Abstract
The crossed intermolecular rhodium-catalyzed [2 + 2 + 2] carbocyclization of carbon and heteroatom tethered 1,6-enynes can be accomplished with symmetrical and unsymmetrical alkynes, to afford the corresponding bicyclohexadienes in an efficient and highly selective manner.
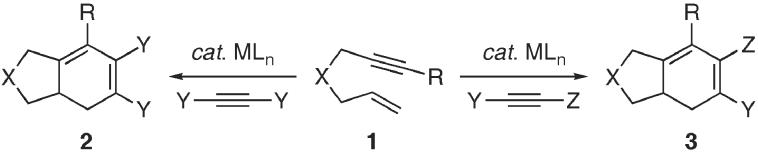
Transition metal-catalyzed carbocyclization reactions provide powerful and expeditious methods for the construction of complex polycyclic systems, which are generally not accessible via classical pericyclic reactions.1 The metal-catalyzed [2 + 2 + 2] reaction of a tethered 1,6-diyne is representative of this class of transformations, and has been employed extensively as a tactic for the construction of various natural product skeletons.2 Despite the myriad studies with 1,6-diynes,3,4 the crossed intermolecular rhodium-catalyzed [2 + 2 + 2] carbocyclization with 1,6-enynes has not been forthcoming.5-7 We envisioned that this type of carbocyclization would facilitate the rapid increase in molecular complexity, through the ability to introduce stereoelectronically orthogonal alkynes in a selective fashion. Herein, we now describe the rhodium-catalyzed intermolecular [2 + 2 + 2] carbocyclization of carbon and heteroatom tethered 1,6-enynes 1 with symmetrical and unsymmetrical alkynes, to afford the corresponding bicyclohexadienes 2 and 3 in a highly efficient and regioselective manner, respectively (Scheme 1).
Scheme 1.
Crossed intermolecular metal-catalyzed [2 + 2 + 2] carbocyclization of 1,6-enynes with symmetrical and unsymmetrical alkynes.
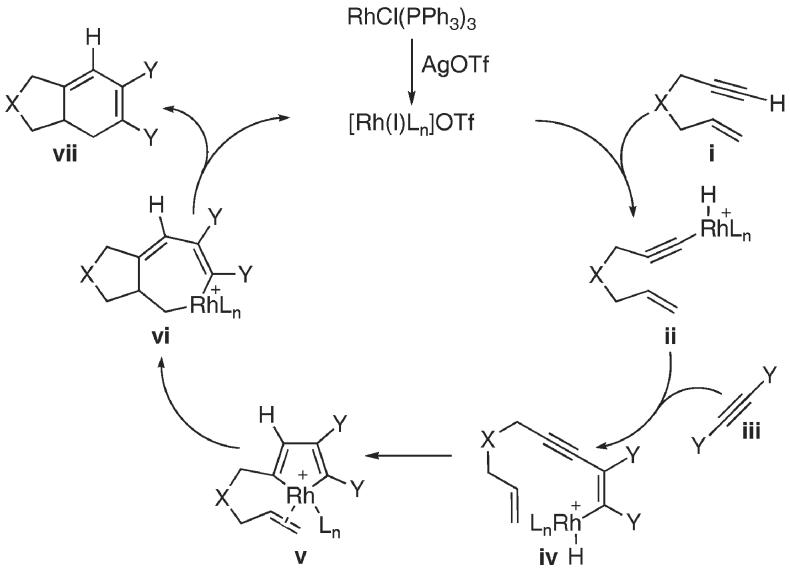
The mechanistic hypothesis for the desired carbocyclization outlined in Scheme 2, draws from the seminal studies of others.2-5 It was anticipated the terminal acetylenic C–H bond of the tethered enyne i should undergo an oxidative insertion to afford ii. Coordination of the free alkyne iii followed by hydrometallation should lead to the formation of iv, en route to the key metallacyclopentadiene v.8 Intramolecular migratory insertion across the tethered alkene, followed by a reductive elimination of metallacycle vi, should then afford the [2 + 2 + 2] carbocyclization adduct vii. We reasoned that selective intermolecular [2 + 2 + 2] carbocyclization would be feasible, since alkyne iii should preferentially undergo hydrometallation with ii, provided the concentration of alkyne iii was such that it would avoid competition with the intramolecular migratory insertion of the alkene in v.
Scheme 2.
Proposed catalytic cycle for the rhodium-catalyzed [2 + 2 + 2] carbocyclization reaction.
