Abstract
Associations between plasma membrane-linked proteins and the actin cytoskeleton play a crucial role in defining cell shape and determination, ensuring cell motility and facilitating cell–cell or cell–substratum adhesion. Here, we present evidence that CEACAM1-L, a cell adhesion molecule of the carcinoembryonic antigen family, is associated with the actin cytoskeleton. We have delineated the regions involved in actin cytoskeleton association to the distal end of the CEACAM1-L long cytoplasmic domain. We have demonstrated that CEACAM1-S, an isoform of CEACAM1 with a truncated cytoplasmic domain, does not interact with the actin cytoskeleton. In addition, a major difference in subcellular localization of the two CEACAM1 isoforms was observed. Furthermore, we have established that the localization of CEACAM1-L at cell–cell boundaries is regulated by the Rho family of GTPases. The retention of the protein at the sites of intercellular contacts critically depends on homophilic CEACAM1–CEACAM1 interactions and association with the actin cytoskeleton. Our results provide new evidence on how the Rho family of GTPases can control cell adhesion: by directing an adhesion molecule to its proper cellular destination. In addition, these results provide an insight into the mechanisms of why CEACAM1-L, but not CEACAM1-S, functions as a tumor cell growth inhibitor.
INTRODUCTION
CEACAM1 is an integral membrane glycoprotein that belongs to the carcinoembryonic antigen (CEA) subfamily within the immunoglobulin (Ig) superfamily (Thompson et al., 1991; Hammarström et al., 1998). The nomenclature of this large gene family has recently been unified: the glycoprotein formerly identified as Bgp/BGP, C-CAM, or CD66a will now be referred to as CEACAM1 (Beauchemin et al., 1999). It contains two or four extracellular Ig-like domains, a transmembrane region and cytoplasmic domain. The unique feature of this CEA family member lies in two isoforms with cytoplasmic domains of either 10 or 71–73 amino acids produced as alternative splicing variants and referred to within as CEACAM1-S (short) or CEACAM1-L (long). The conservation of amino acid sequences of the cytoplasmic domains across species suggests that these two isoforms are functionally important (Barnett et al., 1989; Najjar et al., 1993; Nédellec et al., 1995). CEACAM1 expression patterns have been extensively defined (Odin et al., 1988; Daniels et al., 1996; Prall et al., 1996; Beauchemin and Lin, 1998). The protein is expressed in hepatocytes, epithelial cells of the gastrointestinal tract, and endothelial cells, as well as on B cells, macrophages, natural killer cells, and interleukin 2-activated T cells (Coutelier et al., 1994; Möller et al., 1996; Prall et al., 1996).
CEACAM1 has been implicated in a number of physiological processes. It mediates Ca2+-independent homophilic adhesion (Ocklind and Öbrink, 1982; McCuaig et al., 1992; Cheung et al., 1993; Rojas et al., 1996). This role is instrumental in hepatocyte aggregation as well as in major tissue reorganization observed during embryonic development (Ocklind and Öbrink, 1982; Daniels et al., 1996). Down-regulation of CEACAM1 is an important step in malignant transformation. The expression of CEACAM1 is lost or down-regulated at the adenoma stage in the progression of intestinal malignancies (Neumaier et al., 1993; Rosenberg et al., 1993; Nollau et al., 1997) as well as in liver (Hixson et al., 1985; Tanaka et al., 1997), prostate (Hsieh et al., 1995), endometrial (Bamberger et al., 1998), and 30% of breast cancers (Riethdorf et al., 1997; Huang et al., 1998). Consistently, transfection of CEACAM1-L into colon or prostate carcinoma cells significantly suppressed their tumorigenicity in vitro and in vivo (Hsieh et al., 1995; Kunath et al., 1995). However, transfection of CEACAM1-S, an isoform with a truncated cytoplasmic domain, in the same cellular background did not lead to inhibition of tumor cell growth. The mouse CEACAM proteins are the receptors for all strains of mouse hepatitis viruses (Dveksler et al., 1991; Nédellec et al., 1994), whereas the human CEACAM1 glycoproteins are recognized by bacterial pathogens such as Neisseria gonorrhea (Virji et al., 1996; Gray-Owen et al., 1997). Moreover, the CEACAM1 long cytoplasmic domain is phosphorylated on Ser (Odin et al., 1986; Culic et al., 1992) and Tyr residues (Rees-Jones and Taylor, 1985). Association of CEACAM1-L with protein-tyrosine kinases of the Src family (Brümmer et al., 1995; Skubitz et al., 1995) and the protein-tyrosine phosphatases SH2-containing Tyr phosphatase-1 (SHP-1) and SHP-2 (Beauchemin et al., 1997; Huber et al., 1999) implies an involvement of this glycoprotein in signal transduction. Formisano et al. (1995) have reported that the phosphorylation of CEACAM1-L correlates with internalization of the insulin receptor. In addition, Tyr-phosphorylated CEACAM1-L has been implicated in the activation of Rac1, p65PAK, and Jun kinase in N. gonorrhea-activated neutrophils (Hauck et al., 1998). CEACAM1-L is also Tyr phosphorylated during respiratory bursts in neutrophils (Skubitz et al., 1995).
Despite the fact that intercellular adhesion has been shown to be a major function of CEACAM1 and other members of the CEA family (Rojas et al., 1996), little is known about CEACAM1-mediated adhesion mechanisms. Association of the other classes of cell adhesion molecules such as cadherins and the integrins with the actin cytoskeleton is well established (Geiger, 1989; Takeichi, 1991; Luna and Hitt, 1992; Clark and Brugge, 1995; Gumbiner, 1996). Cytoskeletal association plays a crucial role in defining cell shape and determination, ensuring cell motility and facilitating cell–cell and cell–substratum adhesion (Lauffenburger and Horwitz, 1996; Keely et al., 1998). Here, we report that CEACAM1-L associates with the underlying actin cytoskeleton in mouse CT51 intestinal epithelial cells and Swiss 3T3 fibroblasts. The association is specific for the CEACAM1 long isoform. Localization of CEACAM1-L at sites of cell–cell contact is regulated by members of the Rho family of small GTP-binding proteins and depends on homophilic CEACAM1–CEACAM1 interactions and association with the actin cytoskeleton.
MATERIALS AND METHODS
Cell Culture and Microinjections
Mouse CT51 colon carcinoma cells (Brattain et al., 1980) were established and generously provided by Dr. Michael Brattain (Medical College of Ohio, Toledo, OH). Cells were grown in α-minimal essential medium supplemented with 10% FBS and antibiotics (Life Technologies, Gaithersburg, MD) at 37°C in a humidified atmosphere of 5% CO2. These cells do not endogenously express the CEACAM1 proteins. CT51 stable transfectant cells expressing either the wild-type CEACAM1 proteins or CEACAM1 mutants were generated via retroviral-mediated infections as described (Kunath et al., 1995; Huber et al., 1999) and grown in the presence of 750 μg/ml G418 (Life Technologies). Human embryonic kidney 293 cells were transiently transfected with the CEACAM1-L cDNA as previously described (Huber et al., 1999). The Δ495 CEACAM1-L deletion mutant was originally designed to eliminate a conserved Ser phosphorylation site (Ser-503) as well as sequences surrounding Tyr-515 within the C-terminal region of the cytoplasmic domain. It includes a stop codon at amino acid 496. The Δ472 deletion mutant coincides with the end of the Ceacam1 exon 7 and eliminates the distal half of the cytoplasmic domain (Huber et al., 1999). Experiments were performed with either cell populations or a minimum of two clones from each transfectant cell line.
Swiss 3T3 cells were grown in Dulbecco's modified Eagle's medium supplemented with 5% FBS and antibiotics. Confluent serum-starved Swiss 3T3 cells were prepared as described previously (Lamarche et al., 1996). Briefly, cells were plated in serum onto acid-washed coverslips and 7–10 d later subjected to serum starvation 16 h before microinjections. The CEACAM1-L cDNA, cloned into the eukaryotic expression vector pXM139 (Huber et al., 1999), was microinjected alone (0.2 mg/ml) or in combination with 0.1 mg/ml pRK5-myc-tagged vectors encoding L63RhoA, L61Rac1, L61Cdc42, N17Rac1, or N17Cdc42 or with pEFmyc-C3 transferase cDNAs (Caron and Hall, 1998). The cDNA constructs were microinjected into the nucleus of ∼50 cells over 15 min in CO2-independent medium (18045-088; Life Technologies) using an Eppendorf microinjection system (Lamarche et al., 1996).
Various treatments were applied to the cells. Cytochalasin D (Sigma, St. Louis, MO; 1 μM for 1 h or 0.1 μM for 3–6 h) was added to the medium before fixation of the cells. Fab fragments of the anti-CEACAM1 231 polyclonal antibody or the preimmune serum (McCuaig et al., 1992) were added to the media at 0.25 mg/ml 2 h after the microinjections and incubated with the cells for 3 or 8 h. Serum (20%) was added to serum-starved Swiss 3T3 cells 10 h after microinjections for 30 min. Platelet-derived growth factor (PDGF) and lisophosphatidic acid (LPA) were added 10 h after microinjections at 3 and 20 ng/ml, respectively, for 10 min.
