Abstract
Despite the importance of epithelial cell contacts in determining cell behavior, we still lack a detailed understanding of the assembly and disassembly of intercellular contacts. Here we examined the role of the catalytic activity of the Src family kinases at epithelial cell contacts in vitro. Like E- and P-cadherin, Ca2+ treatment of normal and tumor-derived human keratinocytes resulted in c-Yes (and c-Src and Fyn), as well as their putative substrate p120CTN, being recruited to cell–cell contacts. A tyrosine kinase inhibitor with selectivity against the Src family kinases, PD162531, and a dominant-inhibitory c-Src protein that interferes with the catalytic function of the endogenous Src kinases induced cell–cell contact and E-cadherin redistribution, even in low Ca2+, which does not normally support stable cell–cell adhesion. Time-lapse microscopy demonstrated that Src kinase inhibition induced stabilization of transiently formed intercellular contacts in low Ca2+. Furthermore, a combination of E- and P-cadherin-specific antibodies suppressed cell–cell contact, indicating cadherin involvement. As a consequence of contact stabilization, normal cells were unable to dissociate from an epithelial sheet formed at high density and repair a wound in vitro, although individual cells were still motile. Thus, cadherin-dependent contacts can be stabilized both by high Ca2+ and by inhibiting Src activity in low (0.03 mM) Ca2+ in vitro.
INTRODUCTION
Adherens junctions are among the principal types of cell–cell contacts between epithelial cells. They consist of calcium-dependent transmembrane proteins of the cadherin superfamily, cell–cell adhesion receptors that are linked to the actin cytoskeleton by another group of proteins, the cytoplasmic catenins (reviewed by Cowin and Burke, 1996). The cadherin–catenin multiprotein complexes regulate a variety of fundamental biological processes, including proliferation, differentiation, and cellular invasion (reviewed by Takeichi, 1991, 1993).
Despite the obvious importance of cadherin function as a determinant of cell behavior and cell fate, the regulation of assembly and disassembly of cadherin-mediated cell–cell adhesions and the recruitment of signaling molecules to complexes at these adhesions are not well understood. Recent work has indicated that in epithelial cells, the Rho family of small GTPases, in particular Rho and Rac, are required for the formation of cadherin-mediated adhesions and the stabilization of cadherins at these sites, mediated by their effects on the actin cytoskeleton (Braga et al., 1997). Consistent with a role for actin cytoskeletal remodeling in cadherin-mediated cell–cell adhesion, high extracellular Ca2+ leads to the accumulation of actin at cell–cell contacts (Braga et al., 1997). In addition to the small GTPases, protein kinases may also contribute to the regulation of cadherin-mediated cell–cell adhesions with several reports, suggesting that recruitment of β-catenin is regulated by phosphorylation (Hinck et al., 1994; Balsamo et al., 1996; Rubinfeld et al., 1996).
Control of the disassembly of cadherin-mediated cell–cell adhesions is particularly relevant to cancer development. Early studies identified a clear trend among epithelial cancers, with the more invasive tumor types expressing lower amounts of cadherins (Shimoyama and Hirohashi, 1991; Shiozaki et al., 1991; Takeichi, 1991). In particular, loss of E-cadherin, the major adhesion molecule of epithelia, is often associated with cancer progression (Birchmeier et al., 1993; Birchmeier and Behrens, 1994; Takeichi, 1993). Furthermore, when functional E-cadherin complexes are reestablished in epithelial tumor cells that have lost normal adherens junctions, E-cadherin specifically acts as a suppressor of cancer cell invasion (Frixen et al., 1991; Vleminckx et al., 1991). More recently, a causal role has been demonstrated for loss of E-cadherin-mediated cell–cell adhesion during the adenoma to carcinoma transition in a transgenic model of pancreatic carcinogenesis (Peri et al., 1998), indicating that disruption of cadherin-mediated cell–cell adhesions is required for tumor progression in vivo. In addition, a germ line mutation in the gene encoding E-cadherin, which gives rise to a truncated protein product, was recently shown to be responsible for familial transmission of gastric cancer (Guilford et al., 1998).
There are also several lines of evidence that tyrosine phosphorylation may play a role in disruption of cell–cell adhesions. First, treatment of Madin–Darby canine kidney cells with vanadate leads to a concomitant increase in phosphotyrosine and deterioration of cellular adherens junctions (Volberg et al., 1992). Second, v-Src activity in chick cells and rat 3Y1 cells perturbs cadherin-mediated cell–cell adhesion (Matsuyoshi et al., 1992; Hamaguchi et al., 1993). Third, stimulation of tyrosine phosphorylation of the E-cadherin–β-catenin complex by a temperature-sensitive v-Src protein in Madin–Darby canine kidney cells correlates with loss of epithelial differentiation and gain of invasive potential (Behrens et al., 1993). Fourth, Ras-transformed breast epithelial cells have less developed adherens junctions and increased tyrosine phosphorylation of junction components (Kinch et al., 1995). Fifth, migratory growth factors such as hepatocyte growth factor (or scatter factor) and epidermal growth factor (EGF) induce dispersion or scattering of normal and malignant epithelial cells, most likely by tyrosine phosphorylation of cadherin-associated proteins (Shibamoto et al., 1994; Sato et al., 1995). Furthermore, EGF-induced scattering of the rat bladder carcinoma cell line NBT-11 requires the activity of both Src and Ras, although these apparently induce their scattering effects by distinct mechanisms (Boyer et al., 1997).
Despite the wealth of data that tyrosine phosphorylation in general, and oncogenic Src kinases in particular, can affect the integrity of cadherin-mediated cell–cell adhesions, a recent study has reported that tyrosine phosphorylation and the Src family kinases are associated with the formation of cell–cell adhesions between mouse keratinocytes (Calautti et al., 1998). In particular, keratinocytes derived from fyn-deficient mice are impaired in the formation of cell–cell junctions in vitro, as are epidermal cells in the skin of mice with a double disruption in the src and fyn genes (Calautti et al., 1998). One reason for the apparent discrepancy may lie in the fact that the Src family kinases are multidomain proteins that have adaptor or protein–protein interaction functions involving the Src homology domains, as well as catalytic activity. Because gene disruption leads to ablation of all aspects of cellular protein function, fyn and src null cells have proved useful tools to demonstrate that the protein products of these genes are required for cell–cell junction assembly (Calautti et al., 1998). However, additional, more subtle, interventionist approaches are required to determine the role of specific Src activities in the dynamic regulation of epithelial cell–cell adhesions. This notion is reinforced by the findings that in fibroblasts, where the Src family kinases are predominantly located in cell–extracellular matrix (ECM) focal adhesions, disruption of the src gene leads to reduced cell spreading and impaired focal adhesion assembly (Kaplan et al., 1995), whereas inhibition of the catalytic activity by a dominant interference results in impaired focal adhesion turnover and reduced cell motility (Fincham and Frame, 1998).
These findings prompted us to specifically investigate the role of the catalytic activity of the Src family kinases in human primary epithelial cells, which could be grown in serum-free, low-Ca2+ medium. We found that c-Yes (and the other ubiquitously expressed kinases c-Src and Fyn) and their putative substrate p120CTN were recruited to cadherin-mediated cell–cell contacts in normal epidermal keratinocytes in response to elevating the extracellular Ca2+. Furthermore, pharmacological and molecular inhibition of the Src catalytic activity promoted the stability of cell–cell contacts and recruitment of E-cadherin to regions of contact, even in low Ca2+. In addition, a mixture of E- and P-cadherin-specific antibodies suppressed intercellular contact. Thus, in keratinocytes in vitro, inhibition of the catalytic activity of the Src kinases promotes the stability of cadherin-dependent cell–cell contacts, implying that Src kinase activity is normally required to disassemble cell–cell contacts. Taken together with the findings of Calautti et al. (1998), our data further imply that, although activities of Src other than its kinase may be required for assembly of epithelial cell–cell junctions, the induction of its catalytic activity after recruitment triggers the destabilization and disassembly of cadherin-dependent cell–cell contacts.
MATERIALS AND METHODS
Cell Culture
Normal Human Keratinocytes.
