Abstract
The α1 subunit of Na,K-ATPase is phosphorylated at Ser-16 by phorbol ester-sensitive protein kinase(s) C (PKC). The role of Ser-16 phosphorylation was analyzed in COS-7 cells stably expressing wild-type or mutant (T15A/S16A and S16D-E) ouabain-resistant Bufo α1 subunits. In cells incubated at 37°C, phorbol 12,13-dibutyrate (PDBu) inhibited the transport activity and decreased the cell surface expression of wild-type and mutant Na,K-pumps equally (∼20–30%). This effect of PDBu was mimicked by arachidonic acid and was dependent on PKC, phospholipase A2, and cytochrome P450-dependent monooxygenase. In contrast, incubation of cells at 18°C suppressed the down-regulation of Na,K-pumps and revealed a phosphorylation-dependent stimulation of the transport activity of Na,K-ATPase. Na,K-ATPase from cells expressing α1-mutants mimicking Ser-16 phosphorylation (S16D or S16E) exhibited an increase in the apparent Na affinity. This finding was confirmed by the PDBu-induced increase in Na sensitivity of the activity of Na,K-ATPase measured in permeabilized nontransfected COS-7 cells. These results illustrate the complexity of the regulation of Na,K-ATPase α1 isozymes by phorbol ester-sensitive PKCs and reveal 1) a phosphorylation-independent decrease in cell surface expression and 2) a phosphorylation-dependent stimulation of the transport activity attributable to an increase in the apparent Na affinity.
INTRODUCTION
In animal cells, the Na,K-ATPase uses the energy of ATP hydrolysis for the countertransport of three Na and two K ions and thereby maintains the electrochemical gradients of these ions across the plasma membrane. Because this process is electrogenic, it also participates in the generation of the resting membrane potential. In addition to these general functions, Na,K-ATPase promotes the membrane repolarization in excitable cells, and it provides the driving force for vectorial Na transport in epithelial cells. The enzyme is composed of at least two subunits: the large α subunit with 10 transmembrane segments carries the catalytic and ion transport properties, and the smaller, single-membrane–spanning β subunit is involved in the maturation of Na,K-ATPase and in the modulation of its transport activity.
Because Na,K-ATPase is of crucial importance in many physiological and pathological processes, the elucidation of the mechanisms that regulate its activity is an important issue. It has recently been demonstrated that Na,K-ATPase activity can be controlled by hormones and second messengers independently of Na availability or changes in the rate of subunit synthesis (McGill and Guidotti, 1991; Féraille et al., 1994, 1995; Chibalin et al., 1997; Carranza et al., 1998). This rapid modulation of Na,K-ATPase activity by hormones may be linked to a direct phosphorylation of the Na,K-ATPase α1 subunit by serine/threonine kinases. For instance, in purified enzyme preparations (Bertorello et al., 1991; Feschenko and Sweadner, 1994), in homogenates of Xenopus oocytes (Chibalin et al., 1992), and in intact cells (Middleton et al., 1993; Béguin et al., 1994; Carranza et al., 1996; Feschenko and Sweadner, 1997), protein kinase C (PKC) can phosphorylate the α1 isoforms of Na,K-ATPase and to a much lesser extent the α2 and α3 isoforms (Béguin et al., 1996b). Two PKC phosphorylation sites have been located in the cytoplasmic NH2 terminus of α1 subunits. The first PKC phosphorylation site identified is present in all α1 subunits and was mapped to Ser-16 in an unusual (Ser-Glu-His) PKC motif (Béguin et al., 1994, 1996b). A second rat-specific α1 subunit PKC phosphorylation site was mapped to Ser-23 (Feschenko and Sweadner, 1995; Béguin et al., 1996b). It should be mentioned that Ser-16 and Ser-23 numbered from the initial methionine residue are also named Ser-11 and Ser-18 by other authors according to the removal of the NH2-terminal 5 amino acids during the processing of the α1 chains.
The functional role of PKC phosphorylation is not yet resolved. Indeed, stimulatory, inhibitory, or no effects have been attributed to PKC phosphorylation (Bertorello et al., 1991; Middleton et al., 1993; Fisone et al., 1995; Carranza et al., 1996; Feschenko and Sweadner, 1997; Pedemonte et al., 1997). In view of the multiple mechanisms that affect Na,K pump activity and the possible interplay between different signaling pathways, these conflicting results suggest that tissue-specific factors and uncontrolled experimental conditions may mask the basic function of PKC phosphorylation. In addition, the possibility of specific effects mediated by phosphorylation of either Ser-16 or Ser-23 or both should be considered (Vasilets, 1997). Previous studies focusing on the functional effect of Ser-23 phosphorylation, i.e. the additional rat PKC site, have documented a phosphorylation-dependent inhibition of Na,K-ATPase activity (Belusa et al., 1997; Chibalin et al., 1999). In contrast, the functional role of Ser-16 phosphorylation, i.e., the ubiquitous PKC site, has not yet been defined (Beron et al., 1997).
In the present study, we stably expressed the wild-type Bufo marinus α1 subunit or its T15A/S16A mutant in COS-7 cells. Furthermore, we studied α1-mutants in which the Ser-16 phosphorylated by PKC was replaced by negatively charged amino acids (Asp or Glu), which in other settings was shown to mimic constitutive phosphorylation (Hoffman et al., 1994; Pages et al., 1994). In each cell line, we analyzed the effects of the activation of phorbol ester-sensitive PKCs on the activity of Na,K-ATPase and the cell surface expression of Na,K-pumps under various experimental conditions. The α1 subunit of the Bufo Na,K-ATPase was chosen for transfection because 1) it forms ouabain-resistant Na,K-pumps that can be distinguished from the ouabain-sensitive, endogenous Na,K-pumps of COS cells; and 2) it is efficiently phosphorylated by PKC in intact cells (Béguin et al., 1994). Finally and most importantly, besides a residual 10% phosphorylation on Thr-15, the Bufo α1 subunit is mainly phosphorylated by PKC on Ser-16 (Béguin et al., 1996b), which is representative of all known mammalian α1 subunits.
Our results indeed reveal a complex pattern of regulatory mechanisms that affect Na,K-ATPase activity after stimulation of phorbol ester-sensitive PKCs, which is dependent on experimental conditions and thus may partially account for the contradictory results reported in the literature. However, comparison, under defined experimental conditions, of the functional properties of wild-type α1 subunits, on the one hand, and Ser-16 mutants, on the other hand, clearly demonstrates that PKC phosphorylation of Ser-16 has a basic stimulatory effect on Na,K-ATPase activity, which relies on an increase in the apparent Na affinity.
MATERIALS AND METHODS
Cell Culture and DNA Transfection
COS-7 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with: 10% fetal calf serum, l-glutamine (10−6 M), penicillin (10 IU/ml), and streptomycin (100 μg/ml). COS-7 cells were transfected with the expression vector pRK5 (a gift of J.R. Didsbury, Duke University Medical Center, Durham, NC) containing the cDNA for the wild-type B. marinus α1 subunit or the α1 subunit mutated at Thr-15 and/or Ser-16 (T15A/S16A or S16D-E) or at Ser-943 (S943A), as previously described (Béguin et al., 1994). COS-7 cells stably expressing the wild-type or mutated α1 subunit of B. marinus were selected on the basis of the ouabain resistance of the Bufo enzyme (IC50, ∼5 × 10−5 M) compared with the endogenous Na,K-ATPase of COS-7 cells (IC50, ∼3 × 10−7 M). Two days after transfection, the medium was supplemented with 2.5 × 10−6 M ouabain and changed every 2 d, and 3 wk later, the surviving cell clones were isolated and tested for 1) the presence (wild type) or absence (T15A/S16A or S16D-E) of PKC-dependent phosphorylation of the exogenous α1 subunits (Béguin et al., 1994) and 2) the presence of an ouabain-resistant 86Rb uptake accounting for at least 40% of the total (endogenous and exogenous) Na,K-ATPase-mediated 86Rb uptake (see below). In these stably transfected cells, the presence of functional ouabain-resistant Na,K-ATPase units in the plasma membrane is dependent on the association of the exogenous α1 subunits with the endogenous β subunits.
After stable transfection, COS-7 cells were subsequently grown in medium supplemented with 2.5 × 10−6 M ouabain to maintain the selection pressure. For experiments, cells were grown to confluence and were used between passages 10 and 30.