Antibodies and Immunofluorescence Microscopy
Several antibodies specific for the mouse CEACAM1 protein were used. A rabbit polyclonal antibody (Ab 231), raised against the purified mouse CEACAM1 protein, has previously been described (McCuaig et al., 1992). This antibody recognizes only the first extracellular Ig domain of CEACAM1 (Daniels et al., 1996). Fab fragments of antibody 231 or the preimmune serum were prepared by papain digestion followed by affinity chromatography (McCuaig et al., 1992). CC1 is a monoclonal anti-CEACAM1 antibody that recognizes a conformational-dependent epitope within the first Ig domain of the protein (Wessner et al., 1998). It was generously provided by Dr. K. V. Holmes (University of Colorado Health Sciences Center, Denver, CO). Myc-tagged proteins were detected by the 9E10 anti-myc mAb, which was a generous gift from Dr. Morag Park (Royal Victoria Hospital, Montreal, Québec, Canada). The anti-E-cadherin mAb was purchased from Transduction Laboratories (Lexington, KY). The anti-SHP-2 polyclonal antibody was raised against the C-terminal region of the protein-Tyr phosphatase (Huber et al., 1999). The anti-actin antibody was purchased from Sigma, as were the anti-rabbit and anti-mouse HRP-conjugated antibodies. Anti-rabbit and anti-mouse FITC-conjugated antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). 125I-Labeled goat anti-mouse IgGs were purchased from ICN (Costa Mesa, CA).
Immunofluorescence detection was done essentially as described by Lamarche et al. (1996). In brief, cells were rinsed in PBS, fixed in 4% paraformaldehyde for 10 min, and permeabilized in 0.2% Triton X-100-containing PBS for 5 min. Free aldehyde groups were reduced with 0.5 mg/ml sodium borohydride for 10 min. Coverslips were then incubated with the appropriate primary antibody diluted in PBS for 2 h, washed with PBS, and transferred to a second antibody mixture containing TRITC-conjugated phalloidin (1:1000 dilution; Sigma) for 1 h. Coverslips were mounted with moviol containing p-phenylenediamine (1 mg/ml). The coverslips were examined on either a Zeiss (Thornwood, NY) Axiophot fluorescence microscope or a Bio-Rad (Hercules, CA) confocal microscope.
Detergent Extraction of CEACAM1
Detergent extraction of CEACAM1 was done essentially as described by Neame and Isacke (1993). CT51 cells were grown to subconfluence in six-well dishes, washed with cold PBS, and incubated for 10 min with gentle shaking on ice in a solution containing 15 mM Tris-Cl, pH 7.5, 1 mM CaCl2, 1 mM MgCl2, 150 mM NaCl, 10 μg/ml leupeptin, aprotinin, and phenylmethylsulfonylfluoride as protease inhibitors, and 0.05, 0.1, 0.4,or 1.0% Triton X-100 detergent. The extracted material (400 μl) was collected, and 40 μl of a 10× SDS-sample buffer solution were added. Leftover cellular material on the dishes was rinsed twice with cold PBS and collected by scraping with a rubber policeman in a 440-μl solution of 1× SDS-sample buffer. Samples of the extracted proteins or cellular debris were boiled, separated by SDS-PAGE gels, and assayed by immunoblotting.
For immunofluorescence detection of CEACAM1-L associated with the membrane after detergent extractions, cells were treated with a cytoskeleton (CSK) buffer (10 mM 1,4-piperazinediethanesulfonic acid, pH 7.0, 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, 0.5% Triton X-100, and 10 μg/ml leupeptin, aprotinin, and phenylmethylsulfonylfluoride) for 20 min at 4°C, rinsed in PBS, and fixed with 2% paraformaldehyde for 10 min followed by immunofluorescence detection as described (Royal and Park, 1995).
Immunoprecipitation and Immunoblotting
Immunoprecipitation of CEACAM1-L from cell lyzates was performed using 3 μg of the CC1 mAb IgGs for 2 h at 4°C followed by protein G-Sepharose collection of antibody complexes. Samples of the detergent-extracted proteins or immunoprecipitated proteins were resolved on 8% SDS-PAGE gels and transferred to Immobilon (Millipore, Bedford, MA) membranes. After blocking nonspecific sites for 2 h at 20°C with 5% milk-TBST (10 mM Tris-Cl, pH 8.0, 150 mM NaCl, and 0.05% Tween 20), membranes were incubated with the anti-CEACAM1 polyclonal 231 Ab or the anti-actin Ab for 2 h in 5% milk-TBST buffer at a dilution of 1:1000 (231 Ab) or 1:1000 (anti-actin). Membranes were washed for three times for 10 min each in TBST and incubated for 1 h at 20°C in blocking buffer containing an HRP-conjugated anti-rabbit antibody. After extensive washings, proteins were visualized using an ECL detection system (Amersham, Arlington Heights, IL).
In Vitro Actin Binding Assay
To verify the interaction between CEACAM1-L and filamentous actin (F-actin), in vitro actin binding and cosedimentation assays were performed using an Actin Binding Protein Biochem kit (Cytoskeleton, Denver, CO), as described by the manufacturer. Briefly, 500 μg of monomeric actin, dissolved in a general actin buffer (5 mM Tris-Cl, pH 8.0, 0.2 mM CaCl2, and 0.2 mM ATP) provided in the kit was first polymerized by adding 5 μl of actin polymerization buffer (2.5 M KCl, 100 mM MgCl2, and 50 mM ATP). Subsequently, 5 μg of a GST fusion protein of the CEACAM1-L cytoplasmic domain (Rosenberg et al., 1993) was incubated with 40 μl of the F-actin stock as described by the manufacturer, except that the buffer was adjusted to pH 7.4. After 30 min, proteins were pelleted at 150,000 × g for 1.5 h in an Airfuge (Beckman Instruments, Palo Alto, CA). Proteins present in the resulting pellet and supernatant fractions were resolved by SDS-PAGE gels and stained with Coomassie blue staining. α-Actinin (an actin-binding protein) was used as a control for the F-actin binding, whereas BSA was used as a control for the specificity of the F-actin interaction.
RESULTS
CEACAM1-L Associates with the Actin Cytoskeleton
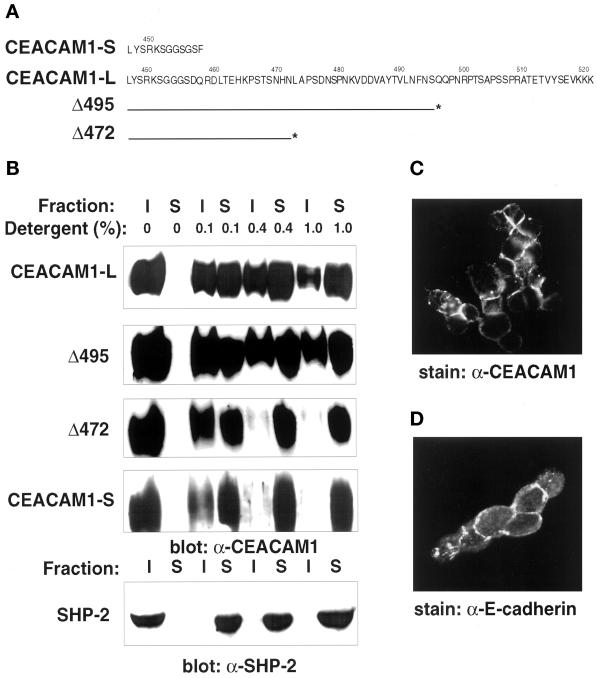
CEACAM1 functions as a cell adhesion molecule in in vitro aggregation assays and has been postulated to play an important role in tissue reorganization (Ocklind and Öbrink, 1982; McCuaig et al., 1992; Cheung et al., 1993; Rojas et al., 1996; Öbrink, 1997). However, the mechanisms leading to alterations in CEACAM1 cellular distribution and functions remained to be clarified. We surmised that its potential association to the cytoskeleton might contribute to its adhesion function. To investigate the existence of an interaction between CEACAM1-L and the actin cytoskeleton in mammalian cells, several approaches were used. Differential detergent solubility assays were first performed. It has been demonstrated that the nonionic detergent Triton X-100 disrupts hydrophobic protein–protein and protein–lipid interactions. Interconnected cytoskeletal proteins, including actin and spectrin, and tightly associated integral membrane proteins remain associated with Triton X-100-insoluble material at Triton X-100 concentrations of ≥0.4% (Tarone et al., 1984; Neame and Isacke, 1993). Proteins that do not associate with the cytoskeleton are found in Triton X-100-soluble fractions. To determine whether the cytoplasmic tail of CEACAM1 was associated with the cytoskeleton, CT51 cells expressing CEACAM1-S,-L or CEACAM1-L deletion mutants (Figure 1A) were subjected to Triton X-100 extractions, and proteins present in the detergent-soluble and -insoluble fractions were identified by immunoblotting. As shown in the immunoblots of Figure 1B, a significant proportion of CEACAM1-L was resistant to 0.4–1.0% Triton X-100 extractions. However, CEACAM1-S was completely extracted from the CT51 cells at a concentration of 0.4% Triton X-100 in four independent experiments using two clones expressing the CEACAM1-S isoform. Thus, under these experimental conditions, CEACAM1-L but not CEACAM1-S appeared to be tightly associated with the underlying cytoskeleton.
