Abstract
Objective
Early deaths from organophosphorus (OP) pesticide self-poisoning result from respiratory failure and cardiovascular collapse. Therapy requires the urgent use of atropine to reverse cholinergic excess, thereby improving respiratory function, heart rate, and blood pressure. We aimed to assess variation in textbook recommendations for early atropinisation and to see whether this variation affected time to stabilisation using model data from 22 severely poisoned patients seen in a Sri Lankan clinical trial.
Methods
We extracted prospectively recorded data on atropine requirements for 22 OP poisoned patients who required intubation but survived to discharge. We did a systematic search for textbook recommendations for initial atropinisation regimens. These regimens were then applied to data from the Sri Lankan patients.
Results
The patients required a mean of 23.4 mg (standard deviation 22.0, range 1-75 mg) atropine to clear the lungs, raise the pulse above 80bpm, and restore systolic blood pressure to more than 80 mmHg. Textbook recommendations varied markedly -atropinisation of an average patient, requiring the mean dose of 23.4mg, would have taken 8 to 1380 mins; atropinisation of a very ill patient, requiring 75mg, would have taken 25 to 4440 mins. Atropinisation was attained most rapidly with a regimen of increasing bolus doses after failure to respond to the previous bolus.
Conclusions
There is great variation in recommendations for atropinisation, with some regimens taking hours and even days to stabilise a patient. The guidelines are very flexible – possibly appropriate for experienced emergency physicians or clinical toxicologists, but completely inappropriate for the inexperienced junior doctors who see most cases worldwide. We recommend that a consensus guideline be developed by appropriate organisations to bring order to this important part of OP therapy, while acknowledging the paucity of data to drive the guidelines.
Introduction
Acute organophosphorus (OP) pesticide poisoning is a major global clinical problem, 1–3 with hundreds of thousands of deaths occurring every year.4,5 Early deaths result from respiratory failure - due to central respiratory depression, neuromuscular junction weakness, bronchorrhoea and bronchospasm - and from cardiovascular collapse.6 Therapy requires urgent administration of sufficient atropine to reverse signs of cholinergic excess (attain ‘atropinisation’),7-9 as well as airway and ventilatory support.10 Current guidelines recommend the use of bolus doses to attain atropinisation, followed by an infusion.8
Carrying out a randomized controlled trial (RCT) of activated charcoal in acute self-poisoning in north central Sri Lanka, we have managed more than 600 OP poisoned patients over 18 months. Major issues with their management have been how to give the loading doses of atropine and for which criteria of atropinisation to aim.11 A review of textbooks found many different regimens with little consensus.
Widely varying regimens of atropinisation have also been reported by doctors seeing OP poisoned patients in different parts of the world. One group infused atropine 0.02-0.08mg/kg over one hour to attain atropinisation.12 Such a regimen would take four hours to administer 20mg of atropine to a severely ill 70kg patient.13
Noting the wide variation found in textbooks, we reviewed texts of clinical toxicology for recommendations on early atropine administration. We aimed to compare the regimens by calculating the time it would take for each recommended regimen to fully atropinise two hypothetical significantly ill patients – one requiring the mean amount of atropine given to severely ill patients in our study and one requiring the highest amount of atropine given to a surviving patient.
Methods
Patients with OP self-poisoning admitted to Anuradhapura General Hospital, a secondary district hospital serving 750,000 people, who had been intubated but survived were identified from the study database. The ingested pesticide was identified by history from patient or relative, or by the bottle being brought into the hospital; clinical features shown by patients were consistent with a diagnosis of OP poisoning. The quantity of atropine given to each patient was noted from entries made in the patient’s records at the time of initial management. All patients were managed following a standard protocol; criteria used for atropinisation are given in Box 1.
Box 1. Target end-points for atropine therapy
Clear chest on auscultation
Heart rate > 80/min
Systolic BP >80mmHg
Pupils no longer pinpoint
Dry axillae
We aimed to attain at least four end-points, including all of the first three, before considering a patient atropinised.
We searched for textbooks of clinical toxicology published between 1993 and 2003 using www.amazon.com and the keywords ‘clinical toxicology’, and ‘poisoning’. The searches produced 2386 and 16848 hits, respectively. The title and accompanying description was reviewed for the first 450 and 800 hits for each search – 200 past the last text found to be a clinical toxicology textbook. The ‘clinical toxicology’ search revealed nine texts,14-22 the ‘poisoning’ search an additional fifteen.23-37 One text27 was an updated concise version of another;28 only the former was therefore included.
