Abstract
Recombinant adenoviral vectors (adenovectors) have been subject to various genetic modifications to improve their transduction efficiency and targeting capacity. Production and purification of adenovectors with modified capsid proteins can be problematic using conventional two-cycle CsCl gradient ultracentrifugation. We have developed a new method for purifying recombinant adenovectors in two steps: iodixanol discontinuous density gradient ultracentrifugation and size-exclusion column chromatography. The purity and infectious activity of adenovectors isolated by either method were comparable. The new method yielded three to four times more adenovectors with RGD-modified fiber proteins than did the conventional CsCl method. For other fiber-modified and wild-type adenovectors, the yields of the two methods were comparable. Thus, the iodixanol-based method can be used not only to improve the production of RGD-modified adenovectors, but also to purify adenovectors with or without fiber modifications. Moreover, the whole procedure can be completed in 3 hours. Therefore, this method is rapid and efficient for production recombination adenovectors, especially those with RGD-modified fibers.
Adenoviral vectors (adenovectors) have high in vivo transduction efficiencies and are easy to construct and produce. They are thus frequently used for in vitro and in vivo gene delivery and for gene therapy in clinical trials [1] . Substantial efforts have been made to characterize adenovirus-host cell interactions and to improve gene delivery by adenovectors, including minimizing vector gene expression, reducing vector related toxicity and immunogenicity, increasing their capacity to accommodate large foreign genes, and increasing their transduction efficiency.
It is now known that adenovirus interacts with host cells by binding to the coxsackievirus and adenovirus receptor (CAR) [2], a cellular receptor for Ad5 and most other adenovirus serotypes. After attachment, the virion is then internalized by endocytosis through the interaction of its penton base with αvβ3 and αvβ5 integrins on the host cell surface [3]. However, there is growing evidence that the expression of CAR is frequently suppressed in various types of cells, including tumor cells and in primary tumors [4,5], resulting in resistance to adenovirus infection [6–8]. Nevertheless, various studies have shown that adenovectors with modified capsid proteins, such as additions of polylysine or RGD sequence to fibers, can effectively infect cells with defective CAR expression [9–11]. Thus, modified adenovectors with increased transduction ability or targeting capacity may be useful for various gene transfer or gene therapy studies or clinical applications.
Unfortunately, technical difficulties may be encountered in production of certain modified adenovectors. We have encountered difficulties when producing certain adenovectors by this method, especially those with RGD-modified fibers or “sticky” virus. For example, we have experienced difficulty in purifying various RGD-modified adenoviral vectors by conventional two-cycle (2x) of CsCl ultracentrifugation because the RGD-modified adenoviral vectors tend to aggregate, especially in the second round of ultracentrifugation, forming floccules without a sharp band. This results in a low yield or even failure of purification. Thus, an alternative method for efficient production of these modified adenovectors is ultimately required.
In this study, we developed a simple and rapid two-step method for efficient purification of RGD-modified adenovectors and conventional adenovectors with wild-type fiber proteins. We used iodixanol as the ultracentrifugation gradient medium because this agent has been successfully used to isolate cells [12,13], organelles [14,15], macromolecules [16,17], and viruses [18–22]. Using iodixanol discontinuous density ultracentrifugation followed by size-exclusion chromatography, we successfully isolated and purified both RGD-modified and non-RGD-modified adenovectors with a degree of purity and infectivity comparable to that of adenovectors purified by traditional 2× CsCl ultracentrifugation. Our new iodixanol/column method saved labor and time and substantially improved the yield of fiber-modified adenovectors.
Materials and methods
Reagents and Chemicals
OptiPrep™, a 60% (w/v) water solution of iodixanol, was obtained from Accurate Chemical & Scientific Corp. (Westbury, NY). Sephadex G100 super fine (SF) was obtained from Amersham Biosciences (Uppsala, Sweden).
Adenoviruses
All RGD-modified (Ad/RGD-eGFP, Ad/RGD-LacZ, and Ad/RGD-TRAIL) and conventional (Ad/CMV-GFP and Ad/CMV-LacZ) adenovectors used in this study were constructed in our laboratory. The expansion, purification, titration, and quality analyses of the adenoviruses were performed and analyzed at the Institutional Vector Core Facility at the University of Texas M. D. Anderson Cancer Center as described previously [23,24].
Cells and cell culture
Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Norcross, GA). All cell culture reagents were obtained from GIBCO (Grand Island, NY). Transformed human embryonic kidney cells (HEK 293 cells) were propagated in Dulbecco’s modified Eagle’s high-glucose medium (DMEM) supplemented with 10% fetal bovine serum and maintained at 37°C in a humidified incubator with 5% CO2. Cells were seeded in 15-cm tissue culture dishes at a density of 2.5 x 106 cells in 20 ml of the medium. When the cell monolayers reached about 60–70% confluency, 2–2.5 days after seeding, virus was thoroughly mixed with fresh media and added to the cells at a multiplicity of infection (MOI) of 1000 viral particles (VPs). The cells were collected when substantial cytopathic effect was observed, 2 to 3 days after infection. Human non-small cell lung cancer (H1299) cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and maintained at 37°C in a humidified incubator with 5% CO2.