Na,K-ATPase-mediated 86Rb Uptake
The transport activity of Na,K-ATPase was measured as the ouabain-sensitive 86Rb uptake under conditions of initial rate. For this purpose, COS-7 cells were seeded on 12 multiwell plates (22-mm-diam wells) and grown to confluence. After removal of the culture medium, cells were washed twice with 1 ml of HEPES-buffered (20 mM, pH 7.4) bicarbonate- and serum-free DMEM. Cells were then preincubated at 37 or at 18°C for 15–30 min after addition of 1 ml of the same medium containing or not activators of protein kinases or/and inhibitors. 86Rb uptake was determined in triplicate samples after addition of 10 μl of DMEM containing tracer amounts of 86RbCl (Amersham, Little Chalfont, United Kingdom; 100 nCi/sample). The K concentration was 5.36 mM during incubation and uptake periods. Incubation was stopped after 1 min (37°C) or 15 min (18°C) by cooling on ice and rapid aspiration of the incubation medium. After three washes with 1 ml of ice-cold washing solution containing 150 mM choline-chloride, 1.2 mM MgSO4, 1.2 mM CaCl2, 2 mM BaCl2, and 5 mM HEPES, pH 7.4, cells were lysed in 0.5 ml of 1% (wt/vol) Na deoxycholate, and 0.4 ml of the lysate were transferred into a counting vial and radioactivity was measured by liquid scintillation. The remaining 0.1 ml of the lysate was used to determine the protein content by the bisinchoninic acid assay (Pierce, Rockford, IL).
The total ouabain-sensitive 86Rb uptake, i.e., the sum of endogenous and exogenous Na,K-ATPase-mediated Rb (K) transport, was calculated as the difference between the mean values measured in triplicate samples incubated with or without 2.5 × 10−3 M ouabain. The Rb (K) transport mediated by the exogenous Na,K pumps containing the Bufo α subunit was calculated as the difference between the mean values measured in triplicate samples incubated with 2.5 × 10−6 or 2.5 × 10−3 M ouabain. When present, ouabain was introduced at the beginning of the preincubation step. 86Rb uptake was calculated as picomoles of Rb (K) × minute−1 × microgram of protein−1. Preliminary experiments have shown that Rb (K) uptake was linear for at least 3 or 20 min at 37 or 18°C, respectively (our unpublished results).
Hydrolytic Activity of Na,K-ATPase
The hydrolytic activity of Na,K-ATPase was estimated by measuring the release of inorganic phosphate from [γ-32P]ATP (Fisone et al., 1994). Na,K-ATPase activity was measured either in situ on permeabilized cells or in crude membrane preparations.
Measurement of Na,K-ATPase activity in permeabilized cells was performed essentially as described previously (Chibalin et al., 1999). COS-7 cells grown to confluence in 25-cm2 flasks were detached by trypsinization. Trypsin was then neutralized by addition of 20 ml of DMEM supplemented with 10% (vol/vol) fetal calf serum. Cells from two flasks were pooled and resuspended in 2 ml of incubation solution containing 120 mM choline-Cl, 5 mM KCl, 4 mM KHCO3, 1 mM CaCl2, 1 mM MgSO4, 0.2 mM KH2PO4, 0.15 mM K2HPO4, 5 mM glucose, 10 mM lactate, 1 mM pyruvate, 4 mM essential and nonessential amino acids, 0.03 mM vitamins, 20 mM HEPES, and 0.1% BSA, pH 7.45 (with KOH). Cells were then separated in two 1-ml aliquots and were preincubated at 37 or 18°C in the absence or presence of phorbol 12,13-dibutyrate (PDBu). Preincubation was stopped by cooling on ice, and cells were permeabilized by a freeze–thaw step at −20°C. Cell aliquots containing 5–15 μg of protein were transferred with 20 μl of incubation solution into 1.5-ml microtubes. After addition of 80 μl of ATPase assay solution (see below), permeabilized cells were incubated for 15 min at 37°C. The reaction was stopped by cooling on ice and addition of 1 ml of 10% (wt/vol) activated charcoal. After mixing and centrifugation, the radioactivity was measured by liquid scintillation on 250-μl aliquots of supernatants, which contain the inorganic phosphate formed from ATP. In each experiment, ATPase activities were determined on four replicates for each condition. Preliminary experiments have shown that preincubation of cells in the absence of Na does not alter the effects of PDBu on Na,K-ATPase activity (our unpublished results). It should be mentioned that the sensitivity of the method did not allow us to measure Na,K-ATPase activity in the presence of Na concentrations <5 mM.
Na,K-ATPase activity was also measured on isolated membranes prepared according to the method of Vilsen (Vasilets et al., 1991) from stably transfected COS-7 cells and made leaky by sonication followed by a freeze–thawing step or by treatment with SDS, which gave similar results. All assays were carried out in the presence of 2.5 μM ouabain to inhibit the endogenous COS-7 cell Na,K-ATPase. In each experiment and for each Na concentration, ATPase activity was determined as described above on four replicates containing 5–10 μg of protein from crude membrane preparations.
The ATPase assay solutions contained various amounts of NaCl (0–140 mM), 10 mM KCl, 5 mM MgCl2, 1 mM EDTA, 100 mM Tris-HCl, 1 mM Tris-ATP, and tracer amounts (5 nCi/μl) of [γ-32P]ATP (DuPont, Boston, MA; 10 Ci/mmol) for measurements of total ATPase activity. For basal Mg-ATPase activity measurements, NaCl and KCl were omitted, and 1 mM ouabain was added. The osmolarity of ATP assay solutions was adjusted by addition of choline-chloride, and the pH of each solution was 7.4. Na,K-ATPase activity was taken after subtracting the mean Mg-ATPase activity from the mean total ATPase activity and was calculated as picomoles of ATP × microgram of protein−1 × hour−1 ± SE.
Cell Surface Biotinylation, Streptavidin Precipitation, and Immunoblot
Changes in cell surface expression of Na,K-ATPase were analyzed by immunoblot after streptavidin precipitation of biotinylated cell surface proteins as described by Gottardi et al. (1995) with slight modifications. For this purpose, COS-7 cells were seeded on 12 multiwell plates (22-mm-diam wells) and grown to confluence. After removal of the culture medium, cells were washed twice with 1 ml of HEPES-buffered (20 mM, pH 7.4) bicarbonate- and serum-free DMEM. Cells were then preincubated at 37 or 18°C for 15–30 min after addition of 1 ml of the same medium containing or not activators of protein kinases or/and inhibitors. Incubation was stopped by cooling on ice and rapid aspiration of the incubation medium. Cells were then washed once with PBS-CM (PBS, 0.1 mM CaCl2, and 1 mM MgCl2) and incubated at 4°C for 1 h with biotinylation buffer (10 mM Tris-HCl, pH 7.5, 2 mM CaCl2, and 150 mM NaCl) containing 1.5 mg/ml biotin (EZ-Link sulfo-NHS-biotin; Pierce). After aspiration of the biotinylation buffer, cells were incubated for 20 min at 4°C in PBS-CM supplemented with 100 mM glycine to quench the unreacted sulfo-NHS-biotin, washed once with PBS-CM, and lysed in 200 μl of 1% (wt/vol) Na-deoxycholate. Equal amounts of proteins (30–50 μg) were added to 100 μl of streptavidin-agarose beads (Immunopure immobilized streptavidin; Pierce) diluted in anti-protease–containing buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA, 10 mg/ml aprotinin, and 50 mg/ml leupeptin) supplemented with 0.5% digitonin and were incubated overnight at 4°C. The beads were then washed once with rinsing solution A (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 5 mM EDTA, and 0.5% digitonin), twice with rinsing solution B (500 mM NaCl, 50 mM Tris-HCl, pH 7.4, 5 mM EDTA, and 0.05% digitonin), three times with rinsing solution C (500 mM NaCl, 20 mM Tris-HCl, pH 7.4, 0.2% BSA, and 0.25% digitonin), and once with 10 mM Tris-HCl, pH 7.4. Samples were then resuspended in 100 μl of sample buffer, heated at 65°C for 15 min, and subjected to 7% SDS-PAGE. After electrotransfer, α1 subunits were revealed using a specific anti-Bufo α1 subunit antibody (a kind gift from Dr. François Verrey, University of Zürich, Zürich, Switzerland). This Bufo anti-α1 antibody did not recognize the endogenous COS-7 cell α subunit or rat or rabbit α subunits. The immunoreactivity was detected by the enhanced chemiluminescence method, according to the manufacturer's instructions (Amersham).
Preliminary experiments have shown that membranes of COS-7 cells are impermeable to sulfo-NHS-biotin, because Hsp27, an abundant cytosolic protein, was only detected by Western blotting (monoclonal antibody from StressGen, Victoria, British Columbia, Canada) in total cellular extracts but not in biotinylated and streptavidin-precipitated samples (our unpublished results).