Human epidermal keratinocytes (HEKs) were prepared from human foreskin tissue essentially as described by Parkinson et al. (1986), except that type Ι S trypsin inhibitor (Sigma, St. Louis, MO) was used instead of FCS-containing medium to neutralize trypsin. The cells were plated in keratinocyte growth medium (KGM; modified MCDB 153 medium [Clonetics, San Diego, CA] containing the following supplements: 0.4% bovine pituitary extract, 10 ng/ml epidermal growth factor, 0.5 μg/ml hydrocortisone, 5 μg/ml insulin, 50 ng/ml amphotericin-B and 50 μg/ml gentamicin). CaCl2 solution was added to give a final Ca2+ concentration of 0.03 mM (low Ca2+) or 1 mM (high Ca2+). HEKs were routinely maintained in low-Ca2+ KGM in a humid 37°C/5% CO2 incubator and were always subcultured before reaching confluence. All experiments were performed using cultures between passages 2 and 5. Cytochalasin D (Sigma) was prepared as a 5 mg/ml stock solution in DMSO and was used at a final concentration of 5 μg/ml. PD162531 (a gift from A. Kraker, Parke-Davis, Ann Arbor, MI) was prepared as a 10 mM stock solution in DMSO and used at a final concentration of 2 μM.
Malignant Human Keratinocytes.
SCC-13 cells (a gift from J.G. Rheinwald, Dana-Farber Cancer Institute, Boston, MA; to E.K.P) were maintained in Dulbecco's modified Eagle's medium (Life Technologies, Gaithersburg, MD) supplemented with 10% FCS, 2 mM l-glutamine, and 0.4 μmg/ml hydrocortisone on lethally irradiated 3T3 feeder cells. SCC-13/ecSrc-KD is a cell clone derived from SCC-13, which can be induced to express kinase-defective (KD) avian c-Src (K295M) with the ecdysone analogue muristerone A (No et al., 1996). Kinase-defective c-Src was cloned as an XbaI fragment from pCA10-K− (a gift from K. Kaplan, Massachusetts Institute of Technology, Boston, MA) into the pIND vector (Invitrogen, San Diego, CA). The resulting plasmid (pIND-295) was introduced into SCC-13 cells in combination with pRXR (Invitrogen) using N-1-(2,3-dioleoyloxy)propyl]-N,N,N,-trimethylammonium methyl sulfate liposomal transfection reagent (Boehringer Mannheim, Indianapolis, IN). Stable cell lines were selected using a combination of 800 μg/ml G418 and 250 μg/ml zeocin and were tested for their ability to express kinase-inactive c-Src in response to induction with 10 μM muristerone A by immunoblotting with avian-specific mAb EC-10 (Upstate Biotechnology, Lake Placid, NY). For experiments involving manipulation of the extracellular Ca2+ concentration, SCC-13 cells were temporarily maintained in KGM. For the experiments to monitor drug selectivity, SCC13 cells were grown in KGM in the absence of EGF for 16 h before stimulation with 2 ng/ml EGF for 10 min.
Time-Lapse Video Microscopy
HEKs were seeded at low density in low-Ca2+ KGM and were treated with 2 μM PD162531 (DMSO for controls) or were transferred to high-Ca2+ medium. The cells were visualized using a phase-contrast microscope equipped with a heated stage, and images were recorded at 10-min intervals using a charge-coupled device camera. The relative rates of cell motility of individual cells were calculated by computer tracking of individual cells that did not come into contact in each frame of the time-lapse series and computing distance traveled in a given time using Openlab software (Improvision, Coventry, United Kingdom). The average speed of a number of cells grown in low Ca2+, low Ca2+ with PD162531, or high Ca2+ was calculated using Excel (Microsoft, Redmond, WA), and the mean speed in low Ca2+ was designated as 100%.
Wound-healing Assay
HEKs or SCC-13/ecSrc-KD cells were grown to confluence in six-well plates (Costar, Cambridge, MA) in low-Ca2+ KGM. Monolayers were wounded using a plastic micropipette tip, and the cells were rinsed with low-Ca2+ KGM before visualization using a phase-contrast microscope. The cells were treated with either 2 μM PD162531 (DMSO for controls) or 10 μM muristerone A or were transferred to high-Ca2+ medium and returned to a 37°C incubator for 16 h before recording wound repair.
Protein Immunoblotting and Immunoprecipitation
Cells were lysed in 10 mM Tris, pH 7.4, 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 10 mM inorganic tetrasodium pyrophosphate, 2 mM PMSF, 100 μM Na3VO4, 0.5 mM NaF, and 0.1% aprotinin (Sigma). Extracts were assayed for protein content using the Micro BCA protein assay kit (Pierce, Rockford, IL) after clarification by high-speed centrifugation at 4°C. Lysates were boiled in high-SDS sample buffer and separated by discontinuous SDS-PAGE under reducing conditions before transfer to nitrocellulose. Proteins were detected by probing with 0.125 μg/ml anti-E-cadherin mAb (Transduction Laboratories, Lexington, KY), 0.25 mg/ml anti-Yes mAb (Transduction Laboratories), 0.1 μg/ml anti-Src mAb 327 (Calbiochem, La Jolla, CA), or 1 μg/ml anti-avian Src mAb EC10 (Upstate Biotechnology) diluted in 5% nonfat milk in PBS/0.2% (vol/vol) Tween 20. Detection of bound antibody was by reaction with horseradish peroxidase-conjugated secondary antibody (Amersham, Little Chalfont, United Kingdom), and visualization was by enhanced chemiluminescence (Amersham).
To test the selectivity of PD162531, SCC-13 keratinocytes were deprived of EGF for 16 h and 2 ng/ml EGF was added back for 10 min. c-Src or EGF receptor was immunoprecipitated using 1 μg of anti-Src mAb 327 or 5 μg of anti-EGF receptor mAb (Upstate Biotechnology), respectively, and immunoprecipitated proteins were collected and washed as described above, separated by 7% SDS-PAGE, and immunoblotted using 1 μg/ml anti-phosphotyrosine PY20 (Transduction Laboratories) in the case of EGF receptor or either 0.1 μg/ml anti-Src mAb 327 or 0.5 μg/ml immunoglobulin G (IgG) specific for c-Src that is phosphorylated at tyrosine 419 of the human sequence (phospho-423-Y; BioSource International, Camarillo, CA).
Immune Complex Kinase Assays
Src Family Kinases.
Lysates were prepared in Src lysis buffer (10 mM 1,4-piperazinediethanesulfonic acid, pH 6.8, 50 mM NaCl, 3 mM MgCl2, 300 mM sucrose, 0.5% Triton X-100, 10 mM sodium pyrophosphate, 2 mM phenylmethylsulfonyl fluoride, 100 μM Na3VO4, and 0.1% aprotinin), and c-Src, c-Yes, and c-Fyn were then immunoprecipitated using 0.5 μg of antisera (c-Src, mAb 327, c-Yes, and c-Fyn; Santa Cruz Biotechnology, Santa Cruz, CA). Immunoprecipitates were washed five times in lysis buffer, followed by one wash in kinase buffer (100 mM 1,4-piperazinediethanesulfonic acid, pH 6.8, 20 mM MnCl2, 10 μM Na3VO4), and then resuspended in 10 μl of kinase buffer. The immune complexes were incubated with [γ-32P]ATP (1 μM, 5 μCi; Amersham; specific activity, 3000 Ci/mmol) and 0.3 mM Src substrate peptide (Upstate Biotechnology) in the presence or absence of 100 nM PD162531. After incubation at 30°C for 10 min, the reaction was stopped by addition of excess unlabeled ATP and chilling on ice. The reaction mixes were spotted onto Whatman (Maidstone, United Kingdom) P81 paper discs, and the discs were then washed three times in 10% trichloroacetic acid. The discs were transferred to scintillation vials, and the radioactivity was determined. Control reactions without peptide were also performed.
EGF Receptor Kinase.