We also searched three library catalogues: Royal Society of Medicine (http://www.rsm.ac.uk/librar/library.htm) using the complex search option, ‘poisoning’ or ‘clinical toxicology’ in the title, for 1993-2003, search restricted to books. 44 texts were retrieved; four texts were relevant, one not previously found.38 British Library (http://www.bl.uk/ catalogues/toppage.html) using ‘all catalogues’ option, ‘poisoning’ or ‘clinical toxicology’ in the title, for 1993-2003. 144 texts were retrieved; 12 texts were relevant, one not previously found.39 National Library of Medicine (http://locatorplus.gov/) using advanced menu search option, ‘poisoning’ or ‘clinical toxicology’ in the title, for 1993-2003, restricting the search to books in English - 471 texts were retrieved; 18 texts were relevant, two not previously found.40,41 One of these texts 41 could not be obtained because it is not yet in print.
Online sources were also checked: INTOX project of the WHO/IPCS (four texts9,42–44); Hypertox (Australia),45 Poisindex (USA),46 Toxbase (UK),47 and Toxinz (New Zealand).48 Interview of colleagues and communication with the AACT central office revealed four other books.10,49–51 Data were also extracted from WHO,52 UK,53 and Australian54 formularies, and from international textbooks of internal medicine.55–57
Regimens for atropine administration in acute OP poisoning were sought in each text. The following information was extracted: administration rate of atropine, criteria for full atropinisation, and maximum amount of atropine that might be required in a severely poisoned patient (tables 1 and 2). Such information was not given in five sources,21,33,35,36,51 which were therefore excluded from the analysis.
Table 1.
Atropine recommendations in textbooks, handbooks, and online databases of clinical toxicology
| Source | Edition/Year | Recommended regimen to attain atropinisation (IV unless stated otherwise) | Markers of atropinisation | Max dose of atropine first 24h |
|---|---|---|---|---|
| Aggarwal 24 | 1st / 1997 | 2-4mg, repeated every 5-15 min | Dry secretions | NG |
| Bryson18 | 3rd / 1996 | 2-4mg, repeat or increase until atropinisation | Drying of secretions, skin and mouth, mydriasis, flushed skin, and tachycardia | 1g |
| Casarett 23 | 7th / 2003 | 5mg, repeated every 20-30 min | No sweating or salivation, flushed skin, mydriasis. | Up to 50mg |
| Dreisbach 30 | 13th / 2001 | 2mg IM, repeat every 3-8 min | Control of “signs of parasympathetic toxicity” | NG |
| Dart 1 40* | 1st / 2000 | 2-4mg, repeated every 5-10 min (or doubling the dose) | Control of pulmonary secretions | “massive amounts” |
| Dart 2 15* | 3rd / 2003 | 2-4 mg, repeated every 2-5 mins, with increasing incremental doses (eg 4-8 mgs) | Control of pulmonary secretions, with clear lungs and adequate oxygenation | NG |
| Fernando 49 | 2nd / 1998 | 2-10mg, then 2mg repeated every 10-15 min | Counteract muscarinic effects on pulse, secretions, pupil | >100mg |
| Flanagan 19 | 1st / 2001 | 2mg, every 5-10 min | Dry mouth, pulse of 70-80bpm, reduction of bronchial secretions | 1-2g |
| Ford17 | 1st / 2001 | 1-2mg; repeated every 5 min doubling the dose | Drying of the tracheobronchial tree and ability to oxygenate the patient | 100s of mg |
| Goldfrank20 | 7th / 2002 | 1-5mg, repeated every 2-3 min | Dry skin and mucous membranes, decreased or absent bowel sounds, tachycardia, reduced secretions, no bronchospasm and usually mydriasis | 1g |
| Gossel 32 | 3rd / 1994 | 2-4mg, repeated every 10-15 min | Diminish bradycardia, salivation, and bronchial secretions; produce signs of atropinisation (mydriasis, tachycardia and dry mouth) | 50mg |
| Haddad16 | 3rd / 1998 | 1-2mg, then 2mg repeated every 15-30 min | Flushing, drying of airway secretions, dilated pupils, increased heart rate. | NG |
| Henry 29 | 1st / 1997 | 2-4mg, repeated every 10 min | Dry mouth, plus pulse >100, pupils dilated | “large amounts” |
| Hypertox 45 | 3.7 / 2003 | 1-2mg, repeated every 5-10 min | No secretions | NG |