Conventional 2x CsCl ultracentrifugation
Infected 293 cells from 25–50 15-cm tissue culture plates were collected and centrifuged in a Beckman CS-6R desktop centrifuge at 1500 rpm for 5 min. The cells were then resuspended in the culture medium without fetal bovine serum and lysed by three freeze-thaw cycles. Cell lysates were clarified to remove cellular debris by centrifugation in a Beckman CS-6R at 3000 rpm for 10 min.
For 2x CsCl ultracentrifugation, the supernatant was layered onto a discontinuous gradient of 1.5 g/ml, 1.35g/ml, and 1.5 g/ml CsCl in phosphate buffered saline (PBS, pH7.5) and centrifuged at 35,000 rpm for 60 min at 10ºC in a Beckman Optima XL-6 using a Beckman SW40Ti swinging bucket rotor. The lower virus band from each tube was collected, pooled, mixed with 1.35 g/ml CsCl, and centrifuged overnight at 35,000 rpm at 10°C in a Beckman SW40Ti swinging bucket rotor. The virus band from each tube was collected and pooled. The final virus pool was then transferred into Slide-A-Lyzer dialysis cuvette (Pierce, Rockford, IL) with molecular weight cutoff of 10,000 and dialyzed extensively against dialysis/storage buffer containing 10mM Tris pH7.5, 10% glycerol, and 1mM MgCl2 . The virus titer (in viral particles/ml) was determined by the absorbency of the dissociated virus at A260 nm [1 A260 nm unit = 1012 viral particles (VPs/ml)]. The titers (in infectious units/ml) were determined by median tissue culture infective dose assay (TCID50).
Iodixanol discontinuous density gradient ultracentrifugation
Adenovirus-infected 293 cell lysates were prepared as described for 2x CsCl ultracentrifugation. Iodixanol was diluted with PBS containing 1 mM MgCl2 and 25 mM KCl. The iodixanol discontinuous density gradient was formed by carefully underlaying 2ml of 15% (w/v) iodixanol in PBS containing 1 M NaCl; 2ml of 25% (w/v) iodixanol; 2 ml 40% (w/v) iodixanol; and 0.5 ml of 54% (w/v) iodixanol. The discontinued gradient was made by carefully underlayering 6.5 ml of clarified cell lysate with 2 ml of 15% iodixanol, 2 ml of 25% iodixanol, 2 ml of 40% iodixanol and 0.5 ml of 54% iodixanol beginning with the densest solution. The gradient was then centrifuge at 35,000 rpm using a Beckman SW40Ti swinging bucket rotor for 1 h at 10 ºC. The clear, sharp band formed by the adenovector in 25%/40% density interface was collected and pooled in as small a volume as possible. The pooled virus/iodaxnol fraction was further purified by size-exclusion chromatography.
Size-exclusion chromatography
The Sephadex G100 SF resin was packed in a sterile 1.5 x 12 cm disposable Econo-Pac polypropylene column (BIO-RAD, Hercules, CA) with maximum bed volume of 20 ml. The packed column was equilibrated with at least three times the column volume of virus dialysis/storage buffer containing 10 mM Tris-Cl (pH7.5), 1 mM MgCl2, and 10% glycerol. Up to 5 ml of the adenovirus/iodaxnol fraction from the iodixanol ultracentrifugation was then applied onto the column without disturbing the resin surface. Adenovirus was eluted with the same equilibration buffer under gravity. During elution, 0.5 ml or 1 ml of each fraction was collected. The first 4 – 5 ml of the eluent was discarded, and the virus was collected from the following 5–6 ml. The A260 of each fraction was determined by ultraviolet (UV) Spectraphotometry. Fractions containing most of the virus were either dialyzed at 4ºC against 1,000 ml of dialysis/storage buffer with agitation overnight or directly aliquoted and stored at −80 ºC.
SDS-PAGE and silver stain analysis
For silver staining, 100 μl of column-purified adenovectors (approximately 1 x 1011 VP) were mixed with 40 μl of Laemmli lysis buffer, boiled for 5 min, and spin for 10 min at 30,000 rpm in an Eppendoff centrifuge. The protein concentration was determined using a MicroBCA assat kit according to protocol provided (Pierce, Rockford, IL). Equal amounts of the supernatant of each sample (normalized by protein concentration) were then separated by 10% SDS-PAGE for 3 hours at 120V. Silver staining of the gel was performed using a ProteoSilver™ Plus silver stain kit according to the manufacturer’s protocol provided (Sigma-Aldrich, Steinheim, Germany).