Lysates were prepared in EGF receptor lysis buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 10 mM sodium pyrophosphate, 2 mM phenylmethylsulphonyl fluoride, 100 μM Na3VO4, and 0.1% aprotinin). The EGF receptor was then immunoprecipitated from clarified lysates with 5 μg anti-EGF receptor mAb (Upstate Biotechnology). The immunoprecipitates were washed twice with HNTG buffer (20 mM HEPES, pH 7.5, 10% glycerol, and 0.1% Triton X-100) containing 500 mM NaCl and then three times with HNTG buffer containing 150 mM NaCl and resuspended in 10 μl of HNTG buffer containing 150 mM NaCl. The immune complexes were incubated with 5 mM MnCl2, 100 μM Na3VO4, and 10 ng/ml EGF on ice for 5 min in the presence or absence of 100 nM PD162531. After addition of [γ-32P]ATP (1 μM, 5 μCi; Amersham; specific activity, 3000 Ci/mmol), the immune complexes were incubated at 30°C for 10 min, and the reaction was then stopped by addition of Laemmli sample buffer (double strength). The products were separated by 7% SDS-PAGE, and phosphorylation of the EGF receptor was detected by autoradiography and quantitated by densitometry.
Confocal Immunofluorescence Microscopy
Cells were grown on chamber slides (Nunc, Naperville, IL) and fixed and permeabilized in methanol for 20 min on ice (HEK and SCC-13). Cells were then blocked with 10% FCS in 120 mM NaCl, 6 mM KCl, 1.2 mM MgCl2, 1 mM CaCl2, and 25 mM HEPES, pH 7.4, and incubated with primary antibodies diluted in this blocking solution for 1 h at room temperature: 1 μg/ml anti-E-cadherin mouse mAb, 2.5 μg/ml anti-p120CTN mAb (Transduction Laboratories), 2.5 μg/ml anti-c-Yes Mab (Transduction Laboratories), 1:100 anti-Src mAb N2-17 ascites fluid (a gift from Dr. T. Hunter, The Salk Institute, San Diego, CA), 1:100 anti-Fyn (Santa Cruz), or 1:100 anti-vinculin (Sigma). Bound antibody was detected using 15 μg/ml FITC-labeled anti-mouse IgG (Jackson ImmunoResearch, Stratatech Scientific, Luton, United Kingdom) and visualization was via a confocal microscope (MRC600; Bio-Rad, Hercules, CA).
Cadherin Blocking Experiments
Cells were grown on chamber slides in 200 μl of medium containing low Ca2+, and either PD162531 was added or cells were transferred to high Ca2+. For 1 h before addition of PD162531, a mixture of anti-E-cadherin and anti-P-cadherin antibodies was added. For blocking experiments the antibodies used were anti-E-cadherin (DECMA-1; Sigma; at 1:20 final dilution) and anti-P-cadherin (NCC-CAD-299; Worthington, Freehold, NJ) at 100 μg/ml final concentration). Cells were visualized by phalloidin staining of their polymerized actin.
RESULTS
Ca2+-induced Translocation of c-Yes (and c-Src and Fyn) to Epithelial Cell–cell Contacts Requires the Actin Cytoskeleton
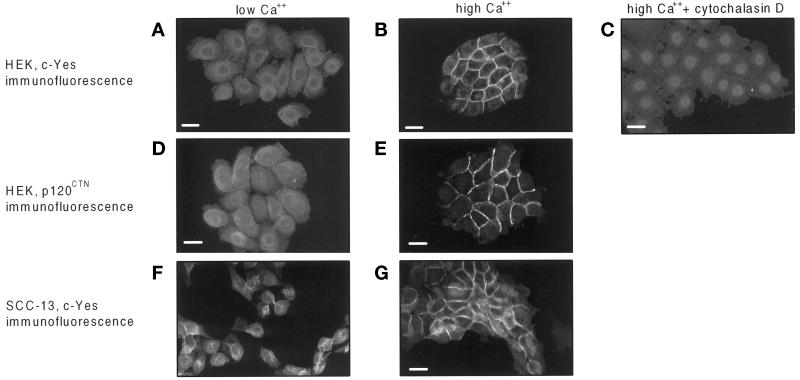
Although c-Src protein expression is required for optimal cell spreading and focal adhesion assembly in fibroblasts (Kaplan et al., 1995), our previous work demonstrated that Src proteins are recruited into newly assembling focal adhesions by an actin-dependent process that does not require Src catalytic activity (Fincham et al., 1996; Fincham and Frame, 1998). In particular, v-Src protein is recruited to cell–ECM focal adhesions in fibroblasts from where it induces focal adhesion turnover during oncogenic transformation and cell motility (Fincham and Frame, 1998). These data, together with the ability of v-Src to induce disruption of cadherin-mediated cell–cell adhesions in epithelial cells (Matsuyoshi et al., 1992; Volberg et al., 1992; Behrens et al., 1993; Hamaguchi et al., 1993; Takeda et al., 1995) and the enrichment of rat liver hepatocyte adherens junctions for Src family kinases (Tsukita et al., 1991), led us to examine the subcellular localization of the endogenous Src kinases in normal and malignant human epithelial cells in low and high Ca2+ and the role of their combined catalytic activities. Specific immunostaining for all three ubiquitous kinases, c-Yes, c-Src, and Fyn, was diffuse in the cytoplasm of normal epidermal keratinocytes maintained in low-Ca2+–containing medium (confocal images shown for c-Yes in Figure 1A). Elevating the extracellular Ca2+ induced c-Yes (and c-Src and Fyn) to translocate to regions of cell–cell contact (shown for c-Yes in Figure 1B) in a manner that was indistinguishable from Ca2+-induced relocalization of both E- and P-cadherin to the cell periphery (our unpublished results). Ca2+-induced translocation of the Src kinases to cell–cell contacts was sensitive to 5 μg/ml cytochalasin D (shown for c-Yes in Figure 1C), implying that their recruitment to contacts required an organized actin cytoskeleton. This, together with the requirement for the cytoskeletal modulators Rho and Rac in Ca2+-induced relocation of E-cadherin (Braga et al., 1997), implies a general role for the actin cytoskeleton in the recruitment of components of cell–cell contacts, including the endogenous Src kinases. In addition to c-Yes (and c-Src and Fyn), their putative substrate p120CTN also underwent Ca2+-induced translocation to cadherin-mediated cell–cell contacts (Figure 1E). We also found that the Src kinases were similarly redistributed in a tumor-derived keratinocyte cell line (SCC-13; Rheinwald and Beckett, 1981). Thus, the Src family kinases undergo Ca2+-induced, actin-dependent translocation to cadherin-mediated cell–cell contacts in normal and tumor-derived human epithelial cells.
Figure 1.
c-Yes and p120CTN translocate to cell–cell adhesions in epithelial cells in response to high Ca2+. Shown are confocal immunofluorescence micrographs of primary human epidermal keratinocytes, HEKs (A–E), and SCC-13 (F and G) cells stained with anti-c-Yes (A, B, C, F, and G) or anti-p120CTN (D and E). HEKs were maintained in low Ca2+ (A and D) or transferred to high Ca2+ for 24 h (B, C, and E). SCC-13 cells were transferred to low-Ca2+–containing medium for 24 h (F) and were subsequently transferred to high Ca2+ for 24 h (G). Cytochalasin D was added to HEKs at a concentration of 5 μmg/ml as they were transferred to high Ca2+ (C). Bars, 25 μm.
Because we, and others, have shown that c-Src and v-Src are translocated to newly forming focal adhesions in fibroblasts (Kaplan et al., 1995; Fincham et al., 1996), we addressed whether c-Yes, (or c-Src or Fyn) could also be detected in keratinocyte cell–matrix (ECM) focal adhesions. For this, we used immunofluorescence confocal microscopy to scan through cells until vinculin-stained focal adhesions at the bottom of the cells were observed in low and high Ca2+ (our unpublished results). When cells stained with c-Yes (or c-Src or Fyn) were similarly scanned, we could not detect any staining in focal adhesion structures (our unpublished results). Thus, the predominant recruitment of these kinases to sites of cell–cell contact in primary human keratinocytes treated with high extracellular Ca2+, and the absence of detectable levels in keratinocyte focal adhesions, is in contrast to the situation in fibroblasts, where the Src kinases are predominantly in cellular focal adhesions. However, we cannot exclude the possibility that c-Yes, c-Src, or Fyn may be present in keratinocyte cell–ECM adhesions at levels that are not detectable by immunofluorescence.