| Jones34 | 1st / 2001 | 2mg, repeated every 10 min | Flushed red skin, tachycardia, dilated pupils, dry mouth | 30mg or more |
| Krieger 38 | 2nd / 2001 | 2-5mg, repeated every 10-20 min | Flushed skin, dry mouth, dilated pupils, bronchodilation, raised heart rate | 3.5g |
| Lall 22 | 1st / 1998 | 2-5mg, repeated every 5-10 min | Control of parasympathetic manifestations, or clearing of rales and drying of pulmonary secretions | NG |
| Leikin 27 | 3rd / 2001 | 2-5mg, repeated every 5-10 min | Dry pulmonary secretions | >100mg |
| Poisindex 46 | 2003 | 2-5mg, repeated every 10-30 min | Clear lungs, no secretions. | NG |
| Olson 14 | 3rd / 1999 | 1-5mg, repeated every 5-10 min | Clear chest, dry secretions, reversal of significant bradycardia | Several grams |
| PAPA 50 | 2nd / 1999 | 1-3mg, repeated every 5-10 min | Dry flushed skin, pupillary size at least 4mm, heart rate >120/min | NG |
| Proudfoot 25 | 2nd / 1993 | 2mg, repeated every 10-30 min | Flushed dry skin, tachycardia, dilated pupils, dry mouth. | NG |
| Proudfoot 31 | 2nd / 1996 | 2mg, repeated to control peripheral muscarinic signs | Control of bronchospasm and bronchorrhoea | 100mg or more |
| Reigart 26 | 5th / 1999 | If GCS normal: 2-4mg, repeated every 15 min. If GCS reduced: ~4-8mg, repeated every 5-15 min | Control of pulmonary secretions | 300mg |
| Seyffart 10 | 4th / 1996 | 1-2mg, repeated or increased in increments every | Abolition of excess bronchial | NG |
| 15-60 min | secretion, dry mouth and skin, flushing | |||
| Toxbase 47 | 2002 | 2mg, repeated every 10-30 min | Reversal of bronchospasm and bronchorrhoea | “very large doses” |
| Toxinz 48 | 2003 | 1-5mg, repeated every 5-30 min | Clear lungs, no secretions. | >200mg |
| WHO PIM 43 | 1986 | 1-2mg, then same or increased dose every 15-30 min | Full atropinisation (signs include dilated pupils, dry mouth, skin flushing) | “several hundred mgs” |
| WHO EHC 42 | 1999 | 2mg, then same or increased dose every 15-30 min | Full atropinisation (signs include dilated pupils, dry mouth, skin flushing) | “several hundred mgs” |
| WHO OP Antidotes 9 | 2002 | 2mg, then same or increased dose every 15-30 min | Reduction in secretions, especially bronchial secretions | NG |
| WHO Treat- ment guide44 | 1999 | 1-2 mg, repeated every 5-10 min | Drying of respiratory secretions | 100mg |
| Viccellio 37 | 2nd / 1998 | 1-2mg, then 2mg repeated every 5-10 min. Larger increments of atropine may be used. | Control of bronchial secretions and bronchospasm | 1g |
These texts had two and three different recommendations, respectively, for atropinisation. The recommendations given for OP poisonings are presented here.
Table 2.
Atropine recommendations in major textbooks of internal medicine and national formularies.
| Source | Edition / Year | Recommended regimen to attain atropinisation | Markers of atropinisation | Max dose of atropine first 24h |
|---|---|---|---|---|
| Australian Medicines Handbook 54 | 4th / 2003 | 2 mg IV repeated as necessary until patient is atropinised, then infusion titrated against clinical effects. | Abolish all secretions | maximum dose may be >50- 100 mg/hour |
| British National Formulary 53 | 46th / 2003 | 2mg, repeated every 5-10 min (IM or IV according to severity) | Dry flushed skin, dilated pupils, tachycardia | NG |
| Davidsons 55 | 19th / 2002 | 2mg, repeated every 10 min | Dry secretions, reversal of bradycardia | 30mg, rarely more |
| Harrisons 56 | 15th / 2001 | 0.5-2mg, repeated every 5-15 min | Dry secretions | NG |
| Oxford Textbook of Medicine 57 | 4th / 2003 | 2mg, repeated every 10-30 min | No bronchorrhoea & bronchospasm, or flushed dry skin, dry mouth, tachycardia | 30mg, occasionally much more |
| WHO Model Formulary 52 | 1st / 2002 | 2mg, repeated every 20-30 min | Flushed dry skin and tachycardia | NG |
These atropine administration rates were then applied to the mean dose of atropine required by the Sri Lankan patients and to the highest dose of atropine required by one patient (75mg).
