Abstract
Boron (B) is essential in plants but often present at low concentrations in the environment. To investigate how plants survive under conditions of B limitation, we conducted a transcriptome analysis and identified NIP5;1, a member of the major intrinsic protein family, as a gene upregulated in B-deficient roots of Arabidopsis thaliana. Promoter–β-glucuronidase fusions indicated that NIP5;1 is strongly upregulated in the root elongation zone and the root hair zone under B limitation, and green fluorescent protein–tagged NIP5;1 proteins localized to the plasma membrane. Expression in Xenopus laevis oocytes demonstrated that NIP5;1 facilitated the transport of boric acid in addition to water. Importantly, two T-DNA insertion lines of NIP5;1 displayed lower boric acid uptake into roots, lower biomass production, and increased sensitivity of root and shoot development to B deficiency. These results identify NIP5;1 as a major plasma membrane boric acid channel crucial for the B uptake required for plant growth and development under B limitation.
INTRODUCTION
Boron (B) is an essential element for higher plants (Marschner, 1995), and B deficiency is an agricultural problem in many parts of the world (Shorrocks, 1997). In general, B-deficiency symptoms first appear in growing regions rather than in mature tissues and generally lead to the rapid cessation of root elongation, reduced leaf expansion, and reduced fertility (Marschner, 1995; Dell and Huang, 1997). Physiological studies have suggested that the primary effect of B deficiency is the reduction of cell expansion (Dell and Huang, 1997). In the last decade, B has been established as essential for cell wall structure and function (O'Neill et al., 2004). In cell walls, B can cross-link pectic polysaccharides through borate–diol bonding of two rhamnogalacturonan II (RG-II) molecules. The borate cross-linked RG-II was shown to be essential for normal plant growth using the Arabidopsis thaliana mur1 mutant and the haploid Nicotiana plumbaginifolia callus mutant nolac-H18, in which the amount of borate cross-linked RG-II is reduced (O'Neill et al., 2001; Iwai et al., 2002). To maintain cell wall biosynthesis and optimal plant development, B has to be continuously delivered to growing tissues from soil through roots and vascular tissues.
B mainly exists as uncharged boric acid [B(OH)3] in solutions at physiological pH and in the absence of interaction with biomolecules. Boric acid is a weak Lewis acid with a pKa of 9.24 [B(OH)3 + H2O =  + H+] (Woods, 1996). On the basis of ether–water partitioning coefficients, the molecular weight, and the number of H bonds of B, Raven (1980) calculated the theoretical lipid permeability coefficient of boric acid to be 8 × 10−6 cm s−1. This relatively high value had been the basis of the widely believed hypothesis that passive diffusion of boric acid across the lipid bilayer represents the major and possibly only mechanism of membrane transport of B. Dordas and Brown (2000) determined the permeability coefficient of boric acid using artificial liposomes consisting of phosphatidylcholine. The estimated value was 4.9 × 10−6 cm s−1, which was similar to the theoretical values obtained by Raven (1980). However, using membranes isolated from squash (Cucurbita pepo) roots, Dordas et al. (2000) determined the permeability coefficients of boric acid to be 3.9 × 10−7 and 2.4 × 10−8 cm s−1 in plasma membrane and plasma membrane–depleted vesicles, respectively. In agreement with these findings, Stangoulis et al. (2001) determined the permeability coefficients of boric acid in the plasma membrane of the giant internodal cells of the charophyte alga Chara corallina to be 4.4 × 10−7 cm s−1. These values were 1 order of magnitude lower than those calculated by Raven (1980) and determined using artificial liposomes (Dordas and Brown, 2000). The lower permeability of plant membranes compared with artificial membranes implied the need of membrane proteins to satisfy a plant's demand of B, especially under B limitation.
+ H+] (Woods, 1996). On the basis of ether–water partitioning coefficients, the molecular weight, and the number of H bonds of B, Raven (1980) calculated the theoretical lipid permeability coefficient of boric acid to be 8 × 10−6 cm s−1. This relatively high value had been the basis of the widely believed hypothesis that passive diffusion of boric acid across the lipid bilayer represents the major and possibly only mechanism of membrane transport of B. Dordas and Brown (2000) determined the permeability coefficient of boric acid using artificial liposomes consisting of phosphatidylcholine. The estimated value was 4.9 × 10−6 cm s−1, which was similar to the theoretical values obtained by Raven (1980). However, using membranes isolated from squash (Cucurbita pepo) roots, Dordas et al. (2000) determined the permeability coefficients of boric acid to be 3.9 × 10−7 and 2.4 × 10−8 cm s−1 in plasma membrane and plasma membrane–depleted vesicles, respectively. In agreement with these findings, Stangoulis et al. (2001) determined the permeability coefficients of boric acid in the plasma membrane of the giant internodal cells of the charophyte alga Chara corallina to be 4.4 × 10−7 cm s−1. These values were 1 order of magnitude lower than those calculated by Raven (1980) and determined using artificial liposomes (Dordas and Brown, 2000). The lower permeability of plant membranes compared with artificial membranes implied the need of membrane proteins to satisfy a plant's demand of B, especially under B limitation.
The pathway of nutrient transport from the root surface to the shoot includes at least two transmembrane transport events: import into epidermal, cortical, or endodermal cells (uptake) and export from pericycle or xylem parenchyma cells into the stelar apoplasm (xylem loading). Physiological studies using sunflower (Helianthus annuus) plants suggested the existence of energy-dependent high-affinity transport systems that are induced at low B supply and established concentration gradients for B in both processes of uptake and xylem loading (Dannel et al., 2002). In Arabidopsis plants, a high-affinity transport system involved in the process of xylem loading has been identified through analysis of the Arabidopsis bor1-1 mutant, which is highly sensitive to B deficiency (Noguchi et al., 1997, 2000). BOR1 is homologous with bicarbonate transporters in animals and is a plasma membrane efflux type B transporter localized in pericycle cells, where it mediates xylem loading of B under B limitation (Takano et al., 2002). After the identification of BOR1, its homolog in Saccharomyces cerevisiae, YNL275w, was also suggested to be a plasma membrane efflux type B transporter (Takano et al., 2002). Furthermore, the closest homolog in mammals, NaBC1, was characterized to be an electrogenic Na+-coupled borate 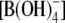 transporter (Park et al., 2004).
transporter (Park et al., 2004).
Besides BOR1 homologs, major intrinsic proteins (MIPs) have been suggested as promising candidates to mediate the membrane transport of B. MIPs function as water-selective or relatively nonselective channels for water and other small uncharged molecules (Tyerman et al., 2002). Plant MIPs cluster into four distinct groups: the tonoplast intrinsic proteins; the plasma membrane intrinsic proteins (PIPs); the small basic intrinsic proteins, recently shown as endoplasmic reticulum membrane channels; and the NOD26-like intrinsic proteins (NIPs), corresponding to close homologs of the soybean (Glycine max) nodulin 26 (NOD26) protein (Johanson et al., 2001; Ishikawa et al., 2005). Boric acid permeation into plasma membrane vesicles from squash roots was partially inhibited (30 to 39%) by mercuric chloride and phloretin, a nonspecific channel blocker (Dordas et al., 2000). This study also showed that expression of maize (Zea mays) PIP1 in Xenopus laevis oocytes resulted in a 30% increase in B uptake into oocytes. Furthermore, B uptake by squash plants was reduced by 40 to 90% in the presence of the channel inhibitors mercuric chloride, phloretin, or 4,4′-diisothiocyanato-stilbene-2-2′-disulfonic acid and by 35 to 54% in the presence of small neutral solutes such as urea and glycerol (Dordas and Brown, 2001). However, the molecular identity of boric acid importers and their physiological significance have not yet been revealed in planta.