Preliminary studies tested this hypothesis by screening various reaction conditions, as outlined in Table 1. Treatment of the enyne 1a (X = NTs, R = H) with silver triflate modified Wilkinson's catalyst in the presence of excess dimethyl acetylenedicarboxylate (Y/Y = CO2Me) in ethanol at 60 °C, furnished the bicyclohexadiene 2a in 17% yield (Entry 1). Additional studies examined the effect of solvent (Entries 1–3), concentration (Entries 3–5), and temperature (Entries 5–7), in an attempt to optimize for the desired carbocyclization reaction. Gratifyingly, the non-coordinating solvent benzene (0.05 M) at 60 °C proved optimum, affording 2a in 95% yield (Entry 5).9 Interestingly, the homodimerization adduct was not observed, whereas the product resulting from the competitive trapping of the metallacyclopentadiene v with a second equivalent of dimethyl acetylenedicarboxylate was produced in varying amounts, depending upon the concentration. Furthermore, the terminal acetylenic proton of the 1,6-enyne 1a proved to be crucial for catalytic activity, since the corresponding substituted derivatives, where R = Me, Ph or TMS, did not afford any of the carbocyclization adduct.3-5,7
Table 1.
Development of the rhodium-catalyzed [2 + 2 + 2] carbocyclization reaction (Scheme 1; 1a where X = NTs, R = H and Y/Y = CO2Me)a
| Entry | Conc. (mol/L) | Solvent | Temp. (°C) | Yield (%)b |
|---|---|---|---|---|
| 1 | 0.10 | EtOH | 60 | 17 |
| 2 | ” | MeCN | ” | 42 |
| 3 | ” | PhH | ” | 80 |
| 4 | 0.50 | ” | ” | 57 |
| 5 | 0.05 | PhH | 60 | 95 |
| 6 | ” | ” | 40 | 48 |
| 7 | ” | ” | 80 | 82 |
All reactions were carried out on a 0.25 mmol reaction scale utilizing 10 mol% of Wilkinson's catalyst [RhCl(PPh3)3] modified with 20 mol% of silver triflate with dimethyl acetylenedicarboxylate (3 equiv.) under an atmosphere of argon.
HPLC yield.
Table 2 outlines the examination of the influence of various tethers and the scope of alkyne substituents that are tolerated. This study demonstrated that nitrogen, carbon, and oxygen containing tethered enynes furnish the corresponding bicyclohexadienes 2a–i in good to excellent yield. Examination of the alkyne scope revealed that the reaction was optimal for the 1,2-disubstituted alkynes bearing electron withdrawing groups, i.e. dimethyl acetylenedicarboxylate, bis-phenyl ketone and bis-dimethylamide, albeit slightly less efficient in the latter case. Interestingly, diphenylacetylene (Y/Y = Ph) furnished only a trace amount of the carbocyclization product, thereby confirming the necessity for a strongly electron withdrawing group on the alkyne.
Table 2.
Scope of the intermolecular rhodium-catalyzed [2 + 2 + 2] carbocyclization reaction (Scheme 1; where R = H)
| 1,6-Enyne 1a | 5,6-Bicyclohexadiene 2 | ||||
|---|---|---|---|---|---|
| Entry | X = | Y/Y = | Yield (%)b | ||
| 1 | TsN | a | CO2Me | a | 92 |
| 2 | ” | ” | COPh | b | 84 |
| 3 | ” | ” | CONMe2 | c | 61 |
| 4 | C(CO2Me)2 | b | CO2Me | d | 85 |
| 5 | ” | ” | COPh | e | 74 |
| 6 | ” | ” | CONMe2 | f | 54 |
| 7 | O | c | CO2Me | g | 84 |
| 8 | ” | ” | COPh | h | 81 |
| 9 | ” | ” | CONMe2 | i | 55 |
All reactions were carried out on a 0.25 mmol reaction scale utilizing 10 mol% of Wilkinson's catalyst [RhCl(PPh3)3] modified with 20 mol% of silver triflate with the requisite 1,2-disubstituted alkyne (3 equiv.) under an atmosphere of argon.9
Isolated yield.
