Abstract
Alterations in the expression of the chemokine, fractalkine (CX3CL1), were examined in the urinary bladder after cyclophosphamide (CYP)-induced cystitis of varying duration: acute (4 hour (hr) or 48 hr), or chronic (10 day). CYP-induced cystitis significantly (p ≤ 0.01) increased fractalkine protein expression in the urinary bladder with acute (48 hr) and chronic CYP-treatment. Western blot analysis also demonstrated significantly (p ≤ 0.01) increased fractalkine expression in the whole urinary bladder with acute (1.5–2.2-fold) and chronic (3-fold) CYP-induced cystitis. Immunohistochemistry for fractalkine-immunoreactivity revealed little fractalkine-IR in control or acute (4 hr) CYP-treated rat urinary bladders except in a vascular bed but showed no co-localization with nerve fibers in the suburothelial plexus in any experimental group. However, expression was significantly (p ≤ 0.001) upregulated in the urothelium with 48 hr or chronic CYP-treatment. Similarly, fractalkine receptor (CX3CR1)-IR was significantly (p ≤ 0.001) upregulated in the urothelium with 48 hr or chronic CYP-treatment. These studies demonstrated upregulation of the chemokine, fractalkine, in the urinary bladder and specifically in the urothelium with CYP-induced cystitis. Chemokines, and specifically, fractalkine, may be another class of neuromodulatory agents upregulated in the urinary bladder that can affect micturition function and sensory processing with cystitis and may represent novel, drug targets for cystitis.
Keywords: inflammation, chemokines, urothelium, western blot, ELISA
Introduction
Recent studies with a chemically (cyclophosphamide, CYP)-induced bladder inflammation model have demonstrated alterations in neurochemical (Vizzard, 2000b, 2001), electrophysiological (Yoshimura and de Groat, 1999) and organizational (Vizzard and Boyle, 1999) properties of micturition reflex elements. These changes suggest marked changes in micturition reflexes with CYP-treatment. These changes may be mediated by chemical mediators (e.g., neurotrophins, cytokines, neuropeptides) produced in the bladder, spinal cord or dorsal root ganglia with cystitis (Vizzard, 2000a, 2000b, 2001; Malley and Vizzard, 2002; Braas et al., 2005).
Chemokines, chemotactic cytokines, are another class of neuromodulatory agents that may contribute to inflammatory-induced changes (Wieseler-Frank et al., 2004; Marchand et al., 2005). Chemokine expression has been demonstrated at sites of inflammation (Garcia et al., 2000; Raychaudhuri et al., 2001; Yamashita et al., 2003; Ahn et al., 2004; Nagarsekar et al., 2005; Sung et al., 2005) and recent studies have focused on a specific chemokine, fractalkine, as a potential mediator of nociceptive facilitation acting as a neuron-to-glial signal (Johnston et al., 2004; Milligan et al., 2004; Verge et al., 2004; Lindia et al., 2005). Fractalkine is the sole member of the CX3C class of chemokines and fractalkine or CX3CL1 binds to only one known receptor (CX3CR1) that in turn, binds only fractalkine (Hughes et al., 2002). Constitutive fractalkine expression has been demonstrated in spinal cord neurons, primary afferent cells in the dorsal root ganglia (Verge et al., 2004) and fractalkine is upregulated in astrocytes and microglia in neuropathic pain models (Verge et al., 2004; Lindia et al., 2005). Expression in peripheral sites includes skin, kidney and endothelial cells (Bazan et al., 1997) where expression is upregulated by tumor necrosis factor-alpha (TNF-α), interleukin-1β, and lipopolysaccharide (Garcia et al., 2000; Ahn et al., 2004; Sung et al., 2005).
Patients with interstitial cystitis, a painful, chronic urinary bladder inflammation syndrome, exhibit urinary frequency, urgency and suprapubic and pelvic pain and pain at low to moderate bladder pressure (Petrone et al., 1995) and an involvement of C-fibers has been suggested (Chancellor and Yoshimura, 2004). We hypothesize that chemokines and specifically, fractalkine, expressed in the urinary bladder may contribute to the neuroplasticity of the lower urinary tract following bladder inflammation and contribute to inflammatory-induced changes including bladder overactivity and changes in sensory processing. The present study determined: (1) fractalkine protein expression in the urinary bladder by enzyme-linked immunoassays (ELISAs) and western blotting after CYP-induced cystitis of varying duration; (2) cellular expression of fractalkine and fractalkine receptor in urinary bladder after CYP-induced cystitis using immunohistochemistry with an emphasis on urothelial expression; and (3) density of fractalkine and fractalkine receptor immunoreactivity in the urothelium after CYP-induced cystitis using image analysis software.