In this study, we demonstrate that NIP5;1, an uncharacterized member of the MIP family, functions as a boric acid channel for B uptake and is crucial for plant growth under B limitation in Arabidopsis.
RESULTS
Identification of NIP5;1 as a Gene Upregulated under B Limitation in Roots
In the search for adaptive plant responses to B deficiency, we screened for genes upregulated in B-deficient Arabidopsis roots. Plants were hydroponically grown for 39 d in the presence of 150 μM B and then transferred to nutrient solution supplied with 150 or 0.3 μM B and incubated for another 3 d. These B-deficient growth conditions slightly affected root elongation but did not induce more severe damage to the plants (data not shown). Total RNA was isolated from the roots and subjected to microarray analysis using Affymetrix GeneChips containing 8300 Arabidopsis genes. Twelve genes turned out to be upregulated greater than threefold under B deficiency (data not shown). To verify the results from the microarray analysis, mRNA levels of these genes were quantified using RT-mediated real-time PCR on total RNA obtained from two independent experiments. Among the 12 candidate genes, only mRNA levels of NIP5;1 were repeatedly upregulated in B-deficient roots. When normalized to those of Elongation Factor 1α, the mRNA levels of NIP5;1 were 12.0 or 12.8 times higher under B-deficient growth conditions than under adequate B supply (data not shown).
B- and Cell Type–Dependent Expression of NIP5;1
To investigate in more detail the regulation of NIP5;1, a time course of mRNA accumulation in roots was examined by RT-mediated real-time PCR analysis (Figure 1A). When plants were grown at 100 μM B (+B) for 34 d and then transferred to medium with 0.1 μM B (−B), NIP5;1 mRNA levels increased within several hours and peaked after 24 h with a 15-fold induction (Figure 1A). Resupply of 100 μM B to plants starved for 24 h (Re +B) decreased NIP5;1 mRNA levels within 24 h. These results clearly demonstrated a B-dependent regulation of NIP5;1 mRNA accumulation in Arabidopsis roots.
Figure 1.
NIP5;1 Expression Is Upregulated in Roots under B Limitation.
(A) B-dependent NIP5;1 mRNA accumulation in roots quantified by RT-mediated real-time PCR analysis. Wild-type plants (Col-0) grown for 34 d supplied with 100 μM boric acid (+B) were transferred to medium containing 0.1 μM boric acid (−B) or resupplied with 100 μM boric acid (Re +B) for 24 h after 24 h of culture at 0.1 μM boric acid. Mean values ± sd from independent plant samples are shown (n = 3).
(B) to (E) GUS staining in NIP5;1 promoter-GUS transgenic plants.
(B) Whole plants of a homozygous T3 line supplied with 0.3 or 100 μM boric acid.
(C) to (E) Root tip (C) and cross sections of the root hair zone (D) and the elongation zone (E) of plants supplied with 0.3 μM boric acid.
Bars = 10 mm (B), 1 mm (C), and 50 μm ([D] and [E]).
We then examined the cell type–specific expression of NIP5;1 in the T2 and T3 generations of transgenic Columbia (Col-0) plants expressing the β-glucuronidase (GUS) gene under the control of the NIP5;1 promoter. In seven independent lines, GUS activity was consistently observed in roots and appeared much stronger in plants grown under low B supply (0.3 μM B) compared with plants grown under high B supply (100 μM B) (Figure 1B). These results suggest that the regulation of transcript accumulation observed by the microarray and RT-PCR analyses is exerted mainly at the level of transcription. GUS activity in the primary root (Figures 1B and 1C) and in lateral roots (Figure 1B) was strongest in the elongation zone and decreased toward the root hair zone and the basal zone. In cross sections of the root hair zone (Figure 1D) and the elongation zone (Figure 1E), GUS activity was observed at high levels in epidermal, cortical, and endodermal cells but weakly in stelar cells. In aboveground tissues, GUS activity was barely detectable, both in vegetative (Figure 1B) and in reproductive (data not shown) growth stages.
Plasma Membrane Localization of NIP5;1
Soybean NOD26, the first identified member of the NIP subfamily in plants, is located at the peribacteroid membrane of nitrogen-fixing symbiosomes in root nodules (Fortin et al., 1987; Weaver et al., 1991). However, subcellular localization of NIPs in nonlegumes has not yet been reported. To investigate the subcellular localization of NIP5;1, green fluorescent protein (GFP) alone or GFP fused either C-terminally (NIP5;1-GFP) or N-terminally (GFP-NIP5;1) to NIP5;1 was expressed in Arabidopsis protoplasts under the control of a cauliflower mosaic virus 35S RNA promoter (Figure 2). GFP alone stained the cytoplasm localized at the cell periphery around a large central vacuole. By contrast, NIP5;1 fusion to GFP confined fluorescence to a fine ring at the extreme cell periphery, indicating plasma membrane localization of the NIP5;1 protein.
Figure 2.
NIP5;1 Localizes to the Plasma Membrane in Arabidopsis Protoplasts.
GFP was fused to NIP5;1 either C-terminally (NIP5;1-GFP) or N-terminally (GFP-NIP5;1) and expressed in protoplasts derived from an Arabidopsis cell culture, or GFP alone was expressed. GFP-derived fluorescence (left), transmission image (middle), and fluorescence superimposed over the transmission image (right) are shown.
Water and Boric Acid Transport by NIP5;1 in Xenopus Oocytes
Among all plant MIPs, NIPs in particular have been shown to transport small uncharged molecules such as glycerol and urea, in addition to water (Rivers et al., 1997; Dean et al., 1999; Guenther and Roberts, 2000; Weig and Jakob, 2000; Ciavatta et al., 2002; Klebl et al., 2003; Cabello-Hurtado and Ramos, 2004; Wallace and Roberts, 2005). In solution at physiological pH, B mainly exists as boric acid, a small uncharged molecule with a molecular radius of 2.573 Å, which is similar in size to urea (2.618 Å) (Dordas and Brown, 2001). These considerations and the B-dependent expression led us to hypothesize that NIP5;1 may function as a boric acid channel.
To test this hypothesis, we expressed NIP5;1 in Xenopus oocytes and first investigated the water transport function by an osmotic swelling assay. Arabidopsis PIP2;1 was used as a control for an efficient water channel (Kammerloher et al., 1994). When transferred from ND96 solution (220 mosmol/kg; see Methods for composition) to a twofold-diluted ND96 solution, PIP2;1-expressing oocytes showed a linear increase of the oocyte volume with time compared with noninjected oocytes, which remained almost unchanged (Figure 3A). By contrast, NIP5;1-expressing oocytes did not show a significant difference in the oocyte volume relative to noninjected oocytes after transfer to the twofold-diluted ND96 solution (data not shown). A greater increase in volume compared with noninjected oocytes was observed only when NIP5;1-expressing oocytes were transferred to a fivefold-diluted ND96 solution (Figure 3B). These results showed that NIP5;1 transports water but much less efficiently than PIP2;1 under our experimental conditions.
