Abstract
The transplantation of animal organs into humans as a way of treating organ failure has been pursued for 100 years. Clinical xenotransplantation, as such, has always failed because the transplanted organ is rejected by the recipient. Recent advances in transplant immunology have revealed some mechanisms underlying the rejection of xenografts, and these discoveries have sparked efforts to use genetic engineering of animals and therapeutics directed at the recipient to overcome this problem. This communication reviews current understanding of the mechanisms of xenograft rejection and efforts to overcome rejection and other hurdles.
Keywords: Xenotransplantation, Organ transplantation, Graft rejection, Acute vascular rejection, Genetic engineering
Introduction
The transplantation of organs and tissues from animals into humans, that is clinical xenotransplantation, has been sought for more than 100 years. When the field of transplantation was begun in the early years of the twentieth century, it was animals and not humans that provided the first organs used for attempts at clinical transplantation [1, 2]. Animals were used as the source of organs then because the transplant surgeons did not think that human organs could be obtained in sufficient numbers and without breeching of ethics. For reasons that are much the same, interest in animal human transplantation persists today. What was not known in 1906 but is widely appreciated today is that transplantation of animal organs into humans is not a surgical challenge; rather it is challenge of overcoming daunting inflammatory and immune hurdles to success. In this brief communication, we shall summarize what is known about the biological barriers to transplanting animal organs into humans and what prospects exist for addressing those barriers. A full and detailed consideration of the biological barriers to xenotransplantation is beyond the scope of this essay; for this, the reader is referred to recent reviews of this subject [3, 4].
Xenotransplantation excites nearly every inflammatory, immune and coagulation pathway. Accordingly, a question to understanding the pathogenesis of rejection and one that surely will determine whether and when xenotransplantation can be applied is whether the inflammatory, immune and coagulation pathways become excited in series or in parallel (Figure 1). If the pathways are excited in series then blocking the first pathway may prevent recruitment of the others and thus allow successful application of xenotransplantation. For example, anti-T cell therapies prevent the inflammation, immunity (humoral) and coagulation characteristically seen in severely rejected organs, thus allowing the prolonged survival of organ allografts and clinical application of allotransplantation. On the other hand, if the broad range of mechanisms reported to attack xenografts act independently then the prospects for inhibiting all successfully must be very low. Work in the field of xenotransplantation in the past several years has brought some encouraging and some discouraging answers to this question.