FPLC chromatography analysis
Fast protein liquid chromatography (FPLC) analysis was performed with a Biologic DuoFlow chromatography system (Bio-Rad Laboratories Inc. Hercules, CA) to evaluate virus purity and iodixanol and cellular protein contamination. A size-exclusion column pre-packed with Superose 12 10/300 GL (Amersham Biosciences AB, SE-751 84 Uppsala, Sweden) was pre-equilibrated in eluent (50mM NaH2PO4 and 0.1M KCl) at a flow rate of 1.5ml/min. After column purification, 100 μl of purified viral samples, with or without dialysis, were diluted 10 folds with the eluent. Chromatography was monitored by UV spectrophotometry at 280 nm. 1.0 ml of sample was injected and analyzed each time.
Biochemical analysis
Green fluorescent protein (GFP) expression in cultured cells was determined by fluorescence microscopy. H1299 cells were cultured as described in Materials and Methods. Cells were seeded in 6-well tissue culture dish at a density of 3.5 x 105 cells/well. After 20 hours, the cells were infected with virus at various multiplicity of infection (MOI) of viral particles (VPs) as indicated. GFP expression was revealed 24 hours after infection under a fluorescence microscope.
Animal experiments
Animal experiments were carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 85–23) and M. D. Anderson Cancer Center’s institutional guidelines.
Four groups of female and male BALB/c mice, three of each tested group, were injected in their tail veins with either PBS (as a mock control) or column-purified Ad/RGD-LacZ (without dialysis) at 1 × 1011, 5 × 1011, or 1 x 1012 VP in 100–200 μl of PBS, respectively. The mice were killed after virus administration, and their liver tissue was collected for β-galactosidase assay and pathologic evaluation. Histopathologic analysis was performed in the histology laboratory of the Department of Veterinary Medicine and Surgery at M. D. Anderson Cancer Center.
Results
Purification of adenovirus by iodixanol gradient ultracentrifugation
To test whether adenovirus can be purified by iodixanol-based ultracentrifugation, we applied adenovirus-infected 293 cell lysates to an iodixanol discontinuous density gradient as described in Material and Methods. We used several RGD-modified recombinant adenovectors that had caused extensive aggregation during CsCl gradient ultracentrifugation (Fig. 1.). This aggregation is not observed in non-modified adenovectors, or in adenovectors whose fiber was modified with 21 polylysines (Ad/K21) or replaced with fiber from adenovirus serotype 35 (Ad35) [25]. All of the tested adenovectors, with or without fiber modifications, formed a clear and sharp band at the 25/40% iodixanol interface after 1 h of ultracentrifugation (Fig. 1). No aggregation or uneven turbidity was observed. Another well-formed band, presumably consisting mainly of cellular proteins, was seen at the 15/25% iodixanol interface (middle band). Cellular debris was exclusively found at the 15% iodixanol/cell lysate interface (upper band).
Fig. 1.

Banding of adenovectors by CsCl (top) and Iodixanol (bottom) ultracentrifugation. CsCl purified samples were photographed after second round of CsCl ultracentrifugation. Iodixanol purified samples were photographed after ultracentrifugation in iodixanol. Numbers 1–6 represent Ad-RGD/TRAIL, Ad-RGD/eGFP, Ad/k21, Ad/S35, Ad/CMV-TRAIL, Ad/CMV-GFP, respectively. The (◂) indicates the band of concentrated adeonoviral particles after ultracentrifugation.
Removal of iodixanol by size-exclusion chromatography
The adenovirus preparation from the iodixanol gradient ultracentrifugation step contained a high concentration of iodixanol. For effective determination of viral particles by absorbance and calculation of particle/infectious unit ratios [23], it was crucial to remove the iodixanol from the purified virus/iodixanol fraction. However, iodixanol was very difficult to remove by simple dialysis under the same conditions used for desalting of CsCl fractions, even using tubing with a molecular weight cutoff of 25,000 Dalton. Moreover, the volume of purified adenovirus expanded substantially during dialysis, presumably because water or small molecules diffused into the dialysis tubing. We therefore attempted to remove the iodixanol from the purified adenovirus by size-exclusion chromatography. Iodixanol has a molecular weight of 1,550 Dalton, which is within the fractionation range of Sephadex G100 SF (1,000–100,000). We also reasoned that the Sephadex G100 SF would provide extra purification by separating the large adenovirus particles from most of the cellular proteins, RNA, and DNA, making a second round of ultracentrifugation unnecessary. Thus, we applied the viral band collected after a single round of iodixanol density gradient ultracentrifugation to a Sephadex G100 SF chromatography column.