Figure 1.
Detergent solubility of CEACAM1 isoforms and deletion mutants. (A) The amino acid sequences of the short (CEACAM1-S) and long (CEACAM1-L) CEACAM1 cytoplasmic domains are presented in one-letter code. Numbers above the sequences indicate the positions in the full-length CEACAM1 protein. The lines below the CEACAM1-L sequence correspond to the C-terminal end of the protein in Δ495 and Δ472 CEACAM1-L deletion mutants. The asterisks correspond to the engineered stop codon. (B) CT51 mouse colon carcinoma cells stably expressing the CEACAM1 proteins were grown to ∼80% confluence and treated directly in the six-well dishes for 10 min at 4°C with buffers containing different concentrations of the Triton X-100 detergent (indicated above the lanes). Triton X-100 soluble (S) or insoluble (I) fractions were separated on 8% SDS-PAGE gels and transferred to Immobilon membranes, and the presence of the CEACAM1 proteins was revealed using the 231 anti-CEACAM1 polyclonal antibody and an ECL detection system. The SHP-2 protein-Tyr phosphatase endogenously expressed in the CT51 cells was used as a control in these experiments. The cells were processed under identical conditions, and the protein was detected with an anti-SHP-2 polyclonal antibody raised against the C-terminal region of the protein. (C) Transfected CT51 cells stably expressing CEACAM1-L were subjected to treatment for 20 min at 4°C with a CSK buffer containing 0.5% Triton X-100 as described in MATERIALS AND METHODS. The cells were fixed in 2% paraformaldehyde and processed for indirect immunofluorescence using the CC1 anti-CEACAM1 mAb (C) or the anti-E-cadherin mAb (D).
To delineate the region within the CEACAM1-L cytoplasmic domain involved in associations with the actin cytoskeleton, we used previously described CEACAM1-L deletion mutants, stably transfected in the CT51 cells (Huber et al., 1999). CEACAM1-L protein containing truncations at residues 518 (Δ518), 510 (Δ510), and 495 (Δ495) (Figure 1A) behaved as the full-length protein in detergent-containing buffers (Figure 1B; our unpublished results). However, truncating the protein further (Δ472) changed the solubility of the mutant protein. It then behaved like the CEACAM1-S protein and was found exclusively in the soluble fraction at a concentration of 0.4% Triton X-100 (Figure 1B). This result indicated that elements within the distal CEACAM1-L cytoplasmic domain, between amino acids 473 and 521, were responsible for tight association with the actin cytoskeleton. The Tyr phosphatase SHP-2, known to be generally soluble within the cytoplasm, was used as a control in these experiments, and upon extraction, as expected, it was found in the soluble fraction, even at low Triton X-100 concentrations (Figure 1B).
The Triton X-100 insolubility of CEACAM1-L was confirmed by immunofluorescence analyses after extraction of cells with a CSK buffer. As demonstrated in Figure 1C, after this treatment, the CEACAM1-L protein remained present at the surface of CT51 cells. This result is consistent with the presence of CEACAM1-L in the insoluble compartment upon detergent extraction (Figure 1B). Under the same experimental conditions, the E-cadherin cell adhesion molecule, endogenously expressed in the same cells, was also found associated with the insoluble compartment (Figure 1D; Takeichi, 1991).
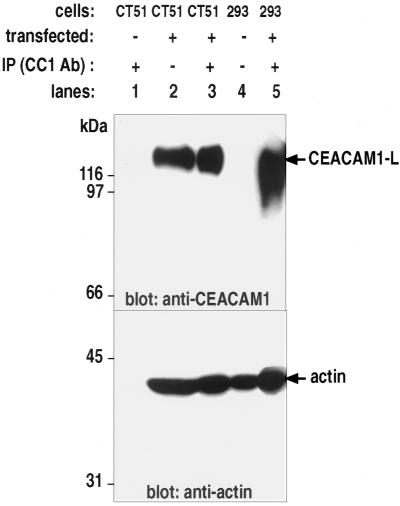
We then verified whether actin could associate with the CEACAM1-L glycoprotein in immune complexes. F-actin is generally associated with the Triton-insoluble pool (Pollard and Cooper, 1982, 1986), and thus its association with membrane proteins may not always be readily detected by coimmunoprecipitation. However, coimmunoprecipitations have previously been used to detect the interaction of E-cadherin with F-actin (Hazan and Norton, 1998). Possibly short fragments of F-actin that either arrested in an early stage of polymerization or that resulted from mechanical shearing of F-actin during extraction allow the detection of F-actin-containing soluble protein complexes (Pollard and Cooper, 1982, 1986, Hazan and Norton, 1998). To this end, mouse CT51 colon carcinoma cells, wild type or those stably transfected with the CEACAM1-L construct, or human embryonic 293 cells transiently transfected with the same cDNA were subjected to cell lysis, and the CEACAM1-L protein was recovered by immunoprecipitation with the CC1 mAb. CT51 stably transfected cells express both CEACAM1-L and actin in appreciable amounts, as seen by immunoblotting these proteins from cells lysates (Figure 2, lane 2). Actin was found specifically associated with CEACAM1-L bound in immune complexes (Figure 2, lane 3). The association appeared specific, because actin was not bound on protein G-Sepharose beads used in the preclearing step (Figure 2, lane 1). Likewise, when CEACAM1-L was transiently transfected in human 293 embryonic kidney cells, actin was found associated with CEACAM1-L in immune complexes (Figure 2, lane 5). As a control, CT51 wild-type cell lysates were subjected to the same immunoprecipitation protocols. In the absence of CEACAM1-L expression (Figure 2, lane 1, top), actin was not detected in immune complexes (Figure 2, lane 1, bottom). Therefore, CEACAM1-L appeared to interact with cytoskeletal proteins.
Figure 2.
Coimmunoprecipitation of actin with CEACAM1-L. The CEACAM1-L cDNA was either stably transfected into mouse CT51 colonic carcinoma cells or transiently transfected into human 293 embryonic kidney cells. Cell lysates were prepared and subjected to preclearing with protein G-Sepharose beads (lane 1). A sample of the total cell lysate was then incubated for 2 h at 4°C with an anti-CEACAM1-specific mAb (CC1). CEACAM1 immune complexes were recovered on protein G-Sepharose beads, and the proteins were separated by SDS-PAGE gels. The membranes were immunoblotted with either an anti-CEACAM1 antibody (top portion) or an anti-actin antibody (bottom portion). (Lane 1) Control immunoprecipitation on a CT51 cell lysate devoid of CEACAM1-L expression. (Lanes 2 and 4) 100 μg of total cell lysate proteins immunoblotted with either the CC1 Ab or the anti-actin Ab. (Lanes 3 and 5) Proteins bound in anti-CEACAM1 immune complexes.
The Binding of CEACAM1-L and Actin Is Indirect
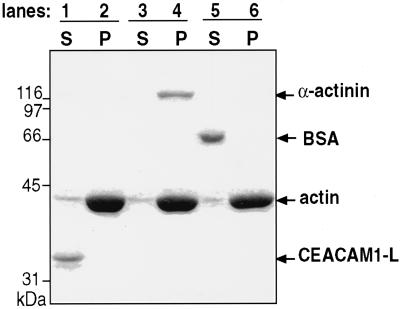
In vivo interactions of many classes of adhesion molecules with the cytoskeleton usually involve a number of adaptor proteins, which function as multiprotein complexes (for review, see Cowin and Burke, 1996). For instance, the cytoplasmic domain of the cadherins mediates interactions with α-, β-, and γ-catenins (Osawa et al., 1989), α-actinin, zyxin, vinculin, and radixin (Luna and Hitt, 1992). The β1 chain of the integrins, as part of focal adhesion complexes, binds talin and α-actinin and connects the molecule to the F-actin multiprotein complex comprising vinculin, paxillin, ezrin, radixin, moesin, zyxin, and other adaptor proteins (Clark and Brugge, 1995; Lauffenburger and Horwitz, 1996; Keely et al., 1998). To verify whether the association of CEACAM1-L to polymerized actin was direct or required a linker protein, we performed cosedimentation assays using a kit purchased from Cytoskeleton. F-actin was incubated with either a GST fusion protein expressing the cytoplasmic domain of CEACAM1-L, α-actinin, or BSA as a control. After centrifugation, the contents of the pellet and supernatant fractions were monitored by SDS-PAGE gels, and the proteins were revealed by Coomassie brilliant blue staining. Incubating the CEACAM1-L cytoplasmic domain with F-actin (Figure 3, lanes 1 and 2) did not lead to the formation of a CEACAM1-L–F-actin complex (Figure 3, lane 2). Combining α-actinin with the CEACAM1-L cytoplasmic domain and F-actin (Figure 3, lanes 3 and 4) did not provoke the formation of a complex, because CEACAM1-L was not found in the pellet (Figure 3, lane 4), although both F-actin and α-actinin were found in this fraction upon ultracentrifugation (Figure 3, lane 4). Under similar experimental conditions, BSA did not bind to F-actin (Figure 3, lane 6). Therefore, the linkage of CEACAM1-L to the cytoskeleton is likely indirect.