Figure 1. The relationship between factors involved in vascular rejection and accommodation.
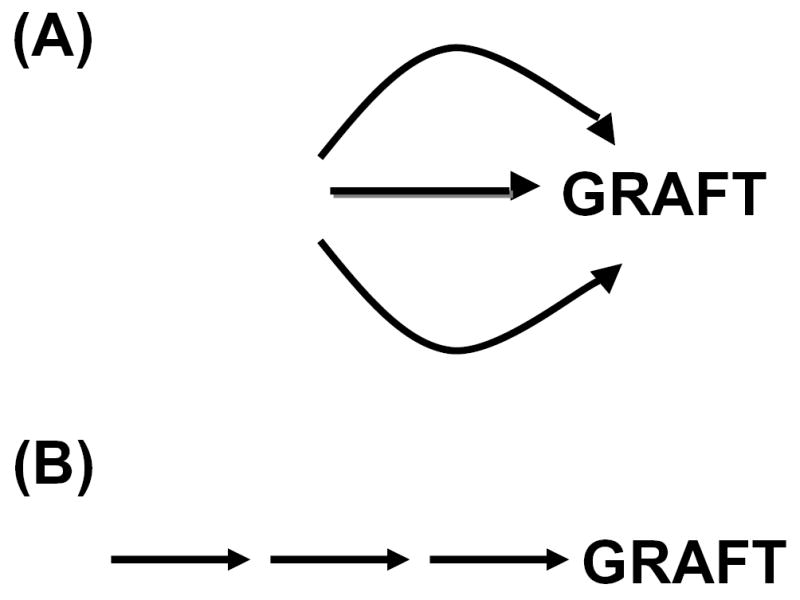
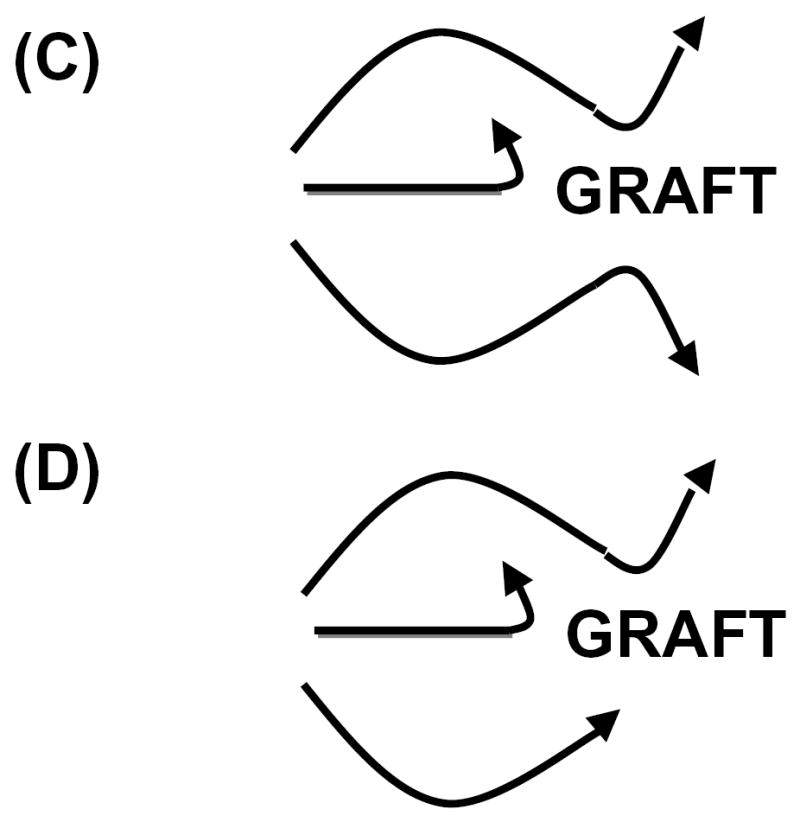
(A) Pathogenic factors act independent to cause graft injury. (B) Pathogenic factors act in series to cause graft injury. (C) Accommodation allows graft to resist various types of injury. (D) Accommodation allows grafts to resist some but not other types of injury.
How many triggers and pathways of injury?
In Table 1 we list some of the putative triggers and pathways through which organ xenografts are damaged. The reader is referred to our recent discussions of this subject [4–8]. As one might expect, the list of pathways grows almost inexorably, as few will publish evidence that a given type of injury does not cause a problem. We reported that the alternative pathway of complement [9] and apoptosis [10] might be less of a problem than had been thought, but reports such as these are far less common (and less noticeable) than reports of new obstacles.
Table 1.
Mechanisms of recognition of xenografts and pathways of injury.
| Factor | Specificity | Pathway | Outcome | References |
|---|---|---|---|---|
| Antibodies | Galα1-3Gal | Direct activation of EC, Complement, Fc receptor | Vascular rejection | [18] |
| Antibodies | MHC and other proteins | Direct activation of EC, Complement, Fc receptor | Vascular rejection | [29] |
| Complement | Bound antibodies | Cytotoxicity, EC activation | Vascular rejection | [49] |
| T cells | MHC | Cytotoxicity, cytokines | Cellular rejection | [50] |
| T cell | All proteins | Cytotoxicity, cytokines | Cellular rejection | [51] |
| NK cells | Non-MHC, Fc, Galα1–3Gal | Cytotoxicity, EC activation, cytokines | Vascular damage | [52] |
| Neutrophils | Unknown | Cytotoxicity | EC activation, vascular damage | [53] |
| Macrophages | Unknown | Cytotoxicity, coagulation | Vascular damage, coagulation | [54] |
| Platelets | VWF | Thrombosis, EC activation | Vascular damage, coagulation | [55] |
| Coagulation | Lack of control | TF | Coagulation, thrombosis | [37] |
Each of the many destructive mechanisms listed in Table 1 has been claimed to potentially act independently to destroy a graft. If those mechanisms act independently, then each would have to be inhibited, potentially by independent therapeutic approaches, for an organ xenograft to persist. The devising of separate therapeutic approaches for each of these pathways would seem a daunting undertaking, and one might well question whether the combination of measures needed would be compatible with life.