Materials and Methods
Adult female Wistar rats (150 – 250g) were purchased from Charles River Canada (St. Constant, Canada). Chemicals used in these studies were purchased from Sigma ImmunoChemicals (St. Louis, MO). Primary antibodies for immunohistochemistry were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies for immunohistochemistry were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Cyclophosphamide (CYP)-induced cystitis
Acute and chronic CYP-induced cystitis rat models were examined in these studies. Chronic CYP (Sigma)-induced cystitis: rats received drug injection (75 mg/kg, intraperitoneal, i.p.) every third day for 10 days. Acute CYP-induced cystitis: rats received a single injection (150 mg/kg, i.p.) and survived for 4 or 48 hr. Control rats received volume-matched injections of saline (0.9%; i.p.) or no treatment. All injections were performed under isoflurane (2%) anesthesia. All experimental protocols involving animal use were approved by the University of Vermont Institutional Animal Care and Use Committee (IACUC # 03-030). Animal care was under the supervision of the University of Vermont’s Office of Animal Care Management in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress or distress.
Tissue Harvesting, sample preparation and assay
Procedures for tissue processing and ELISAs were identical to those described in detail previously. Adult rats were euthanized as above and the bladder (n = 12 for each time point; n = 10 for control) was rapidly dissected and weighed. Individual bladders were solubilized in T-PER Tissue Protein Extraction Reagent (Pierce, Rockford, IL; 1 g tissue/20 ml) with Complete (protease inhibitors cocktail tablets; Roche Diagnostics GmbH, Germany). Bladder tissue was disrupted with a Polytron homogenizer and then centrifuged (10,000 rpm for 5 min). Total protein was determined by the Coomassie Plus Protein Assay Reagent Kit (Pierce). The supernatant was used for fractalkine quantification using commercially available rat specific factalkine/CX3CL1 ELISA kits (DY537; R & D Systems, Minneapolis, MN) in accordance with the manufacturer’s instructions.
Rat Fratalkine/CX3CL1 ELISA
Goat anti-rat fractalkine antibody was adsorbed to microtiter (R&D Systems) plates. After addition of the sample or standard solution, the second antibody (detection antibody) was applied. Sample and standard solutions were run in duplicate. This antibody complex was detected with a horseradish peroxidase-labeled immunoglobulin. Enzyme activity was quantified by the change in optical density, using tetramethyl benzidine as substrate. The fractalkine standard provided with this system generated linear standard curves from 5–2000 pg/ml (r2 = 0.997, P ≤ 0.001). The absorbance values of standards and samples were corrected by subtraction of the background value (absorbance due to nonspecific binding). Samples were diluted to bring the absorbance values onto the linear portion of the standard curve. No samples fell below the minimum detection limits of the assay. Curve fitting of standards and evaluation of fractalkine content of samples was performed using a least squares fit.
Western Blotting for Fractalkine
The bladder was harvested, homogenized and aliquots removed for protein assay as described above. Samples (20 μg) were suspended in sample buffer for fractionation on Tris-Glycine gels (Invitrogen, Carlsbad, CA) and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membranes and efficiency of transfer was evaluated. Membranes were blocked for 1 hour followed by rinsing in Tris-buffered saline + 0.05% Tween (TBST). Membranes were then incubated in rabbit anti-fractalkine (1:1500 in TBST; BioVision, Mountain View, CA)) overnight at 4°C. Washed membranes were incubated in HRP-conjugated goat anti-rabbit IgG (1:5000 in TBST; Jackson, West Grove, PA) for 2 hours at room temperature for enhanced chemiluminescence detection (Amersham, Piscataway, NJ). Blots were exposed to Biomax film (Kodak, Rochester, NY), and developed. The intensity of each band was analyzed (UnScan It; Silk Scientific Inc., Orem, UT) and background intensities subtracted. Recombinant rat fractalkine/ CX3CL1 (2.5 ng; R&D Systems, Minneapolis, MN) was used as a positive control and western blotting of actin (1:20,000; Santa Cruz, Santa Cruz, CA) was used as a loading control. Antibody specificity was confirmed with absorption controls. Preabsorption of fractalkine antisera with appropriate immunogen (1 μg ml−1) reduced staining in blots to background levels.
Immunohistochemistry
Adult rats were euthanized as above and the bladder (n=6 for each time point and control) was rapidly dissected and weighed. Sections of the bladder wall (20 μm) from control and experimental treatments (acute, intermediate, chronic CYP-induced cystitis) were examined for fractalkine and fractalkine receptor immunoreactivity (IR). The tissue was post-fixed in 4% paraformaldehyde, placed in ascending concentrations of sucrose (10–30%) in 0.1 M PBS for cryoprotection, sectioned (20 μm) on a freezing cryostat and directly mounted on gelled (0.5%) microscope slides for on-slide processing as previously described (Vizzard, 1997). Briefly, sections were incubated overnight at room temperature or 72 hr at 4 °C with goat anti-fractalkine (1:1000) or rabbit anti-fractalkine receptor (1:1000) in 1% goat serum and 0.1 M phosphate buffered saline (PBS), and then washed (3 × 10 min) with 0.1 M PBS, pH 7.4. The tissues were then incubated with Cy3-conjugated donkey anti-goat (1:500) or Cy3-conjugated goat anti-rabbit (1:500) secondary antibodies for 2 hr at room temperature. Following washing (3 × 10 min with PBS), the slides were coverslipped with Citifluor (Citifluor Ltd., London). Control sections incubated in the absence of primary or secondary antibody were also processed and evaluated for specificity or background staining levels. In the absence of primary antibody, no positive immunostaining was observed.