Figure 3.
NIP5;1 Transports Boric Acid in Xenopus Oocytes.
(A) to (C) Osmotic swelling assay. NIP5;1 or PIP2;1 mRNA-injected or noninjected oocytes were incubated in ND96 solution (220 mosmol/kg). The initial swelling rates were measured upon immersion in twofold-diluted ND96 solution (A), fivefold-diluted ND96 solution (B), and an isotonic solution containing fivefold-diluted ND96 supplemented with boric acid to adjust the osmolarity to 220 mosmol/kg (C). V indicates the volume at a given time point, and V0 indicates the initial volume. Mean values ± sd are shown (n = 6 to 10).
(D) Boric acid uptake assay. Oocytes injected with mRNA of NIP5;1 and noninjected oocytes were incubated in medium supplemented with 5 mM boric acid. B contents in oocytes were determined. Mean values ± sd are shown (n = 4).
Subsequently, boric acid transport was investigated by transferring oocytes from ND96 solution (220 mosmol/kg) to an isotonic fivefold-diluted ND96 solution supplemented with boric acid to maintain the osmolarity at 220 mosmol/kg. It was presumed that the increase in oocyte volume corresponds to the water influx after the osmotic gradient generated by boric acid uptake. NIP5;1-expressing oocytes showed significant swelling, whereas noninjected oocytes shrunk slightly (Figure 3C). Importantly, PIP2;1-expressing oocytes also failed to show significant swelling under these conditions (Figure 3C) despite their high water transport capacity (Figure 3A). This observation indicated that an endogenous boric acid uptake activity of oocytes is not sufficient to generate an osmotic gradient and subsequent swelling and that NIP5;1, but not PIP2;1, transports boric acid under these conditions.
To independently verify boric acid uptake activity of NIP5;1, B accumulation in oocytes was measured directly. NIP5;1-expressing oocytes accumulated fivefold to ninefold more B than noninjected oocytes when incubated at 5 mM boric acid (Figure 3D). Consistent with the observation from the swelling assay, PIP2;1-expressing oocytes did not accumulate B relative to noninjected oocytes (data not shown).
These results established that NIP5;1 transports boric acid more efficiently and water less efficiently than PIP2;1 in Xenopus oocytes. It cannot be excluded that NIP5;1 transports other substrates in addition to boric acid and water.
Isolation of NIP5;1 T-DNA Insertion Lines
To investigate the function of NIP5;1 in Arabidopsis plants, we obtained two independent mutant alleles for NIP5;1. SALK_122287, a T-DNA insertion line in the Col-0 background, was obtained from the Salk Institute and named nip5;1-1. FLAG_250F08, a T-DNA insertion line in the Wassilewskija (Ws) background, was obtained from the Institut National de la Recherche Agronomique (INRA) and named nip5;1-2. Homozygous lines for each T-DNA insertion were selected by PCR analysis, and the positions of the T-DNA insertion sites were verified by sequencing (Figure 4A). In nip5;1-1, the corresponding T-DNA insertion was localized in the first intron of the NIP5;1 open reading frame (ORF), whereas in nip5;1-2, the T-DNA was located in the predicted promoter region of NIP5;1, 823 bp upstream of the putative translation start site.
Figure 4.
Isolation of T-DNA Insertion Mutants of NIP5;1.
(A) Positions of T-DNA insertions, which were verified by sequencing of PCR products obtained with primers specific for the genomic sequence and for the left border (LB) or right border (RB) of the T-DNA. Large and small black boxes represent exons and other genomic regions, including introns, respectively. The T-DNA is not drawn to scale.
(B) RT-mediated real-time PCR analysis of NIP5;1 mRNA accumulation in roots of NIP5;1 T-DNA insertion lines and corresponding wild-type plants. Plants grown for 11 d on solid medium containing 100 μM boric acid were transferred to medium containing 100 or 0.1 μM boric acid for 24 h. Mean values ± sd are presented for independent reverse transcription reactions followed by real-time PCR using primers from the indicated positions in (A) (n = 3).
Accumulation of NIP5;1 mRNA in the insertion lines and the corresponding wild-type plants was quantified by RT-mediated real-time PCR using primers for amplification of the first and second exons (Figure 4A). The plants were grown on solidified nutrient medium containing 100 μM B for 11 d and then transferred to medium containing 100 or 0.1 μM B for 24 h. The NIP5;1 mRNA levels in roots of nip5;1-1 mutant plants were restricted to 1.2 and 0.8% of those of the Col-0 plants at 100 and 0.1 μM B, respectively (Figure 4B). The mRNA levels in roots of nip5;1-2 mutant plants were 53 and 9.7% of those of wild-type Ws plants at 100 and 0.1 μM B, respectively. Similar to the results obtained in the Col-0 plants grown hydroponically (Figure 1A), NIP5;1 mRNA levels were higher at 0.1 μM B than at 100 μM B, with 13-, 9-, and 14-fold inductions in the Col-0, nip5;1-1, and Ws plants, respectively (Figure 4B). However, in nip5;1-2 mutants, the NIP5;1 mRNA level at 0.1 μM B was reduced to 2.5-fold of that at 100 μM B, suggesting that B-dependent transcriptional regulation of NIP5;1 was inhibited by the T-DNA insertion at 823 bp upstream of the putative translation start site.
nip5;1 Mutants Are Sensitive to B Limitation
Shoot and root growth of the insertion lines was observed during vegetative growth on solidified nutrient medium containing various concentrations of B. Consistent with the general observation in different plant species, root growth of wild-type Arabidopsis plants was more sensitive to B deficiency than shoot growth (Marschner, 1995; Dell and Huang, 1997; Takano et al., 2001) (Figures 5A to 5C). Relative to their corresponding wild-type plants, root and shoot growth of nip5;1-1 and nip5;1-2 plants was reduced when B was supplied at concentrations of <10 or <1 μM, respectively (Figures 5B and 5C). At 0.1 μM B, both insertion lines showed a striking growth retardation, including cessation of main root growth, no development of lateral roots, and no further expansion of rosette leaves (Figure 5A). At 3 μM B, elongation of lateral roots was inhibited and the expansion of rosette leaves was retarded in both insertion lines (Figure 5A). When B was supplied at concentrations of 30 μM or higher, both insertion lines grew similar to the corresponding wild-type plants (Figures 5A to 5C). Notably, the stronger growth depression in nip5;1-1 coincided with the lower level of NIP5;1 transcripts relative to nip5;1-2 (Figure 4B). These observations demonstrate a requirement of NIP5;1 for shoot and root growth of Arabidopsis plants under B-deficient growth conditions.
Figure 5.
Vegetative Growth of nip5;1 Insertion Lines Is Sensitive to B Limitation.