Statistics and Calculations
For comparisons between two means expressed as absolute values, statistical analysis was done by Student's t test for unpaired data (86Rb uptakes) or for paired data (hydrolytic activity of Na,K-ATPase) when appropriate. The Mann–Whitney U test was used for comparisons between two groups for data expressed as fractional changes. Comparison between more than two groups was done by analysis of variance for results expressed as absolute values or by the Kruskal–Wallis test for results expressed as fractional changes, respectively.
Values of the ouabain inhibition of 86Rb uptake were fitted to the following two equations describing either a homogenous population or two independent subpopulations of Na,K-ATPase (Féraille et al., 1993):
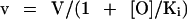 |
1 |
or
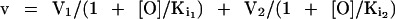 |
2 |
where v is the 86Rb uptake measured at a given concentration of ouabain ([O]); V is the 86Rb uptake measured in the absence of ouabain; and Ki is the apparent inhibition constant for ouabain of the Na,K-ATPase (IC50). Kinetic parameters (V and IC50) were determined from Eqs. 1 and 2 by nonlinear regression analysis using Prism 2.0 software (Graphpad Software, San Diego, CA).
As previously described (Féraille et al., 1995), the Na dependence of the Na,K-ATPase was analyzed using the Hill equation:
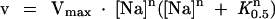 |
3 |
where v is the Na,K-ATPase activity measured at a given concentration of Na ([Na]); Vmax is the maximal Na,K-ATPase activity; K0.5Na is the apparent dissociation constant for Na; and n is the Hill coefficient.
Kinetic parameters were determined by nonlinear regression analysis using Prism 2.0 software. K0.5Na values were compared by Student's t test for unpaired data.
RESULTS
Functional Expression of Ouabain-resistant Na,K-ATPase in Stably Transfected COS-7 Cells
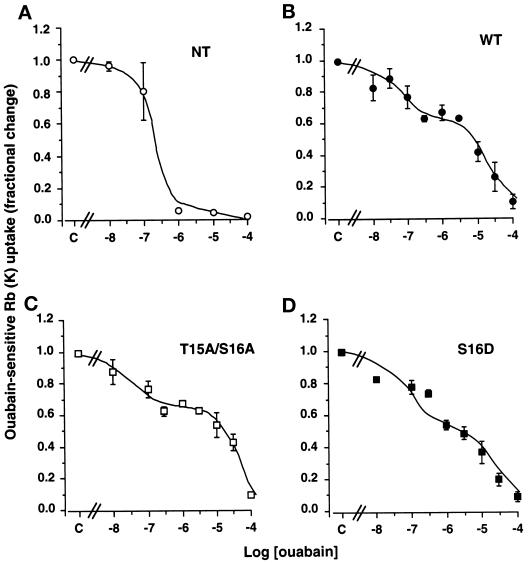
The expression of functional ouabain-resistant Bufo Na,K-ATPase in transfected COS-7 cells was revealed by the dose–inhibition curve of 86Rb uptake by ouabain (from 10−8 to 2.5 × 10−3 M). Figure 1A shows that nontransfected COS-7 cells (NT) exhibit a monophasic inhibition pattern consistent with the presence of a single population of endogenous Na,K-ATPase units. In contrast, COS-7 cells expressing wild-type (WT, Figure 1B) Bufo α1 subunits, T15A/S16A α-mutants (Figure 1C), or S16D α-mutants (Figure 1D) exhibited a bimodal inhibition pattern. The IC50 of the Na,K-ATPase populations with high ouabain sensitivity (2 × 10−8–4 × 10−7 M) and that with low ouabain sensitivity (1–3.5 × 10−5 M) reasonably fit with the IC50 of endogenous COS-7 cell Na,K pumps (Vilsen, 1992) and Bufo Na,K pumps (Jaisser et al., 1993), respectively. These results show that the endogenous β subunits of COS-7 cells associate with exogenous Bufo wild-type or mutant α1 subunits to form functional ouabain-resistant α–β complexes (hybrid pumps), which account for the 40–60% residual 86Rb uptake measured in the presence of 2.5 × 10−6 M ouabain (Figure 1, B–D).
Figure 1.
Stable transfection of COS-7 cells with wild-type or mutant B. marinus α1 subunits results in functional expression of ouabain-resistant Na,K-ATPase. 86Rb uptake was measured under initial rates in COS-7 cells preincubated for 15 min at 37°C in the absence or in the presence of increasing concentrations of ouabain. The Na,K-ATPase-mediated 86Rb uptake was obtained by subtraction of ouabain-insensitive 86Rb uptake measured in the presence of 2.5 × 10−3 M ouabain. Results are expressed as fractional change (with respect to control value, i.e., absence of ouabain) and are means ± SE from three or four independent experiments. (A) The ouabain sensitivity of the endogenous Na,K-ATPase of nontransfected (NT) COS-7 cells was monophasic (IC50 = 2 × 10−7 M) and best described by Eq. 1 (r2 = 0.97; see MATERIALS AND METHODS). (B–D) COS-7 cells transfected with the wild-type (B; WT), T15A/S16A mutant (C), or S16D mutant (D) Bufo α1 subunit cDNAs exhibited a bimodal ouabain inhibition pattern best described by Eq. 2 (r2 = 0.95–0.98; see MATERIALS AND METHODS). The Na,K-ATPase populations with high ouabain sensitivity exhibited IC50 values between 2 × 10−8 and 4 × 10−7 M, and the Na, K-ATPase populations with lower ouabain sensitivity displayed IC50 values between 1 and 3.5 × 10−5 M.
In all subsequent experiments, the exogenous Na,K-ATPase-mediated 86Rb uptake was measured in the presence of 2.5 × 10−6 M ouabain during preincubation of cells as well as incubation with 86Rb.
Effect of PDBu on the Transport Activity of Na,K-ATPase Is Temperature Dependent
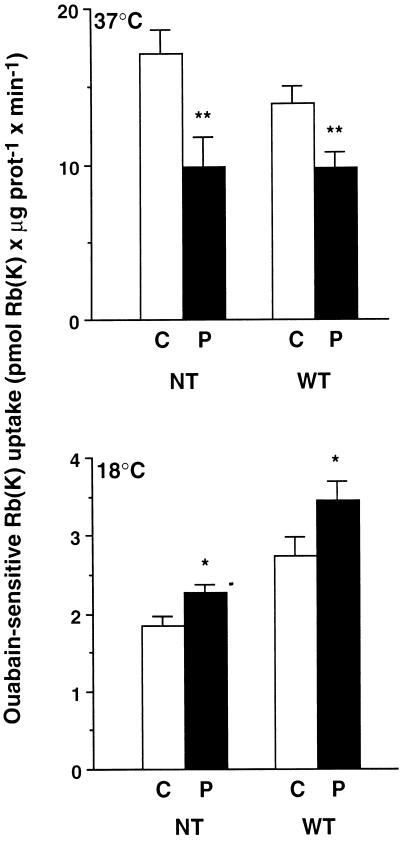
We first determined the effect of the activation of phorbol ester-sensitive PKCs by PDBu on the transport activity of endogenous Na,K-ATPase in nontransfected COS-7 cells and of exogenous Na,K-ATPase in COS-7 cells stably transfected with the wild-type Bufo α1 subunit. As depicted in Figure 2, 10−7 M PDBu for 15 min at 37°C inhibited the endogenous Na,K-ATPase-mediated 86Rb uptake by 46 ± 6% (p < 0.01) and the exogenous Na,K-ATPase-mediated 86Rb uptake by 30 ± 5% (p < 0.01) in nontransfected cells and in cells expressing the wild-type Bufo α1 subunit, respectively. In contrast, when temperature was lowered to 18°C, 10−7 M PDBu for 30 min stimulated the Na,K-ATPase-mediated 86Rb uptake by 29 ± 13% (p < 0.02) in nontransfected cells and by 26 ± 9.5% (p < 0.03) in cells expressing the wild-type Bufo α1 subunit.
Figure 2.
PDBu inhibits or stimulates the transport activity of Na, K-ATPase in COS-7 cells incubated at 37 and 18°C, respectively. Na,K-ATPase-mediated 86Rb uptake was measured under initial rates of influx in nontransfected (NT) COS-7 cells or in cells stably expressing the wild-type (WT) Bufo α1 subunit. In transfected cells, 86Rb uptake was determined in the presence of 2.5 × 10−6 M ouabain, which completely inhibits the endogenous Na, K-ATPase. Cells were incubated without (C, open bars) or with 10−7 M PDBu (P, filled bars) for 15 or 30 min in experiments performed at 37°C (upper panel) and 18°C (lower panel), respectively. Results are expressed as picomoles of Rb (K) × minute−1 × microgram of protein−1 and are means ± SE from 7–20 independent experiments (*, p < 0.05; **, p < 0.01).