Results
Cohort of OP poisoned patients
Between 31st March and 3rd December 2002, 1000 patients with acute poisoning were reviewed on admission to Anuradhapura Hospital. 226 had ingested OP pesticides; a further 44 had ingested an unknown pesticide that required atropine and was most likely an OP (less commonly, carbamates).
Sixty one patients required intubation. Of these patients, 38 died after admission while 23 required intubation but survived to hospital discharge. Data on atropine administration and intubation timing was available in the notes made by the study team at the time of admission for all but one of these patients.
All the patients were seen on admission by a study doctor and 0.6-3mg of atropine administered to patients with signs of cholinergic poisoning. If there was no response after five minutes, this initial dose was doubled until the patient was judged to be stable (for example, 1.2mg initial bolus, then 2.4mg, then 5mg, etc). Criteria for atropinisation are given in Box 1. All patients received pralidoxime 1g qds for 1-3 days.
The 22 patients required a mean of 23.4 mg of atropine on admission (standard deviation 22.0, range 1-75 mg; the patients requiring the lower doses had previously received atropine at a peripheral hospital before transfer). This quantity of atropine given to attain atropinisation does not include the atropine that was subsequently given by infusion. Seventeen patients were intubated on admission; the other five were intubated during the next few days for the intermediate syndrome.
Recommended atropine regimens in textbooks of clinical toxicology
We obtained thirty eight recommendations for atropinisation from clinical toxicology textbooks and electronic sources, national formularies, and international textbooks of internal medicine (tables 1 & 2). All 15–17,20 the texts commonly used by American poison control centers were included (R Soloway, AAPCC, personal communication). Overall, thirty three different recommendations were obtained.
Most sources gave a range of atropine dosages and/or intervals between repeat doses, for example, ‘give 2 to 5mg of atropine every 5-10 minutes’. Using this regimen, the time to give 10 mg varies from 5 minutes after the first dose (5mg every 5 minutes) to 40 minutes after the first dose (2mg every 10 minutes). In this way, a range of times to atropinisation was calculated for each recommendation.
Applying these recommendations to a Sri Lankan patient, intubated but surviving to discharge, and requiring the mean atropine dose of 23.4 mg, between 10 and 1380 minutes were required to give the necessary 23.4 mg (figure 1). For recommendations specifying a range of atropine doses, there was often marked variation in time to atropinisation: eg, using the regimen of Harrison’s textbook (0.5-2mg repeated every 5-15 min),56 atropinisation would have occurred after either 55 or 690 mins depending on whether the larger dose was given every 5 mins or the smaller dose given every 15 mins. Even when given most aggressively, some of the regimens took more than 100 mins to give 23.4 mg.
Figure 1.
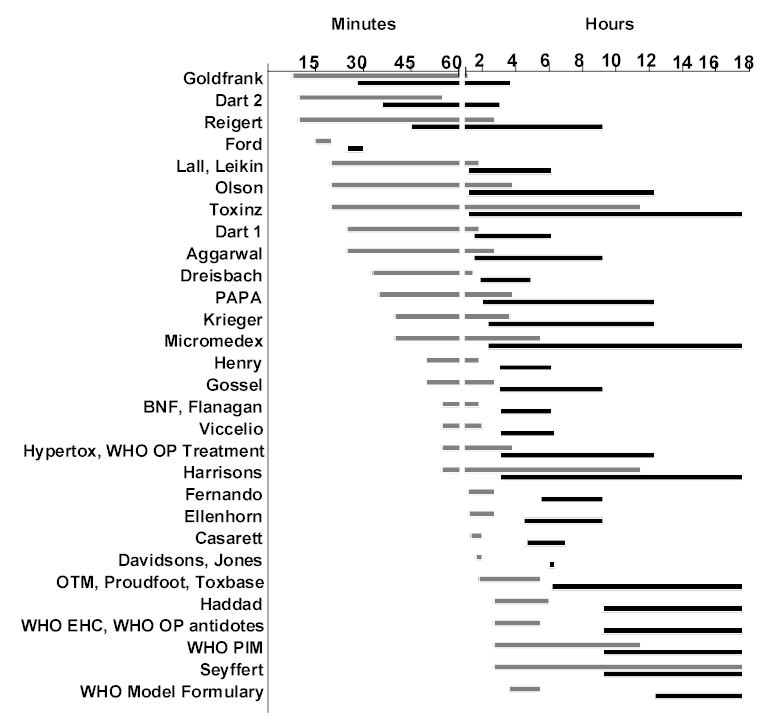
Range of times to give mean (23.4mg; pale bars) and high (75mg; dark bars) atropine loading doses for patients with severe OP poisoning, following instructions from each text. Doses were based on 22 Sri Lankan patients. A range was not given for three regimens18,31,54 and they are not included in this figure. The times are curtailed at 18hrs; eleven regimens took more than 18hrs.