The nip5;1 and corresponding wild-type plants were grown on plates containing various concentrations of boric acid.
(A) Plants after 10 d of growth. Bar = 1 cm.
(B) Fresh weight of aerial portions of 7-d-old plants.
(C) Root length of 7-d-old plants.
(D) Roots after 7 d of growth. Bar = 500 μm.
(E) Longitudinal length of epidermal cells in the root hair zone of 7-d-old plants.
For (B), (C), and (E), mean values ± sd are shown (n = 11 to 16 [B], 10 to 16 [C], and 21 to 53 [E]).
We subsequently analyzed the root phenotype of 7-d-old nip5;1-1 plants in more detail. Root tips of nip5;1-1 were stunted and root hair density was increased dramatically when plants were grown under B deficiency, whereas at adequate B supply these morphological differences disappeared (Figure 5D). A quantitative determination of the longitudinal length of epidermis cells in the root hair zone revealed a substantial reduction of cell length in the insertion line when grown under B limitation. The cell length of the insertion line was reduced to 43 or 66% relative to that of wild-type plants supplied with 0.3 or 1 μM B, respectively (Figure 5E). However, the cell length was similar between the mutant and Col-0 plants at 30 μM B (Figure 5E). Thus, root growth inhibition in B-deficient nip5;1 plants correlated closely with a reduction in cell elongation.
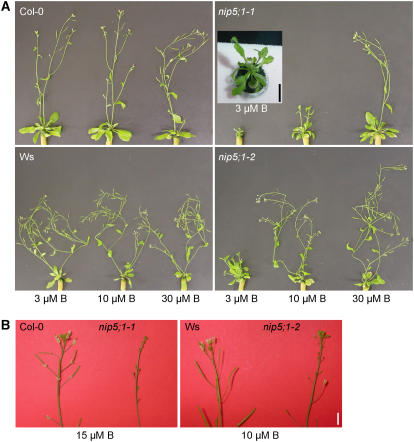
It is widely known that plants are more sensitive to B limitation during reproductive growth than during vegetative growth, and marked seed yield reductions have been reported under B deficiency without other symptoms being expressed during vegetative growth (Dell and Huang, 1997). To investigate the effect of B limitation on reproductive growth, nip5;1 plants were grown hydroponically under long-day conditions and supplied with various concentrations of B (Figure 6). At 3 μM B, rosette leaves were small, misshaped, and wrinkled, and inflorescences did not develop properly in nip5;1-1 plants (Figure 6A). Short internodes and a bushy appearance were observed in nip5;1-1 plants at 10 μM B and in nip5;1-2 plants at 3 μM B (Figure 6A). The development of flowers and siliques was inhibited in nip5;1-1 and nip5;1-2 plants when B was supplied at 15 and 10 μM or lower, respectively (Figures 6A and 6B). However, plants of both insertion lines returned to generative growth and set seeds at the same age as wild-type plants when B was supplied at 30 μM or higher (Figure 6A). These observations demonstrate that NIP5;1 is required for proper reproductive development even under moderate B limitation. The slight differences in the sensitivity to various B levels between the two mutant alleles coincided with their NIP5;1 expression levels (Figure 4B) and might additionally be attributable to the different sensitivity of the two ecotypes to B limitation.
Figure 6.
Reproductive Growth of nip5;1 Insertion Lines Is Sensitive to B Limitation.
The nip5;1 mutants and corresponding wild-type plants were grown hydroponically for 38 d (A) or 44 d (B) and supplied with various concentrations of boric acid. Bars = 1 cm.
B Uptake Is Defective in nip5;1 Insertion Lines
To address the physiological function of NIP5;1 in B transport, B concentrations in roots and shoots of the insertion lines were compared with those of wild-type plants. For this purpose, plants were grown hydroponically under short-day conditions for 37 d with supply of 3, 30, or 150 μM B. At 3 μM B, roots and shoots of nip5;1-1 and nip5;1-2 plants had strongly reduced B concentrations, whereas at 150 μM B, insertion lines accumulated similar amounts of B in their root and shoot tissues as the corresponding wild-type plants (Figures 7A and 7B). The B concentration in nip5;1-1 roots grown at 3 μM B was not determined because of defective root growth. When grown at 3 μM B, B concentrations in roots and shoots of nip5;1-2 plants were 44 and 78% lower than those of Ws plants, respectively. These results showed that B concentrations in both roots and shoots were reduced in nip5;1 plants under limited but not under adequate B supply.
Figure 7.
B Uptake Is Defective in nip5;1 Insertion Lines under B Limitation.
(A) and (B) B accumulation in nip5;1 and corresponding wild-type plants. The plants were grown hydroponically with various concentrations of boric acid for 37 d. B concentrations in roots (A) and shoots (B) of the plants were determined. DW, dry weight. Mean values ± sd are shown (n = 3 to 4). Asterisks show significant differences from the wild-type plants (P < 0.05, Student's t test).
(C) and (D) Time course of B uptake and translocation in the Col-0 wild-type and nip5;1-1 insertion lines. The plants were grown on medium containing 100 μM 11B-enriched boric acid for 27 d and transferred to 100 μM 11B (high B) or 0.1 μM 11B (low B) medium for 24 h. The plants were then exposed to medium containing 10 μM 10B-enriched B. Roots (C) and shoots (D) were harvested at the times indicated, and 10B concentrations in the tissues were determined. DW, dry weight. Mean values ± sd are shown (n = 4).
We subsequently analyzed the time course of B uptake using stable isotopes of B. For this purpose, nip5;1-1 and Col-0 wild-type plants were grown under short-day conditions in the presence of 100 μM 11B-enriched B for 27 d and then subjected to 100 μM 11B-enriched B (high B) or 0.1 μM 11B-enriched B (low B) for 24 h. Then, the plants were exposed to 10 μM 10B-enriched B, and 10B concentrations in roots and shoots were monitored during 120 min (Figures 7C and 7D). In plants pretreated with high B, there was no significant difference in 10B uptake into roots between nip5;1-1 and wild-type plants (Figure 7C). However, in wild-type plants pretreated with low B, 10B uptake into roots was 5.6 times higher than in mutant plants within the first 20 min and saturated after longer incubation times. In the same plants, 10B concentrations in shoots increased continuously during the 120-min time course, whereas they did not increase significantly in wild-type plants pretreated with high B or in nip5;1-1 plants under any conditions (Figure 7D). These results indicated that NIP5;1 is required for efficient B uptake into roots and, hence, for B translocation to the shoot under B limitation.
DISCUSSION
NIP5;1 Mediates B Uptake across the Plasma Membrane
In this study, we describe the molecular identity of a boric acid channel responsible for B import into plant cells. The B-dependent osmotic swelling of NIP5;1-expressing oocytes (Figures 3A to 3C) and the time-dependent B accumulation (Figure 3D) in Xenopus oocytes showed that NIP5;1 mediates the membrane transport of boric acid in a heterologous expression system. Because GFP-tagged NIP5;1 localized to the plasma membrane in Arabidopsis protoplasts (Figure 2), it is reasonable that this transporter acts as a B importer also in planta. A high NIP5;1 promoter activity in root epidermal, cortical, and endodermal cells, particularly in the elongation zone but also in the root hair zone (Figures 1B to 1E), indicated that in Arabidopsis these root zones might contribute most to B uptake from the soil solution. Indeed, B accumulation in roots and shoots of nip5;1 insertion lines was significantly reduced when plants were cultured under low B supply (Figures 7A and 7B). This was consistent with the increased capacity for B accumulation in B-deficient roots and shoots of wild-type plants, whereas B uptake was impaired in the nip5;1-1 insertion line (Figures 7C and 7D). These results clearly demonstrate that NIP5;1 functions as a major boric acid channel in the plasma membrane, being responsible for B uptake into the root under B limitation.