Whole Mount Bladder Preparation
Control and CYP-treated rats were euthanized as above. The urinary bladder was dissected and placed in Krebs solution. The bladder was cut open along the midline and pinned to a sylgard-coated dish. The bladder was incubated for 1.5 hr at room temperature in cold fixative (2% paraformaldehyde + 0.2% picric acid) and urothelium removed as previously described (Zvarova and Vizzard, 2005). Urothelium and bladder musculature were processed for fractalkine immunoreactivity (IR). Whole mounts stained for fractalkine were also stained with antiserum to the pan-neuronal marker, protein gene product (PGP) 9.5. Control (n = 3) and CYP-treated tissues (n = 3 for each group) were incubated overnight at room temperature in a cocktail of fractalkine (as above) plus PGP antiserum (1:1000; Biogenesis, NH) in 1% donkey serum and 0.1M potassium PBS. After washing with 0.1 M PBS, the tissues were incubated with Cy2-donkey anti-rabbit antibody (1:200; Jackson) for PGP immunostaining for 2 hr at room temperature, followed by washing and coverslipping.
Assessment of Positively Stained Urinary Bladder Regions
Staining observed in experimental tissue was compared to that observed from experiment-matched negative controls. Urinary bladder sections exhibiting immunoreactivity that was greater than the background level observed in experiment-matched negative controls were considered positively stained. In this study, we have focused on fractalkine- and fractalkine receptor-IR in the urothelium.
Visualization and quantitative analysis of fractalkine and fractalkine receptor –IR in urothelium
Six to 10 urinary bladder sections from control and experimental groups were examined under an Olympus fluorescence photomicroscope with a multiband filter set for simultaneous visualization of the Cy3 and Cy2 fluorophores. Cy3 was visualized with a filter with an excitation range of 560–596 nm and an emission range from 610–655 nm. Cy2 was viewed by using a filter with an excitation range of 447–501 nm and an emission range from 510–540 nm. Grayscale images acquired in tiff format where imported into Meta Morph image analysis software (version 4.5r4; Universal Imaging, Downingtown, PA). The opened image was first calibrated for pixel size by applying a previously created calibration file. The free hand drawing tool was selected and the urothelium was drawn and measured in total pixels area (Fig. 1A, B). We chose to sample a large area of urothelium because larger sampling is more representative of the total area. A threshold encompassing an intensity range of 100–250 grayscale values was applied to the region of interest (Figure 1) in the least brightly stained condition first. The threshold was adjusted for each experimental series using concomitantly processed negative controls as our guide for setting background fluorescence. The same threshold was subsequently used for all images. We considered immunoreactivity to be positive only when the fractalkine-IR exceeded the established threshold. Percent fractalkine expression above threshold in the total area selected was then calculated. Double-labeling in whole-mount preparations of urothelium or detrusor smooth muscle was assessed by confocal scanning laser microscopy (Bio-Rad Laboratories, CA and Zeiss LSM 510 Meta, Carl Zeiss, Inc., NY) as previously described (Dattilio and Vizzard, 2005). For each z-axis interval (1–2 μm), tissue sections were scanned twice using argon lasers with specific excitation wavelengths and sequential images were captured for computer-generated overlay and analysis.
Figure 1.
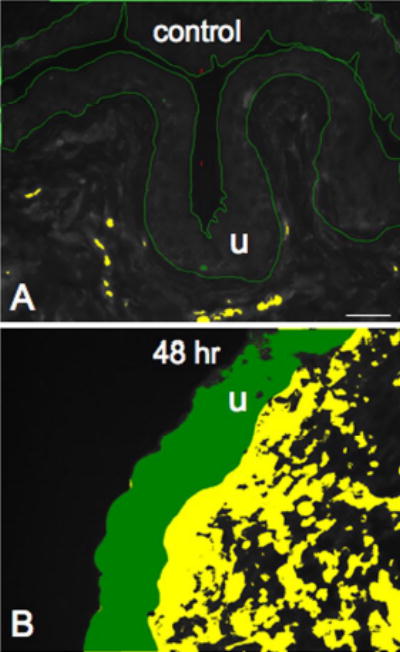
Semi-quantitative analysis of fractalkine or fractalkine receptor-immunoreactivity (IR) in the urothelium after CYP-induced cystitis. Grayscale versions of fractalkine-IR in control urinary bladder (A) or after 48 hr CYP-treatment (B) with the urothelium (u) outlined in green are demonstrated. Both images are thresholded and little fractalkine (absence of green within the outlined region) is above threshold in control compared to significant fractalkine-IR that is above threshold after 48 hr CYP treatment (B, presence of green within the u). Calibration bar represents 50 μm.