Figure 2 shows a typical chromatography profile of a fraction of density gradient-purified Ad/RGD-TRAIL/iodixanol. A total of 4 ml of pooled fractions was applied onto a column manually packed with Sephadex G100 SF and was eluted with virus storage buffer by gravity. The A260 of each fraction was measured by UV spectrophotometry. In a 20-ml resin column, virus particles were first eluted from the column in the 7th ml of eluent. Most of the viral particles were eluted from the column between 7th to 13th ml of eluent. In contrast, iodixanol was eluted from the column after about 16 ml of eluent, indicated by a sharp increase in A260 that quickly increased beyond the reading limit of the spectrophotometer. The A260 of the three 1-ml eluent fractions between the absorbent peaks representing viral particles and iodixanol were almost at background level. These results indicate that iodixanol can be separated from the purified adenovectors, and therefore removed from the adenovirus/iodixanol pool, by Sephadex G100 SF size-exclusion column chromatography.
Fig 2.
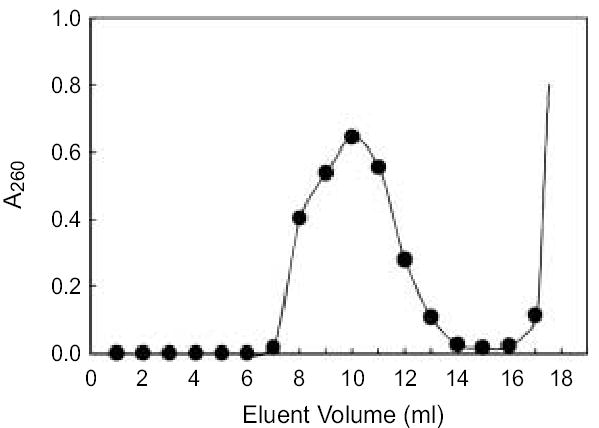
Sephadex G100SF column elution profile. 4 ml of Ad/RGD-TRAIL/ Iodixanol fraction pool were loaded on Sephadex G100 SF column (20 ml bed volume). Virus was eluted by dialysis/storage buffer. Each eluant fraction was collected by 1.0 ml as described.
Purity of adenovirus
To test whether one cycle of iodixanol ultracentrifugation plus Sephadex chromatography can yield sufficient purity of adenovectors for in vitro and in vivo studies, we assessed the purity of adenovirus samples by size-exclusion FPLC analysis monitored by UV detection at 280 nm and by SDS-PAGE silver-stain assays.
The purified adenovectors produced a single, sharp, symmetric peak with a retention time of 7 min on the FPLC profile (Fig. 3B–D), whereas the iodixanol standard produced a single peak with a retention time of about 15 min (Fig. 3A). There was no other detectable peak representing DNA, major protein, and cell debris contamination. Occasionally, however, some preparations of adenovirus had a very small peak at the position representing iodixanol (Fig. 3C), suggesting that some contamination by iodixanol may exist, presumably by over pooling of eluent fractions. Nevertheless, this trace amount of iodixanol contamination can be easily removed by dialysis (Fig. 3D).
Fig 3.
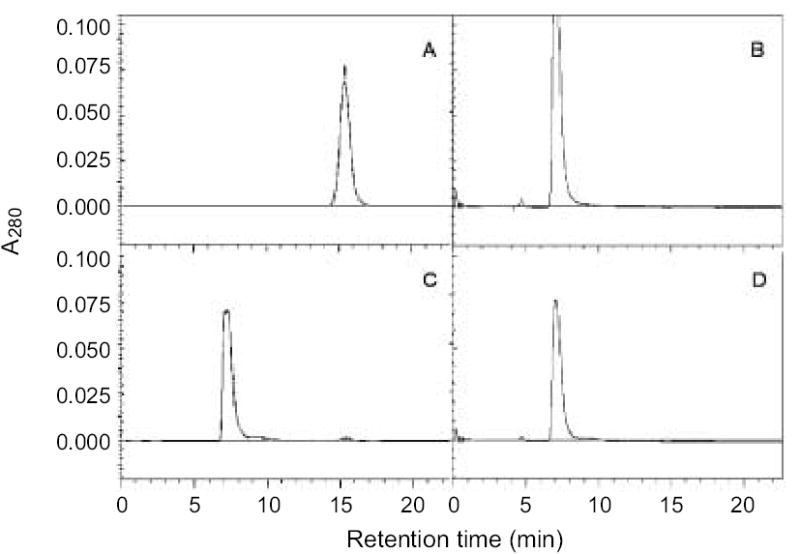
The graphs of FPLC analysis. A size-exclusion Superose TM12 10/300GL column was used for purity analysis. 100 μl of each adenovirus sample purified by either (B) 2x CsCl method or (C, D) iodixanol/column method were analyzed. Each vector was diluted by 10 fold with eluent containing 50mM NaH2PO4 and 0.1mM KCl. 1ml of diluted sample was injected each time and eluted at a flow rate of 1.5 ml/min with UV detection at 280 nm. (A) Iodixanol standard; (B) Ad/CMV-GFP; (C) Ad/RGD-TRAIL without dialysis; and (D) Ad/RGD-TRAIL dialyzed after purification
We also verified the purity of our adenovector samples by SDS-PAGE gel silver stain. Silver staining after SDS-PAGE detected the same viral proteins bands for iodixanol-purified, RGD-modified adenovectors (Ad/RGD-eGFP) and for CsCl-purified, unmodified adenovectors (Fig. 4). The A260/A280 ratios for adenovectors purified by iodixanol-methods were in a range of 1.37 ~ 1.57. In comparison, the ratios for adenovectors purified 2x CsCl banding were in a range of 1.20 ~ 1.40. The cause of this discrepancy in A260/A280 ratio was not clear. A contamination of cellular nucleic acid or iodixanol may lead to a higher A260/A280 ratio. However, our FPLC data showed no detected contamination of cellular DNA or RNA. A trace amount of iodixanol was some times detected by FPLC which could be removed by an additional post-purification dialysis step, a procedure that occasionally reduced A260/A280 ratio. Together, these data indicate that the adenovectors purified by the new method have purity comparable to that of adenovectors obtained by conventional 2× CsCl ultracentrifugation.