GUS activity under the control of the NIP5;1 promoter (Figure 1B), NIP5;1 mRNA levels (Figure 1A), and NIP5;1-dependent B uptake activity (Figures 7C and 7D) were all upregulated in roots under B limitation. These observations suggest that the transcriptional regulation of NIP5;1 expression is a major mechanism for controlling B uptake in Arabidopsis roots.
A Role for NIP5;1 in Plant Growth and Development under B Limitation
Under B limitation, nip5;1 plants exhibited severe growth retardation of roots and shoots (Figures 5 and 6), which was accompanied by reduced B accumulation in the same organs (Figures 7A and 7B). Both insertion lines grew similar to wild-type plants and accomplished their life cycle when a higher concentration of B was supplied (Figures 5 and 6). Therefore, the observed growth retardation was attributed to B deficiency. These physiological data clearly demonstrate a major role of NIP5;1 in B nutrition. However, it cannot be excluded that NIP5;1 takes on other physiological roles (e.g., in the transport of water or of small uncharged molecules other than boric acid).
In shoots of nip5;1 plants, the expansion of rosette leaves, the development of the inflorescence, and flowering were all retarded under B limitation (Figures 5A and 6). Although the extent of B deficiency and threshold levels for B limitation were different, all symptoms were in agreement with those described previously in other plants (Marschner, 1995; Dell and Huang, 1997) or in Arabidopsis wild-type and bor1-1 plants (Noguchi et al., 1997; Takano et al., 2001). In the bor1-1 mutant, which is defective in root-to-shoot translocation of B, growth retardation was observed particularly in shoots (Takano et al., 2001). Because it has been proven that B functions in the structural integrity of the cell wall by dimerization of RG-II (O'Neill et al., 2004), a lower rate of RG-II dimerization in shoot tissues was thought to be responsible for the growth retardation in bor1-1 plants (Noguchi et al., 2003). Similarly, the growth retardation in nip5;1 shoots is best explained by a lower rate of B delivery to the shoots (Figures 7B and 7D) derived from a lower capacity for B uptake into roots (Figures 7A and 7C). Although GUS activity driven by the NIP5;1 promoter was not detected in shoot organs either in the vegetative (Figure 1B) or the reproductive (data not shown) growth stage, NIP5;1 expression restricted to certain cell types in shoot tissues may also have contributed to the observed B-deficiency symptoms in nip5;1 shoots. Indeed, microarray analysis of RNA extracted from tissues at various developmental stages revealed the expression of NIP5;1 in shoot organs such as stem nodes and leaves (Schmid et al., 2005).
In contrast with bor1-1, B-deficient nip5;1 plants also showed severe growth retardation in roots (Figures 5A, 5C, and 5D). In higher plants, the most rapid response to B deficiency is the inhibition or cessation of root elongation in both primary and lateral roots, and this growth effect can be attributed primarily to impaired cell enlargement rather than to impaired cell division (Dell and Huang, 1997). Primary and lateral roots were much shorter than those of wild-type plants under B limitation (Figures 5A and 5C), which correlated with severely reduced cell elongation (Figures 5D and 5E). Consistent with a function of NIP5;1 in boric acid uptake into rapidly growing root cells, NIP5;1 promoter activity was confined mainly to the root elongation zone (Figures 1B and 1C). These findings suggest that under B limitation, NIP5;1-facilitated transport of boric acid across the plasma membrane is a limiting step to supply sufficient B for RG-II dimerization in walls of rapidly elongating root cells. A recent analysis suggested that a BOR1 homolog is expressed in the root elongation zone and required for normal root cell elongation under B limitation (Miwa et al., 2005). Histochemical analysis demonstrated that RG-II is located very close to the plasma membrane in cell walls of immature radish (Raphanus sativus) roots (Matoh et al., 1998). We hypothesize that in elongating cells under B limitation, the boric acid taken up by NIP5;1 is partially converted into the borate anion by the higher symplastic pH. The borate anion is then exported across the plasma membrane to form cis-diol esters with apiosyl residues in RG-II. Moreover, B import by NIP5;1 may be required for other cellular functions of B, such as a structural role in the cytoskeleton (Bassil et al., 2004).
Possible Role of NIPs in the Evolution of Land Plants
The NIP family in Arabidopsis contains nine members that fall into two phylogenetic subclasses. Six NIPs more closely resemble the archetype NOD26 in sequence, whereas three (NIP5;1, NIP6;1, and NIP7;1) are more divergent (Wallace and Roberts, 2004). Functional analysis in Xenopus oocytes showed that NOD26 has a modest osmotic water permeability and the ability to transport uncharged solutes such as glycerol and formamide (Wallace and Roberts, 2005). By contrast, NIP6;1 has an extremely low water permeability but transports glycerol, formamide, and larger solutes such as urea that are impermeable to NOD26. Our results in Xenopus oocytes showed that NIP5;1, which possesses a similar putative selectivity filter to NIP6;1, has low water permeability and transports boric acid (Figure 3). The size and volume of boric acid are similar to those of urea (Dordas and Brown, 2001). These results imply that NIP6;1 is also a boric acid channel. It will be important to investigate the substrate specificity of the NIPs in more detail and the possible involvement of NIPs, especially NIP6;1 and NIP7;1, in B transport in planta.
Although an increasing number of reports suggest essential roles for B in various organisms, seed plants generally require much greater amounts of B for normal growth than other organisms. The B requirement of angiosperms is correlated with the amount of pectin and RG-II in the cell wall (Hu et al., 1996; Matoh et al., 1996). It has been shown that the cell walls of bryophyte gametophytes contain much less borate cross-linked RG-II than the cell walls of tracheophytes, which suggests that the amount of borate cross-linked RG-II increased dramatically during the evolution of vascular plants from their bryophyte-like ancestors (Matsunaga et al., 2004). These authors hypothesized that the acquisition of a B-dependent growth habit and borate cross-linking of RG-II is correlated with the ability of vascular plants to maintain upright growth. NIPs are suggested to have been acquired by horizontal gene transfer of aquaporins (water channels) from bacteria at the origin of plants, and certain amino acids related to substrate specificity were substituted by others during the course of plant evolution (Zardoya et al., 2002). Although it remains to be investigated in which plant species different NIPs transport boric acid, it is possible that the evolution of certain NIPs to efficiently transport boric acid and to support borate cross-linking of RG-II was a prerequisite for the upright growth of vascular plants on B-limiting sites that are distributed all over the world.