Statistics
All values are means ± S.E.M. Comparisons of cytokine protein concentration in urinary bladder samples after acute (4 or 48 hr) or chronic (10 days) CYP-induced cystitis were made using analysis of variance. Percentage data from image analysis were arcsin transformed to meet the requirements of this statistical test. Animals, processed and analyzed on the same day, were tested as a block in the analysis of variance. Two variables were being tested in the analysis: (1) age and (2) the effect of day (i.e., tissue from different postnatal or adult groups of animals were processed on different days). When F ratios exceeded the critical value (p ≤ 0.05), the Dunnett’s post-hoc test was used to compare the control means with each experimental mean.
Figure Preparation
Digital images were obtained using a CCD camera (MagnaFire SP; Optronics; Optical Analysis Corp., Nashua, NH) and LG-3 frame grabber attached to an Olympus microscope (Optical Analysis Corp.). Exposure times were held constant when acquiring images from control and experimental animals processed and analyzed on the same day. Images were imported into Adobe Photoshop 7.0 (Adobe Systems Incorporated, San Jose, CA) where groups of images were assembled and labeled.
Results
Fractalkine Protein Expression in Urinary Bladder with CYP-induced Cystitis
Fractalkine protein expression in whole urinary bladders as determined by ELISAs significantly (p ≤ 0.01) increased (2-fold) with acute (48 hr) CYP treatment and chronic CYP treatment (2.8-fold)(Fig. 2). No change in fractalkine protein expression was observed 4 hr after CYP treatment (Fig. 2). In contrast, western blotting for fractalkine in whole urinary bladder demonstrated significant increases (p ≤ 0.01) in expression at all time points of CYP treatment examined (Fig. 3). The magnitude of increase in fractalkine expression was greatest with chronic CYP treatment (3-fold) and least with acute (48 hr) CYP treatment (1.5-fold).
Figure 2.
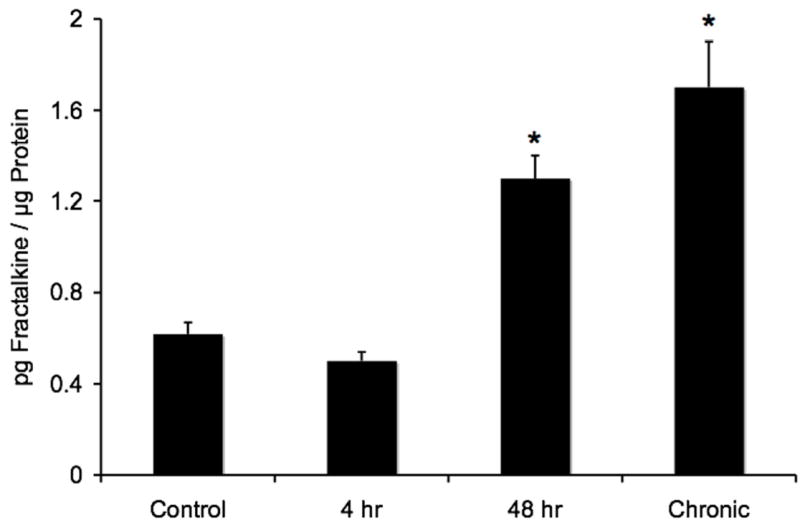
Changes in total urinary bladder fractalkine as detected with immunoassays after different durations of cyclophosphamide (CYP) treatment. Significant increases in total urinary bladder fractalkine were observed after acute (48 hr), or chronic (10 day) CYP treatment compared to control urinary bladder. n = 6 for control and each experimental condition. *, p ≤ 0.01.
Figure 3.