At 37°C, PDBu decreased the ouabain-insensitive 86Rb uptake (as pmol of Rb [K] × μg of protein−1 × min−1 ± SE) from 6.27 ± 0.65 to 4.33 ± 0.51 (p < 0.05) in nontransfected cells and from 10.08 ± 0.81 to 7.86 ± 0.53 (p < 0.05) in transfected cells. In contrast, at 18°C PDBu did not alter the ouabain-insensitive 86Rb uptake both in nontransfected (control, 1.47 ± 0.06; PDBu, 1.60 ± 0.07) and transfected (control, 2.75 ± 0.19; PDBu, 2.56 ± 0.18) cells.
Thus, activation of phorbol ester-sensitive PKCs inhibits or stimulates the transport activity of Na,K-ATPase according to the incubation temperature.
In COS-7 Cells Incubated at 37°C, PDBu Down-regulates Na,K-ATPase Independently of the Phosphorylation of the α1 Subunit at Ser-16
Using the amphibian A6 epithelial cell line, Beron et al. (1997) have reported that activation of phorbol ester-sensitive PKCs induces a Ser-16 phosphorylation-independent down-regulation of cell surface Na,K-ATPase through an increase in fluid phase endocytosis. The following experiments were performed to determine whether a similar mechanism operates in mammalian cells.
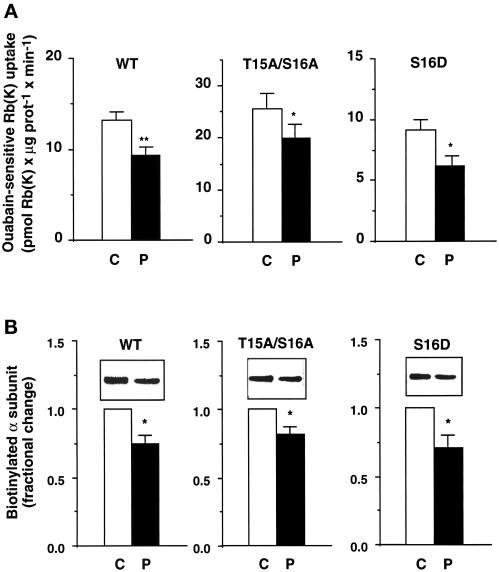
As shown in Figure 3A, in COS-7 cells expressing either the wild-type Bufo α1 subunit (left panel) or its S943A α1-mutant, i.e., the protein kinase A (PKA) phosphorylation site mutant (our unpublished results), 10−7 M PDBu for 15 min at 37°C inhibited the exogenous Na,K-ATPase-mediated 86Rb uptake by 30 ± 5% (p < 0.01) and 31 ± 7% (p < 0.05), respectively. In COS-7 cells expressing the T15A/S16A α1-mutant in which the PKC-dependent phosphorylation is abolished (Béguin et al., 1994, 1996a), PDBu still inhibited the exogenous Na,K-ATPase-mediated 86Rb uptake by 19 ± 3% (p < 0.05; Figure 3A, middle panel). Finally, the ouabain-sensitive 86Rb uptake was inhibited by 33 ± 8% (p < 0.05; Figure 3A, right panel) by PDBu in cells expressing the S16D α1-mutant, which contains a negative charge mimicking constitutive phosphorylation. Similar results were obtained in cells expressing the S16E α1-mutant (our unpublished results).
Figure 3.
In COS-7 cells incubated at 37°C, the effects of PDBu on the transport activity and the cell surface expression of Na, K-ATPase are independent of the phosphorylation of the α1 subunit at Ser-16. COS-7 cells stably expressing the wild-type (WT) Bufo α1 subunit, the T15A/S16A mutant, or the S16D mutant mimicking constitutive phosphorylation were incubated for 15 min at 37°C in the absence (C, open bars) or presence of 10−7 M PDBu (P, filled bars). (A) Exogenous Na,K-ATPase-mediated 86Rb uptake was measured in the presence of 2.5 × 10−6 M ouabain under initial rates of influx. Results are expressed as picomoles of Rb (K) × minute−1 × microgram of protein and are means ± SE from 7–12 independent experiments (*, p < 0.05; **, p < 0.01). (B) The effect of PDBu on the cell surface expression of Bufo α1 subunits was measured by Western blot with anti-Bufo α1 subunit antibody after streptavidin precipitation of the biotinylated cell surface proteins. Insets show a representative experiment, and bars show the quantitation of the relative amounts of cell surface Na,K-ATPase α1 subunits. Results are expressed as fractional change (with respect to control value) and are means ± SE from 6–18 independent experiments (*, p < 0.05).
The effect of PDBu on the cell surface expression of the exogenous Na,K-ATPase was estimated using a biotinylation-streptavidin precipitation assay. Figure 3B shows that 10−7 M PDBu for 15 min at 37°C decreased the number of biotinylated, cell surface–located, wild-type Bufo α1 subunits and T15A/S16A and S16D α1-mutants by 25 ± 6, 18 ± 5, and 29 ± 9%, respectively.
These results demonstrate that, like in amphibian cells (Beron et al., 1997), PDBu down-regulates the cell surface Na,K-ATPase independently of the phosphorylation of its catalytic α1 subunit at Ser-16 in the mammalian COS-7 cell line.
The Inhibition of the Transport Activity of Na,K-ATPase by PDBu at 37°C is Dependent on PKC and Arachidonic Acid Metabolism
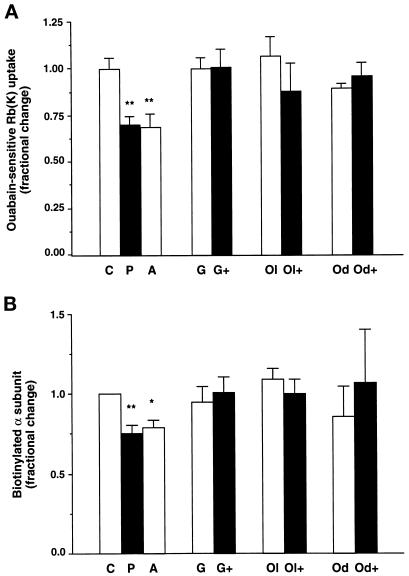
To evaluate the specificity of the effects of PDBu, the ouabain-sensitive 86Rb uptake and the cell surface expression of Na,K-ATPase were studied under conditions in which PKC was inhibited. Preincubation of cells for 15 min at 37°C in the presence of 5 × 10−7 M GF109203X, a specific PKC inhibitor (Toullec et al., 1991), prevented both the PDBu-induced inhibition of the transport activity of the exogenous Na,K-ATPase (Figure 4A) and its decrease in cell surface expression (Figure 4B) in cells expressing the wild-type Bufo α1 subunit. Similar results were obtained in cells expressing the T15A/S16A α1-mutant (our unpublished results). These observations indicate that activation of phorbol ester-sensitive PKC(s) mediates the effects of PDBu.
Figure 4.
In COS-7 cells incubated at 37°C, the effects of PDBu on the transport activity and the cell surface expression of Na,K-ATPase are dependent on PKCs and arachidonic acid metabolism. COS-7 cells expressing the wild-type Bufo α1 subunit were incubated for 30 min at 37°C in the absence (C) or presence of 10−7 M PDBu (P), 5 × 10−6 M arachidonic acid (A), 5 × 10−7 M GF109203X (a PKC inhibitor; G), GF109203X and PDBu (G+), 10−5 M oleoyloxyethylphosphocholine (a phospholipase A2 inhibitor; Ol), oleoyloxyethylphosphocholine and PDBu (Ol+); 17-octadecynoic acid (a cytochrome P450 inhibitor; Od), and 17-octadecynoic acid and PDBu (Od+). (A) Exogenous Na,K-ATPase-mediated 86Rb uptake was measured in the presence of 2.5 × 10−6 M ouabain under initial rates of influx. Results are expressed as fractional change (with respect to control value) and are means ± SE from 6–14 independent experiments (**, p < 0.01). (B) After streptavidin precipitation, the cell surface–expressed Bufo α1 subunits were detected by immunoblotting using a specific anti-Bufo α1 subunit antibody. Results are expressed as fractional change (with respect to control values) and are means ± SE from six or seven independent experiments (*, p < 0.05).