Figure 3.
In vitro actin binding assay. A GST fusion protein of the CEACAM1-L cytoplasmic domain was incubated with F-actin, α-actinin, or BSA, and the protein complexes were sedimented at 150,000 × g. Proteins recovered in either the supernatants or pellets were separated by SDS-PAGE gels and revealed by staining with a Coomassie brillant blue dye. (Lanes 1 and 2) GST-CEACAM1-L and F-actin. (Lanes 3 and 4) GST-CEACAM1-L incubated with F-actin and α-actinin. (Lanes 5 and 6) F-actin incubated with BSA.
Several candidate proteins described as adaptors for other cell adhesion molecules were assayed together with CEACAM1-L using a variety of approaches (such as coimmunoprecipitation, colocalization, and cosedimentation). These were α-actinin, reported as a linker for Ep-CAM (Balzar et al., 1998); β-catenin, which is associated with the cadherins (Osawa et al., 1989); vinculin, known to bind to the cadherin–catenin junctional complex (Weiss et al., 1998); and ezrin, moesin, and radixin, involved in ICAM-2, CD44, and CD43 cytoskeletal associations (Yonemura et al., 1998). The interaction of these proteins with CEACAM1-L was assessed by coimmunoprecipitation, whereas α-actinin binding was evaluated by cosedimentation (Figure 3). None of the proteins tested bound to CEACAM1-L.
Colocalization of CEACAM1-L and Actin in Intestinal Cells
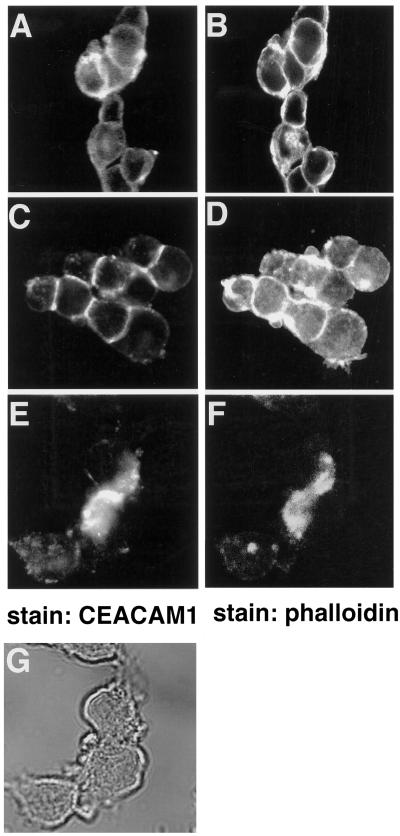
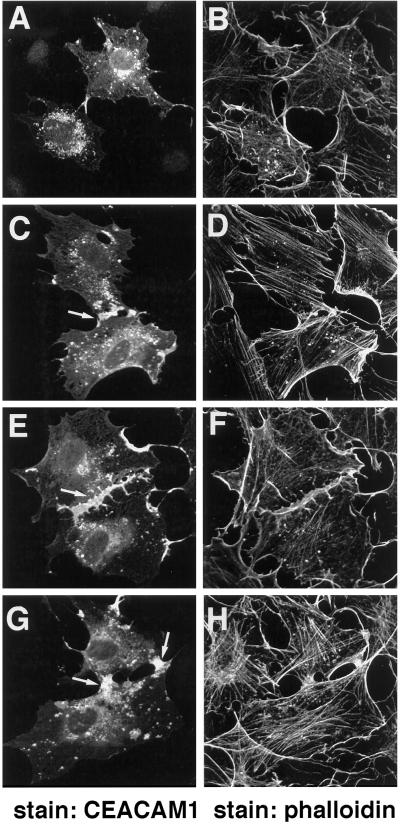
Localization of CEACAM1-L and actin in intestinal CT51 cells was examined. CEACAM1-L stably expressed in the CT51 cells was found at sites of cell–cell contact (Figure 4C), consistent with its role as a cell adhesion molecule (McCuaig et al., 1992; Cheung et al., 1993; Rojas et al., 1996). Phalloidin staining of the same cells revealed predominantly cortical actin (Figure 4D). However, expression of the shorter CEACAM1-S isoform stably transfected into CT51 cells led to a more diffuse staining (Figure 4A), whereas the actin expression remained predominantly cortical (Figure 4B). There were no apparent changes relative to cell–cell contacts between CT51 cell lines transfected with either the CEACAM1-S or CEACAM1-L constructs. The CT51 cells, however, express the E-cadherin cell adhesion molecule (Figure 1D), which also mediates cell–cell binding. Treatment of CT51 CEACAM1-L-expressing cells with 1 μM cytochalasin D, an agent that disrupts the actin filament network (Schliwa, 1982; Cooper, 1987), resulted in the disorganization of the actin cytoskeletal network (Figure 4F), although cellular integrity was unaltered, as judged by phase-constract microscopy (Figure 4G). Interestingly, CEACAM1-L, normally localized at the sites of cell–cell contact, was redistributed in patches after cytochalasin D treatment (Figure 4E). Thus, this result demonstrates a requirement for polymerized actin in maintaining CEACAM1-L at areas of cell–cell contact.
Figure 4.
Localization of the CEACAM1 isoforms in CT51 intestinal cells. CT51 cells stably expressing either the CEACAM1-S (A and B) or CEACAM1-L (C–G) were either untreated (A–D) or treated (E–G) with 1 μM of the cytochalasin D for 1 h and processed for indirect immunofluorescence. CEACAM1 was detected with the CC1 monoclonal antibody and FITC-labeled rabbit anti-mouse secondary antibodies (A, C, and E). Actin in the same cells was revealed using TRITC-conjugated phalloidin (B, D, and F). (G) Phase contrast of cells in E and F.
Rho-like Small GTPases Regulate CEACAM1-L Localization at Site of Cell–Cell Contact
CT51 carcinoma cells are small transformed cells with no defined actin structures. However, actin structures such as lamellipodia, filopodia, and stress fibers are clearly defined in the well-established cellular model represented by the Swiss 3T3 fibroblasts (Hall, 1994). We have previously shown that NIH 3T3 fibroblasts stably transfected with the CEACAM1 cDNA express this protein at the cell surface. In this model, CEACAM1-dependent cell aggregation was inhibited by anti-CEACAM1 Fab fragments, suggesting that CEACAM1 functions as a cell adhesion molecule (McCuaig et al., 1992). Thus, fibroblasts represent an appropriate model to study CEACAM1-mediated cell adhesion.
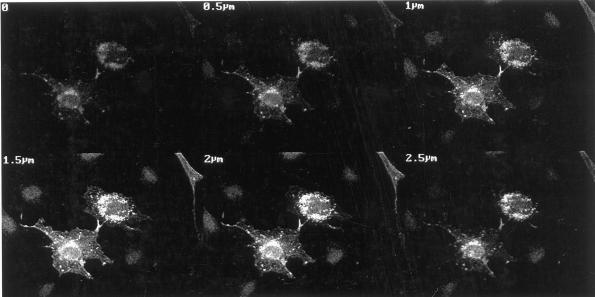
To further investigate CEACAM1-L interactions with specific actin structures, a plasmid encoding CEACAM1-L was microinjected into quiescent serum-starved Swiss 3T3 cells, and CEACAM1-L protein expression was monitored by indirect immunofluorescence. Polymerized actin was visualized by staining the cells with TRITC-phalloidin. Expression of CEACAM1-L was detectable 8 h after microinjection of the cDNA construct with maximum expression after 12 h. The protein was apparent predominantly as patches or clusters (Figure 5A) localized in the cytoplasm as revealed by Z-sectioning of cells using the confocal microscope (Figure 6). This localization of CEACAM1-L in quiescent serum-starved Swiss 3T3 cells was different from that seen in CT51 cells (Figure 4C) or rat NBT II cells, which express CEACAM1-L endogenously (Hunter et al., 1994). Thus, some important pathways regulating the intracellular localization of CEACAM1-L might be inhibited by serum starvation.