A Selective Inhibitor of the Src Family Kinases and a Dominant Inhibitory c-Src Protein Stimulate Cell–Cell Contact in Low Ca2+
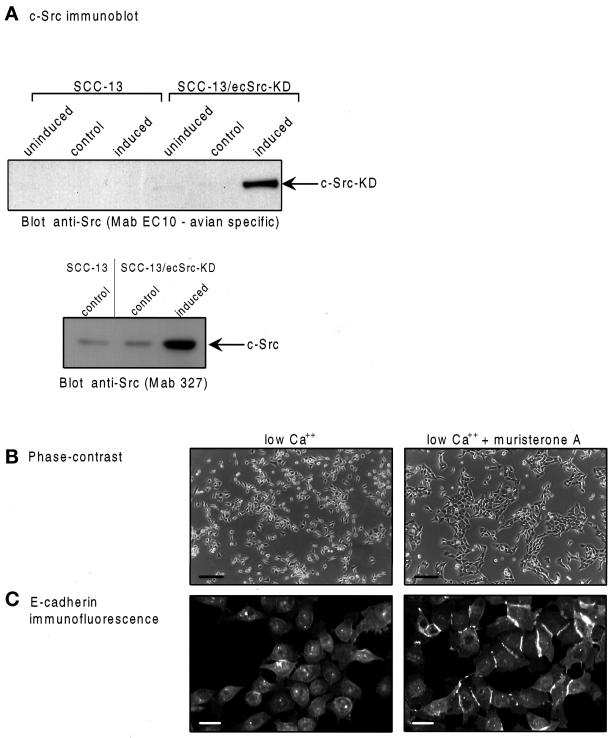
To determine the physiological role of the catalytic activity of the cellular Src kinases in epithelial cells, we inhibited their activity in two ways. First, we used a pharmacological tyrosine kinase inhibitor, PD162531, which selectively inhibits Src family kinases (provided by A. Kraker, Parke-Davis). Treatment of normal epidermal keratinocytes with 2 μM PD162531 in low-Ca2+–containing medium had no effect on cell growth (our unpublished results) but resulted in the formation of tightly packed clusters of cells (Figure 2A, right panel). In addition, treatment of the cells with PD162531 stimulated E-cadherin redistribution from relatively diffuse cytoplasmic staining in low-Ca2+–containing medium (Figure 2B, left panel) to cell–cell contacts induced by the drug (Figure 2B, right panel). Similar effects of PD162531 were observed after treatment of the tumor-derived SCC-13 keratinocytes (our unpublished results). To confirm the selectivity of PD162531 in vivo, we compared its effects on EGF receptor (EGF-R) autophosphorylation (because EGF is the major growth factor present in serum-free KGM medium) and c-Src autophosphorylation, after SCC13 keratinocytes that had been grown in EGF-deprived, low-Ca2+ KGM medium had been treated with 2 ng/ml EGF for 10 min. The addition of 2 μM PD162531 suppressed phosphorylation of c-Src on tyrosine 419, the presumed site of Src autophosphorylation (equivalent to tyrosine 416 in the avian sequence; Smart et al., 1981), as judged by reduced reactivity of immunoprecipitated c-Src with an antibody raised against a peptide containing tyrosine 419 in its phosphorylated form (Figure 2C, upper panel). In contrast, EGF-R tyrosine phosphorylation was not substantially altered by drug treatment (Figure 2C, lower panel). These findings indicate that PD162531 is likely to be a more selective inhibitor of the Src family kinases in vivo than previously used agents, such as herbimycin A, genistein, or tyrphostins. However, although these data are supportive of selectivity against Src in vivo, it is based on the assumption that tyrosine 419 phosphorylation is the result of autophosphorylation, and we note that this has not formally been proven in vivo.
Figure 2.
The Src-selective inhibitor PD162531 induces cell–cell contact and E-cadherin redistribution. (A) Phase-contrast images; (B) E-cadherin confocal immunofluorescence micrographs of HEKs maintained in low Ca2+ and treated with 0.02% DMSO (vol/vol) as controls (left panels) or with 2 μM PD162531 for 24 h (right panels). Bars, (A) 200 μm; (B) 25 μm. (C) SCC-13 tumor-derived keratinocytes, which also responded to PD162531, were grown in KGM serum-free medium and were deprived of exogenous EGF for 16 h, before restimulation with 2 ng/ml EGF (+ EGF) for 10 min in the absence (−) or presence (+) of 2 μM PD162531. Presumed c-Src autophosphorylation was monitored by immunoprecipitating cell lysates with anti-Src (mAb 327) and immunoblotting using anti-Src (phospho-423-Y) as probe (upper left panel). Control immunoprecipitations were blotted with anti-Src (mAb 327; upper right panel). EGF-R autophosphorylation was monitored by immunoprecipitating untreated (− EGF) or EGF-treated (+ EGF) cell lysates with anti-EGF-R and immunoblotting with anti-phosphotyrosine. (D) In vitro kinase activities were measured as described in MATERIALS AND METHODS in the presence and absence of 100 nM PD162531. The kinase activities in the presence of drug are expressed as a percentage of untreated activities.
We also confirmed by immune complex kinase assays that PD162531 potently inhibited the tyrosine kinase activity of all three ubiquitous family members c-Yes, c-Src, and Fyn in vitro, at concentrations that were not inhibitory to EGF-R autophosphorylation in vitro (shown for 100 nM PD162531 in Figure 2D); furthermore, although PD162531 also has some activity against the receptors for both basic fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF), the drug is at least 8 and 50 times more potent at inhibiting the Src family kinases than the bFGF receptor and the PDGF receptor in vitro, respectively (Kraker, Parke-Davis, personal communication). Thus, treatment of normal keratinocytes with a tyrosine kinase inhibitor that exhibits selectivity for the Src family kinases induced cell–cell contact, even in low Ca2+ that does not normally support stable cadherin-mediated cell–cell adhesion (Figure 2, A and B, left panels). That this effect of PD162531 was not mediated by inhibition of either PDGF receptor or bFGF receptor is supported by the facts that cultured keratinocytes do not express the PDGF receptor (Ansel et al., 1993), and that at the concentration used, the drug did not have any effect on cell growth, although bFGF is known to positively influence keratinocyte growth (Sarret et al., 1992).
To complement the pharmacological approach, and to more rigorously test the role of Src kinase activity, we generated SCC-13 cells that expressed an ecdysone-inducible, kinase-inactive c-Src protein (SCC-13/ecSrc-KD). Kinase-inactive mutants of Src have been widely used and effectively block the functioning of the catalytic activity of the endogenous cellular Src family by acting in a dominant-negative manner (Twamley-Stein et al., 1993; Fincham and Frame, 1998). Treatment of SCC-13/ecSrc-KD keratinocytes with 10 μM muristerone A, an analogue of ecdysone, efficiently induced expression of the exogenous avian kinase-inactive c-Src protein (Figure 3A, upper panel). The extent of overexpression was severalfold, as judged by immunoblotting control and muristerone A-treated cells with an antibody (mAb 327) that recognizes both endogenous and exogenous c-Src-KD protein (Figure 3A, lower panel). Muristerone A treatment of these cells in low Ca2+ also induced cell–cell contact, resulting in areas of clustered cells (Figure 3B, right panel), an effect similar to that induced by the Src inhibitor PD162531 in normal keratinocytes (Figure 2A). In contrast, parental SCC-13 cells were unaffected by muristerone A (our unpublished results). Furthermore, muristerone A stimulated redistribution of cytoplasmic E-cadherin in SCC-13/ecSrc-KD cells to regions of cell–cell contact (Figure 3C, right panel). Thus, induction of a dominant-inhibitory, kinase-inactive c-Src protein in tumor-derived keratinocytes had an effect similar to that of the Src-selective inhibitory drug PD162531 in normal keratinocytes, by stimulating the formation of cell–cell contacts at which E-cadherin was present.
Figure 3.