Fig 4.

SDS PAGE silver stain analysis. Adenovirus proteins were deassociated in laemmli lysis buffer, separated by 10% SDS polyacrylamide gel electrophoresis, and silver stained. (M) protein molecular weight marker; (1) CsCl-purified Ad/CMV-GFP; (2) CsCl-purified Ad/RGD-eGFP; and (3) iodixanol/column-purified Ad/RGD-eGFP.
Yield and Biological activity of adenovirus purified by iodixanol
We first determined the viral titers of samples produced either by the iodixanol method or by 2× CsCl banding (Table 1). In general, the final concentrations (VP/ml) and total yields of RGD-modified vectors were three to four times more by the iodixanol method than by 2× CsCl banding. In contrast, the vector concentrations and total yields of conventional adenovectors obtained by these two methods were not markedly different, suggesting that these methods are comparable for purification of unmodified adenovectors. The ratios of VP to infectious units achieved by either method were comparable.
Table 1.
Comparison of iodixanol/column and 2x CsCl ultracentrifugation methods
| Adenovirus | Purified by | Mean VP/mla | Mean IU/mla | Mean VP/IU | Yield (VPs)b |
|---|---|---|---|---|---|
| Ad/RGD-eGFP | 2x CsCl | 5.1 x 1011 (3) | 2.4 x 109 (3) | 212 | 1.7 ± 0.8 x 1012 |
| Iodixanol/column | 2.1 x 1012 (3) | 2.8 x 1010 (3) | 74 | 6.1 ± 1.0 x 1012 | |
| Ad/RGD-TRAIL | 2x CsCl | 7.3 x 1011 (7) | 1.0 x 1010 (7) | 73 | 1.9 ± 0.5 x 1012 |
| Iodixanol/column | 4.1 x 1012 (5) | 6.5 x 1010 (5) | 63 | 1.1 ± 0.5 x 1013 | |
| Ad/K21 | 2x CsCl | 3.7 x 1012 (3) | 3.9 x 1010 (3) | 95 | 1.0 ± 0.4 x 1013 |
| Iodixanol/column | 4.5 x 1012 (2) | 6.5 x 1010 (2) | 70 | 1.1 ± 0.8 x 1013 | |
| Ad/S35 | 2x CsCl | 3.0 x 1012 (3) | 3.3 x 1010 (3) | 90 | 9.3 ± 0.1 x 1012 |
| Iodixanol/column | 3.4 x 1012 (2) | 4.5 x 1010 (2) | 76 | 1.2 ± 0.1 x 1013 | |
| Ad/CMV-GFP | 2x CsCl | 2.6 x 1012 (5) | 2.4 x 1010 (5) | 108 | 1.1 ± 0.5 x 1013 |
| Iodixanol/column | 3.7 x 1012 (3) | 2.9 x 1010 (2) | 127 | 1.2 ± 0.6 x 1013 | |
| Ad/CMV-LacZ | 2 x CsCl | 3.6 x 1012 (3) | 9.9 x 1010 (3) | 36 | 1.4 ± 1.0 x 1013 |
| Iodixanol/column | 4.9 x 1012 (2) | 1.0 x 1011 (2) | 48 | 1.9 ± 0.7 x 1013 |
Abbreviation: IU, infectious unit.
The number in parentheses represents the number of separate vector preparations used for data calculation.
