Abstract
Proteins of the YidC/Oxa1p/ALB3 family play an important role in inserting proteins into membranes of mitochondria, bacteria, and chloroplasts. In Chlamydomonas reinhardtii, one member of this family, Albino3.1 (Alb3.1), was previously shown to be mainly involved in the assembly of the light-harvesting complex. Here, we show that a second member, Alb3.2, is located in the thylakoid membrane, where it is associated with large molecular weight complexes. Coimmunoprecipitation experiments indicate that Alb3.2 interacts with Alb3.1 and the reaction center polypeptides of photosystem I and II as well as with VIPP1, which is involved in thylakoid formation. Moreover, depletion of Alb3.2 by RNA interference to 25 to 40% of wild-type levels leads to a reduction in photosystems I and II, indicating that the level of Alb3.2 is limiting for the assembly and/or maintenance of these complexes in the thylakoid membrane. Although the levels of several photosynthetic proteins are reduced under these conditions, other proteins are overproduced, such as VIPP1 and the chloroplast chaperone pair Hsp70/Cdj2. These changes are accompanied by a large increase in vacuolar size and, after a prolonged period, by cell death. We conclude that Alb3.2 is required directly or indirectly, through its impact on thylakoid protein biogenesis, for cell survival.
INTRODUCTION
The primary reactions of photosynthesis are catalyzed by four protein complexes: photosystem II and photosystem I (PSII and PSI), with their associated light-harvesting systems; the cytochrome b6f complex; and the ATP synthase. These complexes are embedded within the thylakoid membrane of chloroplasts of algae and land plants. Considerable progress has been achieved in determining the subunit composition and structure of these complexes. Genetic and biochemical studies have revealed that biosynthesis of the photosynthetic apparatus depends on concerted interactions between the nuclear and chloroplast genetic systems (Barkan and Goldschmidt-Clermont, 2000). Many nucleus-encoded factors participate in posttranscriptional steps of chloroplast gene expression, including RNA processing, RNA stability, splicing, and translation. Moreover, several identified factors involved in protein membrane routing, insertion, and assembly in the thylakoid membrane are associated with four pathways of membrane integration (Cline and Henry, 1996; Keegstra and Cline, 1999; Jarvis and Robinson, 2004). Some proteins can integrate spontaneously into the membrane cotranslationally or posttranslationally. Others use a pathway related to the Sec system of the bacterial export machinery. The light-harvesting proteins use mostly the GTP-dependent signal recognition particle (SRP) system, whereas other proteins use the TAT pathway, in which the transthylakoid pH gradient is used as its sole energy source but is not mandatory in vivo (Theg et al., 2005). Thus, each of these pathways has its own energy requirements. Additional factors are involved in the assembly of the photosynthetic complexes. The Hcf136 protein is specifically required for the assembly of PSII in Arabidopsis thaliana (Meurer et al., 1998; Plucken et al., 2002). In the case of PSI, several factors specifically required for its assembly have been identified in cyanobacteria, algae, and land plants. They include BtpA (Bartsevich and Pakrasi, 1997; Zak et al., 1999; Zak and Pakrasi, 2000), Ycf3, and Ycf4 (Wilde et al., 1995; Boudreau et al., 1997; Ruf et al., 1997). Ycf3 appears to act as a chaperone that interacts directly and specifically with some of the PSI core subunits during assembly of the PSI complex (Naver et al., 2001). Ycf4 is part of a large complex that may play a role in the initial events of PSI protein assembly (Boudreau et al., 1997). However, the exact mode of action of these assembly factors is still poorly understood.
In yeast, the inner mitochondrial membrane protein Oxa1p has emerged as an important factor for the assembly of the respiratory chain complexes into the inner mitochondrial membrane (Bonnefoy et al., 1994; Hell et al., 1998, 2001). Oxa1p interacts directly with protein translocation intermediates and facilitates their insertion into the membrane. It contains a ribosome binding domain at its C-terminal end that facilitates its cotranslational interaction with mitochondrial translation products (Jia et al., 2003; Szyrach et al., 2003). However, Oxa1p is also involved in inserting nucleus-encoded mitochondrial polypeptides into the inner mitochondrial membrane. A related protein, Oxa2p, functions mainly in posttranslational steps of the assembly of cytochrome oxidase (Funes et al., 2004b). Both proteins have been conserved and are found in mitochondria of fungi, plants, and animals (Preuss et al., 2005). These proteins are related in sequence to the Escherichia coli YidC protein that is involved in membrane protein assembly in bacteria (Samuelson et al., 2000; Scotti et al., 2000). Depending on the substrate protein that is integrated into the membrane, YidC acts either as a separate unit or in connection with the Sec-YEG translocon (Samuelson et al., 2001).
Oxa1p, Oxa2p, and YidC belong to the same protein family as ALB3 in land plants (Luirink et al., 2001; Kuhn et al., 2003). In Arabidopsis, ALB3 was shown to be essential for the assembly of the light-harvesting complex (LHC) in the thylakoid membrane (Moore et al., 2000). Mutants lacking ALB3 have an albino phenotype (Sundberg et al., 1997). In the ac29 mutant of Chlamydomonas reinhardtii, which lacks the Alb3.1 gene, LHC accumulation is decreased by >10-fold (Bellafiore et al., 2002). However, mutants lacking Alb3.1 are less affected than the Arabidopsis alb3 mutant and are still able to grow photoautotrophically (Bellafiore et al., 2002). In vitro experiments revealed that treatment of thylakoid membranes of Arabidopsis with an ALB3 antibody blocks the integration of chlorophyll binding proteins of LHC, indicating a strict correlation between the requirements for ALB3 and SRP (Moore et al., 2003). By contrast, insertion of proteins that are dependent on the Sec or TAT pathways is not prevented under these conditions. However, a direct interaction has been demonstrated between ALB3 and the chloroplast SECY translocase (Klostermann et al., 2002; Pasch et al., 2005). Because the latter is also involved in cotranslational insertion of chloroplast-encoded proteins into the thylakoid membrane (Zhang et al., 2001), these results suggest that ALB3 may also participate in this process. Indeed, studies with C. reinhardtii have revealed a role for Alb3.1 in the assembly of the PSII reaction center polypeptide D1. PSII accumulation is reduced by 2-fold in light-grown cells and by nearly 10-fold in dark-grown cells, and integration of D1 into the core complex of PSII is slowed significantly in the Alb3.1-deficient mutant (Bellafiore et al., 2002; Ossenbuhl et al., 2004). Moreover, use of the yeast split-ubiquitin system has revealed direct interactions between ALB3 and several thylakoid membrane proteins, such as D1, D2, CP43, PsaA, and the ATP synthase subunit CF0III (Pasch et al., 2005).
Another Arabidopsis protein, ARTEMIS, is related to ALB3 (Fulgosi et al., 2002). This protein, which is localized in the chloroplast inner envelope membrane, consists of an N-terminal receptor-like region, a central Gly-rich stretch with a nucleoside triphosphate binding site, and a C-terminal domain resembling YidC/Oxa1p/ALB3. Mutants deficient in ARTEMIS contain chloroplasts that are arrested in the late stages of division. Besides ALB3 and ARTEMIS, four additional genes of this class have been identified in Arabidopsis, one of which is predicted to be targeted to the chloroplast and the other three to mitochondria (Luirink et al., 2001). Little is known about the functions of these genes.
The YidC/Oxa1p/ALB3 proteins contain five to six putative transmembrane domains. Although their sequence identity is relatively low (∼20%), they complement each other to some extent. Thus, YidC partially complements the yeast oxa1 and oxa2 mutants (Preuss et al., 2005), and ALB3 is able to complement a bacterial YidC-deficient mutant (Jiang et al., 2002). Moreover, ARTEMIS can partially replace the function of Oxa1 in the insertion and assembly of mitochondrial membrane proteins (Funes et al., 2004a). However, Oxa1 and Oxa2 do not complement each other, indicating that they perform distinct functions (Saracco and Fox, 2002).
Besides Alb3.1, there is a second Alb3-like gene in C. reinhardtii called Alb3.2 (Bellafiore et al., 2002). Alb3.2 displays slightly greater sequence identity with ALB3 of Arabidopsis than with Alb3.1. It also lacks the receptor-like domain of ARTEMIS. The existence of Alb3.2 in Chlamydomonas raised questions about its function. Given the fact that loss of Alb3.1 leads to a severe reduction of LHC, it seemed likely that these two genes have mostly nonredundant functions. Here, we have characterized Alb3.2 at the functional level using RNA interference (RNAi). We show that this protein plays an essential role in the assembly of the PSII and PSI reaction centers in the thylakoid membrane. The protein is present in limiting amounts, and cells die when it is below a certain threshold level, indicating an essential role for Alb3.2.