Induction of a kinase-defective (KD) c-Src protein also induces cell–cell contact and E-cadherin redistribution. (A) Immunoblot using avian-specific mAb EC10 to detect only exogenous c-Src-KD expression in SCC 13 parental cells and SCC-13/ecSrc-KD transfectant cells that were untreated (uninduced), treated with ethanol alone (control), or treated with 10 μM muristerone A for 24 h (upper panel). Both endogenous and exogneous Src expression were monitored by immunoblotting with anti-Src (mAb 327) that reacts with both human and avian Src (lower panel), (B) Phase-contrast images; (C) E-cadherin confocal immunofluorescence micrographs of SCC-13/ecSrc-KD cells maintained in low Ca2+ and untreated (left panels) or treated with 10 μM muristerone A for 24 h (right panels). Bars, (B) 200 μm; (C) 25 μm.
One explanation for these data is that cell–cell contacts were being transiently assembled and disassembled as cells came into contact during routine keratinocyte cell culture and that this dynamic regulation was perturbed either by Ca2+ addition or by inhibition of Src kinase activity, both stimuli stabilizing transiently assembled cell–cell contacts. If true, this implicates Src catalytic activity in cell–cell contact disassembly in both normal keratinocytes (HEKs) and in malignant keratinocytes that retain expression of E-cadherin (such as SCC-13). Treatment of keratinocytes with PD162531 or muristerone A had no significant effect on cell growth at the concentrations used, and the effect of the Src inhibitor on cell–cell contact was completely reversible (our unpublished results), indicating that Src kinase inhibition was not toxic to cultured keratinocytes.
Inhibiting Src Kinase Activity Stabilizes Cell–Cell Contacts
To test the prediction that Src catalytic activity is required to disassemble transiently formed cell–cell contacts in low Ca2+, we carried out time-lapse phase-contrast imaging of low-density keratinocytes. We found that as cells collided in untreated cultures and formed transient associations, they were subsequently able to free themselves from one another (Figure 4A, upper panel). In contrast, when exposed to the Src-inhibitory drug PD162531 or high Ca2+, cells that collided remained in contact with one another, even after several hours (Figure 4A, middle and lower panels). Quantitation of the time individual cells remained in contact with other cells during a 6-h treatment period is shown (Figure 4B). In addition, if the above explanation is correct, individual cells must remain motile to make contacts and cluster when Src catalytic activity is inhibited. Therefore, using time-lapse microscopy and computer-based tracking of individual cells in sequential time-lapse images, we established that individual cells remained motile when treated with high Ca2+ or with the Src-selective inhibitor PD162531 (Figure 4C, and as exemplified by the cells marked with white arrows that have moved between frames a and b under all three conditions shown, Figure 4A). However, in the presence of the PD162531 Src inhibitor, there was a drop in the rate of cell motility of individual cells by ∼40–50% (Figure 4C). Although the reason for this reduction was not clear, because we were unable to detect any of the ubiquitous, Src kinases in cell–ECM adhesions, this was consistent with previous reports that Src activity is required for optimal cell motility in fibroblasts (Hall et al., 1996; Fincham and Frame, 1998) and suggests that low levels of the Src kinases may be present in focal adhesions or in the smaller focal complexes associated with lamellipodia.
Figure 4.
The Src-inhibitory drug PD162531 stabilizes cell–cell contacts. (A) Phase-contrast time-lapse images of low-density HEKs (in low Ca2+), which were treated with 0.02% DMSO (control, upper panel) or 2 μM PD162531 (middle panel) or transferred to high-Ca2+ medium (lower panel). Treatment with PD162531 or high Ca2+ began at 0 h and was continuous for the duration of the experiment. Individual cells are indicated by arrows. Bars, 50 μm. (B) The mean time that individual keratinocytes grown under different conditions spent in contact with other cells in the culture was determined by observing time-lapse images frame by frame. The duration of each intercellular contact formed by every cell in the time-lapse field (typically ∼20 cells per experiment) was recorded and expressed as a percentage of the experiment time (6 h), and the mean contact time was plotted as percentage of time in contact. Thus, a value of 100% would indicate that all cell–cell contacts persisted for the duration of the experiment. (C) The migration of individual cells that did not come into contact with other cells (typically approximately six cells per experiment) was quantitated using Openlab software, and the mean pixels per frame value for control cells in low Ca2+ was defined as 100%. (D) c-Yes confocal immunofluorescence micrograph of HEKs maintained in low Ca2+. Specifically shown here is the contact region between two cells that have collided. Bars, 25 μm.
Thus, our data indicate that inhibition of Src catalytic activity induced cell clustering, most likely as a result of stabilizing transient cell–cell contacts at which E-cadherin was present (Figures 2 and 3). One potential problem with the conclusion that Src kinase activity is required for disruption of these transiently formed cell–cell contacts is that c-Yes, c-Src, and Fyn were apparently located throughout the cytoplasm of isolated cells in low Ca2+ (Figure 1). However, upon further examination of immunofluorescence staining of c-Yes in cells that had come into contact in low Ca2+, we noted that c-Yes was redistributed to the regions of transient contact (Figure 4D). Thus, although c-Yes was cytoplasmic in isolated cells in low Ca2+, it was located at sites of contact formed as a result of collision between motile cells. Our data indicate that the catalytic activity of the Src family kinases at these sites is required for disruption of transient cell–cell contacts in low Ca2+.
The Stabilization of Cell–Cell Contacts as a Result of Src Kinase Inhibition Involves Cadherins
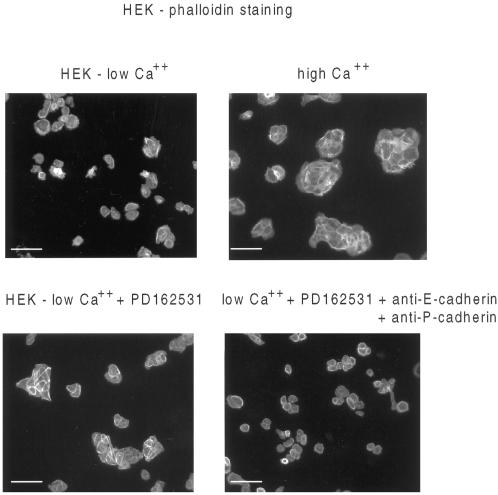
Because E-cadherin redistributed to sites of cell–cell contact upon inhibition of Src catalytic activity, we sought to determine whether the cadherins were involved in contact stabilization. Thus, cells were pretreated for 1 h with a mixture of E- and P-cadherin-specific antibodies. Cell clustering and strong actin staining at the periphery of cells in the clusters were monitored by phalloidin reactivity. Although some random cell clustering occurs in HEK cultures in low Ca2+ (Figure 5, top left panel), tight clustering was dramatically stimulated by transfer to high Ca2+ or by treatment with the PD162531 Src-inhibitory drug (Figure 5, top right and bottom left panels, respectively). However, in the presence of both E- and P-cadherin-specific blocking antibodies, cell–cell contact and cell clustering were suppressed (Figure 5, bottom right panel). This suppressive effect was specific to the cadherin antibodies because equivalent concentrations of nonimmune IgG had no effect on cell clustering, and β1-integrin-specific antibodies induced cell detachment (our unpublished results). These data indicate that the cadherins have a role in the stabilization of the intercellular contacts induced as a result of Src kinase inhibition.
Figure 5.
The contacts stabilized as a result of Src kinase inhibition are cadherin dependent. Immunofluorescence images of cells stained with phalloidin-FITC are shown for HEKs in low Ca2+ (top left), high Ca2+ (top right), and low Ca2+ in the presence of the Src-inhibitory drug PD162531 (bottom panels) in the absence (left panel) and presence of a mixture of E- and P-cadherin-specific antibodies (right panel). Bars 100 μm.