Fortunately, some evidence would suggest that certain of the pathways to injury of xenografts happen in series. Some years ago we found that injury to endothelium may be controlled by transcriptional regulation of IL-1α [11, 12] and more recently that blocking the action of IL-1 prevents or delays graft damage [7]. Consistent with this concept, McGregor et al. [13] recently found that when antibody binding to xenografts is inhibited and the action of complement is controlled, cardiac xenografts appear not to be subject to spontaneous damage by coagulation or inflammation. These results would suggest that, as in allografts, early immune reactions rather than a myriad of inflammatory events determine graft outcome.
Accommodation and the outcome of xenografts
Nearly twenty years ago we noticed that transient control of binding of antibodies to an organ graft causes a change in the graft that allows function and survival in the face of a rekindled humoral response. We used the term accommodation to refer to this apparent resistance to humoral injury and we speculated that this condition might be important for the application of xenotransplantation injury [14]. We suggested too that accommodation might have a broader physiologic role in host defense [15]. If accommodation confers resistance to various forms of tissue injury, it could explain survival of xenografts in the face of multiple independent pathways of injury. Until accommodation can be “diagnosed” and excluded, one cannot conclude that mechanisms of injury act in series.
Galα1-3Gal and the destruction of xenografts
Thirteen years ago, Good et al. [16] reported that a saccharide expressed by pigs and other non-primate mammals was the major target of xenoreactive natural antibodies. Lin et al. showed that anti-Gala1-3Gal antibodies trigger hyperacute and acute vascular rejection of xenografts and that depleting those antibodies can allow accommodation to ensue [17, 18]. These findings raised the possibility specific inhibition of the production of those antibodies or disruption of the synthesis of the saccharide might dramatically improve the outcome of xenografts.
Preventing production of anti-Galα1-3Gal
Various approaches have been taken to inhibiting production of anti-Galα1-3Gal antibodies. Besides attempts at using immunosuppression, some have tried to induce tolerance. One approach involves the administration of swine hematopoietic stems cells into suitably conditioned primates [19]. To date this approach has not been fully evaluated because persistent engraftment of xenogeneic bone marrow cells has proved difficult to achieve [20]. To avoid difficulties in achieving enduring engraftment of these cells, Bracy et al. [21] expressed α1,3-galactosyltransferase, the enzyme that catalyzes synthesis of Gala1-3Gal, in autologous cells, hoping these cells might persist better than xenogeneic cells. This approach has not been reported to succeed in primates.
Preventing expression of Galα1-3Gal and complement activation
Given the difficulties in inducing tolerance to Gala1-3Gal and other antigens, interest became focused on genetic engineering of swine in ways that would make their organs more compatible with human immune system or less susceptible to injury by it. The first approach taken was to express human complement regulatory proteins as the products of transgenes in swine [22]. This approach, particularly expression of decay accelerating factor or membrane co-factor protein, which inhibit complement at the level of C3 or C4, dramatically improved the outcome but did not allow enduring survival of organ xenografts [23, 24].
To the extent that the barrier to xenotransplantation can be reduced to the immunological reaction of antibodies (or natural killer cells) with Galα1-3Gal, an alternative approach to genetic engineering would offer more specificity and perhaps more efficacy—the targeting of the α1,3-galactosyltransferase gene. Targeting of this enzyme was enabled by the development of methods for the cloning of swine [25, 26]. On the other hand, if the barriers extended beyond Galα1-3Gal then Gal-knock out pigs might not provide the “ideal” organs [27]. Recent experiments using Galα1-3Gal-deficient pigs have shown that organs lacking that saccharide can be made to survive for prolonged periods of time (months) but not indefinitely [28, 29], and the outcome may be no better than what can be achieved using organs expressing that sugar [30, 31]. These results suggest that another barrier, perhaps the antibody response to xenogeneic proteins, poses a more difficult hurdle than Galα1-3Gal. The results should stimulate further efforts toward induction of tolerance or accommodation.
Coagulation and thrombosis in xenotransplantation
Organ xenografts are plagued by coagulation and thrombosis. Thrombosis occurs in acute vascular rejection [32] and some have suggested that thrombosis may occur independently of rejection [33-35]. As two potential mechanisms for the latter, porcine von Willebrand factor spontaneously aggregates human platelets [36] and porcine thrombin might interact inefficiently with human thrombinmodulin [37, 38].
