, cells in the CZ are deterred from relocating to the PZ and differentiating. Others have proposed these mechanisms in the past; however, Reddy and Meyerowitz (2005) propose a new mechanism that suggests that cells at the CZ/PZ boundary dedifferentiate and revert to a CZ fate.
Using the live imaging methods described above along with a marker specific to the CZ (a CLV3 promoter driving transcription of GFP [pCLV3:GFP]), the authors were able to examine the effects of CLV3 silencing on CZ size and organization (Figures 1D and 1E). Close examination of the CZ and the junction between the PZ and CZ showed that cells that had already differentiated and moved beyond the CZ began to show pCLV3:GFP activity within 24 h after silencing. These results with the CLV3 marker indicate that the increase in the size of the CZ seen after the loss of CLV3 is due to the dedifferentiation of PZ cells into CZ cells, as suggested by the third mechanism. Live imaging of single cells and their progeny showed that there was an increase in the rate of cell division after expansion of the CZ. However, the effects of the increase in division rate were observed to be greatest outside of the CZ, rather than throughout the IM. This suggests that zone-specific signaling in the IM is involved in expansion as well as being critical for maintaining the balance among the different zones.
These results have shown that CLV3 is involved in maintaining meristem organization both through regulation of cell fate determination as well as regulation of cell division. The implications from these results are that the boundaries between the different zones of the meristem are dynamic and rely on signals from across zone boundaries.
QUESTIONS FOR THE NEXT 10 YEARS
Live imaging can now be used to answer a myriad of questions about SAM organization, maintenance, the positioning and development of new organs, and the signaling mechanisms that regulate these events. Inhibiting the action of a gene or genes and following the consequences on cell behavior and gene expression simultaneously will likely become de rigueur in this literature in the next few years. Below, we highlight a few areas where these methods could be successfully applied.
Determining the Role(s) of Auxin in SAMs
The results of Heisler et al. (2005) add to an increasingly large amount of literature establishing auxin as a regulator of many aspects of meristem function, ranging from regulating gene expression in the CZ, to establishing a boundary between the CZ and the PZ, to playing a direct role in phyllotaxis, to establishing cell fates in organ primordia. How does auxin regulate these diverse processes? These live imaging approaches will also encourage an evaluation of the mechanisms used by auxin in regulating many of the transcription factors described above. Some of the enzymes that synthesize auxin are known, as are several auxin signaling components, and these can be tested for their roles in activation or repression of these transcription factors. Future studies will combine live imaging techniques with perturbation of the function of specific genes to establish a pathway of action for these diverse processes that require auxin.
Several other key areas of focus will be in understanding the mechanisms that regulate the polarity of the PIN proteins and in understanding how the rapid reversals in auxin movement occur. The cytoplasmic kinase PINOID (PID) regulates the polar localization of PIN1 (Friml et al., 2004), although little is known about the details of this regulation. PID could also play a role in the reversals of auxin, as PID-mediated responses could be rapid and not require transcription and translation. Analysis of PIN proteins and auxin movement in meristems in which PID activity is reduced could be useful in understanding how PIN localization occurs.
Unraveling the Complexity of the CLV Signaling Pathway
There are still a number of unanswered questions about the CLV pathway. clv3 mutants display stronger phenotypes than clv1 or clv2 mutants. Is this because of CLV3-mediated feedback regulation in this pathway? It would be relatively simple to compare RNAi phenotypes of CLV1 and CLV2 with that observed for CLV3. The work of Reddy and Meyerowitz (2005) hints at the cause of the clv3 phenotypes. Knockdown of CLV3 affects the fates of cells positioned laterally relative to the CLV3-expressing domain (Figure 1E). Could CLV3 be signaling to other receptors expressed in the CZ/PZ boundary or to the PZ? The LRR RLKs most closely related to CLV1 are BARELY ANY MERISTEM1 (BAM1), BAM2, and BAM3 (DeYoung et al., 2006). All three BAM genes are expressed together in SAMs and are required for their maintenance. Strikingly, BAM1 and BAM2 in particular have expression patterns that partially overlap with CLV1 in the CZ but are more strongly expressed in the PZ (DeYoung et al., 2006), as would be expected for a receptor for a lateral signal originating from the CZ (Figure 1F). Strong clv1 mutants have been observed to be dominant-negative (Dievart et al., 2003), and one possibility is that pathway interference occurs through interactions with the BAM RLKs. It is also possible that additional proteins related to CLV3 (so-called CLV3/ESR-related genes, or CLEs) signal from the CZ to the PZ, as several CLEs are expressed in SAMs (Sharma et al., 2003). With the technology described in these two articles, the CLE and LRR RLK-mediated signaling networks will be more clearly illuminated.