METHODS
Plant Materials
Col-0 and Ws ecotypes of Arabidopsis thaliana were obtained from the ABRC. Information about the T-DNA mutant in the Col-0 ecotype was obtained from the SIGnAL database (Alonso et al., 2003), and the seeds were obtained from the ABRC. Information about the T-DNA mutant in the Ws ecotype was obtained from the FLAGdb/FST (Samson et al., 2002), and the seeds were obtained from the INRA. The genotype of plants was determined by PCR using left and right border–specific primers and gene-specific primers.
Plant Growth Conditions
Plant growth media were prepared according to Fujiwara et al. (1992) and contained various concentrations of B. The growth conditions for vertically placed solidified medium as well as the method of hydroponic plant culture for the microarray experiment, the phenotypic analysis of reproductive growth, and the determination of B accumulation were described previously (Takano et al., 2001). For the time-course analysis of mRNA levels and the tracer experiment, plants were grown on nylon mesh placed on solidified medium and then transferred to hydroponic medium according to Takano et al. (2005). Plants were grown under short-day conditions (10-h/14-h light/dark cycle) for the time-course analysis of mRNA levels, the determination of B accumulation, and the tracer experiments and under long-day conditions (16-h/8-h light/dark cycle) for other experiments. For the tracer experiment using stable isotopes of B, the plants were supplied with 11B-enriched boric acid (99%; Cambridge Isotope Laboratories) instead of boric acid of natural abundance (10B:11B = 19.9:80.1). The plants were grown and supplied with 11B-enriched boric acid and then transferred to medium with 10 μM 10B-enriched boric acid (99%; Cambridge Isotope Laboratories). Preparation of samples and B isotope determination by inductively coupled plasma mass spectrometry were as described previously (Takano et al., 2002).
Microarray Analysis and Real-Time RT-PCR
Arabidopsis GeneChips (Affymetrix) containing 8300 genes were analyzed according to the manufacturer's protocol. Reverse transcription-mediated real-time PCR analysis was performed as described by Ohkama et al. (2002). The primers used in the PCR were 5′-CACCGATTTTCCCTCTCCTGAT-3′ and 5′-GCATGCAGCGTTACCGATTA-3′ for NIP5;1 and 5′-CCTTGGTGTCAAGCAGATGA-3′ and 5′-TGAAGACACCTCCTTGATGATTT-3′ for Elongation Factor 1α.
Promoter-GUS Analysis
The 2.5-kb region upstream of the initiation codon of NIP5;1 was amplified by PCR from BAC clone F24G24 (obtained from the ABRC) using primers 5′-GGTGGATCCGAAAGCAAGCATTCCCTG-3′ and 5′-GAGCCATGGCCAACGTTTTTTTTTTTGGT-3′. Using BamHI and NcoI, the amplified fragment was subcloned into pTF456 carrying GUS and the terminator of the nopaline synthase gene. pTF456 was generated from pBI221 (Jefferson et al., 1987) and carries a modified GUS ORF that contained six extra nucleotides (ATGGTA) encoding Met-Val at the 5′ end of the original GUS ORF to generate a NcoI site. The BamHI–NotI fragment of the resulting plasmid (pMW1) was subcloned into the BamHI–Bsp120-I vector fragment of the binary vector pTkan+ (kindly provided by K. Schumacher). Col-0 plants were transformed via the Agrobacterium tumefaciens–mediated floral dip method (Clough and Bent, 1998) with the NIP5;1 promoter (the 2.5-kb region upstream of the initiation codon)–GUS construct. GUS staining and root cross-sectioning were performed as described by Shibagaki et al. (2002). The sections were counterstained with 0.05% ruthenium red (Sigma-Aldrich) for 30 s.
Expression of GFP-Tagged NIP5;1 in Arabidopsis Protoplasts
The NIP5;1-sGFP fusion was constructed as follows. The NIP5;1 ORF was amplified by PCR from the NIP5;1 cDNA clone RZL55b01, obtained from the Kazusa DNA Research Institute, using primers 5′-AATCTAGAACGTTGGAAATGGCTCCACCGGA-3′ and 5′-GAAAACCATGGCTCCTCCTCCTCCACGACGAAAGCTCCTAACCGGAC-3′. The amplified fragment was A-tailed and cloned into the pGEM-T Easy vector (Promega) according to the manufacturer's instructions. The DNA sequence of the cloned PCR product was confirmed to be error-free. The XbaI–NcoI fragment carrying the NIP5;1 ORF was ligated with the NcoI–EcoRI fragment of pTH2 (Chiu et al., 1996) carrying sGFP, the terminator of the nopaline synthase gene, and the XbaI–EcoRI vector fragment of pBI221 (Jefferson et al., 1987). The resulting plasmid (NIP5;1-sGFP in pBI221) carried a NIP5;1-sGFP fusion gene under the control of the cauliflower mosaic virus 35S RNA promoter. In this construction process, a linker encoding five amino acids (Gly-Gly-Gly-Gly-Ala) was inserted between the NIP5;1 and sGFP ORFs.
The sGFP-NIP5;1 fusion was constructed as follows. The NIP5;1 ORF was amplified by PCR from the NIP5;1 cDNA clone CERES36655, obtained from Ceres, using primers 5′-AAATGTACAAGATGGCTCCACCGGAGGCTGA-3′ and 5′-AACTGCAGACAGCGGCCGCACTCACTTAACGACGAAAGCTC-3′. The amplified fragment was A-tailed and cloned into the pGEM-T Easy vector (Promega). The DNA sequence of the cloned PCR product was confirmed to be error-free. The NIP5;1 ORF was subcloned into pTH2 (Chiu et al., 1996) at the BsrGI and NotI recognition sites. The resulting plasmid (sGFP-NIP5;1 in pUC18) carried a sGFP-NIP5;1 fusion gene under the control of the cauliflower mosaic virus 35S RNA promoter.
Transformation of Arabidopsis protoplasts was performed as described by Liu et al. (2003). As a control, pCF203 vector carrying the GFP gene under the control of the cauliflower mosaic virus 35S RNA promoter was used. Protoplasts were analyzed 2 d after transformation by confocal laser scanning microscopy (Leica TCS-SP).
Expression of NIP5;1 in Xenopus laevis Oocytes
The ORFs of NIP5;1 and PIP2;1 were amplified by PCR from the cDNA clone RZL55b01, obtained from the Kazusa DNA Research Institute and a Col-0 cDNA library (kindly provided by K. Schumacher), respectively. The amplified fragment was A-tailed and cloned into the pGEM-T Easy vector (Promega). Accuracy of the cloned PCR product was confirmed by DNA sequencing. The ORFs were subcloned into pOO2 (Ludewig et al., 2002).
Capped cRNA was transcribed from the plasmids in vitro using the mMessage mMachine kit (Ambion), after linearization of the plasmid with MluI. Xenopus oocytes were removed from adult female frogs by surgery, manually dissected, and defolliculated using 2 mg/mL collagenase (Boehringer Mannheim) and 1 mg/mL trypsin inhibitor (Sigma-Aldrich). Oocytes (Dumont stage V or VI) were injected with 50 nL of cRNA (NIP5;1, 20 to 30 ng/oocyte; PIP2;1, 5 to 10 ng/oocyte). Oocytes were kept at 16°C for 2 to 3 d in ND96. ND96 solution contained 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2 and 5 mM Hepes, pH 7.4.