In various cell types, the inhibitory effect of phorbol esters on Na,K-ATPase activity rely on a PKC-mediated phospholipase A2 (PLA2) activation (Satoh et al., 1993b; Xia et al., 1995) and arachidonic acid metabolism through the cytochrome P450-dependent monooxygenase pathway (Schwartzman et al., 1985; Satoh et al., 1992, 1993a; Chibalin et al., 1998). Therefore, we evaluated the role of this pathway in the down-regulation of Na,K-ATPase by PDBu in COS-7 cells expressing the wild-type Bufo α1 subunit. Preincubation for 15 min at 37°C with 5 × 10−6 M arachidonic acid or 10−7 M PDBu decreased the transport activity (Figure 4A) and the cell surface expression (Figure 4B) of the exogenous Na,K-ATPase to the same extent. On the other hand, the inhibition of PLA2 by 15 min of preincubation at 37°C with 10−5 M oleoyloxyethylphosphocholine prevented the PDBu-induced inhibition of the ouabain-sensitive 86Rb uptake (Figure 4A) and the decrease in cell surface expression of Na,K-ATPase (Figure 4B). Similarly, 10−5 M mepacrine, another PLA2 inhibitor, abolished the effect of PDBu on the transport activity of Na,K-ATPase (as pmol of Rb [K] × μg of protein−1 × min−1: control, 13.2 ± 0.8; PDBu, 9.3 ± 1.0; mepacrine, 12.0 ± 0.8; mepacrine and PDBu, 12.6 ± 0.6). In agreement with these observations, the inhibition of the cytochrome P450-dependent monooxygenase pathway by 15 min of preincubation at 37°C with 10−6 M 17-octadecynoic acid abrogated the effects of PDBu on the transport activity (Figure 4A) and the cell surface expression (Figure 4B) of Na,K-ATPase. In agreement with this result, 10−6 M SKF525A, another structurally unrelated inhibitor of the cytochrome P450-dependent monooxygenase, prevented the PDBu-dependent inhibition of ouabain-sensitive 86Rb uptake (as pmol of Rb [K] × μg of protein−1 × min−1; control, 12.5 ± 1.2; PDBu, 8.6 ± 1.3; SKF, 11.4 ± 1.0; SKF and PDBu, 10.3 ± 1.3).
Altogether, these results may suggest that the PKC-mediated down-regulation of Na,K-ATPase is dependent on PLA2 activation and the subsequent generation of arachidonic acid metabolites through the cytochrome P450-dependent monooxygenase pathway in COS-7 cells.
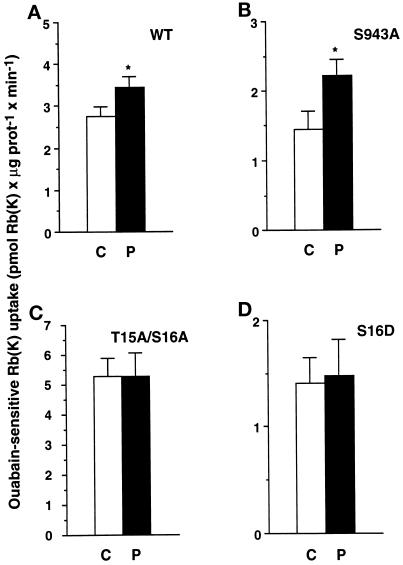
The Stimulation of the Transport Activity of Na,K-ATPase by PDBu at 18°C Is Dependent on Phosphorylation of the α1 Subunit at Ser-16
As shown in Figure 2, lowering the incubation temperature to 18°C revealed a stimulatory effect of PDBu on the ouabain-sensitive 86Rb uptake. The following experiments were therefore performed to determine the role of phosphorylation of the α1 subunit at Ser-16 in the PDBu-induced stimulation of the transport activity of Na,K-ATPase at 18°C. In COS-7 cells expressing either the wild-type Bufo α1 subunit (Figure 5A) or its S943A α1-mutant, i.e., the PKA phosphorylation site mutant (Figure 5B), 10−7 M PDBu for 30 min at 18°C stimulated the exogenous Na,K-ATPase-mediated 86Rb uptake by 26 ± 9% (p < 0.05) and 53 ± 11% (p < 0.05), respectively. The PDBu-induced stimulation of the exogenous Na,K-ATPase-mediated 86Rb uptake was abolished in COS-7 cells that expressed the T15A/S16A α1-mutant (Figure 5C), which is no longer phosphorylated by PKC (Béguin et al., 1994, 1996a). Finally, the ouabain-sensitive 86Rb uptake was not altered by PDBu in cells expressing the S16D α1-mutant (Figure 5D), which contains a negative charge and thus mimics constitutive phosphorylation. Altogether, these results strongly suggest that the stimulation of the ouabain-sensitive 86Rb uptake by PDBu observed at 18°C relies on phosphorylation of the catalytic α1 subunit of Na,K-ATPase at Ser-16.
Figure 5.
In COS-7 cells incubated at 18°C, the PDBu-induced stimulation of the transport activity of the Na,K-ATPase is abolished by mutation of the α1 subunit at Ser-16. Exogenous Na,K-ATPase-mediated 86Rb uptake was measured in the presence of 2.5 × 10−6 M ouabain under initial rates of influx in COS-7 cells stably expressing the wild-type (A, WT) Bufo α1 subunit, the S943A (PKA site) mutant (B), the T15A/S16A mutant (C), or the S16D mutant mimicking constitutive phosphorylation (D). Cells were incubated for 30 min at 18°C in the absence (C, open bars) or in the presence of 10−7 M PDBu (P, filled bars). Results are expressed as picomoles of Rb (K) × microgram of protein−1 × minute−1 and are means ± SE from 7–20 independent experiments (*, p < 0.05).
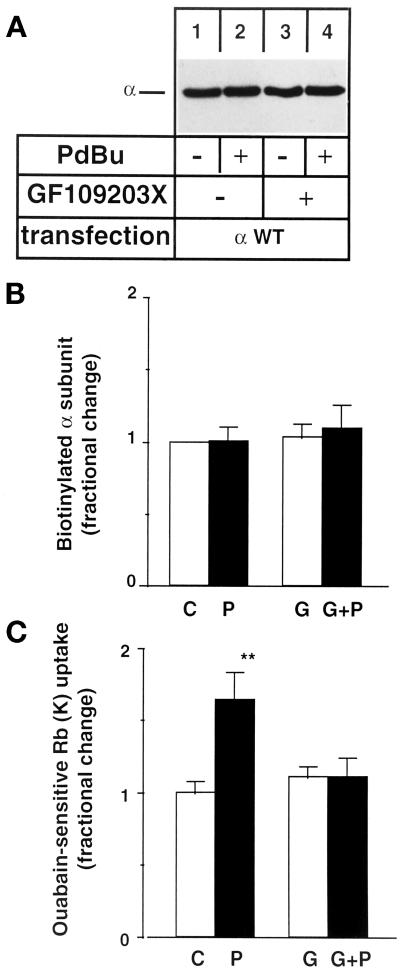
PDBu Does Not Change the Cell Surface Expression of Na,K-ATPase in Cells Incubated at 18°C
Biotinylation assays were performed on intact COS-7 cells expressing wild-type Bufo α1 subunits. Figure 6, A and B, shows that neither 10−7 M PDBu nor 5 × 10−7 M GF109203X, a specific PKC inhibitor, for 30 min at 18°C changed the cell surface expression of exogenous Na,K pumps. In contrast, the PDBu-induced stimulation of the exogenous Na,K-ATPase-mediated 86Rb transport was abolished by GF109203X (Figure 6C).
Figure 6.
Incubation at 18°C prevents the PDBu-induced down-regulation of cell surface Na,K-ATPase. COS-7 cells expressing wild-type Bufo α1 subunit were preincubated for 30 min at 18°C in the absence or in the presence of 10−7 M PDBu (P), 5 × 10−7 M GF109203X (G), or PDBu and GF109203X (G+P) before biotinylation of cell surface proteins (A and B) or measurement of exogenous Na,K-ATPase-mediated 86Rb uptake (C). After streptavidin precipitation, the cell surface–expressed Bufo α1 subunit was detected by immunoblot using a specific anti-Bufo α1 subunit antibody. (A) A representative immunoblot is shown (n = 4). (B) Quantitation of the relative amounts of Na,K-ATPase α1 subunit detected by immunoblotting. Results are expressed as fractional change (with respect to control value) and are means ± SE from four independent experiments. (C) Ouabain-sensitive 86Rb uptake. Results are expressed as fractional change (with respect to control value) and are means ± SE from 11 independent experiments (**, p < 0.01).
This result shows that the PKC phosphorylation-dependent stimulation of the transport activity of Na,K-ATPase observed at 18°C is not due to an increase in the number of active Na,K-pumps located at the cell surface.