Potenital solutions to the problem of coagulation and thrombosis have been studied in rodents. Over-expressing anti-coagulation genes or genetically deleting genes, which cause coagulation, prevents acute vascular rejection in rodent models. Chen et al. [39] found that hearts from mice transgenic for the tissue factor pathway inhibitor or hirudin survived for greater than 100 days after transplantation into rats and resisted acute vascular rejection, whereas non-transgenic hearts were rejected within 3 days. Dwyer et al. [40] found that hearts from transgenic mice expressing human CD39, the major triphosphate diphosphohydrolase which convents ATP and ADP to AMP, which is further degraded to the antithrombotic and anti-inflammatory mediator adenosine, resisted acute vascular rejection. Mendicino at al. [41] found that targeted deletion of Fgl-2, an inducible endothelial cell procoagulant in the donor, prevents acute vascular rejection in a mouse-to-rat cardiac xenotransplantation model. These results would seem to suggest that therapeutics directed at coagulation and thrombosis may have important therapeutic potential in xenotransplantation. However, observations in swine to primate models are less encouraging. Cowan et al. [42] and Byrne et al. [43] found that anti-thrombotic and anticoagulant agents have limited or no impact on the outcome of swine organs transplanted into non-human primates. Hence, whether coagulation and thrombosis would pose hurdles to clinical xenotransplantation independent to rejection and if so how to solve the problem remain unknown.
Xenotransplantation and the risk of infection
Because the animals used as sources of xenografts can be fully characterized, bred and treated in various ways, the risk of transmitting an infectious agent from the graft to the recipient should be much less than the risk in allotransplantation (human-to-human) where the donors are scarce and incompletely characterized. One exception is the risk of transmitting an endogenous retrovirus present in all sources. Such a retrovirus is the porcine endogenous retrovirus or PERV. Nearly ten years ago, Patience et al. [44] reported that PERV could infect human cells. That finding raised alarm that xenotransplantation might not only engender a risk to the recipient of a xenograft but also to society, as a new virus might evolve by mutation or recombination following infection of a human. Happily, while PERV could be found to infect various types of human cells, no survey of human subjects revealed any evidence that infection occurred after xenotransplantation or parenteral exposure to swine cells and tissues [45, 46]. However, these surveys depended on measuring levels of the virus in human versus pig cells and were limited to the analysis of blood cells. Hence we conducted experiments in which human cells were introduced into fetal swine and then later tested for infection by PERV. In fact, we found that PERV did infect human cells in vivo and did so by the fusion of human and swine cells and that the fused cells were infectious, although only to a limited extent, for human cells [47]. This observation provided one explanation for how infection of human cells might have been overlooked and suggested what may be a general mechanism for the spread of viruses between species [48]. However, Galα1-3Gal on PERV can be recognized by anti-Galα1-3Gal antibodies and viral infection blocked in vitro; hence, the transfer of PERV from swine to human cells in pigs may be more relevant to viral transfer from α1,3 galactosyltransferase-knock out pigs than to transfer in from the tissues of pigs expressing that enzyme. Furthermore, whether PERV poses any biological risk is still unknown.
Concluding remarks
Xenotransplantation of organs is met with challenging obstacles. Humoral rejection and the transfer of retroviruses pose difficult hurdles that are still unsolved. However, cell and tissue transplants are not susceptible to humoral rejection, and hence these transplants can be conducted without special measures directed at complement or antibodies. Furthermore, cell and tissue transplants, such as islet or hepatocyte transplants, are generally conducted without removing the failing organ; hence, the recipient is not necessarily harmed by failure of the graft. Given these considerations, xenotransplantation, albeit not of organs, may be closer to clinical application than is generally appreciated.
Footnotes
Supported by operating funds from Canadian Institute of Health Research and by grants from the National Institutes of Health (USA) (HL52297).