RESULTS
Localization of Alb3.2 in the Thylakoid Membrane
The Alb3.2 gene was identified as a homolog of Alb3.1 in the EST database, and the full-length cDNA was isolated (Bellafiore et al., 2002). To determine the cellular location of Alb3.2, a triple FLAG tag was inserted at the C-terminal end of its coding region just upstream of the putative stop codon. The genomic sequence containing the promoter of Alb3.2 was obtained by screening an indexed cosmid library using the EST sequence as a probe. A 9-kb fragment containing the promoter of Alb3.2 and the first four exons was cloned in frame with the FLAG-tagged cDNA. This construct was introduced by transformation into the cell wall–deficient strain cw15, which is particularly suited for isolating intact organelles. Subsequently, an antibody was raised against the C-terminal soluble domain of the protein. It recognizes the protein of 40 kD as well as a FLAG-tagged version of 47 kD (Figure 1A).
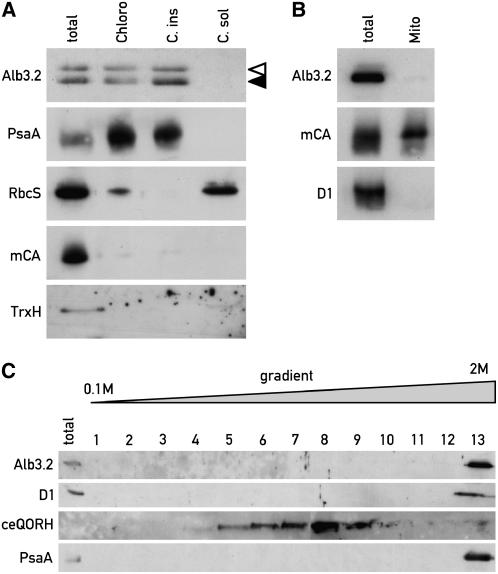
Figure 1.
Localization of Alb3.2 Protein in the Chloroplast Compartment.
(A) Immunoblot analysis of proteins from cw15 cells expressing FLAG-tagged Alb3.2. Extracts from purified chloroplasts (Chloro) and from chloroplast insoluble (C. ins) and soluble (C. sol) fractions were examined. The two bands in the immunoblot with Alb3.2 antiserum correspond to tagged (open arrowhead) and untagged (closed arrowhead) Alb3.2 protein. Samples of 20 μg of protein were loaded in each lane.
(B) Immunoblot analysis of proteins from total cells and purified mitochondria (Mito) of cw15.
(C) Alb3.2 is a thylakoid protein. Thylakoids from cw15 were separated from envelopes by centrifugation on a 0.1 to 2 M sucrose gradient for 4 h at 100,000g. Fractions were tested by immunoblotting.
Filters were incubated with antisera raised against the indicated proteins: Alb3.2, PsaA, RbcS (small subunit of ribulose-1,5-bis-phosphate carboxylase/oxygenase), mCA (mitochondrial carbonic anhydrase), TrxH1 (thioredoxin H), D1, and ceQORH (chloroplast envelope quinone oxidoreductase homolog).
The subcellular localization of Alb3.2 was determined in the cw15 strain expressing FLAG-tagged Alb3.2 to ensure proper localization of the tagged protein. Chloroplasts were isolated, broken, and separated into soluble and insoluble membrane fractions. Each fraction was analyzed by protein immunoblotting. The purity of the fractions was monitored using marker antibodies. Both the tagged and authentic Alb3.2 proteins were found in the chloroplast membrane fraction (Figure 1A). This fraction contains thylakoids, as confirmed by the presence of the PSI reaction center subunit PsaA, but no cytosolic thioredoxin H, mitochondrial carbonic anhydrase, or chloroplast stromal ribulose-1,5-bis-phosphate carboxylase/oxygenase could be detected. Because mitochondrial carbonic anhydrase fractionates with both the soluble and membrane fractions (data not shown), it is unlikely that mitochondrial membrane fragments contaminated the chloroplast preparation. Thus, the tag does not interfere with Alb3.2 protein localization in the chloroplast membrane.
To exclude the possibility that a portion of Alb3.2 is localized in mitochondrial membranes, mitochondria were purified from the cw15 strain. No Alb3.2 was found in the mitochondrial fraction, indicating that Alb3.2 is localized exclusively in the chloroplast (Figure 1B). It is notable that chloroplasts from the strain expressing the tagged version of Alb3.2 were free of contaminating mitochondria after extensive washing of the crude chloroplasts with breaking buffer (Figure 1A). However, a large portion of chloroplasts were broken during these washes, and the yield was low. In most other strains tested, such washes remove a considerable part of mitochondrial contamination, but the extent of removal varies from one strain to another.
Because Alb3.2 is also related to ARTEMIS from Arabidopsis, which has been localized in the inner membrane of the chloroplast envelope (Fulgosi et al., 2002), we tested whether Alb3.2 is associated with chloroplast envelopes. Thylakoids were prepared and separated from the envelope by sucrose density gradient centrifugation as described previously (Perron et al., 1999). Alb3.2 colocalizes with D1 and PsaA in the thylakoid membrane (Figure 1C, fraction 13) and is absent from envelope fractions that were identified using an antibody against ceQORH, a quinone oxidoreductase homolog that is known to be localized in the inner membrane of the chloroplast envelope (Figure 1C, fractions 5 to 9) (Miras et al., 2002). Stacked and unstacked thylakoids were not separated on this gradient, as seen by the cofractionation of the PSII protein D1 and the PSI protein PsaA.
This localization in the thylakoid membrane suggests that Alb3.2 has a function related to thylakoid assembly, similar to C. reinhardtii Alb3.1 and Arabidopsis ALB3, rather than to chloroplast division, like Arabidopsis ARTEMIS.
Alb3.2 Is Associated with Large Molecular Mass Complexes
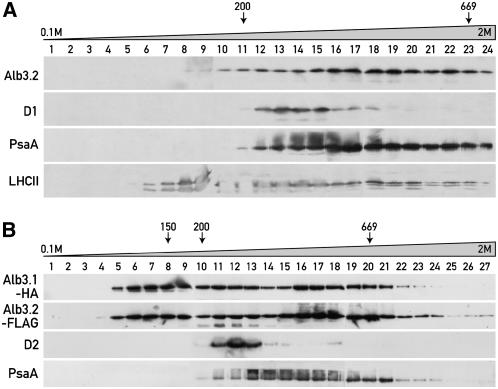
It was shown previously that Alb3.1 is associated with high molecular mass complexes of 100 to 150 and 600 to 700 kD (Bellafiore et al., 2002). To check the presence of Alb3.2 in these or similar complexes, thylakoid membranes from the wild-type strain were solubilized and the complexes were separated by sucrose density gradient centrifugation. The fractions were immunoblotted with αAlb3.2, αD1, αPsaA, and αP11 (LHCII) antibodies (Figure 2A). Alb3.2 signals can be observed from fractions 10 to 24 in a region that includes LHCII complexes, PSI, and PSII. The distribution of Alb3.2 is very similar to that of Alb3.1, as seen in gradients using cells of ac29 expressing both hemagglutinin (HA)-tagged Alb3.1 and FLAG-tagged Alb3.2 (Figure 2B). In contrast with untagged Alb3.2, the tagged protein is also present in the lower molecular mass region. Moreover, a small amount of tagged Alb3.2 is found at the bottom of the gradient, indicating the presence of very large molecular mass complexes or aggregates. These results raise the question of whether Alb3.1 and Alb3.2 are part of the same complexes and whether they may also be associated with the two photosystems.
Figure 2.
Alb3.1 and Alb3.2 Are Part of High Molecular Mass Complexes.
(A) Thylakoid membranes from wild-type cells. The membranes were solubilized with β-dodecyl maltoside and fractionated by centrifugation on a linear 0.1 to 2 M sucrose gradient for 16 h at 170,000g. Fractions were used for immunoblotting with the indicated antibodies.
(B) Thylakoid membranes from the ac29 strain containing HA-tagged Alb3.1 and FLAG-tagged Alb3.2. Membranes were solubilized and fractionated as described for (A). Fractions were used for immunoblotting with the HA, FLAG, D2, and PsaA antibodies.
The lower Alb3.2 band is unspecific, as it is also observed with total protein extracts from an untagged wild-type strain (data not shown). Size markers are indicated.
Alb3.1 and Alb3.2 Are Part of the Same Complex
To test whether Alb3.2 and Alb3.1 interact, coimmunoprecipitations were performed on extracts from the ac29 strain expressing both the FLAG-tagged Alb3.2 and the HA-tagged Alb3.1 proteins. Immunoprecipitation with Alb3.2 antibodies and immunoblotting with HA antibodies revealed the presence of Alb3.1 (Figure 3, left). Although only a small part of the input was immunoprecipitated, the immunoprecipitation was specific, as judged from the absence of a signal when preimmune serum was used. Reciprocally, Alb3.2 (both the tagged and untagged versions) could be detected by immunoblotting of precipitates obtained with HA antibodies (Figure 3, right). In this case, the untagged ac29 (−HA) strain was used as a control. The signals obtained in these immunoprecipitations are weak compared with the input, indicating that only small portions of these two proteins are associated in the same complex. It is not clear whether the interaction between these two proteins is direct or indirect. Attempts to test this possibility using cross-linkers were inconclusive.