The regimen given in Ford’s textbook17 had the least variation in time to atropinisation - 15 and 20 mins for the fastest and slowest administration regimens, respectively.
Twenty four texts indicated that large amounts of atropine might be required for a severely ill patient - specific estimates ranged from 20mg to 3.5g (tables 1 and 2). However, using the fastest regimen suggested by each source, it would take between 25 and 740 minutes (12 h 20 min) to atropinise the Sri Lankan patient who required 75mg (figure 1). With three regimens, it would have taken more than 37 hours to give 75mg using the slower of their recommended dosage rates. Some of the regimens required as many as 70 bolus injections of atropine.
There was also marked variation in the criteria for atropinisation. Fourteen sources used reversal of bronchorrhoea and bronchospasm as their main criteria. Some used pupil size, flushed skin, or heart rate, in the latter case often setting a lower limit of 120bpm.
Discussion
Severe OP pesticide self-poisoning is a major clinical problem in the Asia Pacific region - some hospitals see 500-1000 patients every year with case fatality over 20%. Since OPs are responsible for around two thirds of deaths in most case series of pesticide poisoned patients,3 there are likely to be at least 200,000 deaths every year with these compounds.4,5
Full and early atropinisation is an essential and simple part of early management. Delayed atropinisation can result in death from central respiratory depression, bronchospasm, bronchorrhoea, severe bradycardia and hypotension.6 Animal work suggests that these early deaths may be primarily due to central cholinergic stimulation.58,59 Adoption of a regimen that results in rapid atropinisation to block such stimulation will likely save significant numbers of lives across the developing world where junior doctors manage patients without advice from clinical toxicologists. Recommended regimens must be simple and easily used by such doctors.
Our systematic search of clinical toxicology textbooks revealed multiple guidelines based on little evidence and varying from one source to another. For example, five texts stated that 2-4mg of atropine should be given ‘every 5-10 min’, ‘every 5-15 min’, ‘every 10 min’, ‘every 10-15 min’, or ‘every 15 min’ (tables 1 and 2). It is tempting to wonder whether each new recommendation is simply a tweaked old recommendation, made to look different from its predecessors.
Most importantly, in inexperienced hands, the regimens could result in atropinisation not being reached for many hours – over 11 hours to give 23.4mg in two cases and 23 hours in one case (figure 1) – leaving patients in a dangerously unstable state.
Many texts state that very large amounts of atropine might be required to stabilise a patient but still recommend regimens that take hours to give these amounts. Taking the Sri Lankan patient who required the greatest amount of atropine (75mg, well below the maximum amount stated in most texts – see tables 1 and 2), time to atropinisation varied from 25 min to 740 min (greater than 12hrs) using the most aggressive regimens in each text. Time to atropinisation using some of the less aggressive regimens would have required more than 1.5 days. Note that the mean dose of 23.4 mg and maximum dose of 75mg are conservative estimates since many patients received some atropine at peripheral hospitals, prior to their admission to the study hospital.
There was also variation in the recommended interval between bolus injections – from 5 min to 30 min (tables 1 and 2). Since blood levels peak quickly after IV injection and onset of action is within a few minutes,60 waiting just five minutes for a response before deciding whether to give another dose is probably sufficient.
The regimen that performed best was that of Ford’s textbook.17 An initial bolus of 1-2mg is recommended with subsequent doses doubled every 5 minutes until atropinisation is achieved. This regimen requires no more than 20 minutes to administer 25mg of atropine. It also works well for the rare patient who requires very large amounts of atropine, permitting administration of 75mg in 25-30 minutes. It also works well for patients requiring small doses since the initial bolus can be as little as 1mg. Importantly, it permits little variation in time to atropinisation in the hands of an inexperienced junior doctor. Eight other regimens9,10,15,18,37,40,42,43 suggested the use of larger bolus doses after the first dose but did not specify increasing the amount given with each subsequent dose administered.