Figure 5.
Localization of CEACAM1-L in Swiss 3T3 cells upon physiological activation of Rho-like GTPases. Quiescent Swiss 3T3 cells were serum starved and microinjected with pXM139 encoding the CEACAM1-L protein (0.2 mg/ml) (A and B). Ten hours after microinjections, cells were treated with 20% serum (30 min) (C and D), PDGF (3 ng/ml, 10 min) (E and F), or LPA (20 μg/ml, 10 min) (G and H). (A, C, E, and G) Expression of CEACAM1-L was monitored by indirect immunofluorescence using the CC1 anti-CEACAM1 monoclonal antibody and FITC-labeled rabbit anti-mouse secondary antibodies. (B, D, F, and H) Actin structures were visualized with TRITC-conjugated phalloidin. The arrows in C and G show expression of CEACAM1-L at cell–cell contacts.
Figure 6.
Z sectioning of Swiss 3T3 cells expressing CEACAM1-L. Quiescent serum-starved Swiss 3T3 cells were fixed 10 h after microinjection of pXM139 encoding the CEACAM1-L protein (0.2 mg/ml). Expression of CEACAM1-L was monitored by indirect immunofluorescence using the CC1 anti-CEACAM1 monoclonal antibody and FITC-labeled rabbit anti-mouse secondary antibodies. Z sectioning was performed on a Zeiss 410 confocal microscope along six different planes with intervals of 0.5 μm. The contrast and brightness of the image and the intensity of the laser beam were adjusted to avoid pixel saturation with minimal pinhole opening.
The Rho family of small GTPases has been implicated in the regulation of a wide range of biological processes, including cell motility, cell adhesion, cytokinesis, cell morphology, and growth (Van Aelst and D'Souza-Schorey, 1997; Mackay and Hall, 1998). A major function of the Rho family of small GTP-binding proteins is to control the reorganization of the actin cytoskeleton and the assembly of associated integrin complexes (Hall, 1994). In Swiss 3T3 fibroblasts, activation of Rho leads to the formation of actin stress fibers and focal adhesion complexes (Ridley et al., 1992). Activation of Rac leads to the formation of an actin meshwork at the cell periphery, producing lamellipodia and membrane ruffles (Ridley et al., 1992), whereas Cdc42 activation leads to the formation of filopodia protrusions (Kozma et al., 1995; Nobes and Hall, 1995). Cross-talk between these GTPases has been defined with great details in Swiss 3T3 fibroblasts (Nobes and Hall, 1995; Van Aelst and D'Souza-Schorey, 1997) but less so in other cellular systems. Furthermore, Cdc42 has recently been shown to regulate the localization of basolateral membrane proteins in epithelial Madin–Darby canine kidney cells (Kroschewski et al., 1999).
We considered whether the Rho-like small GTPases were involved in regulation of CEACAM1-L localization and functions. PDGF is a physiological activator of endogenous Rac, shown to induce the formation of lamellipodia or membrane ruffles, whereas LPA activates endogenous Rho and is involved in stress fiber formation (Ridley et al., 1992). We thus examined the effect of serum, PDGF, and LPA on CEACAM1-L expression in quiescent serum-starved Swiss 3T3 cells. These compounds were applied 10 h after microinjection of the CEACAM1-L-encoding vector (period at which the expression of CEACAM1-L can be readily detected). As seen in Figure 5C, treatment of the serum-depleted Swiss 3T3 fibroblasts with serum resulted in the redistribution of some portion of CEACAM1-L at sites of cell–cell contact (arrow). Addition of PDGF or LPA to serum-starved Swiss 3T3 cells also provoked the redistribution of CEACAM1-L to sites in cell–cell boundaries (Figure 5, E and G). These results suggested that physiological activation of endogenous Rac and Rho in Swiss 3T3 cells was inducing the localization of CEACAM1-L to cell–cell contacts.
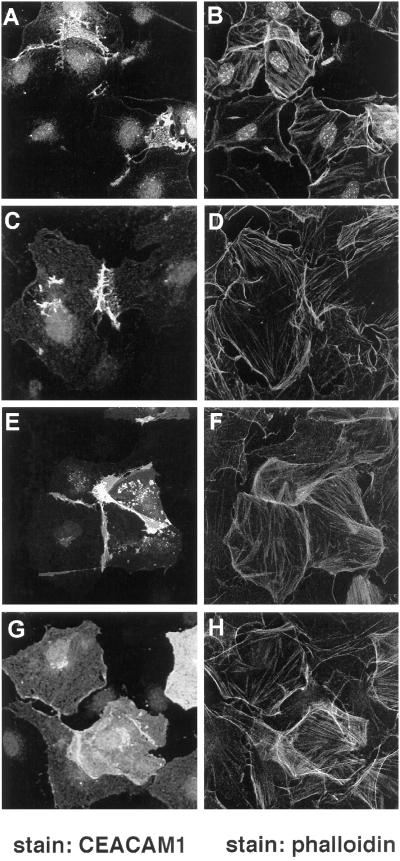
To investigate whether the activation of Rac1, RhoA, and Cdc42 would also result in the localization of CEACAM1-L at cellular contacts, quiescent serum-starved Swiss 3T3 cells were microinjected with expression vectors encoding the constitutively activated mutants of Rac1 (L61Rac1), RhoA (L63RhoA), or Cdc42 (L61Cdc42) together with the CEACAM1-L-expressing vector. Ten to 12 h after microinjection, cells were fixed, and the expression of CEACAM1-L was monitored by indirect immunofluorescence. The expression of the Rho GTPases was also verified using an anti-myc tag antibody, and Rac1, RhoA, and Cdc42 were overexpressed in the same cells expressing CEACAM1-L (our unpublished results). As had been noticed with the activating compounds, when coexpressed with L61Cdc42, L61Rac1, or L63RhoA, CEACAM1-L was found mainly at sites of cell–cell contact (Figure 7, A, C, and E). However, coexpression of CEACAM1-S with the L61Cdc42 GTPase led to scattering of the CEACAM1-S protein over the entire cell surface (Figure 7G), similar to that observed in CT51 cells (Figure 4A). Activation of the GTPases was required for CEACAM1-L localization at sites of cell contacts, because coinjection of CEACAM1-L with dominant-negative mutants of the GTPases (N17Rac1 and N17Cdc42) or a C3-transferase construct (specific for the Rho GTPase) resulted in the absence of CEACAM1-L at cell–cell borders (our unpublished results). CEACAM1-L expression then corresponded to that observed in Figure 5A. This observation is interesting considering the different solubility of the two CEACAM1 isoforms observed with CEACAM1-expressing CT51 cells (Figure 1B).
Figure 7.
Constitutively active mutants of the Rho GTPases relocalize CEACAM1-L at cell–cell boundaries in Swiss 3T3 Cells. Quiescent serum-starved Swiss 3T3 fibroblasts were injected with a pXM139 vector expressing CEACAM1-L (A–F) or CEACAM1-S (G and H) and a pRK5 vector encoding either the myc-tagged constitutively activated L61Cdc42 protein (A and B, G and H), the L61Rac1 protein (C and D), or the L63RhoA protein (E and F). After 12 h of expression, permeabilized cells were stained for CEACAM1-L expression using the 231 anti-CEACAM1 polyclonal antibody and an FITC-conjugated anti-rabbit antibody (A, C, E, and G). Actin was detected using TRITC-conjugated phalloidin (B, D, F, and H).
Rho-like Small GTPases Regulate CEACAM1-L-mediated Homophilic Adhesion
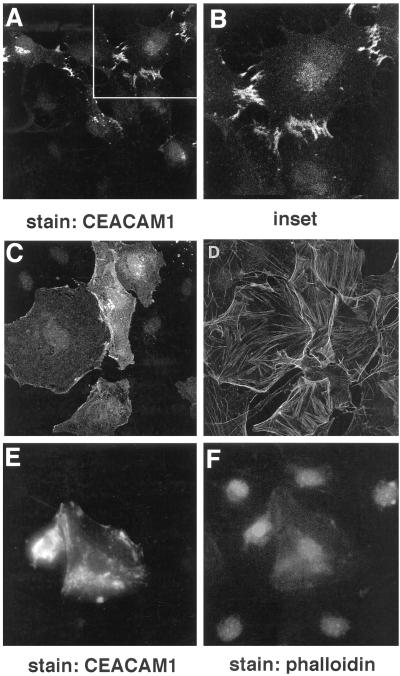
Thus, activated Rho-like small GTPases regulate the localization of the CEACAM1-L to the sites of cell–cell contact. Interestingly, localization of CEACAM1-L at these sites can only be seen when two adjacent cells express the protein. This was in line with the demonstration that CEACAM1-L functions as a homophilic cell adhesion molecule (McCuaig et al., 1992). To determine whether the established contacts between Swiss 3T3 cells are indeed promoted by CEACAM1-L, we incubated the CEACAM1-L-microinjected cells with Fab fragments of an anti-CEACAM1-specific 231 antibody, directed against its extracellular regions (Daniels et al., 1996). Treatment of CEACAM1-expressing NIH 3T3 cells using similar conditions completely inhibited CEACAM1-mediated intercellular aggregation (McCuaig et al., 1992). The Fab fragments were added 2 h after microinjection for 6–8 h, and the expression of CEACAM1-L was visualized by indirect immunofluorescence. As shown in Figure 8C, cells treated with the 231 Fab fragments expressed CEACAM1-L uniformly distributed over the cell surface. Fab fragments prepared from IgGs of the preimmune serum used as a negative control did not inhibit localization of CEACAM1-L at sites of cell–cell contact (Figure 8, A and B). This result suggested that CEACAM1-L indeed mediated intercellular adhesion between Swiss 3T3 fibroblasts. Second, the self-association of CEACAM1-L was necessary for its maintenance at sites of cell–cell contact.