Figure 3.
Alb3.2 Interacts with Alb3.1.
Left, immunoprecipitations (IP) were performed with Alb3.2 antibodies (+Ab) or with preimmune serum (−Ab) with solubilized membranes of the ac29-Alb3.1-HA-Alb3.2-FLAG strain. Right, immunoprecipitations were performed with HA antibodies with solubilized membranes from the strains ac29-Alb3.1-HA-Alb3.2-FLAG (+HA) and ac29 (−HA). The immunoprecipitates were fractionated by PAGE, and immunoblotting (IB) was performed with the indicated antisera. Tagged and untagged Alb3.2 are indicated by open and closed arrowheads, respectively. f.t. refers to flow through.
Alb3.2 Is Associated with PSII Complexes
To test whether Alb3.2 is associated with known photosynthetic complexes, membranes from different photosynthetic mutants were analyzed by blue native (BN) gel electrophoresis because of the better resolution. Total membranes from the wild type and mutants deficient in LHCII and LHCI (ac29), PSII (FuD7), PSI (F15), cytochrome b6f complex (ΔpetD), and ATP synthase (FuD50) were solubilized with β-dodecyl maltoside, and high molecular mass complexes were separated by electrophoresis on a 4 to 14% BN gel.
Immunoblotting showed that a large portion of Alb3.2 migrates near the bottom of the gel, which corresponds most likely to free Alb3.2 (Figure 4A). It appears that the conditions used on the BN gel are harsher than during sucrose gradient centrifugation, in which no free Alb3.2 is detectable (Figure 2A). Alb3.2 is present in two complexes of ∼300 and 600 kD. Moreover, Alb3.2 complexes of larger size are detectable in the F15, ΔpetD, and FuD50 mutants but absent in FuD7 and ac29. These complexes appear to be unstable under the conditions used and are not always detectable. On other BN gels, they are also detected in the wild type. The 300- and 600-kD complexes are both absent in the PSII-deficient FuD7 mutant. The larger complex appears to be reduced in the PSI-deficient mutant F15. These two complexes are present in the other mutants. To test whether they correspond to known photosynthetic complexes, the BN gel blots were probed with antibodies against a subunit of each complex. This revealed that the 300- and 600-kD complexes correspond to the reaction center core PSII monomer and dimer, respectively, because they react with D2 antibodies and are absent in FuD7 (Figure 4A, αD2) (Ossenbuhl et al., 2004). A complex with slower migration than the PSII dimer is detectable at ∼700 kD with PsaA antiserum, corresponding most likely to PSI-LHCI (Figure 4A, αPsaA). It is absent in F15 and strongly reduced in ac29. Instead, a new complex is detectable in ac29 at 300 kD corresponding to PSI without LHCI (Ossenbuhl et al., 2004). This is attributable to the considerable decrease of LHCs in ac29 (Bellafiore et al., 2002; Ossenbuhl et al., 2004). The D2-containing PSII complex is reduced in abundance in ac29, as observed previously (Bellafiore et al., 2002).
Figure 4.
Comigration of Alb3.2 with PSII Complexes on BN Gels.
(A) Total membranes from the wild type and the mutant strains ac29, FuD7, F15, ΔpetD, and FuD50. The membranes were solubilized with β-dodecyl maltoside and fractionated by BN gel electrophoresis. The antibodies used for immunoblots are indicated at top. The arrow indicates the position of the presumed free Alb3.2.
(B) Analysis of Alb3.2 in y-1. The y-1 mutant was grown in darkness and shifted to the light at 0 h. Total membranes were prepared at different times of the greening process and fractionated by BN gel electrophoresis (Gel). Immunoblotting was performed with Alb3.2 antiserum (Alb3.2). The location of the PSII core complex is indicated. CL, growth in continuous light.
(C) Accumulation of proteins during greening of y-1. Total proteins from y-1 and the wild type were extracted during the greening process and fractionated by PAGE. Immunoblotting was performed with antisera raised against the indicated proteins. Rubisco, ribulose-1,5-bis-phosphate carboxylase/oxygenase.
In the mutants lacking PSII or cytochrome b6f complex, several smaller complexes containing PsaA are observed. However, none of these appears to contain Alb3.2. These results suggest that Alb3.2 stably associates with PSII complexes. It is possible that by binding to the PSII core complexes, Alb3.2 catalyzes the formation of PSII-LHCII supercomplexes. Alb3.2 does not comigrate with cytochrome b6f or with the ATP synthase complex, indicating that it does not stably bind to these complexes.
The association of Alb3.2 with PSII was further tested in the y-1 mutant, which is deficient in the light-independent chlorophyll biosynthetic pathway. The y-1 mutant was grown in the dark and subsequently transferred to the light. Under these conditions, PSII is absent in the dark but accumulates gradually in the light, as chlorophyll synthesis resumes (Figure 4B). The corresponding immunoblot shows that there is a similar increase of Alb3.2 comigrating with PSII during the greening phase (Figure 4B). However, in contrast with PSII, the total amount of Alb3.2 remains the same in dark- and light-grown y-1 cells (Figure 4C). The comigration of Alb3.2 with PSII during the induction of PSII strongly suggests that Alb3.2 is associated with PSII. It was shown previously that Alb3.1 facilitates the assembly of D1 in the PSII complex (Ossenbuhl et al., 2004). It is possible that the two Alb3 homologs have complementary roles in PSII assembly.
Alb3.2 Interacts with the Reaction Center Polypeptides of PSI and PSII
To confirm the interactions of Alb3.2 with the photosystems, coimmunoprecipitation experiments were performed. Interactions of tagged Alb3.2-FLAG with the PSII reaction center polypeptides D1 and D2 were detected by reciprocal immunoprecipitations with the corresponding antibodies (Figure 5A). Although tagged Alb3.2 could be immunoprecipitated with D2, the reciprocal immunoprecipitation did not work, possibly because D2 binding may mask the FLAG tag. This immunoprecipitation was possible when the immunoprecipitation was performed with untagged Alb3.2 using Alb3.2 antibodies (Figure 5B). However, in this case, the D1 and D2 antibodies were less efficient than those used in Figure 5A, resulting in a lower yield of immunoprecipitation. No signal was detected with preimmune serum. Furthermore, interaction of Alb3.2 with PsaA could be observed in the untagged strain. Together, these results suggest that a small fraction of Alb3.2 remains associated with PSII and PSI.
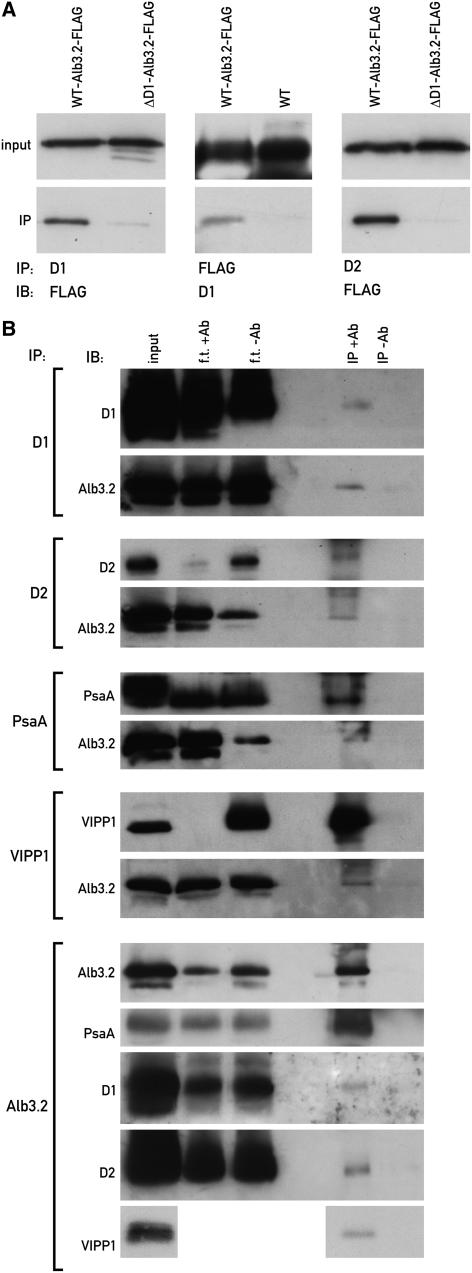
Figure 5.
Alb3.2 Interactions.
(A) Alb3.2 interacts with D1 and D2. Immunoprecipitations like those described for Figure 3 were performed with extracts from the strain containing FLAG-tagged Alb3.2 and, as controls, the wild type and the strain ΔD1-Alb3.2-FLAG (FuD7 lacking psbA transformed with Alb3.2-FLAG). Input refers to an immunoblot (IB) of the extract before immunoprecipitation (IP) with the antiserum indicated for the immunoblot.