Investigating Possible Connections between Auxin and the CLV Signaling Pathway
Based on results from both live imaging studies and from many other studies, the boundary between the CZ and PZ is the site of several signaling pathways (Figure 1F). Could there be crosstalk between these pathways? CLV1 mutants are sensitive to the dose of STM, and STM expression is influenced by auxin. Specifically, it has been hypothesized that competition between the opposing functions of these genes occurs at the CZ/PZ boundary (Clark et al., 1996). Conditional silencing of STM combined with live imaging may help to better understand the relationship between the STM and CLV pathways.
Toward a Universal Model for Meristems
Live imaging studies have established the groundwork for testing whether the genetic networks responsible for SAM maintenance and function are conserved between the different forms of meristems within a plant. Because transformation is possible in many plants, the combination of reporter genes and RNAi for gene silencing can also be applied to other plants to test if there is a common set of mechanisms in plants for generating organs. As described above, the structures of SAMs are generally similar, although there can be differences in the number of cell layers. Recent studies in maize and rice indicate that the function of the CLV pathway may be conserved. Mutations in genes that align most closely with CLV1 and CLV2 produce a suite of phenotypes similar to mutations in clv1 and clv2 in Arabidopsis (Taguchi-Shiobara et al., 2001; Suzaki et al., 2004; Bommert et al., 2005).
Phyllotactic patterns vary between species but also between different stages within a single species. While most Arabidopsis leaves and flowers display spiral phyllotaxis, the first two leaves developing from the seedling in Arabidopsis display a form of phyllotaxy known as decussate, in which pairs of leaves emerge at the same node at right angles to the previous lateral organs (in this case cotyledons). Maize leaves arise on the opposite side of the meristem from the previously formed leaf, a pattern known as distichous phyllotaxy. Examining the regulation of auxin movement and the expression dymamics of primordia patterning genes will reveal which aspects of primordia initiation are conserved and the mechanisms that allow for these different forms of phyllotaxy.
Systems Approaches to Studying Signaling in Meristems
The dynamic nature of auxin movement and response in SAMs has provided challenges that can be met with live imaging and functional studies. Several groups have combined experimental approaches with computer modeling to make predictions about auxin movement and phyllotaxy. A computer simulation of auxin movement revealed that the center of the meristem is predicted to contain substantial amounts of auxin, and experimental analysis confirmed this result (Barbier de Reuille et al., 2006). A second study created a computer simulation of phyllotaxis combining PIN1 localization data and auxin distribution and concentration (Smith et al., 2006). This simulation was able to reproduce the Arabidopsis spiral pattern of phyllotaxis and, with changes in parameters, to generate decussate and distichous phyllotaxy. A third study focused on auxin transport dynamics and feedback relationships between auxin and PIN localization (Jonsson et al., 2006). This model, which adds a mechanical component to simulate cellular growth, can predict the rapid reversals observed in the analysis of Heisler et al. (2005). These three studies provide examples of the power of experimental studies to develop models that then inform further experimental approaches.
Acknowledgments
We apologize to authors whose work we were unable to cite or discuss due to space limitations. We thank Karen Schumaker, Ravishankar Palanivelu, and the reviewers for their insightful comments on the manuscript. Research in the Tax lab is supported by grants from the National Science Foundation (IBN-0347675 and MCB-0418946). A.D. is supported by the National Science Foundation Integrative Graduate Education Research Training Grant Genomics Initiative (DGE-0114420).