Figure 8.
Localization of CEACAM1-L at cell–cell boundaries is inhibited by treatment with anti-CEACAM1 antibodies or cytochalasin D treatment. Quiescent serum-starved Swiss 3T3 fibroblasts were injected with a pXM139 vector expressing CEACAM1-L and a pRK5 vector encoding the myc-tagged constitutively activated L61Cdc42 protein. The cells were treated for 6 h with 250 ng/ml Fab fragments of the anti-CEACAM1 231 polyclonal antibody (C and D) or with Fab fragments of the preimmune serum (A and B) added 2 h after the injections. After 10 h of expression, permeabilized cells were stained for CEACAM1-L expression using the 231 anti-CEACAM1 polyclonal antibody and an FITC-conjugated anti-rabbit antibody (A, C, and E), and actin was detected using TRITC-conjugated phalloidin (B, D, and F). In E and F, 2 h after microinjections, the microinjected cells were treated with cytochalasin D (50 ng/ml) for 6 h and were processed for immunofluorescence at the end of this treatment, as described above. Expression of CEACAM1-L at cell–cell boundaries was not detected, indicating that association with the actin cytoskeleton is necessary for CEACAM1-L localization at sites of cell–cell contact.
As shown in Figure 4, the localization of CEACAM1-L at the epithelial cell–cell boundaries was dependent on the presence of F-actin. To investigate whether this was the case in Swiss 3T3 cells, serum-starved fibroblasts microinjected with L61Cdc42 and CEACAM1-L constructs were incubated for 6 h with 0.1 μM cytochalasin D, added 2 h after the microinjections. This led to the disorganization of the F-actin network as revealed by the phalloidin staining (Figure 8F). Consistent with the results obtained with the transfected CT51 cells (Figure 4E), CEACAM1-L was no longer found at sites of cell–cell contact (Figure 8E). Thus, the interaction of the CEACAM1-L cytoplasmic domain with the actin cytoskeleton is essential for CEACAM1-L localization at cell–cell boundaries.
DISCUSSION
Links between plasma membrane-associated proteins and cytoskeletal elements have been shown to play crucial roles in defining cell shape and determination, ensuring cell motility and facilitating cell–cell or cell–substratum adhesion (Geiger, 1989; Takeichi, 1991; Luna and Hitt, 1992; Tsukita et al., 1992; Gumbiner, 1996). Many adhesion molecules interact with the actin cytoskeleton, some directly, such as the α2 chain of integrins (Kieffer et al., 1995), and others indirectly, such as cadherins, the β1, β2, and β3 integrins (Clark and Brugge, 1995; Lauffenburger and Horwitz, 1996; Keely et al., 1998), L-selectin (Ben-Ze'ev, 1997), and EpCam (Balzar et al., 1998). In this report, we present several lines of evidence demonstrating that CEACAM1-L is associated with the actin cytoskeleton. Previous reports had suggested that such a connection might exist. For instance, several proteins coprecipitated with CEACAM1 extracted from intestinal brush border membranes, one of which was thought to be actin (Hansson et al., 1989). Similarly, CEACAM1/actin colocalization has been documented in NBTII cells (Hunter et al., 1994).
The interaction between actin and CEACAM1-L was first established using detergent solubility and coimmunoprecipitation assays. Detergent extraction of CEACAM1 from CT51 cells showed that the distal region of CEACAM1-L mediates the tight association with the cytoskeleton (Figure 1). Treatment of the CEACAM1-L-expressing CT51 and Swiss 3T3 cells with cytochalasin D, a filamentous actin-disrupting agent, affected not only the actin cytoskeleton but also the subcellular localization of CEACAM1-L: the protein, previously localized at cell–cell boundaries, was redistributed in clusters that contained both CEACAM1-L and polymerized actin (Figures 4, E and F, and 8, E and F). Finally, CEACAM1-L was shown to colocalize with cortical actin in epithelial cells (Figure 4, C and D). Together, the data described above demonstrated the interaction of CEACAM1-L with the actin cytoskeleton. However, the association of CEACAM1-L and actin does not appear to be direct. It remains possible that their interaction might depend on certain physiological conditions or post-translational modifications of the proteins that were not reproduced in in vitro binding assays. Nonetheless, the anchorage of the CEACAM1-L to F-actin is essential for its subcellular localization and, thus, for CEACAM1-L-mediated intercellular adhesion.
The Swiss 3T3 fibroblasts represent an established model for studying the relationship between the actin cytoskeleton and the functions of small GTPases (Hall, 1994). These small GTP-binding proteins control diverse biological processes such as cytoskeletal organization, gene transcription, and adhesion (for review, see Mackay and Hall, 1998). It has been established that Rho and Rac regulate the formation of integrin adhesion complexes in mammalian fibroblasts (Ridley et al., 1992; Hotchin and Hall, 1995; Mackay et al., 1997). Furthermore, in epithelial cells, Rho and Rac are required for the assembly of cadherin-based adherens junctions (Braga et al., 1997), whereas Cdc42 has been shown to regulate the fidelity of membrane transport (Kroschewski et al., 1999). Because the cytoplasmic domain of CEACAM1-L is associated with the actin cytoskeleton, we investigated the involvement of the Rho family of small GTPases in CEACAM1-L localization. Microinjection of the CEACAM1-L-encoded plasmid alone into confluent serum-starved Swiss 3T3 cells leads to the localization of this protein in patches, which accumulated in the cytoplasm, as confirmed by confocal microscopy Z sectioning (Figure 6). However, when constitutively activated Rho, Rac, and Cdc42 were coinjected with CEACAM1-L, the glycoprotein was then predominantly localized at the sites of cell–cell contact (Figure 7). Notably, such localization of CEACAM1-L was observed only when adjacent cells expressed this protein. CEACAM1-L was then engaged in intercellular adhesion, because treatment with the Fab fragments directed against the extracellular regions of CEACAM1-L led to the disruption of CEACAM1-L-mediated cell–cell contact (Figure 8C). It also altered the localization of the protein, whereby CEACAM1-L in Fab-treated cells was no longer found at the sites of cell–cell contact but distributed over the entire cell surface (Figure 8C). This observation suggests that activated GTPases direct CEACAM1-L to sites of cell–cell contacts where it is locked in by homophilic interactions of its adhesion domain (the first Ig extracellular domain). Tight association of CEACAM1-L with the cytoskeleton also contributes to the localization of CEACAM1-L, because CEACAM1-S, which is not tightly bound to the cytoskeleton, was uniformly distributed over the cell surface. In addition, treatment with cytochalasin D led to the relocalization of CEACAM1-L in epithelial cells (Figure 4, E and F) and Swiss 3T3 cells (Figure 8, E and F). A similar situation has been noticed with another adhesion molecule, Ep-CAM: its cytoplasmic domain and the presence of F-actin were also necessary for its localization at the cell–cell boundaries (Balzar et al., 1998).
Three fundamental mechanisms have been described that contribute to targeting of membrane proteins to their proper cellular destination (for review, see LeGall, 1995). In epithelial cells, these include 1) diffusive restriction by the tight junctions, 2) immobilization by domain-specific interactions with the cytoskeleton, and 3) intracellular sorting and polarized delivery of proteins and lipids to the cell surface. A number of studies have implicated the members of the Rho family in various membrane-trafficking processes. In yeast, Cdc42 has been shown to localize in the vicinity of secretory vesicles found at the site of bud emergence (Ziman et al., 1993). In mammalian cells, it has been suggested that Cdc42 may play a role in cell morphogenesis by acting on targets in the Golgi that affect polarized growth at the plasma membrane (Erickson et al., 1996; McCallum et al., 1996). Furthermore, Rac and Rho play a role in endocytic trafficking and in the regulation of secretory vesicle transport (Van Aelst and D'Souza-Schorey, 1997). Presumably, CEACAM1-L patches observed in serum-starved Swiss 3T3 cells could be consistent with Golgi or secretory vesicle localization, but this will need further confirmation. Whether CEACAM1-L localization at sites of cell–cell contact is promoted through one of the above mechanisms also needs to be further defined.