Criteria for atropinisation varied widely among sources - some texts recommended giving atropine until pupils were dilated and pulse more than 120-140/min. Since patients die from respiratory failure and/or cardiogenic shock, we think it more important to reverse bronchospasm and bronchorrhoea, and improve systolic blood pressure and heart rate, than to dilate pupils or produce a tachycardia. Furthermore, both dilated pupils and tachycardia can result from stimulation of nicotinic ACh receptors, and tachycardia result from low total peripheral resistance with a partially compensatory high cardiac output.61
There is currently little clear evidence for selecting atropine endpoints. Giving atropine to reverse signs attributable to specific muscarinic (M) receptor subtypes may not reverse OP effects at other receptors. In particular, since current endpoints do not include a CNS endpoint, it is possible that atropinisation is not reversing the CNS cholinergic syndrome, which may have significant consequences for preventing early death from OP poisoning.58,59
Excess atropine can be dangerous. Endpoints such as pulse rates >120/min and dilated pupils suggest that the patients are being given too much atropine. Atropine toxicity causes confusion, agitation, and hyperthermia.7 Such effects are major problems in the hot, non-air conditioned wards of the tropical developing world where most patients present. Agitated patients in ambient temperatures greater than 35C, not sweating because of atropine, can become very hot. The situation is exacerbated by alcohol or alcohol-withdrawal induced agitation. Patients can die from atropine-induced hyperthermia and cardiac arrest (Eddleston, unpublished).
A further problem with fast heart rates is ischaemic heart disease in elderly patients. We have noted patients with fairly mild poisoning who died in ICU from myocardial infarctions after being given atropine to keep their heart rate at 120-140 bpm. We therefore prefer criteria for atropinisation to concentrate on clearing lungs, raising systolic blood pressure, and increasing the heart rate to just 80-100bpm.
Conclusions
This review of the clinical toxicology reference sources reveals a variety of recommendations for atropinisation, many of which would delay its attainment for hours. In addition, some sources used criteria for atropinisation that would cause atropine toxicity rather than resolution of the poisoning. Evidence for the recommendations is weak – there has been only one comparative study of different atropine regimens and this used historical rather than parallel group controls.62 This situation is not unique to OPs - rather it appears to be true for all forms of pesticide poisoning, if not all of clinical toxicology.63
In light of the importance of OP pesticide poisoning worldwide, we call upon clinical toxicology associations to work with the WHO to review the evidence for atropine administration and to produce and disseminate a simple guideline that will be useful for junior doctors faced by this severe form of poisoning across the world. Given the paucity of existing evidence, strategies should be developed for performing clinical studies to determine the optimal dosing regimen of atropine that rapidly and safely achieves atropinisation in these patients.
Acknowledgments
We thank the members of the Ox-Col Collaboration Poisoning Team for their superb work on the RCT, the medical and nursing staff of the study hospitals for their support, and Nida Besbelli, Lewis Nelson, Ladislaus Szinicz, Peter Eyer, and the journal referees for their critique. ME is a Wellcome Trust Career Development Fellow, funded by grant GR063560MA from the Tropical Interest Group. The South Asian Clinical Toxicology Research Collaboration is funded by a Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant GR071669MA.
References
- 1.Jeyaratnam J. Acute pesticide poisoning: a major global health problem. Wld Hlth Statist Quart. 1990;43:139–44. [PubMed] [Google Scholar]
- 2.van der Hoek W, Konradsen F, Athukorala K, Wanigadewa T. Pesticide poisoning: a major health problem in Sri Lanka. Soc Sci Med. 1998;46:495–504. doi: 10.1016/s0277-9536(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 3.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. Q J Med. 2000;93:715–31. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- 4.Gunnell D, Eddleston M. Suicide by intentional ingestion of pesticides: a continuing tragedy in developing countries. Int J Epidemiol. 2003;32:902–9. doi: 10.1093/ije/dyg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328:42–4. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballantyne B, Marrs TC. Overview of the biological and clinical aspects of organophosphates and carbamates. In: Clinical and experimental toxicology of organophosphates and carbamates, 0 edn. Oxford, Butterworth heinemann, 1992:3-14.
- 7.Heath AJW, Meredith T. Atropine in the management of anticholinesterase poisoning. In: Clinical and experimental toxicology of organophosphates and carbamates Oxford, Butterworth Heinemann, 1992:543-54.