The yield of recombinant adenovectors in each row was normalized to 6 x 108 cells from 25 15-cm plates. Data were expressed as mean ± standard deviation.
We also compared transgene expression by the iodixanol- and CsCl-purified adenovectors. GFP expression in cultured cells infected with CsCl-purified Ad/RGD-eGFP was substantially lower than that in cells infected with iodixanol-purified Ad/RGD-eGFP, while GFP expression was very similar in cultured cells infected with conventional Ad/CMV-GFP purified by either method (Fig. 5). These results were consistent with our observations of lower yield and quality of fiber-modified adenovectors produced by the CsCl method.
Fig. 5.

H1299 cells were seeded in 6-well tissue culture dishes at a density of 3.5 x 105 cells/well. Infection was started at the indicated multiplicity of infection (MOI) of viral particles (VPs) 20 hours after seeding. GFP expression was determined using fluorescent microscope.
In our in vivo experiments, up to 1 × 1012 VPs (the highest dose tested) were safely infused into the tail veins of mice without causing death or apparent physical changes. Histopathologic examination of liver sections of mice systemically treated with iodixanol-purified Ad/RGD-LacZ at doses between 1 × 1011 to 1 × 1012 VP showed changes that are commonly observed in animals treated with E-deleted adenovectors, including mild portal triaditis, mild hepatocellular degeneration (cytomegaly and karyomegaly), and apoptosis in 1–3% of hepatocytes. Similar histopathologic changes have been observed in animals treated with CsCl-purified adenovectors at doses of less than 1 × 1011 VP [24,26], suggesting that adenovectors purified by iodixanol are at least as safe as those purified by the CsCl method.
Discussion
In addition to the conventional 2× CsCl method, a number of column-based procedures have been reported for the purification of adenovectors, including ion-exchange [27–31], metal chelation [30], size-exclusion [27,29–31], and affinity [28, 30] chromatography. Although these column-based methods may improve scalability, recovery rate, and sometimes purity of production, they are usually complicated and require multiple steps with more contingent and harsh elution conditions [27, 28, 30, 31] that can be time-consuming and costly. Conventional 2× CsCl gradient centrifugation has therefore remained a common method for mid-scale production of adenovirus in academic laboratories.
In the present study, we developed an iodixanol-based gradient centrifugation method as an alternative to the CsCl method. Our iodixanol method prevented adenovector aggregation during centrifugation, a difficulty encountered during purification of RGD-modified adenovector by CsCl gradient centrifugation, and increased the yield of RGD-modified adenovectors by three- to four-fold compared with the CsCl method. We also tested whether the similar aggregation problem would be encountered when using CsCl or iodixanol gradient centrifugation to purify adenovectors with other modifications, such as fibers bearing polylysine modification or fiber bing replaced with that from adenovirus serotype 35 (Ad/K21 and Ad/S35). We did not see vector aggregation in either purification methods. Purification by 2x CsCl banding or by iodixanol methods resulted in a similar yield and purity of non-modified or Ad/K21 and Ad/S35 vectors. The reason that caused aggregation in RGD-modified adenovectors during CsCl banding is not known, possibly due to altered interactions between virus-virus or virus-cellular proteins. Nevertheless, aggregation of adeno-associated virus (AAV) vectors during CsCl ultracentrifugation was reported previously by others [25]. Use of iodixanol discontinuous gradient centrifugation was reported to solve the aggregation problem of AAV observed during purification by CsCl method and significantly improve infectious titer and yield of AAV vectors [18, 22].
As an iso-osmolal nonionic hydrophilic contrast agent, iodixanol has been used safely as an imaging medium in clinics for intravascular imaging diagnosis of diseases of the heart, brain, kidney, blood vessels and other major organs [32,33]. This agent is also stable in various conditions, both in glass and polypropylene bottles, with a recommended shelf-life of at least 36 months when stored at room temperature and protected from light. As a nonionic density gradient medium, iodixanol has been successfully applied to isolate cells, organelles, macromolecules and viruses. Iodixanol has several potential advantages over other iodinated density gradient media. First its aqueous solutions are iso-osmotic up to a density of 1.32 g/ml and it is capable of forming self-generating gradients in 1 to 3 h. Second, iodixanol is essentially inert; it has shown no measurable cytotoxicity or side effects against any biological molecules assayed thus far in its presence [34]. Thus, trace amounts of iodixanol in adenovirus preparations should not have any notable adverse biological effects. However, we found that iodixanol interfered markedly with the titration of adenoviral particles based on A260. Thus, removing iodixanol from the adenovector preparation was required to ensure precise measurements. We achieved this by size exclusion through a Sephadex resin column. Direct dialysis was not effective in removing large amounts of iodixanol but was able to remove trace amounts remaining after Sephadex chromatography.