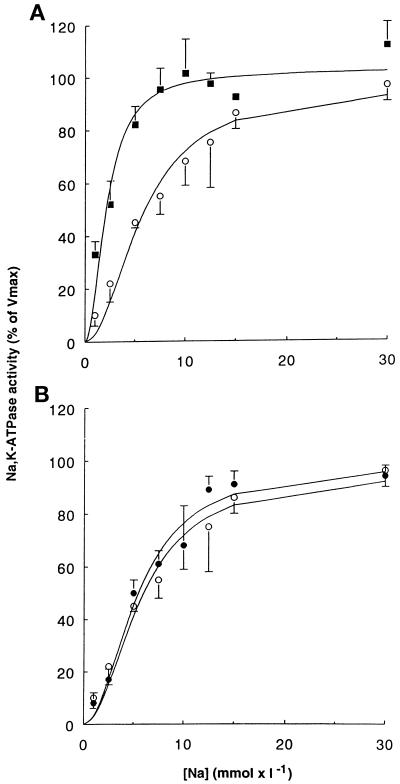
α1-Mutants Mimicking Ser-16 Phosphorylation Increase the Apparent Na Affinity of Na,K-ATPase
The absence of alteration in cell surface expression of Na,K-pumps in cells incubated with PDBu at 18°C may indicate that the Ser-16 phosphorylation-dependent stimulation of the transport activity of Na,K-ATPase could be achieved by an alteration of the functional properties of preexisting pumps.
We therefore analyzed the Na activation curves of the hydrolytic activity of the exogenous Na,K-ATPase in crude membranes from transfected COS-7 cells expressing the wild-type Bufo α1 subunit or its S16D and S16E mutants. The results show that compared with wild-type α1 subunits, expression of S16D (Figure 7A) and S16E (our unpublished results) α-mutants induced a significant leftward shift of the Na activation curve of the hydrolytic activity of the exogenous Na,K-ATPase. This finding reflects an increase in the apparent Na affinity of the Na,K pumps containing the constitutive phosphorylation mutant α subunits (K0.5Na [mM ± SE]: wild type, 6.72 ± 0.63; S16D, 2.34 ± 0.42*; S16E, 4.82 ± 0.25*; *p < 0.01). Figure 7B shows that expression of the T15A/S16A α-mutant that suppresses the phosphorylation site does not alter the apparent Na affinity of the exogenous Na,K-ATPase (K0.5Na, 6.32 ± 0.68). This observation supports the notion of a specific effect of negatively charged residues that mimic the effect of phosphorylation. These results strongly suggest that phosphorylation of the Na,K-ATPase α1 subunit on Ser-16 induces an increase in the apparent Na affinity of the enzyme, which can account for the stimulation of the cation transport activity of Na,K-ATPase in response to phorbol ester-sensitive PKC(s) activation in intact cells.
Figure 7.
A mutant mimicking phosphorylation of the α1 subunit at Ser-16 (S16D) increases the apparent Na affinity of Na,K-ATPase. The Na activation of the hydrolytic activity of the hybrid Na,K pumps was measured in permeabilized membranes isolated from stably transfected COS-7 cells. Results are expressed as the percentage of maximal activity and are means ± SE from four to seven independent experiments. (A) Na activation curves of Na,K-ATPase from cells expressing the wild-type Bufo α1 subunit (open circles) or the S16D α-mutant (closed squares). (B) Na activation curves of Na,K-ATPase from cells expressing the wild-type Bufo α1 subunit (open circles) or the T15A/S16A α-mutant (closed circles). The activity of exogenous Na,K-ATPase measured in the presence of 30 mM Na was 586 ± 9 (n = 6), 314 ± 47 (n = 4), and 631 ± 85 (n = 5) pmol of ATP × h−1 × μg of protein−1 for the wild-type, the S16D mutant, and the T15A/S16A mutant Na,K-pumps, respectively.
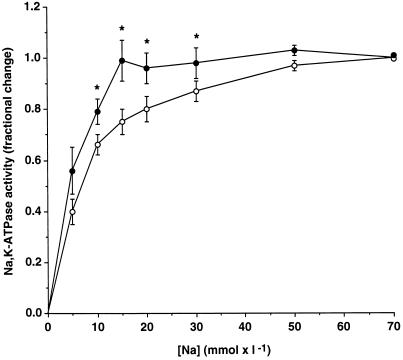
PDBu Increases the Na Sensitivity of the Hydrolytic Activity of Na,K-ATPase in Permeabilized COS-7 Cells
The following experiments were designed to assess whether in addition to the down-regulation of cell surface Na,K-ATPase, PDBu also increases the apparent Na affinity of the enzyme in nontransfected COS-7 cells preincubated at 37°C. After 15 min of preincubation at 37°C in the absence or the presence of 10−7 M PDBu, cells were permeabilized, and the hydrolytic activity of Na,K-ATPase was measured in the presence of increasing concentrations of Na (from 0 to 70 mM). In agreement with the down-regulation of cell surface Na,K-pumps (see Figure 3B), PDBu inhibited the maximal hydrolytic activity of Na,K-ATPase measured in the presence of a saturating Na concentration (as pmol of ATP × μg of protein−1 × h−1: control, 484 ± 26; PDBu, 348 ± 34; p < 0.005). However, Figure 8 shows that PDBu also increased the Na sensitivity of the Na,K-ATPase, as shown by the leftward shift of the Na activation curve. Indeed, the maximal Na,K-ATPase activity is reached in the presence of 15 and 50 mM Na in control and PDBu-treated cells, respectively. Therefore, PDBu decreases the maximal activity and increases the Na sensitivity of Na,K-ATPase in COS-7 cells incubated at 37°C.
Figure 8.
PDBu increases the Na sensitivity of Na,K-ATPase in permeabilized COS-7 cells. After preincubation in the absence (C, open bars) or presence of 10−7 M PDBu (P, filled bars) at 37°C, COS-7 cells were permeabilized by freeze–thawing. The hydrolytic activity of Na,K-ATPase was measured in permeabilized cells in the presence of increasing Na concentrations (from 0 to 70 mM). Results are expressed as a fraction of the maximal Na,K-ATPase activity and are means ± SE from 12 independent experiments (* p < 0.05). The maximal Na,K-ATPase activity was 484 ± 26 and 348 ± 34 pmol of ATP × μg of protein−1 × h−1 ± SE for control and PDBu-treated cells, respectively.
DISCUSSION
Expression of an ouabain-resistant wild-type Na,K-ATPase α1 subunit or its T15A/S16A and S16D mutants in COS-7 cells permitted us to inhibit the endogenous Na,K pumps and to study the direct relationship existing between α1 subunit Ser-16 phosphorylation and modulation of Na,K-ATPase activity. The present study provides evidence that in addition to the previously described Ser-16 phosphorylation-independent down-regulation of cell surface Na,K-ATPase (Beron et al., 1997), the activation of phorbol ester-sensitive PKC(s) induces a Ser-16 phosphorylation-dependent increase in the apparent Na affinity of Na,K-ATPase.
In agreement with the findings of Beron et al. (1997) in A6 epithelial cells, our study shows that the inhibition Na,K-ATPase by phorbol esters in COS-7 cells incubated at 37°C relies on a down-regulation of cell surface Na,K-pumps and is independent of Ser-16 phosphorylation (see Figure 3). Indeed, these effects of phorbol esters are not altered in cells expressing the T15A/S16A α-mutant, which is not phosphorylated. Furthermore, expression of an α-mutant in which Ser-16 is substituted by a negatively charged amino acid (Asp or Glu) mimicking permanent phosphorylation of the α subunit did not prevent either the inhibition of Na,K-ATPase or its decrease in cell surface expression in response to PDBu.
The decrease in activity and cell surface expression of Na,K-ATPase observed after phorbol ester treatment at 37°C is likely to be mediated by a PKC-dependent PLA2 activation and subsequent metabolism of the generated free arachidonic acid through the cytochrome P-450-dependent monooxygenase (CP-450) pathway. Indeed, these effects are prevented by the inhibition of PKC, of PLA2, and of CP-450 (see Figure 4). In addition, the effects of PDBu are mimicked by arachidonic acid. Several lines of evidence support the physiological importance of this observation: 1) PKC can directly phosphorylate and activate PLA2 (Nemenoff et al., 1993); and 2) in various cells, including renal epithelial cells, the PKC-dependent inhibition of Na,K-ATPase activity relies on PLA2 activation and arachidonic metabolism through CP-450 (Schwartzman et al., 1985; Satoh et al., 1993b; Xia et al., 1995).