- 8.Johnson MK, Jacobsen D, Meredith TJ, Eyer P, Heath AJW, Ligtenstein DA, Marrs TC, Szinicz L, Vale JA, Haines JA. Evaluation of antidotes for poisoning by organophosphorus pesticides. Emergency Medicine. 2000;12:22–37. [Google Scholar]
- 9.International Programme on Chemical Safety. Antidotes for poisoning by organophosphorus pesticides. Monograph on atropine. http://www.intox.org/databank/documents/antidote/antidote/atropine.htm 2002. Ref Type: Electronic Citation
- 10.Seyffart G. Poison index. The treatment of acute intoxication, 4 edn. Miami, FL, PABST, 1996.
- 11.Eddleston M, Dawson AH, Karalliedde L, Dissanayake W, Hittarage A, Azher S, Buckley NA. Early management after self-poisoning with an organophosphorus or carbamate pesticide. Crit.Care . 2004. Ref Type: In Press [DOI] [PMC free article] [PubMed]
- 12.Sungur M, Guven M. Intensive care management of organophosphate insecticide poisoning. Critical Care. 2001;5:211–5. doi: 10.1186/cc1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eddleston M, Roberts D, Buckley N. Management of severe organophosphorus pesticide poisoning. Critical Care. 2002;6:259. doi: 10.1186/cc1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson KR. Poisoning and drug overdose, 3 edn. Stamford, CT, Appleton & Lange, 1999.
- 15.Dart RC. Medical Toxicology, 3 edn. Philadelphia, Lippincott Williams & Wilkins, 2003.
- 16.Haddad LM, Shannon MW, Winchester JF. Clinical management of poisoning and drug overdose, 3 edn. W B Saunders, 1998.
- 17.Ford MD, Delaney KA, Ling LJ, Erickson T. Clinical toxicology WB Saunders, 2000.
- 18.Bryson PD. Comprehensive review in toxicology for emergency clinicians, 3 edn. Washington, DC, Taylor & Francis, 1997.
- 19.Flanagan RJ, Jones AL. Antidotes. Principles and applications London, Taylor & Francis, 2001.
- 20.Goldfrank L, Flomenbaun N, Lewin N, Howland MA, Hoffman R, Nelson L. Goldfrank's Toxicologic emergencies, 7 edn. McGraw-Hill Professional, 2002.
- 21.Loomis TA. Loomis's Essentials of Toxicology, 4 edn. San Diego, Academic Press, 1996.
- 22.Lall SB, Peshin SS, Khattar S. Essentials of clinical toxicology Bombay, Narosa Publishing House, 1998.
- 23.Casarett LJ, Klaassen CD, Watkins JB. Casarett and Doull's Essentials of Toxicology. The basic science of poisons, 7 edn. New York, McGraw-Hill, 2003.
- 24.Aggarwal P, Wali JP. Diagnosis and management of common poisoning Delhi, Oxford University Press, 1997.
- 25.Proudfoot AT. Acute poisoning: diagnosis and management Oxford, Butterworth-Heinemann, 1993.
- 26.Reigart JR, Roberts JR. Recognition and management of pesticide poisonings Washington, DC, U.S Environmental Protection Agency, 1999.
- 27.Leikin JB, Paloucek FP. Poisoning and toxicology handbook, 3 edn. Hudson, OH, Lexi-Comp Inc, 2001.
- 28.Leikin JB, Paloucek FP. Poisoning and toxicology compendium Hudson, OH, Lexi-Comp Inc, 1998.
- 29.Henry J, Wiseman H. Management of poisoning. A handbook for health care workers Geneva, WHO/UNEP/ILO, 1997.
- 30.True B-L, Dreisbach RH. Dreisbach's handbook of poisoning: prevention, diagnosis and treatment, 13 edn. CRC Press-Parthenon Publishers, 2001.
- 31.Proudfoot AT. Pesticide poisoning. Notes for the guidance of medical practitioners, 2 edn. London, HMSO, 1996.
- 32.Gossel TA, Bricker JD. Principles of clinical toxicology, 3 edn. New York, Raven Press, 1994.
- 33.Massaro EJ. Handbook of human toxicology ?, CRC press, 1997.
- 34.Jones AL, Dargan PI. Pocketbook of toxicology London, Churchill Livingstone, 2001.
- 35.Wexler P. Encyclopedia of toxicology San Diego, Academic Press, 1998.
- 36.Descotes J. Human toxicology Amsterdam, Elsevier, 1996.
- 37.Viccellio P. Emergency toxicology, 2 edn. Philadelphia, PA, Lippincott-Raven, 1998.
- 38.Krieger RI, Doull J. Handbook of pesticide toxicology, 2 edn. San Diego, Academic Press, 2001.
- 39.Shankar A. Handbook of poisoning Bombay, Bhalani, 1994.