Both iodixanol and the Sephadex G resin described in this report are inexpensive and commercially available. No special pretreatment for crude adenoviral samples was required, as described in previously reported column-base methods, such as nuclease treatment, ultrafiltration and condensation [28, 30]. Virus particles can be eluted from column by gravity directly using virus storage buffer, and therefore dialysis step is optional. In addition, the entire procedure, including ultracentrifugation and chromatography, can be completed in 3 h. Furthermore, adenovectors produced by this method were comparable to those isolated by the CsCl method in terms of purity, infectivity, and safety. Thus, our new iodixanol-based method is a simple, economic, efficient alternative for producing recombinant adenovectors, especially those with RGD-modified capsid, “sticky” vectors that are difficult to purify by conventional means.
Acknowledgments
We thank Pierrette Lo for editorial review. This work was supported in part by National Cancer Institute grants RO1 CA 092487-01A1 (to B.F.), RO1 CA 098582-01A1 (to B.F.), P01 CA78778-01A1 (to JAR), Lung Cancer SPORE (2P50-CA70907), Core Grant (CA 16672); by a grant from the Tobacco Settlement Funds as appropriated by the Texas State Legislature (Project 8); by the W. M. Keck Foundation, and by Lockton Grant Matching Funds.
References
- 1.Anonymous. Human gene marker/therapy clinical protocols (complete updated listing) Hum Gene Ther. 2001;12:2251–2337. doi: 10.1089/10430340152710586. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 3.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 4.Okegawa T, Pong RC, Li Y, Bergelson JM, Sagalowsky AI, Hsieh JT. The mechanism of the growth-inhibitory effect of coxsackie and adenovirus receptor (CAR) on human bladder cancer: a functional analysis of car protein structure. Cancer Res. 2001;61:6592–6600. [PubMed] [Google Scholar]
- 5.Kim M, Sumerel LA, Belousova N, Lyons GR, Carey DE, Krasnykh V, Douglas JT. The coxsackievirus and adenovirus receptor acts as a tumour suppressor in malignant glioma cells. Br J Cancer. 2003;88:1411–1416. doi: 10.1038/sj.bjc.6600932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmi S, Geertsen R, Mezzacasa A, Peter I, Dummer R. The presence of human coxsackievirus and adenovirus receptor is associated with efficient adenovirus-mediated transgene expression in human melanoma cell cultures. Hum Gene Ther. 1998;9:2363–2373. doi: 10.1089/hum.1998.9.16-2363. [DOI] [PubMed] [Google Scholar]
- 7.Rauen KA, Sudilovsky D, Le JL, Chew KL, Hann B, Weinberg V, Schmitt LD, McCormick F. Expression of the coxsackie adenovirus receptor in normal prostate and in primary and metastatic prostate carcinoma: potential relevance to gene therapy. Cancer Res. 2002;62:3812–3818. [PubMed] [Google Scholar]
- 8.Wickham TJ. Targeting adenovirus. Gene Ther. 2000;7:110–114. doi: 10.1038/sj.gt.3301115. [DOI] [PubMed] [Google Scholar]
- 9.Roelvink PW, Mi LG, Einfeld DA, Kovesdi I, Wickham TJ. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- 10.Wickham TJ, Roelvink PWDE, Brough DE, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 11.Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel D, Rubbi CP, Rickwood D. Use of density perturbation to isolate immunologically distinct populations of cells. J Immunol Methods. 1993;163:241–251. doi: 10.1016/0022-1759(93)90128-t. [DOI] [PubMed] [Google Scholar]
- 13.Ruedl C, Rieser C, Bock G, Wick G, Wolf H. Phenotypic and functional characterization of CD11c+ dendritic cell population in mouse Peyer's patches. Eur J Immunol. 1996;26:1801–1806. doi: 10.1002/eji.1830260821. [DOI] [PubMed] [Google Scholar]
- 14.Graham J, Ford T, Rickwood D. The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol. Anal Biochem. 1994;220:367–373. doi: 10.1006/abio.1994.1351. [DOI] [PubMed] [Google Scholar]
- 15.Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basi NS, Rebois RV. Rate zonal sedimentation of proteins in one hour or less. Anal Biochem. 1997;251:103–109. doi: 10.1006/abio.1997.2255. [DOI] [PubMed] [Google Scholar]