References
- Aida, M., Ishida, T., and Tasaka, M. (1999). Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: Interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126 1563–1570. [DOI] [PubMed] [Google Scholar]
- Barbier de Reuille, P., Bohn-Courseau, I., Ljung, K., Morin, H., Carraro, N., Godin, C., and Traas, J. (2006). Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proc. Natl. Acad. Sci. USA 103 1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton, M.K., and Poethig, R.S. (1993). Formation of the shoot apical meristem in Arabidopsis thaliana: An analysis of development in the wild type and in the shoot meristemless mutant. Development 119 823–831. [Google Scholar]
- Benkova, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertova, D., Jurgens, G., and Friml, J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602. [DOI] [PubMed] [Google Scholar]
- Bommert, P., Lunde, C., Nardmann, J., Vollbrecht, E., Running, M., Jackson, D., Hake, S., and Werr, W. (2005). thick tassel dwarf1 encodes a putative maize ortholog of the Arabidopsis CLAVATA1 leucine-rich repeat receptor-like kinase. Development 132 1235–1245. [DOI] [PubMed] [Google Scholar]
- Brand, U., Fletcher, J.C., Hobe, M., Meyerowitz, E.M., and Simon, R. (2000). Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289 617–619. [DOI] [PubMed] [Google Scholar]
- Chen, Q., Atkinson, A., Otsuga, D., Christensen, T., Reynolds, L., and Drews, G.N. (1999). The Arabidopsis FILAMENTOUS FLOWER gene is required for flower formation. Development 126 2715–2726. [DOI] [PubMed] [Google Scholar]
- Clark, S.E., Jacobsen, S.E., Levin, J.Z., and Meyerowitz, E.M. (1996). The CLAVATA and SHOOT MERISTEMLESS loci competitively regulate meristem activity in Arabidopsis. Development 122 1567–1575. [DOI] [PubMed] [Google Scholar]
- Clark, S.E., Running, M.P., and Meyerowitz, E.M. (1993). CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119 397–418. [DOI] [PubMed] [Google Scholar]
- Clark, S.E., Running, M.P., and Meyerowitz, E.M. (1995). CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121 2057–2067. [Google Scholar]
- Clark, S.E., Williams, R.W., and Meyerowitz, E.M. (1997). The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89 575–585. [DOI] [PubMed] [Google Scholar]
- Cutler, S., and McCourt, P. (2005). Dude, where's my phenotype? Dealing with redundancy in signaling networks. Plant Physiol. 138 558–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung, B.J., Bickle, K.L., Schrage, K.J., Muskett, P., Patel, K., and Clark, S.E. (2006). The CLAVATA1-related BAM1, BAM2 and BAM3 receptor kinase-like proteins are required for meristem function in Arabidopsis. Plant J. 45 1–16. [DOI] [PubMed] [Google Scholar]
- Dievart, A., Dalal, M., Tax, F.E., Lacey, A.D., Huttly, A., Li, J., and Clark, S.E. (2003). CLAVATA1 dominant-negative alleles reveal functional overlap between multiple receptor kinases that regulate meristem and organ development. Plant Cell 15 1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, R.C., Betzner, A.S., Huttner, E., Oakes, M.P., Tucker, W.Q., Gerentes, D., Perez, P., and Smyth, D.R. (1996). AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, J.C., Brand, U., Running, M.P., Simon, R., and Meyerowitz, E.M. (1999). Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283 1911–1914. [DOI] [PubMed] [Google Scholar]
- Friml, J., et al. (2004). A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306 862–865. [DOI] [PubMed] [Google Scholar]
- Galweiler, L., Guan, C., Muller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282 2226–2230. [DOI] [PubMed] [Google Scholar]
- Grandjean, O., Vernoux, T., Laufs, P., Bellcram, K., Mizukami, Y., and Traas, J. (2004). In vivo analysis of cell division, cell growth, and differentiation at the shoot apical meristem in Arabidopsis. Plant Cell 16 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler, M.G., Ohno, C., Das, P., Sieber, P., Reddy, G.V., Long, J.A., and Meyerowitz, E.M. (2005). Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 15 1899–1911. [DOI] [PubMed] [Google Scholar]
- Jeong, S., Trotochaud, A.E., and Clark, S.E. (1999). The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson, H., Heisler, M.G., Shapiro, B.E., Meyerowitz, E.M., and Mjolsness, E. (2006). An auxin-driven polarized transport model for phyllotaxis. Proc. Natl. Acad. Sci. USA 103 1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayes, J.M., and Clark, S.E. (1998). CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 125 3843–3851. [DOI] [PubMed] [Google Scholar]
- Long, J.A., Moan, E.I., Medford, J.I., and Barton, M.K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379 66–69. [DOI] [PubMed] [Google Scholar]
- Lyndon, R.F. (1998). The Shoot Apical Meristem: Its Growth and Development. (Cambridge, UK: Cambridge University Press).