Swelling Assay
Swelling of oocytes was monitored with a Leica binocular microscope equipped with a charge-coupled device camera. Photographs were taken from the cross-sectional area of oocytes using Metamorph SPOT software (Universal Imaging). NIH image 1.62 was used to determine oocyte diameters. Volumes of oocytes were calculated assuming spherical geometry. Oocytes incubated in ND96 (220 mosmol/kg) were transferred to five- or two-times-diluted ND96 (44 and 110 mosmol/kg, respectively) for water transport assays or to an isotonic solution containing five-times-diluted ND96 supplemented with boric acid to adjust the osmolarity to 220 mosmol/kg for boric acid transport assays.
Boric Acid Uptake Measurement in Oocytes
Oocytes were incubated in ND96 supplemented with 5 mM boric acid. Six oocytes were sampled as a batch and rinsed five times with ice-cold ND96. The oocytes were digested with concentrated nitric acid, and B contents were determined using inductively coupled plasma mass spectrometry as described previously (Takano et al., 2002).
Measurement of Root Cell Length
Roots from 7-d-old plants were stained with 10 μg/mL propidium iodide (Molecular Probes) for 10 min and imaged with a laser scanning confocal microscope (LSM 510; Carl Zeiss) using 543-nm excitation and >585-nm detection. The longitudinal cell length of epidermal cells in the root hair zone was measured using ImageJ software (National Institutes of Health).
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for NIP5;1 and PIP2;1 are At4g10380 and At3g53420, respectively.
Acknowledgments
We thank C. Brancato (Universität Tübingen) for protoplast transformation, H. Hanaoka, K. Miwa (both University of Tokyo), and K.E. Ile (University of North Carolina) for critical reading, and I. Kasajima (University of Tokyo) for technical assistance. We acknowledge K. Schumacher (Universität Tübingen), Y. Niwa (University of Shizuoka), the ABRC, the INRA, Ceres, and the Kazusa DNA Research Institute for providing materials. This work was supported in part by grants from the Japanese Society of Promotion of Science (to J.T.) and by Grants-in-Aid for Scientific Research (B) and Scientific Research on Priority Areas from the Ministry of Education, Culture, Sport, Science, and Technology of Japan, the 21st Century COE Program, and the Rice Genome Research Project (to T.F.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Toru Fujiwara (atorufu@mail.ecc.u-tokyo.ac.jp).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.106.041640.
References
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Bassil, E., Hu, H., and Brown, P.H. (2004). Use of phenylboronic acids to investigate boron function in plants. Possible role of boron in transvacuolar cytoplasmic strands and cell-to-wall adhesion. Plant Physiol. 136 3383–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello-Hurtado, F., and Ramos, J. (2004). Isolation and functional analysis of the glycerol permease activity of two new nodulin-like intrinsic proteins from salt stressed roots of the halophyte Atriplex nummularia. Plant Sci. 166 633–640. [Google Scholar]
- Chiu, W.L., Niwa, Y., Zeng, W., Hirano, T., Kobayashi, H., and Sheen, J. (1996). Engineered GFP as a vital reporter in plants. Curr. Biol. 6 325–330. [DOI] [PubMed] [Google Scholar]
- Ciavatta, V.T., Morillon, R., Pullman, G.S., Chrispeels, M.J., and Cairney, J. (2001). An aquaglyceroporin is abundantly expressed early in the development of the suspensor and the embryo proper of loblolly pine. Plant Physiol. 127 1556–1567. [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Dannel, F., Pfeffer, H., and Römheld, V. (2002). Update on boron in higher plants—Uptake, primary translocation and compartmentation. Plant Biol. 4 193–204. [Google Scholar]
- Dean, R.M., Rivers, R.L., Zeidel, M.L., and Roberts, D.M. (1999). Purification and functional reconstitution of soybean nodulin 26. An aquaporin with water and glycerol transport properties. Biochemistry 38 347–353. [DOI] [PubMed] [Google Scholar]
- Dell, B., and Huang, L. (1997). Physiological response of plants to low boron. Plant Soil 193 103–120. [Google Scholar]
- Dordas, C., and Brown, P.H. (2000). Permeability of boric acid across lipid bilayers and factors affecting it. J. Membr. Biol. 175 95–105. [DOI] [PubMed] [Google Scholar]
- Dordas, C., and Brown, P.H. (2001). Evidence for channel mediated transport of boric acid in squash (Cucurbita pepo). Plant Soil 235 95–103. [Google Scholar]
- Dordas, C., Chrispeels, M.J., and Brown, P.H. (2000). Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol. 124 1349–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin, M.G., Morrison, N.A., and Verma, D.P. (1987). Nodulin-26, a peribacteroid membrane nodulin is expressed independently of the development of the peribacteroid compartment. Nucleic Acids Res. 15 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara, T., Hirai, M.Y., Chino, M., Komeda, Y., and Naito, S. (1992). Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol. 99 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther, J.F., and Roberts, D.M. (2000). Water-selective and multifunctional aquaporins from Lotus japonicus nodules. Planta 210 741–748. [DOI] [PubMed] [Google Scholar]
- Hu, H.N., Brown, P.H., and Labavitch, J.M. (1996). Species variability in boron requirement is correlated with cell wall pectin. J. Exp. Bot. 47 227–232. [Google Scholar]
- Ishikawa, F., Suga, S., Uemura, T., Sato, M.H., and Maeshima, M. (2005). Novel type aquaporin SIPs are mainly localized to the ER membrane and show cell-specific expression in Arabidopsis thaliana. FEBS Lett. 579 5814–5820. [DOI] [PubMed] [Google Scholar]
- Iwai, H., Masaoka, N., Ishii, T., and Satoh, S. (2002). A pectin glucuronyltransferase gene is essential for intercellular attachment in the plant meristem. Proc. Natl. Acad. Sci. USA 99 16319–16324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson, U., Karlsson, M., Johansson, I., Gustavsson, S., Sjovall, S., Fraysee, L., Weig, A.R., and Kjellbom, P. (2001). The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 126 1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerloher, W., Fischer, U., Piechottka, G.P., and Schaffner, A.R. (1994). Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 6 187–199. [DOI] [PubMed] [Google Scholar]
- Klebl, F., Wolf, M., and Sauer, N. (2003). A defect in the yeast plasma membrane urea transporter Dur3p is complemented by CpNIP1, a Nod26-like protein from zucchini (Cucurbita pepo L.), and by Arabidopsis thaliana delta-TIP or gamma-TIP. FEBS Lett. 547 69–74. [DOI] [PubMed] [Google Scholar]
- Liu, L.H., Ludewig, U., Gassert, B., Frommer, W.B., and von Wirén, N. (2003). Urea transport by nitrogen-regulated tonoplast intrinsic proteins in Arabidopsis. Plant Physiol. 133 1220–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Ludewig, U., von Wirén, N., and Frommer, W.B. (2002). Uniport of
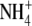 by the root hair plasma membrane ammonium transporter LeAMT1;1. J. Biol. Chem. 277 13548–13555. [DOI] [PubMed] [Google Scholar]
by the root hair plasma membrane ammonium transporter LeAMT1;1. J. Biol. Chem. 277 13548–13555. [DOI] [PubMed] [Google Scholar] - Marschner, H. (1995). Mineral Nutrition of Higher Plants, 2nd ed. (San Diego, CA: Academic Press).