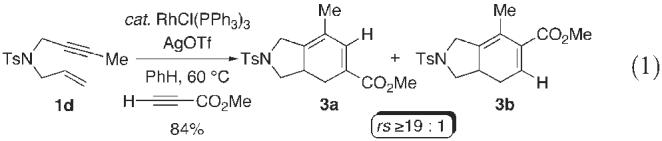
In order to further demonstrate the potential synthetic utility of this transformation and the importance of a terminal alkyne, we elected to examine the regioselective version utilizing an unsymmetrical alkyne (eqn. 1).10 Treatment of the 1,6-enyne 1d with methyl propiolate in the presence of Wilkinson's catalyst modified with silver triflate, furnished the azabicycles 3a/3b in 84% yield, in a highly regioselective manner .11 The regioselective outcome for this transformation was confirmed by X-ray crystallographic analysis of 3a.§
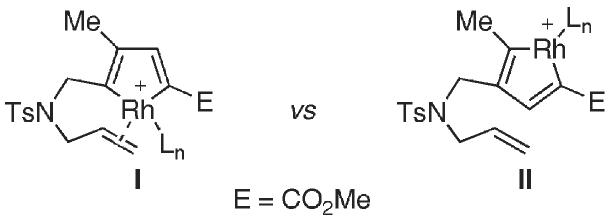
The origin of the excellent regioselectivity can be rationalized by considering the putative metallacycle intermediates, as outlined in Fig. 1. We assume that provided the metallacycle intermediates are formed in an analogous manner outlined in Scheme 2, the 1,6-enyne 1d with methyl 2-propiolate should lead to the formation of I and II, in which II would be unable to undergo the intramolecular carbocyclization due to geometrical constraints.12 The efficiency of the carbocyclization and absence of homodimerization products are consistent with the notion that either the metallacycle formation is reversible, allowing the equilibration of I and II, or that I is formed exclusively. Additional studies are now underway to elucidate the origin of the regioselectivity.
Fig. 1.
Analysis of the plausible regioisomeric metallacycle intermediates.
In conclusion, we have developed a crossed intermolecular rhodium(I)-catalyzed [2 + 2 + 2] carbocyclization reaction with carbon and heteroatom tethered 1,6-enynes using symmetrical 1,2-disubstituted alkynes. This study demonstrates that a terminal alkyne is crucial for good reactivity, and the nature of the bis-carbonyl 1,2-substituted alkyne impacts on the overall efficiency of this transformation. Moreover, this study also illustrates that analysis of the putative metallacycle provides insight into controlling the regioselective intermolecular rhodium-catalyzed [2 + 2 + 2] carbocyclization with terminal alkynes. Finally, this work provides novel and exciting possibilities for the synthesis of bicyclohexadiene derivatives that are applicable to target directed synthesis.
We sincerely thank the National Institutes of Health (GM58877) for generous financial support. We also thank Johnson and Johnson for a Focused Giving Award, and Pfizer Pharmaceuticals for the Creativity in Organic Chemistry Award (PAE).
Supplementary Material
Footnotes
Electronic supplementary information (ESI) available: experimental procedures, X-ray crystallographic analysis of 3a, and spectral data (IR, 1H and 13C-NMR) including High Resolution MS for 2a–i and 3a. See http://www.rsc.org/suppdata/cc/b5/b505383h/index.sht
Crystal structure data for 3a: C18H21NO4S, colorless, monoclinic, P21/c, a = 5.9474(8) Å, b = 19.586(2) Å, c = 14.4749(18) Å, β = 97.260(4)°, V = 1672.6(4) Å3, Z = 4, T = 119(2) K, ρcalc = 1.380 Mg/m3, μ = 0.216 mm−1, GOF = 0.864, R(F) = 0.0496 and wR(F2) = 0.1169 for 2166 observed reflections I > 4. CCDC 269096. See http://www.rsc.org/suppdata/cc/b5/b505383h/index.sht for crystallographic data in CIF or other electronic format.