Inhibiting Src Catalytic Activity Suppresses Repair of a Wounded Epithelial Monolayer
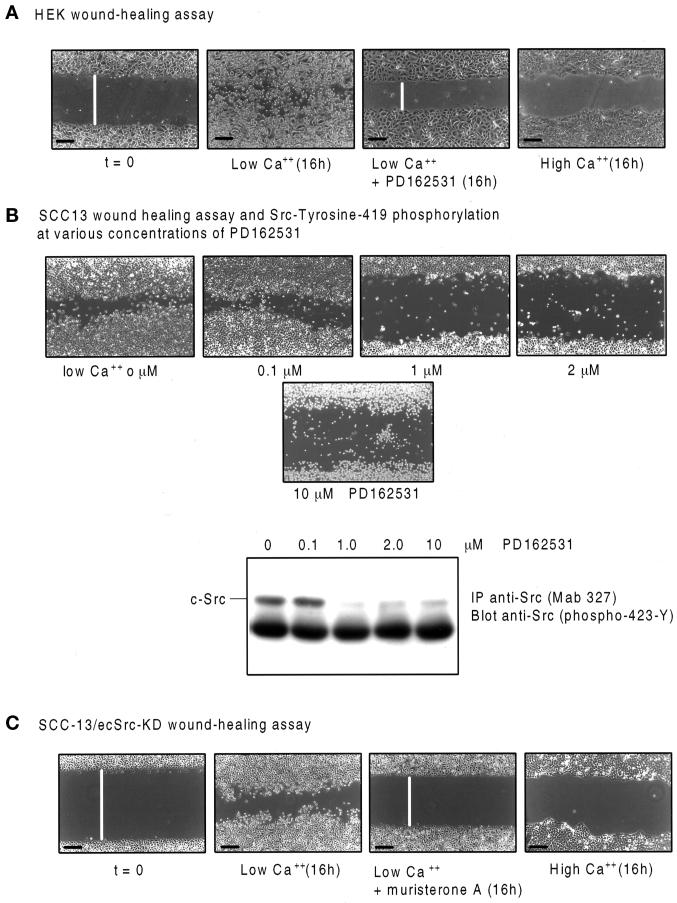
We next tested whether inhibition of the endogenous Src kinases affected the ability of cells present at the edge of a wounded keratinocyte monolayer to break their intercellular contacts and move freely out of the epithelial sheet and repair the wound. Confluent keratinocyte monolayers were wounded, and cell migration into the denuded area of individual cultures that were equivalently wounded was monitored microscopically. Untreated HEKs had extensively migrated into the wound as individual rounded cells after 16 h [Figure 6A, low Ca2+ (16 h)]. Wound repair was unaffected by the presence of mitomycin C (our unpublished results), indicating that cell division was not a major component of the wound repair process. In contrast, HEKs treated with the Src-selective inhibitor PD162531, or with high extracellular Ca2+, were substantially impaired in their ability to migrate as individual cells into the wound [Figure 6A, PD162531 (16h) and high Ca2+ (16h), respectively], although the denuded area was narrowed as a consequence of forward movement of the edge of the epithelial sheet [compare low Ca2+ (16h) with PD162531 (16h)]. Thus, because individual keratinocytes were motile under conditions that did not permit wound repair (Figure 4), we conclude that it was the freeing of individual cells from their epithelial neighbors in the sheet that was impaired in cells treated with the PD162531 Src inhibitor or with high extracellular Ca2+. Dose–response analysis confirmed that drug-induced impairment of wound repair occurred at the same concentration range of PD162531 as inhibition of Src Tyrosine-419 phosphorylation (Figure 6B), consistent with the drug having its biological effects via Src inhibition. This was further supported by the impaired wound repair by individual cells dissociating from the epithelial sheet, which was observed in confluent SCC-13/ecSrc-KD cell cultures treated with muristerone A or with high Ca2+ (Figure 6C). These data indicate that the catalytic activity of the endogenous cellular Src kinases is required to induce disruption of established cell–cell contacts that have formed in an epithelial sheet at high density and is necessary for cell dispersion and efficient repair of a wounded keratinocyte monolayer in vitro.
Figure 6.
The Src-inhibitory drug PD162531 and overexpression of the dominant-negative c-Src protein suppress keratinocyte wound healing in vitro. Phase-contrast photomicrographs show four individual HEK monolayers (A) or SCC-13/ecSrc-KD cells (B and C) grown to confluence in low Ca2+ and then wounded (example shown at t = 0) and either treated for 16 h (16h) with 0.02% DMSO (low Ca2+, 0 μM), 0.1, 1, 2, or 10 μM PD162531 as indicated (low Ca2+ + PD162531), or 10 μM muristerone A [low Ca2+ muristerone A (16h)] or transferred to high Ca2+ for 16 h [high Ca2+ (16h)]. Bars, 200 μm. White bars represent relative distances between the two edges of apposing epithelial sheets. Presumed c-Src autophosphorylation at the indicated drug concentrations was monitored by immunoprecipitating cell lysates with anti-Src (mAb 327) and immunoblotting using anti-Src (phospho-423-Y) as probe (B).
DISCUSSION
Cell motility is controlled, at least in part, by a cycle of cell attachment and detachment. In epithelial sheets where the cells themselves, rather than the underlying matrix, bear most of the mechanical stress, this almost certainly involves the assembly and disassembly of intercellular contacts. There is considerable circumstantial evidence implicating tyrosine phosphorylation in the disassembly of cadherin-mediated cell–cell contacts. Much of this evidence is derived from experiments using the v-Src oncoprotein, which induces tyrosine phosphorylation of β-catenin, as well as p120CTN (Reynolds et al., 1989) and E-cadherin (Papkoff, 1997), with concomitant loss, or substantial weakening, of cadherin-mediated adhesion (Matsuyoshi et al., 1992; Behrens et al., 1993; Hamaguchi et al., 1993; Shibamoto et al., 1994; Takeda et al., 1995). In addition, growth factors such as EGF and hepatocyte growth factor induce tyrosine phosphorylation and dispersion or scattering of responsive epithelial cells (Weidner et al., 1991; Shibamoto et al., 1994). However, to date there is no direct experimental evidence that tyrosine kinases are important for the E-cadherin-expressing tumor cell disaggregation that is required for invasion, and the identity of putative endogenous cellular kinases that might be directly responsible has not been established.
In the work presented here, we provide evidence that the catalytic activities of one or more of the endogenous cellular Src family kinases are required for the disassembly of cell–cell contacts during their dynamic regulation in normal and malignant human keratinocytes in vitro. In normal skin in vivo, disruption of epithelial cell–cell contacts is relevant to wounded epidermis, in which c-Yes locates at regions of cell–cell contact (Kreuger et al., 1991). In cultured human keratinocytes treated with high extracellular Ca2+, c-Yes (and c-Src and Fyn), as well as their putative substrate p120CTN, were recruited to cell–cell adhesions (Figure 1) in a manner that was indistinguishable from the translocation of both E- and P-cadherin. Furthermore, Ca2+-induced translocation of the Src kinases was blocked when the actin cytoskeleton was disrupted by cytochalasin D (Figure 1), implying that their recruitment to cadherin-mediated cell–cell adhesions is an actin-dependent process, which requires reorganization of the actin cytoskeleton. Consistent with this, Dsrc41, the Drosophila close relative of vertebrate c-Src, colocalizes with actin fibers and DE-cadherin in vivo during the development of Drosophila eyes (Takahashi et al., 1996). One possibility, previously suggested by Braga et al. (1997), is that the Rho family of small GTPases induces the recruitment of cytoplasmic proteins to regions of contact between epithelial cells, mediated by their effects on the actin cytoskeleton. The possibility that c-Yes and the other Src kinases are recruited in this way is consistent with our previous findings that recruitment of v-Src to its site of action at the cell periphery of fibroblasts is also an actin-dependent process that requires the activity of Rho proteins (Fincham et al., 1996). However, in contrast to fibroblasts, we were unable to detect any of the ubiquitous cellular Src family kinases in keratinocyte focal adhesions.