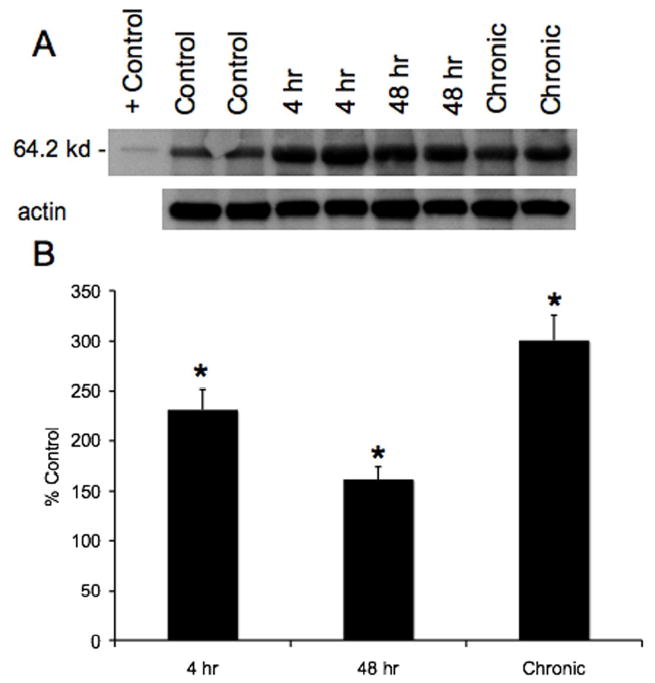
A. Representative example of a western blot of whole urinary bladder (20 μg) for fractalkine expression in control rats and those treated with cyclophosphamide (CYP) for varying duration. Actin staining was used as a loading control and recombinant rat fractalkine/CX3CL1 (2.5 ng) was used as a positive control. B. Histogram of relative fractalkine band density in all groups examined (n = 8 for each) normalized to actin staining presented as a percentage of control fractalkine expression. Fractalkine receptor expression in urinary bladder is significantly increased at all time points of CYP-induced cystitis examined. *, p ≤ 0.01.
Fractalkine-and Fractalkine Receptor Immunoreactivity (IR) in Urinary Bladder with CYP-Induced Cystitis
The expression of fractalkine-IR was virtually absent in urinary bladder sections from control (Fig. 4A) and acute (4 hr; Fig. 4B) CYP treatment. On occasion, some fractalkine-IR was present in the lamina propria but no fractalkine-IR was observed in the detrusor smooth muscle. With acute (48 hr; Fig. 4C) and chronic CYP-treatment (Fig. 4D), fractalkine-IR was observed primarily in the urothelium and some diffuse staining was observed in the lamina propria. Staining in the lamina propria may be parts of a vascular bed that was more thoroughly visualized in whole mount preparations of the urinary bladder (see below). Little if any staining was observed in the detrusor smooth muscle with CYP-treatment. Similarly, the pattern of fractalkine receptor-IR paralleled that observed with fractalkine. Expression of fractalkine receptor was not observed in the urothelium or detrusor smooth muscle in control (Fig. 5A) or acute CYP (4 hr; Fig. 5B) treated rats. With acute (48 hr; Fig. 5C) and chronic CYP-treatment (Fig. 5D), expression of fractalkine receptor was now observed in the urothelium with some diffuse staining in the lamina propria. We chose to focus the quantitation of fractalkine and fractalkine receptor expression in the urothelium because expression was dramatically upregulated in the urothelium with CYP-treatment. Quantitation of fractalkine (Fig. 6A) and fractalkine receptor (Fig. 6B) expression in the urothelium significantly (p ≤ 0.001) increased in the urothelium with acute (48 hr; Fig. 6) and chronic CYP-treatment (Fig. 6).
Figure 4.
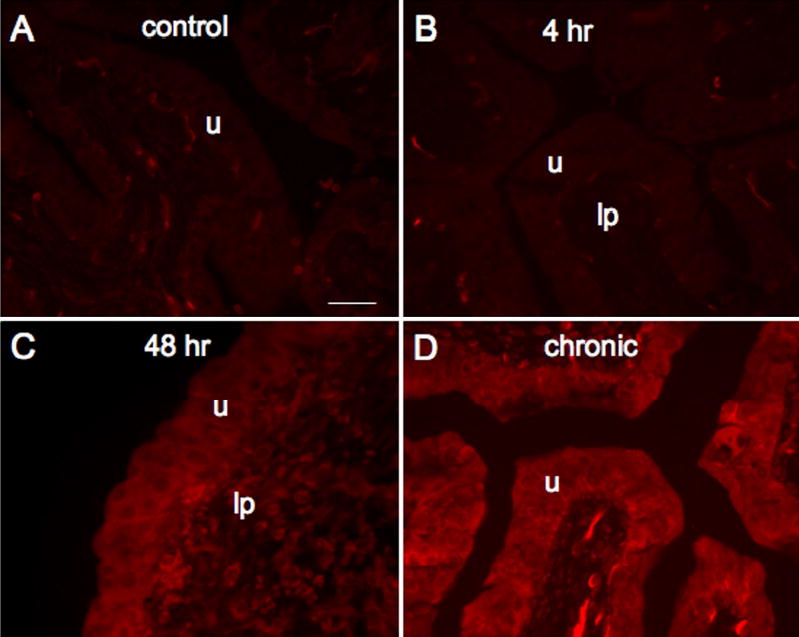
Fluorescence images of fractalkine expression in urinary bladder sections of control (A), 4 hr (B), 48 hr (C) and chronic (D) CYP treated rats. For all images, exposure times were held constant and all tissues were processed simultaneously. In control and 4 hr CYP treated rats, little if any fractalkine expression was visible in the urothelium (u; A, B) but some diffuse fractalkine-immunoreactivity was visible in the lamina propria (lp; B). CYP treatment (48 hr, C; chronic, D) upregulated expression of fractalkine in the urothelium in all layers (apical, intermediate and basal) of the urothelium. Calibration bar represents 50 μm.