- 17.Graham JM, Higgins JA, Gillott T, Taylor T, Wilkinson J, Ford T, Billington D. A novel method for the rapid separation of plasma lipoproteins using self-generating gradients of iodixanol. Atherosclerosis. 1996;124:125–135. doi: 10.1016/0021-9150(96)05797-8. [DOI] [PubMed] [Google Scholar]
- 18.Hermens WT, ter,Brake O, Dijkhuizen PA, onnemans MA, Grimm D, Kleinschmidt JA, Verhaagen J. Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous system. Hum Gene Ther. 1999;10:1885–1891. doi: 10.1089/10430349950017563. [DOI] [PubMed] [Google Scholar]
- 19.Moller-Larsen A, Christensen T. Isolation of a retrovirus from multiple sclerosis patients in self-generated Iodixanol gradients. J Virol Methods. 1998;73:151–161. doi: 10.1016/s0166-0934(98)00052-4. [DOI] [PubMed] [Google Scholar]
- 20.Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantly M, Muzyczka N, Byrne BJ. Sustained secretion of human alpha-1 -antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci U S A. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao Y, Olson NH, Xu W, Anderson DL, Rossmann MG, Baker TS. Assembly of a tailed bacterial virus and its genome release studied in three dimensions. Cell. 1998;95:431–437. doi: 10.1016/s0092-8674(00)81773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 23.Anonymous. Assessment of adenoviral vector safety and toxicity: report of the National Institutes of Health Recombinant DNA Advisory Committee. Hum Gene Ther. 2002;13:3–13. doi: 10.1089/10430340152712629. [DOI] [PubMed] [Google Scholar]
- 24.Ji L, Bouvet M, Price RE, Roth JA, Fang B. Reduced toxicity, attenuated immunogenicity and efficient mediation of human p53 gene expression in vivo by an adenovirus vector with deleted E1–E3 and inactivated E4 by GAL4-TATA promoter replacement. Gene Ther. 1999;6:393–402. doi: 10.1038/sj.gt.3300825. [DOI] [PubMed] [Google Scholar]
- 25.Yotnda P, Zompeta C, Heslop HE, Andreeff M, Brenner MK, Marini F. Comparison of the Efficiency of Transduction of Leukemic Cells by Fiber-Modified Adenoviruses. Hum Gene Ther. 2004;15:1229–1242. doi: 10.1089/hum.2004.15.1229. [DOI] [PubMed] [Google Scholar]
- 26.Kagawa S, Pearson SA, Ji L, Xu K, McDonnell TJ, Swisher SG, Roth JA, Fang B. A binary adenoviral vector system for expressing high levels of the proapoptotic gene bax. Gene Ther. 2000;7:75–79. doi: 10.1038/sj.gt.3301048. [DOI] [PubMed] [Google Scholar]
- 27.Blanche F, Cameron B, Barbot A, Ferrero L, Guillemin T, Guyot S, Somarriba S, Bisch D. An improved anion-exchange HPLC method for the detection and purification of adenoviral particles. Gene Ther. 2000;7:1055–1062. doi: 10.1038/sj.gt.3301190. [DOI] [PubMed] [Google Scholar]
- 28.Green AP, Huang JJ, Scott MO, Kierstead TD, Beaupre I, Gao GP, Wilson JM. A new scalable method for the purification of recombinant adenovirus vectors. Hum Gene Ther. 2002;13:1921–1934. doi: 10.1089/10430340260355338. [DOI] [PubMed] [Google Scholar]
- 29.Haruna I, Yaoi H, Kono R, Watanabe I. Separation of adenovirus by chromatography on DEAE-Cellulose. Virology. 1961;13:264–267. doi: 10.1016/0042-6822(61)90065-4. [DOI] [PubMed] [Google Scholar]
- 30.Huyghe BG, Liu X, Sutjipto S, Sugarman BJ, Horn MT, Shepard HM, Scandella CJ, Shabram P. Purification of a type 5 recombinant adenovirus encoding human p53 by column chromatography. Hum Gene Ther. 1995;6:1403–1416. doi: 10.1089/hum.1995.6.11-1403. [DOI] [PubMed] [Google Scholar]
- 31.Kamen A, Henry O. Development and optimization of an adenovirus production process. J Gene Med 6 Suppl. 2004;1:S184–S192. doi: 10.1002/jgm.503. [DOI] [PubMed] [Google Scholar]
- 32.Fountaine H, Harnish P, Andrew E, Grynne B. Safety, tolerance, and pharmacokinetics of iodixanol injection, a nonionic, isosmolar, hexa-iodinated contrast agent. Acad. Radiol 3 Suppl. 1996;3:S475–S484. doi: 10.1016/s1076-6332(05)80362-9. [DOI] [PubMed] [Google Scholar]
- 33.Spencer CM, Goa KL. Iodixanol. A review of its pharmacodynamic and pharmacokinetic properties and diagnostic use as an x-ray contrast medium. Drugs. 1996;52:899–927. doi: 10.2165/00003495-199652060-00013. [DOI] [PubMed] [Google Scholar]
- 34.Ford T, Graham J, Rickwood D. Iodixanol: a nonionic iso-osmotic centrifugation medium for the formation of self-generated gradients. Anal Biochem. 1994;220:360–366. doi: 10.1006/abio.1994.1350. [DOI] [PubMed] [Google Scholar]