(B) Alb3.2 interacts with PsaA and VIPP1. Similar immunoprecipitations of solubilized membranes of wild-type cells were performed with the indicated antisera (+Ab) or, as controls, with preimmune serum (−Ab). The input and flow through (f.t.) represent 10% of the proteins used for the immunoprecipitations. The immunoprecipitates were separated by PAGE and immunoblotted. Immunoblotting was performed with the indicated antisera.
Other Partners of Alb3.2
Interestingly, VIPP1 (for vesicle-inducing protein) was also coimmunoprecipitated with Alb3.2 (Figure 5B). VIPP1 was originally identified as an envelope protein involved in lipid transfer in land plants (Li et al., 1994). Later, it was shown to be implicated in plastid vesicle formation (Kroll et al., 2001; Westphal et al., 2001). In Chlamydomonas, VIPP1 was localized in the stroma, the thylakoids, and the low density chloroplast membranes and was shown to be a major target of the Hsp70B/Cdj2 chaperone pair (Liu et al., 2005). Although VIPP1 was shown to coimmunoprecipitate with Hsp70B and Cdj2, we detected no interaction between Alb3.2 and Hsp70B or Cdj2 by immunoprecipitation (data not shown). This interaction with VIPP1 suggests a role for Alb3.2 in thylakoid membrane maintenance. Alternatively, Alb3.2 might be required for VIPP1 insertion in the thylakoid membrane, or the reverse may be true.
To further characterize Alb3.2 complexes and identify novel partners, large-scale immunoprecipitations were performed with solubilized thylakoid membranes from the FLAG-tagged Alb3.2 strain with FLAG antiserum. The immunoprecipitated proteins were subjected to mass spectrometry sequencing. As expected, several internal peptides from Alb3.2 were detected. Also, several LHC proteins were identified (Lhcbm3, Lhcbm4, and Lhcbm5) as well as AtpA, AtpB, and PsbC (data not shown). However, we were not able to confirm the association of Alb3.2 with AtpA and AtpB by BN gel analysis (Figure 4A) and reciprocal immunoprecipitations (data not shown). Moreover, neither PSI assembly factors Ycf3 and Ycf4 nor SRP54 and its cpFtsY receptor and cpSecY, which are involved in protein integration into thylakoid membranes, could be identified among the proteins coimmunoprecipitated with tagged Alb3.2.
To test whether Alb3.2 is involved in the integration of nascent protein chains into the thylakoid membrane, its association with polysomes was tested using the cw15 strain containing Alb3.2-FLAG (data not shown). No Alb3.2 was detected in the polysomal fraction, indicating either that its association with polysomes is very labile or that it acts at a later step of assembly, as proposed previously for Alb3.1 (Ossenbuhl et al., 2004).
Reduction of Alb3.2 by RNAi Leads to a Decrease of PSII and PSI and to Cell Death
To gain further insights into the function of Alb3.2, the level of this protein was reduced using the recently developed RNAi cosilencing system, in which it is possible to select for cells in which RNAi is occurring (Rohr et al., 2004). A 300-bp DNA fragment from the 3′ end of the Alb3.2 coding region with lowest homology with Alb3.1 was cloned in the sense and antisense orientations between the Maa7 inverted repeat. This construct was introduced into wild-type cells by transformation. The Maa7 gene encodes the β subunit of Trp synthase, which converts the indole analog 5-fluoroindole (5-FI) into the toxic Trp analog 5-fluorotryptophan. Transformed cells were first selected on paromomycin plates containing 2.5 μM 5-FI and subsequently transferred to plates containing 5, 10, 15, and 20 μM 5-FI. All plates were kept in the dark for 3 weeks between the transfers, because 5-FI is light-sensitive. After 3 weeks at each 5-FI concentration, the level of Alb3.2 was checked by immunoblotting. In the transformant Alb3.2_4, the amount of Alb3.2 started to decline with 15 μM 5-FI and reached <50% of wild-type levels with 20 μM 5-FI. Further increase of the 5-FI concentration had negative effects on PSI. The cells could be maintained for only 2 to 3 weeks on 20 μM 5-FI, because they died after the second transfer. However, they could be maintained on 5 μM 5-FI. Under these conditions, the level of Alb3.2 is not affected. RNAi of Alb3.2 can be induced by transferring these cells progressively to higher 5-FI concentrations. The lethality is not attributable to the RNAi system used, because another RNAi strain in which the FLP gene of Chlamydomonas was silenced could be maintained indefinitely under the same conditions (Falciatore et al., 2005). Therefore, Alb3.2 seems to have an essential function in the cell.
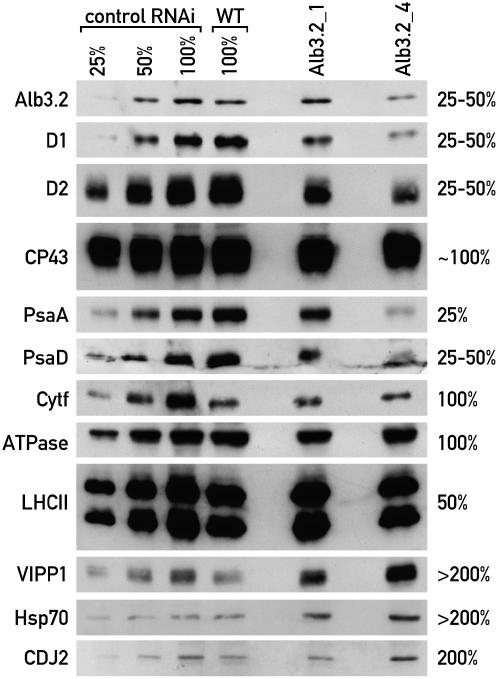
The levels of several subunits of the photosynthetic complexes were examined in the two transformants Alb3.2_1 and Alb3.2_4, in which Alb3.2 accumulated to 60 to 80% and to 25 to 40% relative to wild-type cells, respectively, in the presence of 20 μM 5-FI (Figure 6). To correct for possible effects of 5-FI on the accumulation of the photosynthetic proteins, the RNAi strain targeted for FLP and grown on 20 μM 5-FI was used as a control (Falciatore et al., 2005). With the exception of cytochrome f, VIPP1, and Cdj2, this strain accumulated the same amount of protein as the wild type. In the more severely affected transformant Alb3.2_4, the levels of the PSII D1 and D2 proteins were reduced to ∼25%. By contrast, the level of CP43 containing the peripheral antenna of PSII was less reduced. The PSI proteins PsaA and PsaD accumulated to 25 to 50% compared with wild-type cells. On the contrary, the levels of cytochrome b6f and ATP synthase subunits were barely affected compared with the wild type. Although the amount of cytochrome f was increased in the FLP RNAi strain, this increase was most likely attributable to the decrease of FLP and not to 5-FI, because the level of cytochrome f was the same in Alb3.2_1, Alb3.2_4, and the wild type. The LHCII proteins accumulated to ∼50 to 75% in the RNAi strain, with a similar reduction in the chlorophyll level. Thus, the photosynthetic complexes that Alb3.2 interacts with are decreased in the RNAi strain, whereas the two other complexes are not affected. Interestingly, the levels of VIPP1, another partner of Alb3.2, and chloroplast Hsp70 and its cochaperone Cdj2 were increased in the RNAi strain.
Figure 6.
Depletion of Alb3.2 by RNAi Leads to the Reduction of the Photosynthetic Complexes PSII and PSI.
Wild-type cells were transformed with a plasmid carrying an inverted repeat of Alb3.2 cDNA using the cosilencing system described by Rohr et al. (2004). RNAi transformants were selected on 2.5 μM 5-FI and then transferred subsequently at 3-week intervals to 5, 10, 15, and 20 μM 5-FI. The transformants were always grown in the dark because 5-FI is light-sensitive. Total proteins (10 μg) from two independent transformants, Alb3.2_1 and Alb3.2_4, grown on 20 μM 5-FI for 2 weeks were used for PAGE and immunoblotted with several antibodies, as indicated. CDJ2, chloroplast DNAJ-like protein; VIPP1, vesicle-inducing protein. Serial dilutions from the FLP RNAi strain (control RNAi) (Falciatore et al., 2005) grown in the same conditions were used for quantification together with the wild type to correct for possible effects of 5-FI. Protein levels of Alb3.2_4 relative to the wild type are indicated at right. This experiment was repeated three times.
Examination of the RNAi transformants by electron microscopy revealed that the vacuoles are considerably enlarged in the Alb3.2_4 strain. The effect is attributable specifically to the depletion of Alb3.2, because the less affected strain Alb3.2_1 and another RNAi transformant targeted for FLP (Falciatore et al., 2005) did not display this phenotype (Figure 7). Measurements of the areas of vacuoles in 21 randomly chosen cell sections revealed that the vacuolar area represented 60 and 11% of the total cell area in Alb3.2_4 and FLP cells, respectively. Therefore, depletion of Alb3.2 not only affects the accumulation of the photosynthetic complexes of the thylakoid membranes but also has a pronounced effect on the organization of other cell compartments. In some cases, the vacuoles contain small organelles, most likely mitochondria that are presumably destined for degradation (Figure 7).