The Ser-16 phosphorylation-dependent stimulation of the transport activity of a representative α1-β Na,K-ATPase isozyme complex demonstrated in COS-7 cells incubated at 18°C (see Figure 5) is most likely mediated by a change in its apparent Na affinity. Indeed, in permeabilized COS-7 cells, PDBu increases the Na sensitivity of Na,K-ATPase, and this effect is large enough to fully account for the PDBu-induced increase in ouabain-sensitive Rb (K) uptake measured in intact cells (see Figure 8). Because under these experimental conditions the transmembrane ion gradients are abolished and the intracellular and extracellular Na concentrations are equal and constant, the latter observation implies that PDBu increased the apparent Na affinity of the fraction of Na,K pumps that remained active at the cell surface. The present results confirm the previously described increase in the apparent Na affinity of Na,K-ATPase in response to PKC activation in isolated proximal convoluted tubules (Féraille et al., 1995). The PDBu-induced increase in apparent Na affinity of Na,K-ATPase most likely relies on Ser-16 phosphorylation, because this effect is reproduced by α1-mutants mimicking constitutive Ser-16 phosphorylation (see Figure 7A). This effect of Ser-16 phosphorylation on the apparent Na affinity of Na,K-ATPase is in agreement with the results of Logvinenko et al. (1996), who showed that in vitro phosphorylation of purified Na,K-ATPase by PKC shifts the conformational equilibrium of the Na,K pump toward E1, i.e., the Na conformation. These observations are consistent with earlier studies showing that the α1 subunit NH2-terminal domain is involved in conformational changes of the enzyme. Indeed, tryptic cleavage of the α1 subunit in the E1 conformation occurring between Lys-30 and Glu-31 (Jorgensen and Collins, 1986) or truncations of the NH2 terminus by site-directed mutagenesis (Wierzbicki and Blostein, 1993; Wang et al., 1996) displace the E1–E2 conformational equilibrium in direction of the E1 conformation through an increased rate of potassium deocclusion (Wierzbicki and Blostein, 1993), which may account for the increased apparent Na affinity of Na,K-ATPase (Jorgensen and Collins, 1986).
Altogether, our results indicate that the down-regulation of cell surface Na,K-ATPase in response to phorbol esters masks the intrinsic functional effect of Ser-16 phosphorylation of the Na,K-ATPase α subunit in COS-7 cells incubated at 37°C. This observation is in agreement with a growing number of studies, which report stimulation of Na,K-ATPase activity in response to PKC activation (Lynch et al., 1986; Hootman et al., 1987; Gupta et al., 1991; Féraille et al., 1995; Pedemonte et al., 1997) but contrasts with findings on rat α1 subunits in which PKC-dependent phosphorylation inhibits (Belusa et al., 1997) or does not alter (Feschenko and Sweadner, 1997) Na,K-ATPase activity. However, our study and these former studies (Belusa et al., 1997; Feschenko and Sweadner, 1997) cannot be directly compared, because we specifically studied the role of the ubiquitous Ser-16 phosphorylation site, whereas others focused on the role of the rat-specific Ser-23 phosphorylation site. Indeed, among higher vertebrates, the rat α1 subunit is the only one that exhibits two PKC phosphorylation sites: the ubiquitous site on Ser-16 and an additional site on Ser-23 (Feschenko and Sweadner, 1995; Béguin et al., 1996b), accounting for 80% of in vitro PKC phosphorylation in this species (Feschenko and Sweadner, 1995). These two phosphorylation sites might be targets for different PKC isozymes and/or produce different physiological effects. Ser-23 is indeed located within a consensus PKC site lying within the lysine cluster, whereas Ser-16 is part of a novel unconventional PKC phosphorylation site (Béguin et al., 1996b). This hypothesis is indirectly supported by data from Vasilets (1997), which show that the transport activity of rat α1–β complexes expressed in Xenopus oocytes is inhibited, whereas that of the endogenous, Xenopus α1–β complexes, which were previously shown to be exclusively phosphorylated on Ser-16 (Béguin et al., 1996b), are stimulated by injection of purified rat PKC.
In conclusion, our results show that phosphorylation of the α subunit of Na,K-ATPase on Ser-16 may stimulate its activity through an increase in apparent Na affinity. Thus, phosphorylation of the Na,K-ATPase α1 subunit on Ser-16 in response to the activation of phorbol ester-sensitive PKC(s) is likely to play a critical role in the homeostasis of intracellular monovalent cation concentration as well as in repolarization of excitable cells and vectorial ion transport by epithelial cells. In addition, the present study provides evidence that in some cells, the activity of Na,K-ATPase can be controlled by an additional mechanism, which alters membrane trafficking and changes the cell surface expression of Na,K pumps independently of Ser-16 phosphorylation of the α1 subunit.
ACKNOWLEDGMENTS
We thank Dr. François Verrey for the kind gift of specific anti-Bufo α1 subunit antibody. This work was supported in part by Swiss National Science Foundation grants 31-40386.94 and 31-50643.97 to H.F. and E.F. and 31-42954.95 to K.G. and by a grant from the Foundation Carlos and Elsie de Reuter to H.F. and E.F.
Abbreviations used:
- AEBSF
4-(2-aminoethyl)-benzenesulfonyl fluoride
- DMEM
Dulbecco's modified Eagle's medium
- PDBu
phorbol 12,13-dibutyrate
- PKA
protein kinase A
- PKC
protein kinase C
- PLA2
phospholipase A2
REFERENCES
- Béguin P, Beggah AT, Chibalin AV, Burgener-Kairuz P, Jaisser F, Mathews PM, Rossier BC, Cotecchia S, Geering K. Phosphorylation of the Na,K-ATPase α-subunit by protein kinase A and C in vitro and in intact cells. Identification of a novel motif for PKC-mediated phosphorylation. J Biol Chem. 1994;269:24437–24445. [PubMed] [Google Scholar]
- Béguin P, Beggah AT, Cotecchia S, Geering K. Adrenergic, dopaminergic, and muscarinic receptor stimulation leads to PKA phosphorylation of Na-K-ATPase. Am J Physiol. 1996a;270:C131–C137. doi: 10.1152/ajpcell.1996.270.1.C131. [DOI] [PubMed] [Google Scholar]
- Béguin P, Peitsch MC, Geering K. α1 but not α2 or α3 isoforms of Na,K-ATPase are efficiently phosphorylated in a novel protein kinase C motif. Biochemistry. 1996b;35:14098–14108. doi: 10.1021/bi960516o. [DOI] [PubMed] [Google Scholar]
- Belusa R, Wang Z-M, Matsubara T, Sahlgen B, Dulubova I, Nairn AC, Ruoslahti E, Greengard P, Aperia A. Mutation of the protein kinase C phosphorylation site on rat α 1 Na+,K+-ATPase alters regulation of intracellular Na+ and pH and influences cell shape and adhesiveness. J Biol Chem. 1997;272:20179–20184. doi: 10.1074/jbc.272.32.20179. [DOI] [PubMed] [Google Scholar]
- Beron J, Forster I, Béguin P, Geering K, Verrey F. Phorbol 12-myristate 13-acetate down-regulates Na,K-ATPase independent of its protein kinase C site: decrease in basolateral cell surface area. Mol Biol Cell. 1997;8:387–398. doi: 10.1091/mbc.8.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorello AM, Aperia A, Walaas SI, Nairn AC, Greengard P. Phosphorylation of the catalytic subunit of Na+,K+-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci USA. 1991;88:11359–11362. doi: 10.1073/pnas.88.24.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranza ML, Féraille E, Favre H. Protein kinase C-dependent phosphorylation of the Na+,K+-ATPase α-subunit in rat kidney cortical tubules. Am J Physiol. 1996;271:C136–C143. doi: 10.1152/ajpcell.1996.271.1.C136. [DOI] [PubMed] [Google Scholar]
- Carranza ML, Rousselot M, Chibalin AV, Bertorello AM, Favre H, Féraille E. Protein kinase A induces recruitment of active Na+,K+-ATPase units to the plasma membrane of rat proximal convoluted tubule. J Physiol. 1998;511:235–243. doi: 10.1111/j.1469-7793.1998.235bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibalin AV, Katz AI, Berggren P-O, Bertorello AM. Receptor-mediated inhibition of renal Na+-K+-ATPase is associated with endocytosis of its α- and β-subunits. Am J Physiol. 1997;273:C1458–C1465. doi: 10.1152/ajpcell.1997.273.5.C1458. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Féraille E, Berggren P-O, Bertorello AM. Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser18 in the rat α-subunit and is responsible for the decreased activity in epithelial cells. J Biol Chem. 1999;274:1920–1927. doi: 10.1074/jbc.274.4.1920. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Vasilets LA, Hennekes H, Pralong D, Geering K. Phosphorylation of Na,K-ATPase α-subunits in microsomes and in homogenates of Xenopus oocytes resulting from the stimulation of protein kinase A and protein kinase C. J Biol Chem. 1992;267:22378–22384. [PubMed] [Google Scholar]