When normal epidermal keratinocytes were treated with an Src-selective tyrosine kinase inhibitor, PD162531, or tumor-derived keratinocytes were induced to express a dominant-negative, kinase-defective c-Src protein, the cells clustered as a consequence of the formation of cell–cell contacts (Figures 2 and 3, respectively), an effect that was accompanied by translocation of E- and P-cadherin to the sites of intercellular contact. Thus, both pharmacological and molecular inhibition of the endogenous cellular Src family kinases were sufficient to induce the formation of cell–cell contacts at which the cadherins were present, even in low Ca2+. Using time-lapse microscopy, we demonstrated that when keratinocytes in low Ca2+ came into contact as they moved around in low-density culture, they formed transient cell–cell adhesions that were rapidly disassembled as cells broke apart. In cultures treated with the Src-inhibitory drug PD162531, or with high Ca2+, cells also formed cell–cell contacts, but they were impaired in their ability to sever these contacts (Figure 4). The finding that c-Yes accumulated at transiently formed cell–cell contacts in low Ca2+ from its cytoplasmic location in isolated cells (Figure 4) is consistent with the proposed role in the regulation of cell–cell contacts. Furthermore, we demonstrated that a combination of E- and P-cadherin-specifc antibodies was able to suppress contact formation induced by inhibition of cellular Src catalytic activity (Figure 5). Thus, our data provide the first clear demonstration that cellular Src activity at cadherin-dependent cell–cell contacts plays an important role in contact disruption. However, because all three ubiquitous members of the Src family are similarly located at intercellular contacts and are inhibited by both the Src-inhibitory drug and dominant-negative c-Src protein, we are unable to conclude whether individual members of the Src family proteins have unique functions at intercellular contacts.
Because the experiments presented here were carried out using adherent keratinocytes, we were unable to draw conclusions about the relative adhesive strength of intercellular contacts formed by inhibition of Src kinase activity and elevation of the extracellular Ca2+. Although it would be possible, in principal, to measure adhesive strength between keratinocytes in suspension, this would not necessarily relate to the strength or mode of regulation of intercellular contacts formed between adherent keratinocytes in which the cellular cytoskeleton and adhesive properties are substantially different. This would be particularly true if the effects of inhibiting Src kinase activity were mediated by altered regulation of cytoskeletal dynamics. Thus, because the adhesive strength is unclear, we have used “contacts” and not “adhesions” to describe the cadherin-dependent cell–cell junctions formed as a result of Src kinase inhibition. One possibility is that such contact formation is a prerequisite for Ca2+-dependent, cadherin-mediated adhesion, and that the state of catalytic activation of one or more of the Src family kinases at these contact sites determines whether transient contacts are disassembled or are stabilized before formation of stronger cadherin-mediated adhesions.
Because we, and others, have shown that the Src kinases are important components of the pathways that regulate cell motility in fibroblasts (Hall et al., 1996; Fincham and Frame, 1998), we also examined whether cells that formed stable cell–cell contacts as a result of Src inhibition were able to migrate into a wound in vitro. Normal keratinocytes treated with the PD162531 Src inhibitor, or tumor-derived keratinocytes induced to overexpress kinase-defective c-Src, were unable to break free from their epithelial neighbors and migrate as individual cells into the denuded area of a confluent cell sheet (Figure 6). We confirmed using time-lapse microscopy that individual cells remained motile when the PD162531 Src inhibitor was present, although the rate of cell motility was reduced by ∼40–50%, presumably as a result of interference with a relatively small amount of the Src kinases present in focal adhesions or focal complexes. Thus, inhibition of the endogenous cellular Src kinase activity in keratinocytes leads to substantially impaired wound repair in vitro, at least in part as a result of the stabilization of cell–cell contacts.
Epithelial cancer cells can be divided into two classes with respect to cadherin-mediated cell–cell adhesion. One class have dysfunctional adhesions as a consequence of genetic loss or mutation of at least one adhesion component, and many of these cells have undergone an epithelial to mesenchymal transition; consequently, they are relatively unrestrained in dissociating from the tumor and have the potential to invade freely into surrounding tissue. The other class have retained normal levels of functional cell–cell adhesion components, yet many such cells are still able to free themselves from the stable epithelia and invade and metastasize. Although the mechanisms used by the latter class of tumor cells to deregulate stable cadherin-mediated contacts are not well understood, we have also found that release of individual cancer cells from interaction with their neighbors requires the activity of the Src family kinases at sites of cell–cell adhesion (Figure 6) and is a prerequisite for growth factor-induced invasion of epithelial cancer cells from a monolayer into Matrigel in vitro (our unpublished results). Taken together with the findings of others, we postulate that migratory growth factors that induce epithelial cell dispersion do so by stimulating Src-induced disassembly of cadherin-mediated cell–cell contacts, a likely requirement for epithelial cancer cell invasion. In this way, the aberrant regulation of a normal Src-mediated process in epithelial cells may contribute to invasive behavior. In this regard, activating mutations of the c-src gene have recently been associated with late-stage human colorectal cancers (Irby et al., 1999).
The mechanisms by which Src-induced tyrosine phosphorylation induces cadherin-mediated contact disassembly are not known, although it seems likely that phosphorylation of one or more crucial substrates triggers disruption. Although β-catenin is a candidate for a triggering substrate, its role remains unclear because v-Src-induced weakening of cadherin-mediated adhesions can occur in cells in which β-catenin is not involved in adhesion (Takeda et al., 1995). Other candidate substrates include the ERM proteins (ezrin/radixin/moesin) and ZO-1, which have been implicated as determinants of cadherin-dependent adhesion strength (Takeda et al., 1995; Imamura et al., 1999), and p120CTN, which has recently been shown to bind to the juxtamembrane intracellular domain of C-cadherin that is responsible for cadherin clustering and adhesion strengthening (Yap et al., 1998). Although it is not yet clear how p120CTN contributes to cadherin clustering, it is possible that tyrosine phosphorylation may negatively regulate some p120CTN-induced clustering function. Another possibility is that the catalytic activity of the Src kinases at cadherin-mediated cell contacts disrupts or destabilizes the actin cytoskeleton that is required for adhesion maintenance (Braga et al., 1997). In support of the latter possibility, there is evidence that inhibition of serine/threonine kinases inhibits epithelial cell–cell junction dissociation by influencing the contractility of associated cytoskeleton (Citi et al., 1994). In addition to the mechanism of Src kinase-induced disruption of cell–cell contacts, it remains to be established whether Src activity at cadherin-mediated adhesions influences the downstream signaling pathways that originate at these intracellular sites.
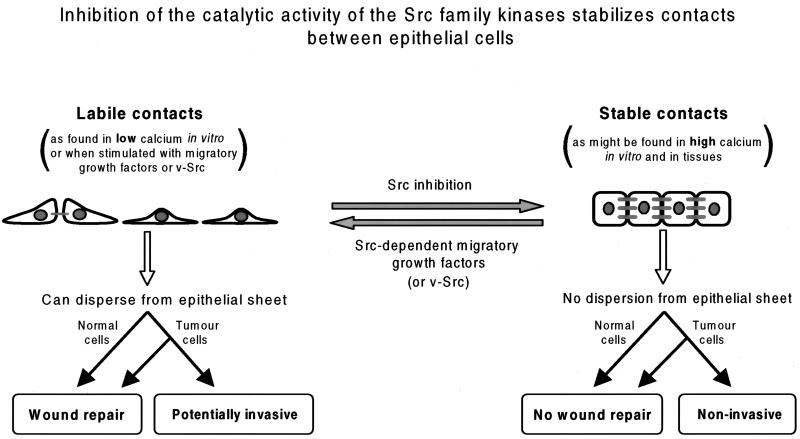
In conclusion, our results demonstrate that the catalytic activity of the endogenous cellular Src family kinases disrupts epithelial cell–cell contacts during their dynamic regulation in low Ca2+ in vitro and is also required to free cells from the constraints of their epithelial connections during in vitro wound repair. In contrast, the study by Calautti et al., (1998) demonstrated that cells lacking c-Src and Fyn are impaired at forming cell–cell junctions in vitro and in vivo. This is summarized in the model in Figure 7. Taken together, these findings indicate that in an analogous manner to focal adhesion regulation in fibroblasts, aspects of Src function other than the catalytic activity, most likely adaptor functions associated with the Src homology domains, are required for epithelial cell–cell junction assembly, whereas the kinase activity is required for subsequent cell–cell contact disruption. This implies that the Src kinases are recruited to cadherin-dependent cell–cell contacts as they form, and subsequent stimulus-induced enzymatic activation may trigger cell–cell contact disassembly. Our work further predicts that if clinically useful anticancer drugs that specifically target the kinase activity of the Src family are developed, these will be of particular benefit in the treatment of potentially invasive tumors that have not lost functional cadherins or catenins.