- Matoh, T., Kawaguchi, S., and Kobayashi, M. (1996). Ubiquity of a borate rhamnogalacturonan II complex in the cell walls of higher plants. Plant Cell Physiol. 37 636–640. [Google Scholar]
- Matoh, T., Takasaki, M., Takabe, K., and Kobayashi, M. (1998). Immunocytochemistry of rhamnogalacturonan II in cell walls of higher plants. Plant Cell Physiol. 39 483–491. [Google Scholar]
- Matsunaga, T., Ishii, T., Matsumoto, S., Higuchi, M., Darvill, A., Albersheim, P., and O'Neill, M.A. (2004). Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes, and bryophytes. Implications for the evolution of vascular plants. Plant Physiol. 134 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa, K., Takano, J., Seki, M., Shinozaki, K., and Fujiwara, T. (2005). Arabidopsis BOR2, an efflux-type boron transporter, is essential for root elongation under boron deficiency. Plant Cell Physiol. 46 (suppl.), S83. [Google Scholar]
- Noguchi, K., Dannel, F., Pfeffer, H., Römheld, V., Hayashi, H., and Fujiwara, T. (2000). Defect in root-shoot translocation of boron in Arabidopsis thaliana mutant bor1-1. J. Plant Physiol. 156 751–755. [Google Scholar]
- Noguchi, K., Ishii, T., Matsunaga, T., Kakegawa, K., Hayashi, H., and Fujiwara, T. (2003). Biochemical properties of the cell wall in the Arabidopsis mutant bor1-1 in relation to boron nutrition. J. Plant Nutr. Soil Sci. 166 175–178. [Google Scholar]
- Noguchi, K., Yasumori, M., Imai, T., Naito, S., Matsunaga, T., Oda, H., Hayashi, H., Chino, M., and Fujiwara, T. (1997). bor1-1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiol. 115 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkama, N., Takei, K., Sakakibara, H., Hayashi, H., Yoneyama, T., and Fujiwara, T. (2002). Regulation of sulfur-responsive gene expression by exogenously applied cytokinins in Arabidopsis thaliana. Plant Cell Physiol. 43 1493–1501. [DOI] [PubMed] [Google Scholar]
- O'Neill, M.A., Eberhard, S., Albersheim, P., and Darvill, A.G. (2001). Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294 846–849. [DOI] [PubMed] [Google Scholar]
- O'Neill, M.A., Ishii, T., Albersheim, P., and Darvill, A.G. (2004). Rhamnogalacturonan II: Structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 55 109–139. [DOI] [PubMed] [Google Scholar]
- Park, M., Li, Q., Shcheynikov, N., Zeng, W., and Muallern, S. (2004). NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol. Cell 16 331–341. [DOI] [PubMed] [Google Scholar]
- Raven, J.A. (1980). Short- and long-distance transport of boric acid in plants. New Phytol. 84 231–249. [Google Scholar]
- Rivers, R.L., Dean, R.M., Chandy, G., Hall, J., Roberts, D.M., and Zeidel, M.L. (1997). Functional analysis of nodulin 26, an aquaporin in soybean root nodule symbiosomes. J. Biol. Chem. 272 16256–16261. [DOI] [PubMed] [Google Scholar]
- Samson, F., Brunaud, V., Balzergue, S., Dubreucq, B., Lepiniec, L., Pelletier, G., Caboche, M., and Lecharny, A. (2002). FLAGdb/FST: A database of mapped flanking insertion sites (FSTs) of Arabidopsis thaliana T-DNA transformants. Nucleic Acids Res. 30 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, M., Davison, T.S., Henz, S.R., Pape, U.J., Demar, M., Vingron, M., Schölkopf, B., Weigel, D., and Lohmann, J. (2005). A gene expression map of Arabidopsis thaliana development. Nat. Genet. 37 501–506. [DOI] [PubMed] [Google Scholar]
- Shibagaki, N., Rose, A., McDermott, J.P., Fujiwara, T., Hayashi, H., Yoneyama, T., and Davies, J.P. (2002). Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J. 29 475–486. [DOI] [PubMed] [Google Scholar]
- Shorrocks, V.M. (1997). The occurrence and correction of boron deficiency. Plant Soil 193 121–148. [Google Scholar]
- Stangoulis, J.C.R., Reid, R.J., Brown, P.H., and Graham, R.D. (2001). Kinetic analysis of boron transport in Chara. Planta 213 142–146. [DOI] [PubMed] [Google Scholar]
- Takano, J., Miwa, K., Yuan, L., von Wirén, N., and Fujiwara, T. (2005). Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl. Acad. Sci. USA 102 12276–12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano, J., Noguchi, K., Yasumori, M., Kobayashi, M., Gajdos, Z., Miwa, K., Hayashi, H., Yoneyama, T., and Fujiwara, T. (2002). Arabidopsis boron transporter for xylem loading. Nature 420 337–340. [DOI] [PubMed] [Google Scholar]
- Takano, J., Yamagami, M., Noguchi, K., Hayashi, H., and Fujiwara, T. (2001). Preferential translocation of boron to young leaves in Arabidopsis thaliana regulated by the BOR1 gene. Soil Sci. Plant Nutr. 47 345–357. [Google Scholar]
- Tyerman, S.D., Niemietz, C.M., and Bramley, H. (2002). Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 25 173–194. [DOI] [PubMed] [Google Scholar]
- Wallace, I.S., and Roberts, D.M. (2004). Homology modeling of representative subfamilies of Arabidopsis major intrinsic proteins. Classification based on the aromatic/arginine selectivity filter. Plant Physiol. 135 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, I.S., and Roberts, D.M. (2005). Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry 44 16826–16834. [DOI] [PubMed] [Google Scholar]
- Weaver, C.D., Crombie, B., Stacey, G., and Roberts, D.M. (1991). Calcium-dependent phosphorylation of symbiosome membrane-proteins from nitrogen-fixing soybean nodules—Evidence for phosphorylation of nodulin-26. Plant Physiol. 95 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weig, A.R., and Jakob, C. (2000). Functional identification of the glycerol permease activity of Arabidopsis thaliana NLM1 and NLM2 proteins by heterologous expression in Saccharomyces cerevisiae. FEBS Lett. 481 293–298. [DOI] [PubMed] [Google Scholar]
- Woods, W.G. (1996). Review of possible boron speciation relating to its essentiality. J. Trace Elem. Exp. Med. 9 153–163. [Google Scholar]
- Zardoya, R., Ding, X., Kitagawa, Y., and Chrispeels, M.J. (2002). Origin of plant glycerol transporters by horizontal gene transfer and functional recruitment. Proc. Natl. Acad. Sci. USA 99 14893–14896. [DOI] [PMC free article] [PubMed] [Google Scholar]