Figure 7.
Depletion of Alb3.2 Leads to an Increase in Vacuolar Size.
(A) Wild type (cw15).
(B) RNAi strain Alb3.2_4.
(C) RNAi strain Alb3.2_1.
(D) RNAi control strain FLP.
(E) Vacuole of Alb3.2_4 containing mitochondria.
Cells were prepared for transmission electron microscopy as described in Methods. C, chloroplast; M, mitochondria; N, nucleus; V, vacuole. Bars = 1 μm.
DISCUSSION
Complementary Roles for Alb3.2 and Alb3.1
The Alb3.1 and Alb3.2 proteins share 37% sequence identity and 54% sequence similarity (Bellafiore et al., 2002). Both proteins are closely related to ALB3 and to the C-terminal part of ARTEMIS of Arabidopsis. All of these proteins belong to the Oxa1/YidC protein family found in mitochondria, plastids, and bacteria, which is mainly involved in the insertion of proteins into membranes. Here, we have shown that Alb3.2 is localized in the thylakoid membrane like Alb3.1. Both proteins interact directly or indirectly with each other based on their association with complexes of similar size and on coimmunoprecipitation experiments. The situation appears to be different for the two Arabidopsis ALB3 homologs. ALB3 was localized on thylakoid membranes, although envelopes were not clearly separated from thylakoid membranes in that work (Sundberg et al., 1997). By contrast, ARTEMIS was localized on the inner envelope membrane (Fulgosi et al., 2002).
It was shown previously that in the ac29 mutant of C. reinhardtii, in which the Alb3.1 gene is deleted, LHC proteins are drastically reduced (Bellafiore et al., 2002). Moreover, there is a 10-fold decrease in D1 levels in dark-grown Chlamydomonas cells, although the decrease is less pronounced for light-grown cells. Because of the lack of a Chlamydomonas mutant affected in Alb3.2, we used RNAi technology to assess the role of this protein. Even a modest decrease in Alb3.2 has strong effects on the accumulation of PSII and PSI subunits, suggesting that the Alb3.2 protein may be present in limiting amounts for the assembly of these complexes. Alb3.2 appears to be associated with the reaction center polypeptides of PSII, as revealed by the coimmunoprecipitations with D1 and D2 (Figure 5), by the greening experiment with the y-1 mutant (Figure 4B), and by the comparative BN gel analysis of thylakoid membrane complexes from the wild type and a PSII-deficient mutant (Figure 4). Further evidence comes from recent studies with the yeast split-ubiquitin system, which revealed direct interactions between ALB3 of Arabidopsis and the reaction center subunits of PSII and PSI (Pasch et al., 2005). In contrast with the Alb3.1-deficient mutant, in the Alb3.2 RNAi strain, the amounts of PSII and PSI proteins are more reduced than the LHC protein level, suggesting that Alb3.1 and Alb3.2 act in different ways. Similar differences have been observed for the homologs Oxa1/Oxa2 in yeast, in which one protein is essential for cell survival and the other is specifically involved in protein insertion into membranes (Funes et al., 2004b; Preuss et al., 2005).
In contrast with Oxa1, Alb3.2 is not stably associated with polysomes. Alb3.2 interacts with Alb3.1 and with proteins of PSI and PSII. It was shown that Alb3.1 can be coimmunoprecipitated with D1 protein (Ossenbuhl et al., 2004). These data suggest a chaperone function rather than a role in protein integration. A possible function of Alb3.2 that would explain its association with the PSII core complex is that it is required for the formation of PSII-LHCII supercomplexes.
Whereas most factors involved in the assembly of photosynthetic complexes in Chlamydomonas usually act on a single complex, this is not the case for Alb3.2. This factor appears to have a wider role than Alb3.1, based on the observation that in the RNAi strains the levels of both PSII and PSI are diminished. By contrast, the level of PSI is unaffected by the loss of Alb3.1.
Alb3.2 Is Essential for Cell Survival in Chlamydomonas
Photosynthetic function is dispensable for Chlamydomonas when acetate is provided to the growth medium (Harris, 1989). Thus, mutants with lesions in the photosynthetic apparatus can be maintained and grown readily in the presence of acetate. Therefore, analysis of mutants of Chlamydomonas provides a means to distinguish between functions that are required only for photosynthetic activity and those that are required for cell survival. In the case of Alb3.2, which is involved in thylakoid protein assembly, it is surprising that even a modest depletion of this protein to 25 to 50% of wild-type levels by RNAi has such wide effects and ultimately leads to cell death, even under conditions that are permissive for photosynthetic mutants of C. reinhardtii. In this respect, Alb3.2 differs drastically from Alb3.1, which is partially dispensable for photoautotrophic growth and fully dispensable for mixotrophic growth (Bellafiore et al., 2002). If Alb3.2 were uniquely required for the assembly of PSII and PSI and the light-harvesting system, cells of the RNAi strain would be able to grow on acetate-containing medium for a prolonged period. This is clearly not the case, which strongly suggests that Alb3.2 has an additional role that is essential for cell survival.
Electron microscopy revealed that the vacuoles are markedly enlarged in the RNAi strains. These vacuoles appear to engulf small organelles and presumably cytoplasmic components that are likely to be degraded. This is reminiscent of autophagy, a process during which cytoplasmic components are delivered to the vacuole for recycling in yeast, animal, and plant cells during periods of developmental remodeling and under conditions of nutrient starvation (Thompson and Vierstra, 2005). However, such a process has not yet been described for Chlamydomonas, although treatment of this alga with rapamycin resulted in a pronounced increase in the size of vacuoles, which resembled an autophagy-like process (Crespo et al., 2005). Interactions between chloroplasts and vacuoles have been observed (Park et al., 1999; Komine et al., 2000). In particular, a pathway for degradation through transfer of proteins from the chloroplast to vacuoles in Chlamydomonas has been suggested, based on the finding that several chloroplast proteins were found in dense granules in cytoplasmic vacuoles.
How the depletion of a single thylakoid protein triggers such a massive reorganization of the cytoplasmic compartment in Chlamydomonas is not yet clear. It is interesting that inactivation of the YME1 gene, coding for an ATP-dependent metalloprotease located in the inner mitochondrial membrane in yeast, causes mitochondrial damage that leads to DNA escape from the mitochondria to the nucleus (Campbell and Thorsness, 1998). This process appears to be mediated by the degradation of the abnormal mitochondria by the vacuole. It is possible that a decrease of Alb3.2 leads to the misfolding of thylakoid complexes. This damage may trigger an extensive recycling of chloroplast proteins and their transfer to vacuoles for degradation by an unknown pathway.
An interesting observation is the interaction of Alb3.2 with VIPP1 and the overproduction of VIPP1 and of the Hsp70/Cdj2 chaperone pair in the Alb3.2 RNAi strains. One possible explanation for this is that Alb3.2 accumulation in the thylakoid membranes depends on VIPP1, especially given the fact that the targeting and integration of Alb3.2 have not yet been elucidated. The increase of VIPP1 caused by the depletion of Alb3.2 may represent a stress response, with a compensatory effort of the chloroplast to maintain the integrity of its thylakoid system. Because VIPP1 is present in both the stroma and chloroplast membranes of Chlamydomonas (Liu et al., 2005), it could interact directly with Alb3.2, as indicated by the coimmunoprecipitations of VIPP1 and Alb3.2 (Figure 5B). It is possible that the primary function of Alb3.2 is to maintain thylakoid membrane integrity and that it acts preferentially on PSII and PSI assembly. If the action of Alb3.2 is restricted to the thylakoid compartment, our results strongly suggest that this compartment is not only involved in photosynthetic activity but participates in essential cellular pathways and/or signaling chains, which remain to be identified.
METHODS
Cells and Culture Conditions
The Escherichia coli strain DH10-b was used for cloning, maintenance, and propagation of plasmids. Cells were grown in Luria-Bertani medium at 37°C. Ampicillin at 100 μg/mL was added where appropriate.
Chlamydomonas reinhardtii wild-type and mutant strains were used. The cell wall–deficient cw15 strain can be lysed gently and was used for cell fractionation studies. The ac29 strain lacks Alb3.1 (Bellafiore et al., 2002). It was complemented with an HA-tagged Alb3.1 construct. Moreover, a FLAG-tagged Alb3.2 construct was introduced into this strain to test for interactions between Alb3.1 and Alb3.2. In some cases, the cw15 strain expressing Alb3.1-HA and Alb3.2-FLAG was used for cell fractionation experiments (Figure 1). The strains were grown on Tris-acetate-phosphate (TAP) plates (Harris, 1989) at 25°C in continuous light (40 μE·m−2·s−1 for the wild type, 6 μE·m−2·s−1 for photosynthetic mutants) or in the dark. For transformations with a plasmid containing the paromomycin resistance cassette, paromomycin was added to 10 μg/mL.