References
- 1.JABOULAY M. De reins au pli du coude par soutures arterielles et veineuses. Lyon Med. 1906;107:575–577. [Google Scholar]
- 2.ULLMAN E. Tissue and organ transplantation. Ann Surg. 1914;60:195–219. doi: 10.1097/00000658-191408000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CASCALHO M, PLATT JL. Xenotransplantation and other means of organ replacement. Nat Rev Immunol. 2001;1:154–160. doi: 10.1038/35100578. [DOI] [PubMed] [Google Scholar]
- 4.CASCALHO M, PLATT JL. The immunological barrier to xenotransplantation. Immunity. 2001;14:437–446. doi: 10.1016/s1074-7613(01)00124-8. **This paper reviews the immunologic barriers to xenotransplantation. [DOI] [PubMed] [Google Scholar]
- 5.KHALPEY Z, KOCH CA, PLATT JL. Xenograft transplantation. Anesethesiol Clin North America. 2004;22:871–875. doi: 10.1016/j.atc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 6.WILLIAMS JM, HOLZKNECHT ZE, PLUMMER TB, LIN SS, BRUNN GJ, PLATT JL. Acute vascular rejection and accommodation: divergent outcomes of the humoral response to organ transplantation. Transplantation. 2004;78:1471–1478. doi: 10.1097/01.tp.0000140770.81537.64. [DOI] [PubMed] [Google Scholar]
- 7.SAADI S, TAKAHASHI T, HOLZKNECHT RA, PLATT JL. Pathways to acute humoral rejection. Am J Pathol. 2004;164:1073–1080. doi: 10.1016/S0002-9440(10)63194-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.OGATA K, PLATT JL. Cardiac xenotransplantation: future and limitations. Cardiology. 2004;101:144–155. doi: 10.1159/000075995. [DOI] [PubMed] [Google Scholar]
- 9.PLATT JL, VERCELLOTTI GM, LINDMAN BJ, OEGEMA TR, JR, BACH FH, DALMASSO AP. Release of heparan sulfate from endothelial cells. Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990;171:1363–1368. doi: 10.1084/jem.171.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HOLZKNECHT ZE, KUYPERS KL, PLUMMER TB, et al. Apoptosis and cellular activation in the pathogenesis of acute vascular rejection. Circ Res. 2002;91:1135–1141. doi: 10.1161/01.res.0000046236.20251.fa. [DOI] [PubMed] [Google Scholar]
- 11.SAADI S, HOLZKNECHT RA, PATTE CP, STERN DM, PLATT JL. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182:1807–1814. doi: 10.1084/jem.182.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SAADI S, HOLZKNECHT RA, PATTE CP, PLATT JL. Endothelial cell activation by pore forming structures: pivotal role for IL-1α. Circulation. 2000;101:1867–1873. doi: 10.1161/01.cir.101.15.1867. [DOI] [PubMed] [Google Scholar]
- 13.MCGREGOR CG, TEOTIA SS, BYRNE GW, et al. Cardiac xenotransplantation: progress toward the clinic. Transplantation. 2004;78:1569–1575. doi: 10.1097/01.tp.0000147302.64947.43. [DOI] [PubMed] [Google Scholar]
- 14.PLATT JL, VERCELLOTTI GM, DALMASSO AP, et al. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990;11:450–456. doi: 10.1016/0167-5699(90)90174-8. **This paper provides the first suggestion that genetic engineering and accommodation might be applied in xenotransplantation. [DOI] [PubMed] [Google Scholar]
- 15.KOCH CA, KHALPEY ZI, PLATT JL. Accommodation: preventing injury in transplantation and disease. J Immunol. 2004;172:5143–5148. doi: 10.4049/jimmunol.172.9.5143. [DOI] [PubMed] [Google Scholar]
- 16.GOOD AH, COOPER DKC, MALCOLM AJ, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24:559–562. **This paper first mentions Gala1-3Gal as a potential target of rejection in xenotransplantation. [PubMed] [Google Scholar]
- 17.LIN SS, KOOYMAN DL, DANIELS LJ, et al. The role of natural anti-Galα1-3Gal antibodies in hyperacute rejection of pig-to-baboon cardiac xenotransplants. Transpl Immunol. 1997;5:212–218. doi: 10.1016/s0966-3274(97)80040-8. [DOI] [PubMed] [Google Scholar]
- 18.LIN SS, HANAWAY MJ, GONZALEZ-STAWINSKI GV, et al. The role of anti-Galα1-3Gal antibodies in acute vascular rejection and accommodation of xenografts. Transplantation (Rapid Communication) 2000;70:1667–1674. doi: 10.1097/00007890-200012270-00002. [DOI] [PubMed] [Google Scholar]
- 19.SACHS DH, SABLINSKI T. Tolerance across discordant xenogeneic barriers. Xenotransplantation. 1995;2:234–239. [Google Scholar]
- 20.SABLINSKI T, EMERY DW, MONROY R, et al. Long-term discordant xenogeneic (porcine-to-primate) bone marrow engraftment in a monkey treated with porcine-specific growth factors. Transplantation. 1999;67:972–977. doi: 10.1097/00007890-199904150-00007. [DOI] [PubMed] [Google Scholar]
- 21.BRACY JL, SACHS DH, IACOMINI J. Inhibition of xenoreactive natural antibody production by retroviral gene therapy. Science. 1998;281:1845–1847. doi: 10.1126/science.281.5384.1845. [DOI] [PubMed] [Google Scholar]
- 22.MCCURRY KR, KOOYMAN DL, ALVARADO CG, et al. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nat Med. 1995;1:423–427. doi: 10.1038/nm0595-423. **This paper reports the first transplants using genetically engineered pigs as a source of organs. [DOI] [PubMed] [Google Scholar]
- 23.COZZI E, YANNOUTSOS N, LANGFORD GA, PINO-CHAVEZ G, WALLWORK J, WHITE DJG: Effect of transgenic expression of human decay-accelerating factor on the inhibition of hyperacute rejection of pig organs. In: Xenotransplantation: the transplantation of organs and tissues between species. 2nd ed. Cooper DKC, Kemp E, Platt JL and White DJG, (Eds), Springer, Berlin (1997):665–682.