The data presented here demonstrate that two isoforms of CEACAM1 (CEACAM1-L and CEACAM1-S) differ in their subcellular localization and association with the actin cytoskeleton. CEACAM1-L is tightly associated with the cytoskeleton and localizes to the sites of cell–cell contacts, whereas CEACAM1-S is a soluble protein scattered over the cell surface. Previous reports demonstrated that CEACAM1-L, but not CEACAM1-S, functions as a tumor suppressor (Hsieh et al., 1995; Kunath et al., 1995). However, the exact mechanisms of CEACAM1-L-mediated tumor suppression have not been established. The difference in subcellular localization and solubility of CEACAM1 isoforms may contribute to our understanding of the mechanisms of CEACAM1-L tumor suppressor function.
In conclusion, results presented herein provide evidence on the role of the small GTPases of the Rho family in control of cellular adhesion: by directing a cell surface molecule to its proper cellular destination. However, the complexity of the signaling pathways used for this and the downstream effectors of CEACAM1-L will need to be established.
ACKNOWLEDGMENTS
We are grateful to the following colleagues who provided either reagents or equipment as well as advice and critical reading of the manuscript: Dr. Morag Park (Molecular Oncology Group, Royal Victoria Hospital, Montreal, Québec, Canada) for the anti-myc antibodies, use of the microinjection system, advice throughout the course of this work, and critical reading of the manuscript; Dr. André Veillette (McGill Cancer Centre) for critical discussions and reading of the manuscript; Dr. Kathryn V. Holmes (Department of Microbiology, University of Colorado Health Sciences Center) for the CC1 anti-CEACAM1 mAb and critical reading of the manuscript; Drs. Jacques Huot and Jacques Landry (Center de Recherche Hotel-Dieu de Québec, Québec, Canada) for critical reading of the manuscript; Dr. Danny Baranes (Department of Anatomy and Cell Biology, McGill University) for discussions and help with the confocal microscope; Dr. Gordon Shore (Department of Biochemistry, McGill University) for use of equipment; and Pedro Rodriguez and Dr. Bénédicte Fournès (McGill Cancer Center) for help with in vitro binding assays and fluorecence microscopy and helpful discussions. This work was supported by grants from the Medical Research Council of Canada to N.B. and N.L.-V. N.L.-V. is a Junior Scholar and N.B. is a Senior Scholar from the Fonds de la Recherche en Santé du Québec.
REFERENCES
- Balzar M, Bakker HAM, Briaire-deBruijn IH, Fleuren GJ, Warnaar SO, Litvinov SV. Cytoplasmic tail regulates the intercellular adhesion function of the epithelial cell adhesion molecule. Mol Cell Biol. 1998;18:4833–4843. doi: 10.1128/mcb.18.8.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger AM, Riethdorf L, Nollau P, Naumann M, Erdmann I, Götze J, Brümmer J, Schulte HM, Wagener C, Löning T. Dysregulated expression of CD66a (BGP, C-CAM), an adhesion molecule of the CEA family, in endometrial cancer. Am J Pathol. 1998;152:1401–1406. [PMC free article] [PubMed] [Google Scholar]
- Barnett T, Kretschmer A, Austen DA, Goebel SJ, Hart JT, Elting JJ, Kamarck ME. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989;108:267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin N, et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–249. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- Beauchemin N, Kunath T, Robitaille J, Chow B, Turbide C, Daniels E, Veillette A. Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene. 1997;14:783–790. doi: 10.1038/sj.onc.1200888. [DOI] [PubMed] [Google Scholar]
- Beauchemin N, Lin SH. Role of C-CAM as a tumor suppressor. In: Stanners CP, editor. Cell Adhesion and Communication Mediated by the CEA Family. Amsterdam: Harwood Academic Publishers; 1998. pp. 155–175. [Google Scholar]
- Ben-Ze'ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997;9:99–108. doi: 10.1016/s0955-0674(97)80158-5. [DOI] [PubMed] [Google Scholar]
- Braga VMM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattain MG, Stobel-Stevens J, Fine D, Webb M, Sarrif AM. Establishment of different mouse colonic carcinoma cell lines with different metastatic properties. Cancer Res. 1980;40:2142–2146. [PubMed] [Google Scholar]
- Brümmer J, Neumaier M, Göpfert C, Wagener C. Association of pp60c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family down-regulated in colorectal carcinomas. Oncogene. 1995;11:1649–1655. [PubMed] [Google Scholar]
- Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Cheung PH, Luo W, Qiu Y, Zhang X, Earley K, Millirons P, Lin S-H. Structure and function of C-CAM1. J Biol Chem. 1993;268:24303–24310. [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutelier J-P, Godfraind C, Dveksler GS, Wysocka M, Cardellichio CB, Noëll H, Holmes KV. B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for the murine coronavirus. Eur J Immunol. 1994;24:1383–1390. doi: 10.1002/eji.1830240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P, Burke B. Cytoskeleton-membrane interactions. Curr Opin Cell Biol. 1996;8:56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- Culic O, Huang QH, Flanagan D, Hixson D, Lin SH. Molecular cloning and expression of a new rat liver Cell-CAM 105 isoform. Biochem J. 1992;285:47–53. doi: 10.1042/bj2850047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels E, Létourneau S, Turbide C, Kuprina N, Rudinskaya T, Yazova AC, Holmes KV, Dveksler GS, Beauchemin N. Biliary glycoprotein 1 expression during embryogenesis: correlation with events of epithelial differentiation, mesenchymal-epithelial interactions, absorption, and myogenesis. Dev Dyn. 1996;206:272–290. doi: 10.1002/(SICI)1097-0177(199607)206:3<272::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Dveksler GS, Pensiero MN, Cardellichio CB, Williams RK, Jiang GS, Holmes KV, Dieffenbach CW. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991;65:6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JW, Zhang CJ, Kahn RA, Cerione RA. Mammalian Cdc42 is a Brefeldin A sensitive compound in the Golgi apparatus. J Biol Chem. 1996;271:26850–26854. doi: 10.1074/jbc.271.43.26850. [DOI] [PubMed] [Google Scholar]
- Formisano P, Najjar SM, Gross CN, Philippe N, Oriente F, Kern-Buell CL, Accili D, Gorden P. Receptor-mediated internalization of insulin. J Biol Chem. 1995;270:24073–24077. doi: 10.1074/jbc.270.41.24073. [DOI] [PubMed] [Google Scholar]
- Geiger B. Cytoskeleton-associated cell contacts. Curr Opin Cell Biol. 1989;1:103–109. doi: 10.1016/s0955-0674(89)80045-6. [DOI] [PubMed] [Google Scholar]
- Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 1997;16:3435–3445. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hammarström S, Olsen A, Teglund S, Baranov V. The nature and expression of the human CEA family. In: Stanners CP, editor. Cell Adhesion and Communication Mediated by the CEA Family. Amsterdam: Harwood Academic Publishers; 1998. pp. 1–30. [Google Scholar]
- Hansson M, Blilstad I, Öbrink B. Cell-surface location and molecular properties of Cell-CAM 105 in intestinal epithelial cells. Exp Cell Res. 1989;181:63–74. doi: 10.1016/0014-4827(89)90182-1. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Meyer TF, Lang F, Gulbins E. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signaling pathway. EMBO J. 1998;17:443–454. doi: 10.1093/emboj/17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan RB, Norton L. The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton J. Biol Chem. 1998;273:9078–9084. doi: 10.1074/jbc.273.15.9078. [DOI] [PubMed] [Google Scholar]
- Hixson DC, McEntire KD, Öbrink B. Alterations in the expression of a hepatocyte cell adhesion molecule by transplantable rat hepatocellular carcinomas. Cancer Res. 1985;45:3742–3749. [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J-T, Luo W, Song W, Wang Y, Kleinerman DI, Van NT, Lin S-H. Tumor suppressive role of an androgen-regulated epithelial cell adhesion molecule (C-CAM) in prostate carcinoma cell revealed by sense and antisense approaches. Cancer Res. 1995;55:190–197. [PubMed] [Google Scholar]
- Huang J, Simpson JF, Glackin C, Riethorf L, Wagener C, Shively JE. Expression of biliary glycoprotein (CD66a) in normal and malignant breast epithelial cells. Anticancer Res. 1998;18:3203–3212. [PubMed] [Google Scholar]
- Huber M, Izzi L, Grondin P, Turbide C, Houde C, Kunath T, Veillette A, Beauchemin N. The C-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J Biol Chem. 1999;274:335–344. doi: 10.1074/jbc.274.1.335. [DOI] [PubMed] [Google Scholar]