Figure 7.
Model depicting the likely biological consequences of modulating Src kinase activity in E-cadherin-expressing epithelial cells. The weakening of intercellular contact stability may facilitate processes such as epithelial wound repair or cancer cell invasion.
ACKNOWLEDGMENTS
We thank John Wyke, Val Fincham, Malcolm Finbow, and John Pitts for their helpful comments on this work and for critical reading of the manuscript. Thanks also to K. Kaplan for the plasmid-encoding kinase-inactive c-Src, to A. Malliri for help with time lapse, to A. Kraker and Parke-Davis for the PD162531 tyrosine kinase inhibitor (and for allowing us access to information on its selectivity), and to T. Hunter and N. Carter for the N2–17 mAb against c-Src. This work was supported by the Cancer Research Campaign, United Kingdom (M.C.F., E.K.P., C.P., A.W.W., and G.W.M.) and by the Medical Research Council, United Kingdom (V.G.B. and D.W.O.).
REFERENCES
- Ansel JC, Yiesman JP, Olerud JE, Kreuger JG, Krane JF, Tara DC, Shipley GD, Gilbertson D, Usui ML, Hart CE. Human keratinocytes are a major source of cutaneous platelet-derived growth factor. J Clin Invest. 1993;92:671–678. doi: 10.1172/JCI116636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo J, Leung T, Ernst H, Zanin MKB, Hoffman S, Lilien J. Regulated binding of a PTP1B-like phosphatase to N-cadherin: control of cadherin-mediated adhesion by dephosphorylation of β-catenin. J Cell Biol. 1996;134:801–813. doi: 10.1083/jcb.134.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Vaket L, Friis R, Winterhager E, Van Roy F, Mareel M, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expresssion in carcinomas; role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Weidner KM, Hulsken J, Behrens J. Molecular mechanisms leading to cell junction (cadherin) deficiency in invasive carcinomas. Semin Cancer Biol. 1993;4:231–239. [PubMed] [Google Scholar]
- Boyer B, Roche S, Denoyelle M, Thiery JP. Src and Ras are involved in separate pathways in epithelial cell scattering. EMBO J. 1997;16:5904–5913. doi: 10.1093/emboj/16.19.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VMM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti E, Cabodi S, Stein PL, Hatzfeld M, Kedersha N, Paolo Dotto G. Tyrosine phosphorylation and Src family kinases control keratinocyte cell–cell adhesion. J Cell Biol. 1998;141:1449–1465. doi: 10.1083/jcb.141.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S, Volberg T, Bershadsky AD, Denisenko N, Geiger B. Cytoskeletal involvement in the modulation of cell–cell junctions by the protein kinase inhibitor H-7. J Cell Sci. 1994;107:683–692. [PubMed] [Google Scholar]
- Cowin PM, Burke B. Cytoskeletal-membrane interactions. Curr Opin Cell Biol. 1996;8:56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, Frame MC. The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J. 1998;17:81–92. doi: 10.1093/emboj/17.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham VJ, Unlu M, Brunton VG, Pitts JD, Wyke JA, Frame MC. Translocation of Src kinase to the cell periphery is mediated by the actin cytoskeleton under the control of the Rho family of small G-proteins. J Cell Biol. 1996;135:1551–1564. doi: 10.1083/jcb.135.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W. E-cadherin-mediated cell–cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- Hall CL, Lange LA, Prober DA, Zhang S, Turley EA. Pp60(c-Src) is required for cell locomotion regulated by the hyaluronin receptor RHAMM. Oncogene. 1996;13:2213–2224. [PubMed] [Google Scholar]
- Hamaguchi M, Matsuyoshi N, Ohnishi Y, Gotoh B, Takeichi M, Nagai Y. P60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J. 1993;12:307–314. doi: 10.1002/j.1460-2075.1993.tb05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Nelson JW, Papkoff J. Wnt-1 modulates cell–cell adhesion in mammalian cells by stabilizing β-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Itoh M, Maeno Y, Tsukita S, Nagafuchi A. Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irby RB, Mao W, Coppola D, Kang J, Lobeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187–190. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- Kaplan KB, Bibbins KB, Swedlow JR, Arnaud M, Morgan DO, Varmus HE. Association of the amino-terminal half of c-Src with focal adhesions alters their properties and is regulated by phosphorylation of tyrosine. EMBO J. 1994;13:4745–4756. doi: 10.1002/j.1460-2075.1994.tb06800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of Ras-transformed breast epithelia. J Cell Biol. 1995;130:461–471. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger J, Zhao Y-H, Murphy D, Sudol M. Differential expression of p62c-yes in normal, hyperplastic and neoplastic human epidermis. Oncogene. 1991;6:933–940. [PubMed] [Google Scholar]
- Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell–cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- No D, Yao T-P, Evans RM. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci USA. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J. Regulation of complexed and free catenin pools by distinct mechanisms. Differential effects of Wnt-1 and v-Src. J Biol Chem. 1997;272:4536–4543. [PubMed] [Google Scholar]
- Parkinson EK, Hume WJ, Potten CS. The radiosensitivity of cultured mouse and human keratinocytes. Int J Radiat Biol. 1986;50:717–726. doi: 10.1080/09553008614551111. [DOI] [PubMed] [Google Scholar]
- Peri A-K, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3b to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;262:1731–1733. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Sarret Y, Woodley DT, Grigsby K, Wynn K, O'Keefe EJ. Human keratinocyte locomotion: the effect of selected cytokines. J Invest Dermatol. 1992;98:12–16. doi: 10.1111/1523-1747.ep12493517. [DOI] [PubMed] [Google Scholar]
- Sato C, Tsuboi R, Shi C-M, Rubin JS, Ogawa H. Comparative study of hepatocyte growth factor/scatter factor and keratinocyte growth factor effects on human keratinocytes. J Invest Dermatol. 1995;104:958–963. doi: 10.1111/1523-1747.ep12606221. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F. Tyrosine phosphorylation of β-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun. 1994;1:295–305. doi: 10.3109/15419069409097261. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Hirohashi S. Expression of E- and P-cadherin in gastric carcinomas. Cancer Res. 1991;51:2185–2192. [PubMed] [Google Scholar]
- Shiozaki H, et al. Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol. 1991;139:17–23. [PMC free article] [PubMed] [Google Scholar]
- Smart JE, Opermann AP, Czernilofsky AP, Purchio AF, Erikson RL, Bishop JM. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homolog (pp60c-src) Proc Natl Acad Sci USA. 1981;78:6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F, Endo S, Kojima T, Saigo K. Regulation of cell–cell contacts in developing Drosophila eyes by Dsrc41, a new, close relative of vertebrate c-src. Genes Dev. 1996;10:1645–1656. doi: 10.1101/gad.10.13.1645. [DOI] [PubMed] [Google Scholar]
- Takeda H, Nagafuchi A, Yonemura S, Tsukita S, Behrens J, Birchmeier W, Tsukita S. V-Src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and β-catenin is not required for the shift. J Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Akiyama T, Yamanashi Y, Yamamoto T, Tsukita S. Specific proto-oncogenic tyrosine kinases of src family are enriched in cell-to-cell adherens junctions where the level of tyrosine phosphorylation is elevated. J Cell Biol. 1991;113:867–879. doi: 10.1083/jcb.113.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley-Stein GM, Pepperkok R, Ansorge W, Courtneidge SA. The Src family tyrosine kinases are required for platelet-derived growth factor-mediated signal transduction in NIH3T3 cells. Proc Natl Acad Sci USA. 1993;90:7696–7700. doi: 10.1073/pnas.90.16.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleminckx K, Vakaet L, Mareel M, Friers W, Van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Volberg T, Zick Y, Dror R, Sabanay I, Gilon C, Levitzki A, Geiger B. The effect of tyrosine-specific phosphorylation on the assembly of adherens-type junctions. EMBO J. 1992;11:1733–1742. doi: 10.1002/j.1460-2075.1992.tb05225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner KM, et al. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci USA. 1991;88:7001–7005. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]