Cloning of the Midigene
The Alb3.2 cDNA was obtained as described (Bellafiore et al., 2002). A triple FLAG tag was inserted in frame just before the stop codon to create the tagged cDNA in pKS. To insert the tag, an NruI site was constructed by PCR.
A cosmid containing Alb3.2 was identified by hybridizing a labeled fragment from the cDNA to DNA from a Chlamydomonas cosmid library (Purton and Rochaix, 1994). A 14-kb NotI fragment containing the whole gene was subcloned.
A 9-kb XbaI-BamHI fragment containing the promoter, the first four introns, and the first five exons from the genomic fragment was cloned in frame with the tagged cDNA in a vector containing the paromomycin resistance cassette (midigene) (Sizova et al., 2001). This construct was transformed into cw15, ac-29, FuD7, and F14 strains by the glass bead transformation method (Kindle, 1990). It was also used for cotransformation with the Alb3.1-HA midigene into cw15 and ac-29.
Purification of Recombinant Alb3.2 from E. coli and Production of an Antiserum
A DNA fragment containing the last 387 nucleotides of the Alb3.2 open reading frame coding for a soluble protein fragment was amplified using the primers 5′-CCGGAATTCAACAACCTGCTGTCCACCGGC-3′ and 5′-GGCTCAGATTATGCGGAGTTATCCTTGCC-3′. Using the newly created restriction sites, the fragment was cloned in frame with a 6xHis tag into pHIS8-3 TEV1. The His fusion protein was expressed in E. coli BL21 after induction with 0.4 mM isopropylthio-β-galactoside for 3 h at 37°C. It was purified on a Ni2+ column (nickel-nitrilotriacetic acid agarose; Qiagen).
For production of the antiserum, 200 μg of gel-purified His-tagged recombinant protein was injected in a rabbit six times at 3-week intervals. Blood was collected 10 d after each injection. In the experiments reported, the fifth bleed was used at a dilution of 1:10,000.
Preparation of Total Protein, Chloroplast Fractions, and Mitochondrial Extracts
Total protein was prepared by resuspending a frozen cell pellet (2 × 107 to 5 × 107 cells) in 200 μL of lysis buffer (50 mM Tris-HCl, pH 8, 2% SDS, 10 mM EDTA, and Sigma-Aldrich protease inhibitors). After incubation at room temperature for 1 h, the insoluble material was removed by centrifugation and the supernatant was used for immunoblot analysis.
Chloroplasts were prepared by resuspending a cell pellet from the cw15 strain (2 × 109 cells) in 20 mL of breaking buffer (300 mM sorbitol, 50 mM HEPES-KOH, pH 7.2, 2 mM EDTA, pH 8, and 1 mM MgCl2). Cells were broken for 1 min on ice by adding saponin (Sigma-Aldrich) to 0.25%. After removal of unbroken cells and two to three washes with breaking buffer to remove mitochondria, crude chloroplasts were loaded onto a discontinuous 75 to 45% Percoll gradient in chloroplast gradient buffer (300 mM sorbitol, 30 mM Tris, pH 7.9, and 10 mM MgCl2). Chloroplasts were collected at the interphase of the two gradient steps and washed with gradient buffer. They were then resuspended in lysis buffer and treated as the total cell extracts.
To isolate membrane and soluble fractions, chloroplasts were resuspended in lysis buffer without SDS and sonicated five times for 10 s. The total extracts were centrifuged for 30 min at 100,000g at 4°C. The supernatant contains soluble proteins. The pellet (membrane fraction) was washed twice with washing buffer (50 mM Tris, pH 8, 10 mM EDTA, and 0.4 M sucrose), resuspended in lysis buffer, and treated as total extracts. Mitochondria were prepared as described (Eriksson et al., 1995), resuspended in lysis buffer, and treated as total extracts.
Envelope membranes were separated from thylakoid membrane using pellets of 1 × 107 to 2 × 107 cells that were resuspended in ACA buffer (750 mM aminocaproic acid, 50 mM BisTris, pH 7, and 0.5 mM EDTA) and broken in a French press at 400 p.s.i. Total membranes were collected by centrifugation at 15,000g for 15 min. Membranes were resuspended in envelope gradient buffer (50 mM HEPES-KOH, pH 7.8, 2 mM MgCl2, and Sigma-Aldrich inhibitors) and loaded on a 0.1 to 2 M sucrose gradient. After centrifugation for 4 h at 100,000g, fractions of 1 mL were taken, denatured, and analyzed by protein immunoblotting.
The concentrations of all protein extracts were determined using the bicinchoninic acid assay (Sigma-Aldrich).
Isolation of Photosynthetic Complexes and Immunoblotting
Thylakoid membranes were isolated by resuspending a frozen cell pellet (2 × 109 cells) of cw15 or the cw15 strain containing the Alb3.1-HA midigene and the Alb3.2-FLAG midigene in 0.3 M sucrose, 5 mM HEPES-KOH, pH 7.5, 5 mM MgCl2, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Membranes were collected by centrifugation and washed with 0.3 M sucrose, 1 mM HEPES-KOH, pH 7.5, 5 mM EDTA, and 1 mM PMSF. The membrane pellet was resuspended using a homogenizer in 1.8 M sucrose, 1 mM HEPES-KOH, pH 7.5, 5 mM EDTA, and 1 mM PMSF and overlaid with 1 volume of 1.3 M sucrose and 1 volume of 0.5 M sucrose in the same buffer. After centrifugation at 80,000g for 1 h, the thylakoids float at both interphases of the gradient. They were collected, washed with 1 mM HEPES-KOH, pH 7.5, 5 mM EDTA, and 1 mM PMSF, and resuspended to 0.8 μg/μL chlorophyll in the same buffer.
To obtain the photosynthetic complexes, thylakoid membranes were solubilized in 0.9% β-dodecyl maltoside (Calbiochem) for 10 min. Insoluble material was removed by centrifugation, and the supernatant was loaded onto a continuous sucrose gradient (0.1 to 2 M) in 20 mM Tricine, pH 7.5, and 0.05% β-dodecyl maltoside. After centrifugation for 16 h at 170,000g, three green bands were visible in the gradient. One-milliliter fractions were taken and analyzed by immunoblot analysis. The signals were visualized be enhanced chemiluminescence (ECL system) (Durrant, 1990).
Membrane Extraction and BN Gel Electrophoresis
Wild-type and mutant cultures were grown under 6 μE of light to 2 × 106 to 5 × 106 cells/mL. Pellets of 1 × 107 to 2 × 107 cells were resuspended in ACA buffer (see above) and broken by vortexing with glass beads (400 to 600 μm; Sigma-Aldrich) for 2 min. Unbroken cells and glass beads were removed, and total membranes were collected by centrifugation at 15,000g for 15 min. For BN gel electrophoresis, membranes were resuspended to 0.8 μg/μL chlorophyll in ACA buffer and solubilized as described above. Ten micrograms of chlorophyll was loaded onto a 4 to 14% gradient BN gel as described (Ossenbuhl et al., 2004). Electrophoresis was performed at 100 V (24-h migration) or 12 mA (6-h migration). For immunoblotting, the gel was washed in transfer buffer (25 mM Tris, pH 8, 250 mM Gly, 0.1% SDS, and 20% methanol) for 2 × 30 min to remove remaining Coomassie Brilliant Blue G 250. Proteins were transferred to a polyvinylidene difluoride membrane, and immunoblotting was performed using the ECL system.
Coimmunoprecipitations
Membranes were extracted and solubilized as described. All further steps were performed at 4°C. To preclear the solubilized membranes, 50 μL per 100 μg of chlorophyll-washed protein G agarose beads (Roche) was added for 2 h at room temperature. For immunoprecipitation of the HA epitope, 50 μL per reaction of anti-HA affinity matrix (Sigma-Aldrich) was prepared according to the manufacturer's instructions and added to the 500 mg of chlorophyll-solubilized membranes. Binding was performed overnight at 4°C. For immunoprecipitation of the other proteins, 5 μL of antiserum was added and binding was performed for 2 h at 4°C. Fifty microliters of washed protein G agarose beads was added to the mix. Further binding was performed for 2 h at 4°C. All beads were washed six times in TBS-BSA (100 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.05% BSA), and the bound proteins were eluted in 40 μL of 2× SDS loading dye (100 mM Tris-HCl, pH 6.8, 4% SDS, 0.2% bromphenol blue, and 20% glycerol) for 30 min at room temperature. Thirty microliters of solubilized membranes and immunoprecipitated proteins was analyzed by protein gel immunoblotting.
For large-scale immunoprecipitation, 1 mg of chlorophyll was used with 100 μg of anti-FLAG affinity matrix (Sigma-Aldrich). The proteins were separated on a 12% gel, and the bands were cut for sequencing.