- 24.COZZI E, BHATTI F, SCHMOECKEL M, et al. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation. 2000;70:15–21. [PubMed] [Google Scholar]
- 25.LAI L, KOLBER-SIMONDS D, PARK KW, et al. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 26.PHELPS CJ, KOIKE C, VAUGHT TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MIYATA Y, PLATT J. Xeno: still stuck without a-Gal. Nat Biotechnol. 2003;21:359–360. doi: 10.1038/nbt0403-359. [DOI] [PubMed] [Google Scholar]
- 28.KUWAKI K, TSENG YL, DOR FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. **This paper reports the first use of Gala1-3Gal-deficient pigs as a source of cardiac xenografts. [DOI] [PubMed] [Google Scholar]
- 29.YAMADA K, YAZAWA K, SHIMIZU A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. **This paper reports the first use of Gala1-3Gal-deficient pigs as a source of renal xenografts. [DOI] [PubMed] [Google Scholar]
- 30.MCGREGOR CG, DAVIES WR, OI K, et al. Cardiac xenotransplantation: Recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130:844–e841. doi: 10.1016/j.jtcvs.2005.04.017. **This paper reports the outstanding outcome of xenografts that express Gala1-3Gal and no evidence of spontaneous, uncontrollable coagulation independent of rejection. [DOI] [PubMed] [Google Scholar]
- 31.CHEN G, QIAN H, STARZL T et al.: Induced anti-non-Gal antibodies lead to acute humoral xenograft rejection in baboons using alpha1, 3-galactosyltransferase gene-knockout pigs as kidney donors. Nat. Med. (In Press). **This paper cautions that eliminating Gala1-3Gal, the main target in pigs of the human antibody response, may not improve the outcome of xenografts.