Figure 5.
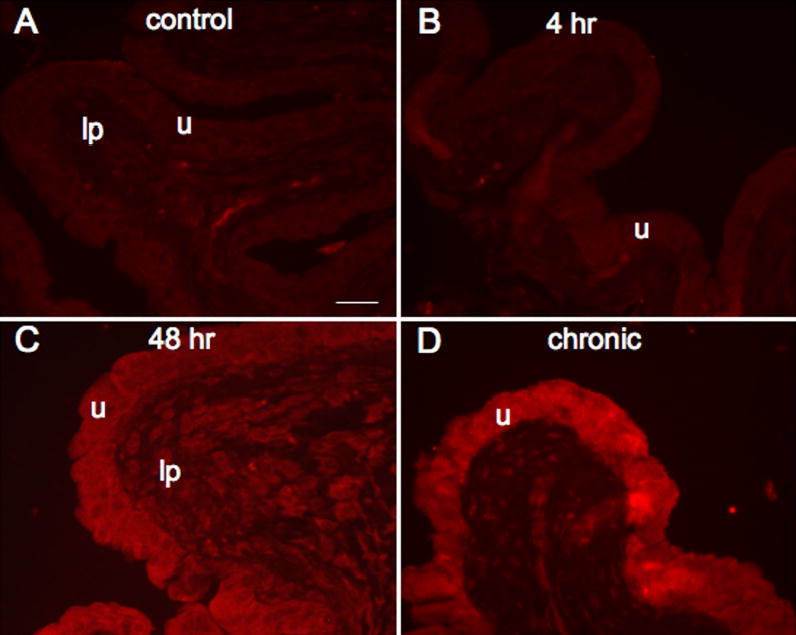
Fluorescence images of fractalkine receptor expression in urinary bladder sections of control (A), 4 hr (B), 48 hr (C) and chronic (D) CYP treated rats. For all images, exposure times were held constant and all tissues were processed simultaneously. In control and 4 hr CYP treated rats, little if any fractalkine receptor expression was visible in the urothelium (u; A, B) but some diffuse fractalkine-immunoreactivity was visible in the lamina propria (lp; A). CYP treatment (48 hr, C; chronic, D) upregulated expression of fractalkine receptor in the urothelium in all layers (apical, intermediate and basal). In normal or inflamed urinary bladder, expression of fractalkine receptor in the detrusor smooth muscle was not obvious. Calibration bar represents 50 μm.
Figure 6.
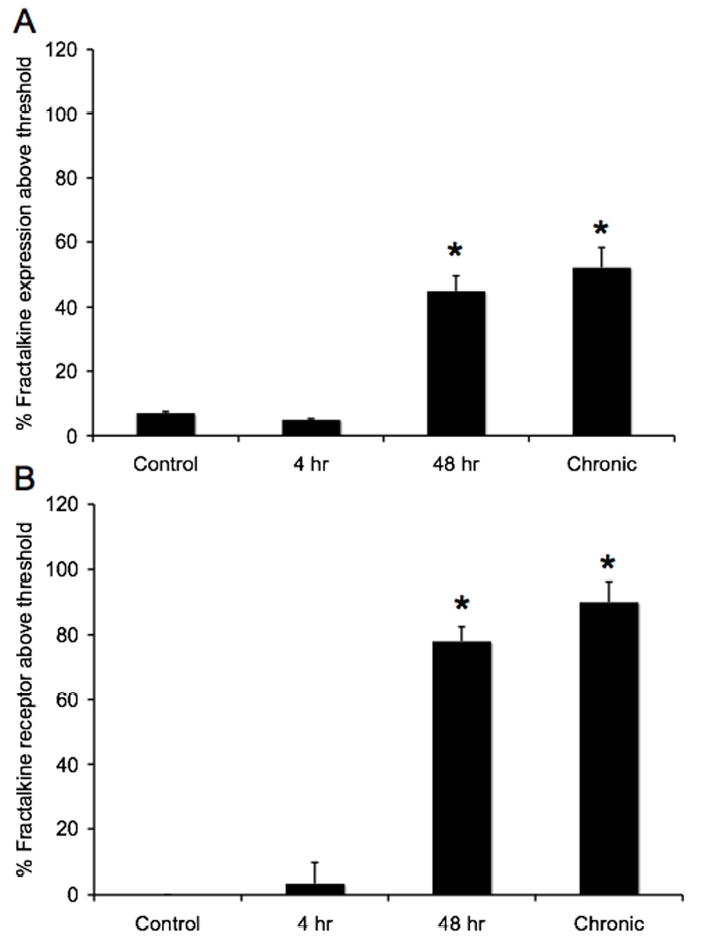
Histograms of the percent of fractalkine (A) or fractalkine receptor (B) expression above threshold in the urothelium of control (n = 6) or CYP-treated rats (n = 8). CYP treatment (48 hr and chronic) significantly (p ≤ 0.001) upregulated the percent of fractalkine- (A) and fractalkine receptor- (B) immunoreactivity present in the urothelium.