- Hunter I, Lindh M, Öbrink B. Differential regulation of C-CAM isoforms in epithelial cells. J Cell Sci. 1994;107:1205–1216. doi: 10.1242/jcs.107.5.1205. [DOI] [PubMed] [Google Scholar]
- Keely P, Parise L, Juliano R. Integrins and GTPases in tumor cell growth, motility and invasion. Trends Cell Biol. 1998;8:101–106. doi: 10.1016/s0962-8924(97)01219-1. [DOI] [PubMed] [Google Scholar]
- Kieffer JD, Plopper G, Ingber DE, Hartwig JH, Kupper TS. Direct binding of F actin to the cytoplasmic domain of the alpha 2 integrin chain in vitro. Biochem Biophys Res Commun. 1995;217:466–474. doi: 10.1006/bbrc.1995.2799. [DOI] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- Kunath T, Ordoñez-Garcia C, Turbide C, Beauchemin N. Inhibition of colonic tumor cell growth by biliary glycoprotein. Oncogene. 1995;11:2375–2382. [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenström P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- LeGall AH, Yeaman C, Muesch A, Rodriguez-Boulan E. Epithelial cell polarity: new perspectives. Semin Nephrol. 1995;15:272–284. [PubMed] [Google Scholar]
- Luna EJ, Hitt AL. Cytoskeleton-plasma membrane interactions. Science. 1992;258:955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Mackay DJG, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- Mackay JG, Esch F, Furthmayr H, Hall A. Rho- and Rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ERM proteins. J Cell Biol. 1997;138:927–938. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SJ, Wu WJ, Cerione RA. Identification of a putative effector for Cdc42Hs with high sequence similarity to the RasGAP-related protein IQGAP1 and a Cdc42Hs binding partner with similarity to IQGAP2. J Biol Chem. 1996;271:21732–21737. doi: 10.1074/jbc.271.36.21732. [DOI] [PubMed] [Google Scholar]
- McCuaig K, Turbide C, Beauchemin N. mmCGM1a: a mouse carcinoembryonic antigen gene family member, generated by alternative splicing, functions as an adhesion molecule. Cell Growth Differ. 1992;3:165–174. [PubMed] [Google Scholar]
- Möller MJ, Kammerer R, Grunert F, von Kleist S. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell subpopulation. Int J Cancer. 1996;65:740–745. doi: 10.1002/(SICI)1097-0215(19960315)65:6<740::AID-IJC5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Najjar SM, Accili D, Philippe N, Jernberg J, Margolis R, Taylor SI. pp120/ecto-ATPase, an endogenous substrate of the insulin receptor tyrosine kinase, is expressed as two variably spliced isoforms. J Biol Chem. 1993;268:1201–1206. [PubMed] [Google Scholar]
- Neame SJ, Isacke CM. The cytoplasmic tail of CD44 is required for basolateral localization in epithelial MDCK cells but does not mediate association with the detergent-insoluble cytoskeleton of fibroblasts. J Cell Biol. 1993;121:1299–1310. doi: 10.1083/jcb.121.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nédellec P, Dveksler GS, Daniels E, Turbide C, Chow B, Basile AA, Holmes KV, Beauchemin N. Bgp2, a new member of the carcinoembryonic antigen-related gene family, encodes an alternative receptor for mouse hepatitis viruses. J Virol. 1994;68:4525–4537. doi: 10.1128/jvi.68.7.4525-4537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nédellec P, Turbide C, Beauchemin N. Characterization and transcriptional activity of the mouse biliary glycoprotein 1 gene, a carcinoembryonic antigen-related gene. Eur J Biochem. 1995;231:104–114. doi: 10.1111/j.1432-1033.1995.tb20676.x. [DOI] [PubMed] [Google Scholar]
- Neumaier M, Paululat S, Chan A, Matthaes P, Wagener C. Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc Natl Acad Sci USA. 1993;90:10744–10748. doi: 10.1073/pnas.90.22.10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nollau P, Scheller H, Kona-Horstmann M, Rohde S, Hagenmuller F, Wagener C, Neumaier M. Expression of CD66a (human C-CAM) and other members of the carcinoembryonic antigen family of adhesion molecules in human colorectal adenomas. Cancer Res. 1997;57:2354–2357. [PubMed] [Google Scholar]
- Öbrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol. 1997;9:616–626. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocklind C, Öbrink B. Intercellular adhesion of rat hepatocytes. J Biol Chem. 1982;257:6788–6795. [PubMed] [Google Scholar]
- Odin P, Asplund M, Busch C, Öbrink B. Immunohistochemical localization of cell-CAM105 in rat tissues: appearance in epithelia, platelets, and granulocytes. J Histochem Cytochem. 1988;36:729–739. doi: 10.1177/36.7.3290331. [DOI] [PubMed] [Google Scholar]
- Odin P, Tingström A, Öbrink B. Chemical characterization of Cell-CAM 105, a cell-adhesion molecule isolated from rat liver membranes. Biochem J. 1986;236:559–568. doi: 10.1042/bj2360559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Methods to characterize actin filament networks. Methods Enzymol. 1982;85:211–233. doi: 10.1016/0076-6879(82)85022-2. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Prall F, Nollau P, Neumaier M, Haubeck HD, Drzeniek Z, Helmchen U, Löning T, Wagener C. CD66a (BGP), an adhesion molecule of the carcinoembryonic antigen family, is expressed in epithelium, endothelium, and myeloid cells in a wide range of normal human tissues. J Histochem Cytochem. 1996;44:35–41. doi: 10.1177/44.1.8543780. [DOI] [PubMed] [Google Scholar]
- Rees-Jones RW, Taylor SI. An endogenous substrate for the insulin receptor-associated tyrosine kinase. J Biol Chem. 1985;260:4461–4467. [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Riethdorf L, Lisboa BW, Henkel U, Naumann M, Wagener C, Loning T. Differential expression of CD66a (BGP), a cell adhesion molecule of the carcinoembryonic antigen family, in benign, premalignant and malignant lesions of the human mammary gland. J Histochem Cytochem. 1997;45:957–963. doi: 10.1177/002215549704500705. [DOI] [PubMed] [Google Scholar]
- Rojas M, DeMarte L, Screaton RA, Stanners CP. Radical differences in functions of closely related members of the human carcinoembryonic antigen gene family. Cell Growth Differ. 1996;7:655–662. [PubMed] [Google Scholar]
- Rosenberg M, Nédellec P, Jothy S, Fleiszer D, Turbide C, Beauchemin N. The expression of mouse biliary glycoprotein, a carcinoembryonic antigen-related gene, is down-regulated in malignant mouse tissues. Cancer Res. 1993;53:4938–4945. [PubMed] [Google Scholar]
- Royal I, Park M. Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- Schliwa M. Action of cytochalasin D on cytoskeletal networks. J Cell Biol. 1982;92:79–91. doi: 10.1083/jcb.92.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz KM, Campbell KD, Ahmed K, Skubitz APN. CD66 family members are associated with tyrosine kinase activity in human neutrophils. J Immunol. 1995;151:5382–5390. [PubMed] [Google Scholar]
- Tanaka K, Hinoda Y, Takahashi H, Sakamoto H, Nakajima Y, Imai K. Decreased expression of biliary glycoprotein in hepatocellular carcinomas. Int J Cancer. 1997;74:15–19. doi: 10.1002/(sici)1097-0215(19970220)74:1<15::aid-ijc3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Tarone G, Ferracini R, Galetto G, Comoglio P. A cell surface integral membrane glycoprotein of 85,000 mol wt (gp85) associated with Triton X-100-insoluble cell skeleton. J Cell Biol. 1984;99:512–519. doi: 10.1083/jcb.99.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Invest. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- Tsukita Sh, Tsukita Sa, Nagafuchi A, Yonemura S. Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol. 1992;4:834–839. doi: 10.1016/0955-0674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Virji M, Makepeace K, Ferguson DJP, Watt SM. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of phatogenic neisseriae. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- Weiss EE, Kroemker M, Rudiger AH, Jockusch BM, Rudiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between alpha-catenin and vinculin. J Cell Biol. 1998;141:755–764. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessner DR, Shick PC, Lu JH, Cardellichio CB, Gagneten SE, Beauchemin N, Holmes KV, Dveksler GS. Mutational analysis of the virus and monoclonal antibody binding sites in MHVR, the cellular receptor of the murine coronavirus mouse hepatitis virus strain MHV-59. J Virol. 1998;72:1941–1948. doi: 10.1128/jvi.72.3.1941-1948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol. 1998;140:885–895. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, Preuss D, Mulholland J, O'Brien J, Botstein D, Johnson DI. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in control of cell polarity. Mol Cell Biol. 1993;4:1307–1316. doi: 10.1091/mbc.4.12.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]