- 32.LIN SS, WEIDNER BC, BYRNE GW, et al. The role of antibodies in acute vascular rejection of pig-to-baboon cardiac transplants. J Clin Invest. 1998;101:1745–1756. doi: 10.1172/JCI2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.BUHLER L, BASKER M, ALWAYN IPJ, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70:1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 34.COWAN PJ, AMINIAN A, BARLOW H, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in nonimmunosuppressed baboons. Transplantation. 2000;69:2504–2515. doi: 10.1097/00007890-200006270-00008. [DOI] [PubMed] [Google Scholar]
- 35.KUWAKI K, KNOSALLA C, DOR FJ, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4:363–372. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 36.MAZZUCATO M, DE MARCOL, PRADELLA P, MASOTTI A, PARETI FI. Porcine von Willebrand factor binding to human platelet GPIb induces transmembrane calcium influx. Thromb Haemost. 1996;75:655–660. [PubMed] [Google Scholar]
- 37.LAWSON JH, PLATT JL. Molecular barriers to xenotransplantation. Transplantation. 1996;62:303–310. doi: 10.1097/00007890-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 38.LAWSON JH, DANIELS L, PLATT JL. The evaluation of thrombomodulin activity in porcine to human xenotransplantation. Transplant Proc. 1997;29:884–885. doi: 10.1016/s0041-1345(96)00192-3. [DOI] [PubMed] [Google Scholar]
- 39.CHEN D, WEBER M, MCVEY JH, et al. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant. 2004;4:1958–1963. doi: 10.1111/j.1600-6143.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 40.DWYER KM, ROBSON SC, NANDURKAR HH, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004;113:1440–1446. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MENDICINO M, LIU M, GHANEKAR A, et al. Targeted deletion of Fgl-2/fibroleukin in the donor modulates immunologic response and acute vascular rejection in cardiac xenografts. Circulation. 2005;112:248–256. doi: 10.1161/CIRCULATIONAHA.105.534271. [DOI] [PubMed] [Google Scholar]
- 42.COWAN PJ, AMINIAN A, BARLOW H, et al. Protective effects of recombinant human antithrombin III in pig-to-primate renal xenotransplantation. Am J Transplant. 2002;2:520–525. doi: 10.1034/j.1600-6143.2002.20605.x. [DOI] [PubMed] [Google Scholar]
- 43.BYRNE GW, SCHIRMER JM, FASS DN, et al. Warfarin or low-molecular-weight heparin therapy does not prolong pig-to-primate cardiac xenograft function. Am J Transplant. 2005;5:1011–1020. doi: 10.1111/j.1600-6143.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 44.PATIENCE C, TAKEUCHI Y, WEISS RA. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 45.PATIENCE C, PATTON GS, TAKEUCHI Y, et al. No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet. 1998;352:699–701. doi: 10.1016/S0140-6736(98)04369-4. [DOI] [PubMed] [Google Scholar]
- 46.PARADIS K, LANGFORD G, LONG Z, et al. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 47.OGLE BM, BUTTERS KB, PLUMMER TB, et al. Spontaneous fusion of cells between species yields transdifferentiation and retroviral in vivo. FASEB J. 2004;18:548–550. doi: 10.1096/fj.03-0962fje. **This paper provides the first evidence of transfer of the endogenous retrovirus of pigs from swine to human cells in vivo. [DOI] [PubMed] [Google Scholar]
- 48.OGLE BM, CASCALHO M, PLATT JL. Biological implications of cell fusion. Nat Rev Mol Cell Biol. 2005;6:567–575. doi: 10.1038/nrm1678. [DOI] [PubMed] [Google Scholar]
- 49.DALMASSO AP, VERCELLOTTI GM, FISCHEL RJ, BOLMAN RM, BACH FH, PLATT JL. Mechanism of complement activation in the hyperacute rejection of porcine organs transplanted into primate recipients. Am J Pathol. 1992;140:1157–1166. [PMC free article] [PubMed] [Google Scholar]
- 50.YAMADA K, SACHS DH, DERSIMONIAN H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155:5249–5256. [PubMed] [Google Scholar]
- 51.DORLING A, LOMBARDI G, BINNS R, LECHLER RI. Detection of primary direct and indirect human anti-porcine T cell responses using a porcine dendritic cell population. Eur J Immunol. 1996;26:1378–1387. doi: 10.1002/eji.1830260630. [DOI] [PubMed] [Google Scholar]
- 52.INVERARDI L, SAMAJA M, MOTTERLINI R, MANGILI F, BENDER JR, PARDI R. Early recognition of a discordant xenogeneic organ by human circulating lymphocytes. J Immunol. 1992;149:1416–1423. [PubMed] [Google Scholar]
- 53.AL-MOHANNA F, COLLISON K, PARHAR R, et al. Activation of naive xenogeneic but not allogeneic endothelial cells by human naive neutrophils: a potential occult barrier to xenotransplantation. Am J Pathol. 1997;151:111–120. [PMC free article] [PubMed] [Google Scholar]
- 54.BLAKELY ML, VAN DER WERF WJ, BERNDT MC, DALMASSO AP, BACH FH, HANCOCK WW. Activation of intragraft endothelial and mononuclear cells during discordant xenograft rejection. Transplantation. 1994;58:1059–1066. [PubMed] [Google Scholar]
- 55.BUSTOS M, SAADI S, PLATT JL. Platelet-mediated activation of endothelial cells: implications for the pathogenesis of transplant rejection. Transplantation. 2001;72:509–515. doi: 10.1097/00007890-200108150-00025. [DOI] [PubMed] [Google Scholar]