Mass Spectrometry
In-gel digestion of proteins before mass spectrometry was performed as described (Eichacker et al., 2004). Mass spectrometric analysis was performed on a Micromass Q-TOF-I hybrid mass spectrometer equipped with an orthogonal nanoelectrospray ionization source operating in the positive electrospray ionization mode. Samples were loaded into medium nanoelectrospray capillaries (Protana) and positioned in the source. Acquired mass spectra were processed using Mass-Lynx versions 3.4 and 3.5 software. For calibration, standard solutions of sodium iodide (2 μg/μL) and rubidium iodide (0.05 μg/μL) were dissolved in 50% propan-2-ol (according to the Q-TOF user's guide).
RNAi
The RNAi system for C. reinhardtii using tandem inverted repeats for selection of silencing was used to create mutants deficient in Alb3.2 (Rohr et al., 2004). Two fragments from the end of the open reading frame were amplified by PCR, creating new restriction sites (5′-CGGAATTCTGGAGTCGACGCTGTCGCT-3′, 5′-GCTCTAGAAGCACCGGCATCACCAGGTA-3′, or 5′-GCTCTAGAGGCTGCTGATGATTTTC-3′), and cloned as an inverted repeat into the vector pNE537 in the inverted repeat for the Maa7 gene.
Cells from cw15 were transformed with this construct, kept for 2 d in liquid culture (TAP + 1.5 mM Trp) in dim light, and then plated onto TAP plates containing 2.5 μM 5-FI, 10 μg/μL paromomycin, and 1.5 mM Trp. The plates were kept in the dark because 5-FI is light-sensitive. At intervals of 3 weeks, they were transferred to stepwise increasing 5-FI concentrations of 5, 10, 15, and 20 μM.
For analysis, cells were taken directly from the plates after 3 weeks and resuspended in buffer for protein or RNA extraction or in medium for electron microscopy.
Electron Microscopy
The cells were transferred from agar plates containing 20 μM 5-FI and Trp to liquid TAP medium containing 1.5 mM Trp at a concentration of 2 × 106 cells/mL, in which they were allowed to grow for 4 d. Whole cells were directly fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7, at room temperature for 2 h. The cells were then pelleted by centrifugation, washed in cacodylate, and postfixed in 1% OsO4 in cacodylate for 1 h at room temperature. They were rinsed in buffer and further fixed for 1 h in 1% aqueous uranyl acetate. Samples were washed in water, dehydrated in a graded ethanol series, and embedded in Epon 812. Ultrathin sections 60 nm thick were cut and stained classically with 2% aqueous uranyl acetate, then in Reynold's lead citrate. Sections were viewed with a Phillips EM 10 transmission electron microscope. Surface areas were measured using Leica Twin Image analysis software.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AAM11662 (Alb3.1), AAM49792 (Alb3.2), and AAUO6582 (VIPP1).
Acknowledgments
We thank N. Roggli for preparing the figures, S. Lemaire for antibodies, Anne Utz for assistance in electron microscopy, and M. Goldschmidt-Clermont for helpful comments. This work was supported by Grant 3100-0667763.02 from the Swiss National Foundation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Jean-David Rochaix (jean-david.rochaix@molbio.unige.ch).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.038695.
References
- Barkan, A., and Goldschmidt-Clermont, M. (2000). Participation of nuclear genes in chloroplast gene expression. Biochimie 82 559–572. [DOI] [PubMed] [Google Scholar]
- Bartsevich, V.V., and Pakrasi, H.B. (1997). Molecular identification of a novel protein that regulates biogenesis of photosystem I, a membrane protein complex. J. Biol. Chem. 272 6382–6387. [DOI] [PubMed] [Google Scholar]
- Bellafiore, S., Ferris, P., Naver, H., Gohre, V., and Rochaix, J.D. (2002). Loss of Albino3 leads to the specific depletion of the light-harvesting system. Plant Cell 14 2303–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy, N., Chalvet, F., Hamel, P., Slonimski, P.P., and Dujardin, G. (1994). OXA1, a Saccharomyces cerevisiae nuclear gene whose sequence is conserved from prokaryotes to eukaryotes controls cytochrome oxidase biogenesis. J. Mol. Biol. 239 201–212. [DOI] [PubMed] [Google Scholar]
- Boudreau, E., Takahashi, Y., Lemieux, C., Turmel, M., and Rochaix, J.D. (1997). The chloroplast ycf3 and ycf4 open reading frames of Chlamydomonas reinhardtii are required for the accumulation of the photosystem I complex. EMBO J. 16 6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, C.L., and Thorsness, P.E. (1998). Escape of mitochondrial DNA to the nucleus in yme1 yeast is mediated by vacuolar-dependent turnover of abnormal mitochondrial compartments. J. Cell Sci. 111 2455–2464. [DOI] [PubMed] [Google Scholar]
- Cline, K., and Henry, R. (1996). Import and routing of nucleus-encoded chloroplast proteins. Annu. Rev. Cell Dev. Biol. 12 1–26. [DOI] [PubMed] [Google Scholar]
- Crespo, J.L., Diaz-Troya, S., and Florencio, F.J. (2005). Inhibition of target of rapamycin signaling by rapamycin in the unicellular green alga Chlamydomonas reinhardtii. Plant Physiol. 139 1736–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant, I. (1990). Light-based detection of biomolecules. Nature 3436 297–298. [DOI] [PubMed] [Google Scholar]
- Eichacker, L.A., Granvogl, B., Mirus, O., Muller, B.C., Miess, C., and Schleiff, E. (2004). Hiding behind hydrophobicity. Transmembrane segments in mass spectrometry. J. Biol. Chem. 279 50915–50922. [DOI] [PubMed] [Google Scholar]
- Eriksson, M., Gardestrom, P., and Samuelsson, G. (1995). Isolation, purification, and characterization of mitochondria from Chlamydomonas reinhardtii. Plant Physiol. 107 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falciatore, A., Merendino, L., Barneche, F., Ceol, M., Meskauskiene, R., Apel, K., and Rochaix, J.D. (2005). The FLP proteins act as regulators of chlorophyll synthesis in response to light and plastid signals in Chlamydomonas. Genes Dev. 19 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulgosi, H., Gerdes, L., Westphal, S., Glockmann, C., and Soll, J. (2002). Cell and chloroplast division requires ARTEMIS. Proc. Natl. Acad. Sci. USA 99 11501–11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes, S., Gerdes, L., Inaba, M., Soll, J., Herrmann, J.M., Fulgosi, H., Westphal, S., and Glockmann, C. (2004. a). The Arabidopsis thaliana chloroplast inner envelope protein ARTEMIS is a functional member of the Alb3/Oxa1/YidC family of proteins. FEBS Lett. 569 89–93. [DOI] [PubMed] [Google Scholar]
- Funes, S., Nargang, F.E., Neupert, W., and Herrmann, J.M. (2004. b). The Oxa2 protein of Neurospora crassa plays a critical role in the biogenesis of cytochrome oxidase and defines a ubiquitous subbranch of the Oxa1/YidC/Alb3 protein family. Mol. Biol. Cell 15 1853–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, E.H. (1989). The Chlamydomonas Source Book: A Comprehensive Guide to Biology and Laboratory Use. (San Diego, CA: Academic Press). [DOI] [PubMed]
- Hell, K., Herrmann, J.M., Pratje, E., Neupert, W., and Stuart, R.A. (1998). Oxa1p, an essential component of the N-tail protein export machinery in mitochondria. Proc. Natl. Acad. Sci. USA 95 2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell, K., Neupert, W., and Stuart, R.A. (2001). Oxa1p acts as a general membrane insertion machinery for proteins encoded by mitochondrial DNA. EMBO J. 20 1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis, P., and Robinson, C. (2004). Mechanisms of protein import and routing in chloroplasts. Curr. Biol. 14 R1064–R1077. [DOI] [PubMed] [Google Scholar]
- Jia, L., Dienhart, M., Schramp, M., McCauley, M., Hell, K., and Stuart, R.A. (2003). Yeast Oxa1 interacts with mitochondrial ribosomes: The importance of the C-terminal region of Oxa1. EMBO J. 22 6438–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, F., Yi, L., Moore, M., Chen, M., Rohl, T., Van Wijk, K.J., De Gier, J.W., Henry, R., and Dalbey, R.E. (2002). Chloroplast YidC homolog Albino3 can functionally complement the bacterial YidC depletion strain and promote membrane insertion of both bacterial and chloroplast thylakoid proteins. J. Biol. Chem. 277 19281–19288. [DOI] [PubMed] [Google Scholar]
- Keegstra, K., and Cline, K. (1999). Protein import and routing systems of chloroplasts. Plant Cell 11 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle, K.L. (1990). High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 87 1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann, E., Droste Gen Helling, I., Carde, J.P., and Schunemann, D. (2002). The thylakoid membrane protein ALB3 associates with the cpSecY-translocase in Arabidopsis thaliana. Biochem. J. 368 777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine, Y., Eggink, L.L., Park, H., and Hoober, J.K. (2000). Vacuolar granules in Chlamydomonas reinhardtii: Polyphosphate and a 70-kDa polypeptide as major components. Planta 210 897–905. [DOI] [PubMed] [Google Scholar]