Fractalkine expression in whole-mounts of urothelium and detrusor smooth muscle
To determine if fractalkine-IR was present in the suburothelial plexus, whole mount preparations of urinary bladder were prepared to aid in the visualization of the nerve plexus. In the urothelium/lamina propria whole mount (Fig. 7A) or detrusor whole mount (Fig. 7B), fractalkine-IR was observed in an extensive vascular bed throughout either whole mount preparation. No vessels were observed that lacked fractalkine-IR. Fractalkine-IR in the vascular bed was present in control and CYP-treated rats with no change in fractalkine-IR being observed with cystitis. Fractalkine-IR in the vascular bed in the whole mount preparations was intensely stained in all groups examined. The pattern of fractalkine-IR in the vascular bed was distinct from that observed with PGP 9.5 staining of the suburothelial plexus (Fig. 7A). In some instances, PGP labeled nerve fibers were in close proximity to the fractalkine-IR vascular bed (Fig. 7A). No fractalkine-IR was observed in the suburothelial plexus in any experimental group examined (Fig. 7A, B).
Figure 7.
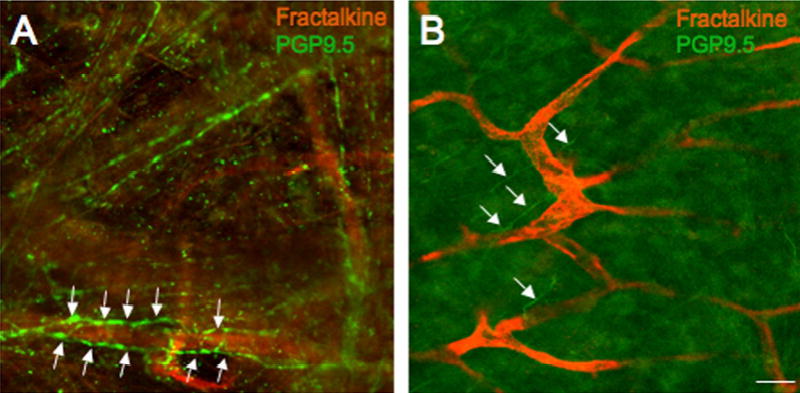
Fluorescence photographs of fractalkine-IR (red) in a vascular bed and PGP 9.5-IR nerve fibers (green) in the suburothelial plexus in whole mount preparations of the urothelium/lamina propria (A) or detrusor smooth muscle (B). Intense fractalkine-IR was present in the vascular bed in the urinary bladder in all experimental groups examined (A, control; B, 4 hr CYP-treatment). Extensive PGP 9.5-IR nerve fibers were present abundantly in the urothelium/lamina propria (A, green) and were more diffuse in the in detrusor smooth muscle whole mount (B, arrows). In no instance, was fractalkine-IR colocalized with PGP9.5-IR nerve fibers in the suburothelial plexus but PGP 9.5 nerve fibers were observed in close proximity to the fractalkine-IR vascular bed (A, arrows). Calibration bar represent 80 μm.
Discussion
Changes in the expression of the chemokine, fractalkine (CX3CL1), were examined in the urinary bladder after acute (4 hour (hr) or 48 hr) or chronic (10 day) cyclophosphamide (CYP) treatment. CYP-induced cystitis significantly increased fractalkine protein expression in the urinary bladder with acute (48 hr) and chronic CYP-treatment. Immunohistochemistry for fractalkine-immunoreactivity (IR) revealed little fractalkine-IR in control or acute (4 hr) CYP-treated rat urinary bladders. However, expression was significantly upregulated in the urothelium with 48 hr or chronic CYP-treatment. Similarly, fractalkine receptor (CX3CR1)-IR was significantly upregulated in the urothelium with 48 hr or chronic CYP-treatment. Whole mount preparations of urothelium/lamina propria or detrusor smooth muscle showed prominent fractalkine-IR in a vascular bed in all experimental conditions but fractalkine-IR did not colocalize with nerve fibers in the suburothelial plexus. Chemokines may represent another group of neuromodulatory agents that can contribute to altered urinary bladder and sensory processing with cystitis.