- Mayer, K.F., Schoof, H., Haecker, A., Lenhard, M., Jurgens, G., and Laux, T. (1998). Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95 805–815. [DOI] [PubMed] [Google Scholar]
- McConnell, J.R., Emery, J., Eshed, Y., Bao, N., Bowman, J., and Barton, M.K. (2001). Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411 709–713. [DOI] [PubMed] [Google Scholar]
- Okada, K., Ueda, J., Komaki, M.K., Bell, C.J., and Shimura, Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuga, D., DeGuzman, B., Prigge, M.J., Drews, G.N., and Clark, S.E. (2001). REVOLUTA regulates meristem initiation at lateral positions. Plant J. 25 223–236. [DOI] [PubMed] [Google Scholar]
- Reddy, G.V., Heisler, M.G., Ehrhardt, D.W., and Meyerowitz, E.M. (2004). Real-time lineage analysis reveals oriented cell divisions associated with morphogenesis at the shoot apex of Arabidopsis thaliana. Development 131 4225–4237. [DOI] [PubMed] [Google Scholar]
- Reddy, G.V., and Meyerowitz, E.M. (2005). Stem-cell homeostasis and growth dynamics can be uncoupled in the Arabidopsis shoot apex. Science 310 663–667. [DOI] [PubMed] [Google Scholar]
- Reinhardt, D., Mandel, T., and Kuhlemeier, C. (2000). Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, D., Pesce, E.R., Stieger, P., Mandel, T., Baltensperger, K., Bennett, M., Traas, J., Friml, J., and Kuhlemeier, C. (2003). Regulation of phyllotaxis by polar auxin transport. Nature 426 255–260. [DOI] [PubMed] [Google Scholar]
- Satina, S., and Blakeslee, A.F. (1941). Periclinal chimeras in Datura stramonium in relation to the development of the leaf and flower. Am. J. Bot. 28 862–871. [Google Scholar]
- Satina, S., Blakelsee, A.F., and Avery, A.G. (1940). Demonstration of the three germ layers in the shoot apex of Datura by means of induced polyploidy in periclinal chimeras. Am. J. Bot. 27 895–905. [Google Scholar]
- Sawa, S., Watanabe, K., Goto, K., Kanaya, E., Morita, E.H., and Okada, K. (1999). FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev. 13 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof, H., Lenhard, M., Haecker, A., Mayer, K.F., Jurgens, G., and Laux, T. (2000). The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100 635–644. [DOI] [PubMed] [Google Scholar]
- Sharma, V.K., Ramirez, J., and Fletcher, J.C. (2003). The Arabidopsis CLV3-like (CLE) genes are expressed in diverse tissues and encode secreted proteins. Plant Mol. Biol. 51 415–425. [DOI] [PubMed] [Google Scholar]
- Smith, R.S., Guyomarc'h, S., Mandel, T., Reinhardt, D., Kuhlemeier, C., and Prusinkiewicz, P. (2006). A plausible model of phyllotaxis. Proc. Natl. Acad. Sci. USA 103 1301–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, R.N., and Derman, H. (1975). Flexibility in ontogeny as shown by the contribution of the shoot apical layers to leaves of periclinal chimaeras. Am. J. Bot. 66 47–58. [Google Scholar]
- Stewart, R.N., Meyer, F.G., and Dermen, H. (1972). Camellia + ‘Daisy Eagleson’, a graft chimera of Camellia sasaqua and C. japonica. Am. J. Bot. 59 515–524. [Google Scholar]
- Suzaki, T., Sato, M., Ashikari, M., Miyoshi, M., Nagato, Y., and Hirano, H.-Y. (2004). The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development 131 5649–5657. [DOI] [PubMed] [Google Scholar]
- Szymkowiak, E.J., and Sussex, I.M. (1992). The internal meristem layer (L3) determines floral meristem size and carpel number in tomato periclinal chimeras. Plant Cell 4 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymkowiak, E.J., and Sussex, I.M. (1996). What chimeras can tell us about plant development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 351–376. [DOI] [PubMed] [Google Scholar]
- Taguchi-Shiobara, F., Yuan, Z., Hake, S., and Jackson, D. (2001). The fascinated ear2 gene encodes a leucine-rich repeat receptor-like protein that regulates shoot meristem proliferation in maize. Genes Dev. 15 2755–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada, S., Hibara, K., Ishida, T., and Tasaka, M. (2001). The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation. Development 128 1127–1135. [DOI] [PubMed] [Google Scholar]
- Ulmasov, T., Murfett, J., Hagen, G., and Guilfoyle, T.J. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, J.G. (1952). Structure of the angiosperm apex. Nature 169 458–459. [Google Scholar]
- Vernoux, T., Kronenberger, J., Grandjean, O., Laufs, P., and Traas, J. (2000). PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development 127 5157–5165. [DOI] [PubMed] [Google Scholar]
- Weigel, D., Alvarez, J., Smyth, D.R., Yanofsky, M.F., and Meyerowitz, E.M. (1992). LEAFY controls floral meristem identity in Arabidopsis. Cell 69 843–859. [DOI] [PubMed] [Google Scholar]
- Woodward, A.W., and Bartel, B. (2005). A receptor for auxin. Plant Cell 17 2425–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]