- 40.Dart RC, Hurlbut KM, Kuffner EK, Yip L. The five minute toxicology consult Philadelphia, Lippincott Williams & Williams, 2000.
- 41.Barile FA. Clinical toxicology: principles and mechanisms Boca Raton, CRC Press, 2003.
- 42.International Programme on Chemical Safety. Environmental health criteria 63. Organosphosphorus insecticides: a general introduction Geneva, WHO, 1986.
- 43.International Programme on Chemical Safety. Poisons information monograph G001. Organophosphorus pesticides Geneva, WHO, 1990.
- 44.International Programme on Chemical Safety. Treatment guide for the cholinergic syndrome. http://www.intox.org/databank/documents/treat/treate/trt15_e.htm . 1999. Ref Type: Electronic Citation
- 45.Hypertox. Organophosphorus poisoning. HYPERTOX [database online].Accessed 2003 September.Available from: URL: http://www.hypertox.com [3.7]. 2003. Ref Type: Electronic Citation
- 46.Poisindex. Organophosphorus poisoning. www . 2003. Ref Type: Electronic Citation
- 47.National Poisons Information Service U. Organophosphorus insecticides. TOXBASE . 2002. Ref Type: Electronic Citation
- 48.University of Otago. Organophosphorus poisoning. TOXINZ [database online].Accessed 2003 September.Available from: URL: http://www.toxinz.com .2003. 2003. Ref Type: Electronic Citation
- 49.Fernando R. Management of acute poisoning, 2 edn. Colombo, Sri Lanka, National Poisons Information Centre, 1998.
- 50.Pakistan agricultural pesticides association. Quick reference for treatment of pesticide poisoning, 2 edn. Karachi, Pakistan, PAPA, 1999.
- 51.Gupta SK. Emergency toxicology. Management of common poisons New Delhi, Narosa, 2002.
- 52.WHO Model Formulary Geneva, World Health Organization, 2002.
- 53.British National Formulary, Number 46 (September 2003), 0 edn. London, British Medical Association and Royal Pharmaceutical Society of Great Britain, 2003.
- 54.Australian medicines handbook, 4 edn. Canberra, Australian Government Printing Office, 2003.
- 55.Haslett C, Chilvers ER, Boon NA, Colledge N, Hunter JA. Davidson's Principles and practice of medicine, 19 edn. Edinburgh, Churchill Livingstone, 2002.
- 56.Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL. Harrison's Principles of internal medicine, 15 edn. McGraw-Hill Professional, 2001.
- 57.Warrell DA, Cox TM, Firth JD, Benz EJ. Oxford Textbook of Medicine, 4 edn. Oxford, Oxford University Press, 2003.
- 58.Niemegeers CJ, Awouters F, Lenaerts FM, Vermeire J, Janssen PA. Prevention of physostigmine-induced lethality in rats. A pharmacological analysis. Archives Internationales de Pharmacodynamie et de Therapie. 1982;259:153–65. [PubMed] [Google Scholar]
- 59.Bird SB, Gaspari RJ, Dickson EW. Early death due to severe organophosphate poisoning is a centrally mediated process. Acad Emerg Med. 2003;10:295–8. doi: 10.1111/j.1553-2712.2003.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 60.Ali-Melkkila T, Kanto J, Lisalo E. Pharmacokinetics and related pharmacodynamics of anticholinergic drugs. Acta Anaesthesiol Scan. 1993;37:633–42. doi: 10.1111/j.1399-6576.1993.tb03780.x. [DOI] [PubMed] [Google Scholar]
- 61.Buckley NA, Dawson AH, Whyte IM. Organophosphate poisoning. Peripheral vascular resistance - a measure of adequate atropinization. J Toxicol Clin Toxicol. 1994;32:61–8. doi: 10.3109/15563659409000431. [DOI] [PubMed] [Google Scholar]
- 62.Sunder Ram J, Kumar SS, Jayarajan A, Kuppuswamy G. Continuous infusion of high doses of atropine in the management of organophosphorus compound poisoning. J Assoc Physicians India. 1991;39:190–3. [PubMed] [Google Scholar]
- 63.Buckley NA, Karalliedde L, Dawson A, Senanayake N, Eddleston M. Where is the evidence for the management of pesticide poisoning - is clinical toxicology fiddling while the developing world burns? J Toxicol Clin Toxicol. 2004;42:113–6. doi: 10.1081/clt-120028756. [DOI] [PMC free article] [PubMed] [Google Scholar]