- Chibalin AV, Zierath JR, Katz AI, Berggren P-O, Bertorello AM. Phosphatidylinositol 3-kinase-mediated endocytosis of renal Na+,K+-ATPase α subunit in response to dopamine. Mol Biol Cell. 1998;9:1209–1220. doi: 10.1091/mbc.9.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féraille E, Carranza ML, Buffin-Meyer B, Rousselot M, Doucet A, Favre H. Protein kinase C-dependent stimulation of Na+-K+-ATPase in rat proximal convoluted tubules. Am J Physiol. 1995;268:C1277–C1283. doi: 10.1152/ajpcell.1995.268.5.C1277. [DOI] [PubMed] [Google Scholar]
- Féraille E, Carranza ML, Rousselot M, Favre H. Insulin enhances sodium sensitivity of Na-K-ATPase in isolated rat proximal convoluted tubule. Am J Physiol. 1994;267:F55–F62. doi: 10.1152/ajprenal.1994.267.1.F55. [DOI] [PubMed] [Google Scholar]
- Féraille E, Vogt B, Rousselot M, Barlet-Bas C, Cheval L, Doucet A, Favre H. Mechanism of enhanced Na-K-ATPase activity in cortical collecting duct from rats with nephrotic syndrome. J Clin Invest. 1993;91:1295–1300. doi: 10.1172/JCI116328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feschenko MS, Sweadner KJ. Conformation-dependent phosphorylation of Na,K-ATPase by protein kinase A and protein kinase C. J Biol Chem. 1994;269:30436–30444. [PubMed] [Google Scholar]
- Feschenko MS, Sweadner KJ. Structural basis for species-specific differences in the phosphorylation of Na,K-ATPase by protein kinase C. J Biol Chem. 1995;270:14072–14077. doi: 10.1074/jbc.270.23.14072. [DOI] [PubMed] [Google Scholar]
- Feschenko MS, Sweadner KJ. Phosphorylation of Na,K-ATPase by protein kinase C at Ser18 occurs in intact cells but does not result in direct inhibition of ATP hydrolysis. J Biol Chem. 1997;272:17726–17733. doi: 10.1074/jbc.272.28.17726. [DOI] [PubMed] [Google Scholar]
- Fisone G, et al. Identification of the phosphorylation site for cAMP-dependent protein kinase on Na+,K+-ATPase and effects of site-directed mutagenesis. J Biol Chem. 1994;269:9368–9373. [PubMed] [Google Scholar]
- Fisone G, Snyder GL, Fryckstedt J, Caplan MJ, Aperia A, Greengard P. Na+,K+-ATPase in the choroid plexus. Regulation by serotonin/protein kinase C pathway. J Biol Chem. 1995;270:2427–2430. doi: 10.1074/jbc.270.6.2427. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Dunbar LA, Caplan MJ. Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am J Physiol. 1995;268:F285–F295. doi: 10.1152/ajprenal.1995.268.2.F285. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ruderman NB, Cragoe EJ, Sussman I. Endothelin stimulates Na-K-ATPase activity by a protein kinase C-dependent pathway in rabbit aorta. Am J Physiol. 1991;261:H38–H45. doi: 10.1152/ajpheart.1991.261.1.H38. [DOI] [PubMed] [Google Scholar]
- Hoffman PW, Ravindran A, Hunagir RL. Role of phosphorylation in desensitization of acetylcholine receptors expressed in Xenopus oocytes. J Neurosci. 1994;14:4185–4195. doi: 10.1523/JNEUROSCI.14-07-04185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hootman SR, Brown ME, Williams JA. Phorbol esters and A23187 regulate Na-K pump activity in pancreatic acinar cells. Am J Physiol. 1987;252:G499–G505. doi: 10.1152/ajpgi.1987.252.4.G499. [DOI] [PubMed] [Google Scholar]
- Jaisser F, Horisberger JD, Rossier BC. Primary sequence and functional expression of a novel β subunit of the P-ATPase gene family. Pflügers Arch. 1993;425:446–452. doi: 10.1007/BF00374871. [DOI] [PubMed] [Google Scholar]
- Jorgensen PL, Collins JH. Tryptic and chemotryptic cleavage sites in sequence of α-subunit of (Na++K+)-ATPase from outer medulla of mammalian kidney. Biochim Biophys Acta. 1986;860:570–576. doi: 10.1016/0005-2736(86)90555-9. [DOI] [PubMed] [Google Scholar]
- Logvinenko NS, Dulubova I, Fedosova N, Larsson SH, Nairn AC, Esmann M, Greengard P, Aperia A. Phosphorylation by protein kinase C of serine-23 of the α-1 subunit of rat Na+,K+-ATPase affects its conformational equilibrium. Proc Natl Acad Sci USA. 1996;93:9132–9137. doi: 10.1073/pnas.93.17.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CJ, Wilson PB, Blackmore PF, Exton JH. The hormone-sensitive hepatic Na-pump. J Biol Chem. 1986;261:14551–14556. [PubMed] [Google Scholar]
- McGill DL, Guidotti G. Insulin stimulates both the α1 and the α2 isoforms of the rat adipocyte (Na,K) ATPase. J Biol Chem. 1991;266:15824–15831. [PubMed] [Google Scholar]
- Middleton JP, Khan WA, Collinsworth G, Hannun YA, Medford RM. Heterogeneity of protein kinase C-mediated rapid regulation of Na/K-ATPase in kidney epithelial cells. J Biol Chem. 1993;268:15958–15964. [PubMed] [Google Scholar]
- Nemenoff RA, Winitz S, Qian N-X, Van Putten V, Johnson GL, Heasley LE. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase, and protein kinase C. J Biol Chem. 1993;268:4960–4964. [PubMed] [Google Scholar]
- Pages G, Brunet A, L'Allemain G, Pouyssegur J. Constitutive mutant and putative regulatory serine phosphorylation site of mammalian MAP kinase kinase (MEK1) EMBO J. 1994;13:3003–3010. doi: 10.1002/j.1460-2075.1994.tb06599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte CH, Pressley TA, Lokhandwala MF, Cinelli AR. Regulation of Na,K-ATPase transport activity by protein kinase C. J Membr Biol. 1997;155:219–227. doi: 10.1007/s002329900174. [DOI] [PubMed] [Google Scholar]
- Satoh T, Cohen HT, Katz AI. Intracellular signaling in the regulation of renal Na-K-ATPase. J Clin Invest. 1992;89:1496–1500. doi: 10.1172/JCI115740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Cohen HT, Katz AI. Different mechanisms of renal Na-K-ATPase regulation by protein kinases in proximal and distal nephron. Am J Physiol. 1993a;265:F399–F405. doi: 10.1152/ajprenal.1993.265.3.F399. [DOI] [PubMed] [Google Scholar]
- Satoh T, Cohen HT, Katz AI. Intracellular signaling in the regulation of renal Na-K-ATPase. J Clin Invest. 1993b;91:409–415. doi: 10.1172/JCI116215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman M, Ferreri NR, Carroll MA, Songu-Mize E, McGiff JC. Renal cytochrome P-450-related arachidonate metabolite inhibits (Na+K)ATPase. Nature. 1985;314:620–622. doi: 10.1038/314620a0. [DOI] [PubMed] [Google Scholar]
- Toullec D, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Vasilets LA. Diversity of regulatory phosphorylation of the Na+/K+-ATPase from mammalian kidneys and Xenopus oocytes by protein kinases: characterization of the phosphorylation site for protein kinase C. Cell Physiol Biochem. 1997;7:1–18. doi: 10.1111/j.1749-6632.1997.tb52326.x. [DOI] [PubMed] [Google Scholar]
- Vasilets LA, Omay HS, Ohta T, Noguchi S, Kawamura M, Schwartz W. Stimulation of the Na+/K+ pump by external [K+] is regulated by voltage-dependent gating. J Biol Chem. 1991;266:16285–16288. [PubMed] [Google Scholar]
- Vilsen B. Functional consequences of alterations to Pro-328 and Leu-332 located in the 4th transmembrane segment of the α-subunit of the rat kidney Na+,K+-ATPase. FEBS Lett. 1992;314:301–307. doi: 10.1016/0014-5793(92)81494-7. [DOI] [PubMed] [Google Scholar]
- Wang X, Jaisser F, Horisberger J-D. Role in cation translocation of the N-terminus of the α-subunit of the Na+-K+ pump of Bufo. J Physiol. 1996;491:579–594. doi: 10.1113/jphysiol.1996.sp021241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki W, Blostein R. The amino-terminal segment of the catalytic subunit of kidney Na,K-ATPase regulates the potassium deocclusion pathway of the reaction cycle. Proc Natl Acad Sci USA. 1993;90:70–74. doi: 10.1073/pnas.90.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Kramer RM, King GL. Identification of the mechanism for the inhibition of Na+,K+-adenosine triphosphatase by hyperglycemia involving activation of protein kinase C and cytosolic phospholipase A2. J Clin Invest. 1995;96:733–740. doi: 10.1172/JCI118117. [DOI] [PMC free article] [PubMed] [Google Scholar]