- Kroll, D., Meierhoff, K., Bechtold, N., Kinoshita, M., Westphal, S., Vothknecht, U.C., Soll, J., and Westhoff, P. (2001). VIPP1, a nuclear gene of Arabidopsis thaliana essential for thylakoid membrane formation. Proc. Natl. Acad. Sci. USA 98 4238–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, A., Stuart, R., Henry, R., and Dalbey, R.E. (2003). The Alb3/Oxa1/YidC protein family: Membrane-localized chaperones facilitating membrane protein insertion? Trends Cell Biol. 13 510–516. [DOI] [PubMed] [Google Scholar]
- Li, H.M., Kaneko, Y., and Keegstra, K. (1994). Molecular cloning of a chloroplastic protein associated with both the envelope and thylakoid membranes. Plant Mol. Biol. 25 619–632. [DOI] [PubMed] [Google Scholar]
- Liu, C., Willmund, F., Whitelegge, J.P., Hawat, S., Knapp, B., Lodha, M., and Schroda, M. (2005). J-domain protein CDJ2 and HSP70B are a plastidic chaperone pair that interacts with vesicle-inducing protein in plastids 1. Mol. Biol. Cell 16 1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink, J., Samuelsson, T., and de Gier, J.W. (2001). YidC/Oxa1p/Alb3: Evolutionarily conserved mediators of membrane protein assembly. FEBS Lett. 501 1–5. [DOI] [PubMed] [Google Scholar]
- Meurer, J., Plucken, H., Kowallik, K.V., and Westhoff, P. (1998). A nuclear-encoded protein of prokaryotic origin is essential for the stability of photosystem II in Arabidopsis thaliana. EMBO J. 17 5286–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miras, S., Salvi, D., Ferro, M., Grunwald, D., Garin, J., Joyard, J., and Rolland, N. (2002). Non-canonical transit peptide for import into the chloroplast. J. Biol. Chem. 277 47770–47778. [DOI] [PubMed] [Google Scholar]
- Moore, M., Goforth, R.L., Mori, H., and Henry, R. (2003). Functional interaction of chloroplast SRP/FtsY with the ALB3 translocase in thylakoids: Substrate not required. J. Cell Biol. 162 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, M., Harrison, M.S., Peterson, E.C., and Henry, R. (2000). Chloroplast Oxa1p homolog albino3 is required for post-translational integration of the light harvesting chlorophyll-binding protein into thylakoid membranes. J. Biol. Chem. 275 1529–1532. [DOI] [PubMed] [Google Scholar]
- Naver, H., Boudreau, E., and Rochaix, J.D. (2001). Functional studies of Ycf3: Its role in assembly of photosystem I and interactions with some of its subunits. Plant Cell 13 2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenbuhl, F., Gohre, V., Meurer, J., Krieger-Liszkay, A., Rochaix, J.D., and Eichacker, L.A. (2004). Efficient assembly of photosystem II in Chlamydomonas reinhardtii requires Alb3.1p, a homolog of Arabidopsis ALBINO3. Plant Cell 16 1790–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H., Eggink, L., Roberson, R.W., and Hoober, K. (1999). Transfer of proteins from the chloroplast to vacuoles in Chlamydomonas reinhardtii: A pathway for degradation. J. Phycol. 35 528–538. [Google Scholar]
- Pasch, J.C., Nickelsen, J., and Schunemann, D. (2005). The yeast split-ubiquitin system to study chloroplast membrane protein interactions. Appl. Microbiol. Biotechnol. 69 440–447. [DOI] [PubMed] [Google Scholar]
- Perron, K., Goldschmidt-Clermont, M., and Rochaix, J.D. (1999). A factor related to pseudouridine synthases is required for chloroplast group II intron trans-splicing in Chlamydomonas reinhardtii. EMBO J. 18 6481–6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plucken, H., Muller, B., Grohmann, D., Westhoff, P., and Eichacker, L.A. (2002). The HCF136 protein is essential for assembly of the photosystem II reaction center in Arabidopsis thaliana. FEBS Lett. 532 85–90. [DOI] [PubMed] [Google Scholar]
- Preuss, M., Ott, M., Funes, S., Luirink, J., Herrmann, J.M., Nargang, F.E., and Neupert, W. (2005). Evolution of mitochondrial oxa proteins from bacterial YidC. Inherited and acquired functions of a conserved protein insertion machinery. J. Biol. Chem. 280 13004–13011. [DOI] [PubMed] [Google Scholar]
- Purton, S., and Rochaix, J.D. (1994). Complementation of a Chlamydomonas reinhardtii mutant using a genomic cosmid library. Plant Mol. Biol. 24 533–537. [DOI] [PubMed] [Google Scholar]
- Rohr, J., Sarkar, N., Balenger, S., Jeong, B.R., and Cerutti, H. (2004). Tandem inverted repeat system for selection of effective transgenic RNAi strains in Chlamydomonas. Plant J. 40 611–621. [DOI] [PubMed] [Google Scholar]
- Ruf, S., Kossel, H., and Bock, R. (1997). Targeted inactivation of a tobacco intron-containing open reading frame reveals a novel chloroplast-encoded photosystem I-related gene. J. Cell Biol. 139 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson, J.C., Chen, M., Jiang, F., Moller, I., Wiedmann, M., Kuhn, A., Phillips, G.J., and Dalbey, R.E. (2000). YidC mediates membrane protein insertion in bacteria. Nature 406 637–641. [DOI] [PubMed] [Google Scholar]
- Samuelson, J.C., Jiang, F., Yi, L., Chen, M., de Gier, J.W., Kuhn, A., and Dalbey, R.E. (2001). Function of YidC for the insertion of M13 procoat protein in Escherichia coli: Translocation of mutants that show differences in their membrane potential dependence and Sec requirement. J. Biol. Chem. 276 34847–34852. [DOI] [PubMed] [Google Scholar]
- Saracco, S.A., and Fox, T.D. (2002). Cox18p is required for export of the mitochondrially encoded Saccharomyces cerevisiae Cox2p C-tail and interacts with Pnt1p and Mss2p in the inner membrane. Mol. Biol. Cell 13 1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti, P.A., Urbanus, M.L., Brunner, J., de Gier, J.W., von Heijne, G., van der Does, C., Driessen, A.J., Oudega, B., and Luirink, J. (2000). YidC, the Escherichia coli homologue of mitochondrial Oxa1p, is a component of the Sec translocase. EMBO J. 19 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizova, I., Fuhrmann, M., and Hegemann, P. (2001). A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene 277 221–229. [DOI] [PubMed] [Google Scholar]
- Sundberg, E., Slagter, J.G., Fridborg, I., Cleary, S.P., Robinson, C., and Coupland, G. (1997). ALBINO3, an Arabidopsis nuclear gene essential for chloroplast differentiation, encodes a chloroplast protein that shows homology to proteins present in bacterial membranes and yeast mitochondria. Plant Cell 9 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyrach, G., Ott, M., Bonnefoy, N., Neupert, W., and Herrmann, J.M. (2003). Ribosome binding to the Oxa1 complex facilitates co-translational protein insertion in mitochondria. EMBO J. 22 6448–6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theg, S.M., Cline, K., Finazzi, G., and Wollman, F.A. (2005). The energetics of the chloroplast Tat protein transport pathway revisited. Trends Plant Sci. 10 153–154. [DOI] [PubMed] [Google Scholar]
- Thompson, A.R., and Vierstra, R.D. (2005). Autophagic recycling: Lessons from yeast help define the process in plants. Curr. Opin. Plant Biol. 8 165–173. [DOI] [PubMed] [Google Scholar]
- Westphal, S., Soll, J., Vothknecht, U.C., Kroll, D., Meierhoff, K., Bechtold, N., Kinoshita, M., and Westhoff, P. (2001). A vesicle transport system inside chloroplasts VIPP1, a nuclear gene of Arabidopsis thaliana essential for thylakoid membrane formation. FEBS Lett. 506 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde, A., Hartel, H., Hubschmann, T., Hoffmann, P., Shestakov, S.V., and Borner, T. (1995). Inactivation of a Synechocystis sp strain PCC 6803 gene with homology to conserved chloroplast open reading frame 184 increases the photosystem II-to-photosystem I ratio. Plant Cell 7 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak, E., Norling, B., Andersson, B., and Pakrasi, H.B. (1999). Subcellular localization of the BtpA protein in the cyanobacterium Synechocystis sp. PCC 6803. Eur. J. Biochem. 261 311–316. [DOI] [PubMed] [Google Scholar]
- Zak, E., and Pakrasi, H.B. (2000). The BtpA protein stabilizes the reaction center proteins of photosystem I in the cyanobacterium Synechocystis sp. PCC 6803 at low temperature. Plant Physiol. 123 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., Paakkarinen, V., Suorsa, M., and Aro, E.M. (2001). A SecY homologue is involved in chloroplast-encoded D1 protein biogenesis. J. Biol. Chem. 276 37809–37814. [DOI] [PubMed] [Google Scholar]