Notes and references
- 1.Aubert C, Buisine O, Malacria M. Chem. Rev. 2002;102:813. doi: 10.1021/cr980054f. For a recent review on metal-catalyzed carbocyclization reactions involving 1,n-enynes, see: and pertinent references cited therein. [DOI] [PubMed] [Google Scholar]
- 2.(a) Malacria M, Aubert C, Renaud JL. In: Science of Synthesis: Houben-Weyl Methods for Molecular Transformations. Lautens M, Trost BM, editors. Vol. 1. Georg Thieme Verlag; New York: 2001. pp. 439–530. For recent reviews on metal-mediated [2 + 2 + 2] cyclizations, see: [Google Scholar]; (b) Varela J, Saá C. Chem. Rev. 2003;103:3787. doi: 10.1021/cr030677f. [DOI] [PubMed] [Google Scholar]; (c) Fujiwara M, Ojima I. In: Modern Rhodium-Catalyzed Organic Reactions. Evans PA, editor. 2005. pp. 129–150. Ch. 7. and pertinent references cited therein. [Google Scholar]
- 3.(a) Grigg R, Scott R, Stevenson P. J. Chem. Soc., Perkin Trans. 1988;1:1357. For pioneering work on the rhodium-catalyzed intermolecular [2 + 2 + 2] carbocyclization using tethered 1,6-diynes and 1,6-enynes, see: [Google Scholar]; (b) Grigg R, Scott R, Stevenson P. J. Chem. Soc., Perkin Trans. 1988;1:1365. [Google Scholar]
- 4.(a) McDonald FE, Zhu HYH, Holmquist CR. J. Am. Chem. Soc. 1995;117:6605. For some recent examples of intermolecular rhodium-catalyzed [2 + 2 + 2] carbocyclizations of tethered 1,6-diynes, see: [Google Scholar]; (b) Witulski B, Stengel T. Angew. Chem., Int. Ed. 1999;38:2426. [PubMed] [Google Scholar]; (c) Nishiyama H, Niwa E, Inoue T, Ishima Y, Aoki K. Organometallics. 2002;21:2572. [Google Scholar]; (d) Kinoshita H, Shinokubo H, Oshima K. J. Am. Chem. Soc. 2003;125:7784. doi: 10.1021/ja035438o. and pertinent references cited therein. [DOI] [PubMed] [Google Scholar]
- 5.Oh CH, Sung HR, Jung SH, Lim YM. Tetrahedron Lett. 2001;42:5493. For a recent example of a rhodium-catalyzed [2 + 2 + 2] dimerization of 1,6-enynes, see: [Google Scholar]
- 6.(a) Trost BM, Tanoury GJ.J. Am. Chem. Soc 19871094753.For examples of an intermolecular metal-catalyzed [2 + 2 + 2] carbocyclization with various 1,6-enynes using symmetrical alkynes, see:Pd: [Google Scholar]; (b) Kezuka S, Okado T, Niou E, Takeuchi R.Org. Lett 200571711Ir: [DOI] [PubMed] [Google Scholar]
- 7.(a) Ohshita J, Furumori K, Matsuguchi A, Ishikawa M. J. Org. Chem. 1990;55:3277. [Google Scholar]; (b) Field LD, Ward AJ, Turner P. Aust. J. Chem. 1999;52:1085. [Google Scholar]
- 8.(a) Tanaka K, Fu GC. J. Am. Chem. Soc. 2001;123:11492. doi: 10.1021/ja011907f. For a trans-hydrometalation with rhodium, see: [DOI] [PubMed] [Google Scholar]; (b) Tanaka K, Fu GC. Angew. Chem., Int. Ed. 2002;41:1607. doi: 10.1002/1521-3773(20020503)41:9<1607::aid-anie1607>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Representative procedure for the rhodium-catalyzed [2 + 2 + 2] carbocyclization: Wilkinson's catalyst (23.1 mg, 10 mol%) and silver triflate (12.8 mg, 20 mol%) were suspended in anhydrous benzene (4.0 mL) under an atmosphere of argon. The catalyst was then placed in a preheated oil bath at 60 °C for ca. 5 minutes, before dimethyl acetylenedicarboxylate (100.6 mg, 0.75 mmol) was added via a tared microliter syringe. In a separate flask, enyne 1a (62.3 mg, 0.25 mmol) was dissolved in anhydrous benzene (0.5 mL), and placed in the 60 °C bath for ca. 5 minutes, before being transferred via Teflon® cannula to the preformed catalyst solution (2 × 0.25 mL rinse). The reaction mixture was allowed to stir at 60 °C for ca. 12 hours under an inert atmosphere of argon (t.l.c. control), before being concentrated in vacuo to afford the crude product as a brown solid. Purification by flash chromatography (gradient elution 20–40% ethyl acetate/hexanes) furnished the azabicycle 2a (90 mg, 92%) as a white crystalline solid (mp 139–148 °C, dec.).
- 10.Chang C-A, King JA, Jr., Vollhardt KPC. J. Chem. Soc., Chem. Commun. 1981:53. For an example of an intermolecular cobalt-mediated [2 + 2 + 2] carbocyclization with various 1,6-enynes using an unsymmetrical alkyne, see: [Google Scholar]
- 11.The alkyne was added via syringe pump to avoid competitive dimerization of the enyne 1d, which proved problematic for this particular carbocyclization.
- 12.Tanaka K, Shriasaka K. Org. Lett. 2003;5:4697. doi: 10.1021/ol035963s. For an example of complementary regiochemistry in metallacycle formation, see: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.