Interstitial cystitis (IC) is a chronic inflammatory bladder disease syndrome characterized by urinary frequency, urgency, suprapubic and pelvic pain (Petrone et al., 1995; Driscoll and Teichman, 2001). Although the etiology and pathogenesis of IC are unknown, numerous theories including; infection, autoimmune disorder, toxic urinary agents, deficiency in bladder wall lining and neurogenic causes have been proposed (Petrone et al., 1995; Ho et al., 1997; Johansson et al., 1997; Driscoll and Teichman, 2001; Sant and Hanno, 2001). We have hypothesized that pain associated with IC involves an alteration of visceral sensation/bladder sensory physiology. Altered visceral sensations from the urinary bladder (i.e., pain at low or moderate bladder filling) that accompany IC (Petrone et al., 1995; Ho et al., 1997; Johansson et al., 1997; Driscoll and Teichman, 2001; Sant and Hanno, 2001) may be mediated by many factors including changes in the properties of peripheral bladder afferent pathways such that bladder afferent neurons respond in an exaggerated manner to normally innocuous stimuli (allodynia). These changes may be mediated, in part, by inflammatory changes in the urinary bladder. Among potential mediators of inflammation, neurotrophins (e.g., nerve growth factor) have been implicated in the peripheral sensitization of nociceptors (Steers and de Groat, 1988; Lindsay and Harmar, 1989; Dray, 1995; Dinarello, 1997; Dinarello, 1998; Mason et al., 2001). Pro-inflammatory cytokines also cause sensitization of polymodal C-fibers (Dray, 1995) and facilitate A-beta input to the spinal cord (Baba et al., 1999; Yoshimura and de Groat, 1999). Previous studies from this laboratory (Malley and Vizzard, 2002) have suggested that cytokines produced in the urinary bladder after CYP-induced cystitis may also contribute to this sensitization process.
The present study adds chemokines and specifically, fractalkine, to the list of potential mediators that may contribute to altered micturition reflexes and sensory processing after cystitis. In addition to its role as an adhesion molecule and chemotactic agent to T cells and monocytes (Fong et al., 1998; Haskell et al., 2000), recent studies have suggested roles for fractalkine and its receptor in the establishment and/or maintenance of neuropathic pain (Verge et al., 2004) and other studies have suggested the involvement of chemokines in inflammatory and neuropathic pain (Marchand et al., 2005). Recent studies (Verge et al., 2004) have demonstrated that intrathecal fractalkine produces thermal hyperalgesia and mechanical allodynia whereas intrathecal administration of a CX3CR1 (fractalkine receptor) antagonist inhibits or reverses neuropathic pain (Watkins et al., 2001; Watkins and Maier, 2002; Milligan et al., 2004). In addition, fractalkine has been suggested to be a neuron-to-glial signal that could facilitate nociception (Milligan et al., 2004; Watkins et al., 2005).
Previous studies have demonstrated fractalkine expression in endothelial cells (venous and arterial) and upregulation by tumor necrosis factor (TNF)-α, nuclear factor kappa B (NF-κB), lipopolysaccharide and interleukin (IL)-1β (Garcia et al., 2000; Ahn et al., 2004; Sung et al., 2005). Similarly, in this study, we demonstrate constitutive fractalkine expression in a vascular bed throughout the urinary bladder whose appearance does not appear to be regulated by CYP-induced cystitis. In addition, we believe that this study is the first to demonstrate upregulation of fractalkine and fractalkine receptor in the urothelium after bladder inflammation. Previous studies have demonstrated constitutive expression and upregulation of fractalkine in rodent primary afferent cells in the dorsal root ganglia under neuropathic pain conditions (Verge et al., 2004). Urothelial cells share a number of similarities with sensory neurons and the urothelium has been suggested by Birder et al. (Birder et al., 2001; Birder, 2005b, 2005a) to have ‘neuronal-like’ properties. Urothelial cells express a number of receptors and ion channels similar to those found in sensory neurons (Birder et al., 2001; Murray et al., 2004). Thus, the present study adds to the growing list of similarities between urothelial cells and sensory neurons and urothelial cells may actively participate in the sensing and transducing of inflammatory signals to the central nervous system (Birder, 2005b, 2005a). Despite constitutive expression of fractalkine-IR in rodent DRG cells (Verge et al., 2004), we did not observe colocalization of fractalkine-IR with PGP 9.5 labeled nerve fibers in the suburothelial plexus from any experimental group examined. Absence of colocalization may be related to the use of the whole mount bladder preparation that was harvested from rats that were not transcardially perfused. This may have limited our ability to detect low fractalkine-IR in the urinary bladder nerves. Future studies will determine if fractalkine-IR is present in bladder afferent cells in the lumbosacral DRG harvested from perfused rats.
Previous studies (Malley and Vizzard, 2002) from this laboratory have demonstrated robust changes in a number of urinary bladder cytokines including IL-1-β, IL-2, IL-4, IL-6 and more modest changes in TNF-α/ or TNF-β with CYP-induced cystitis. Similarly, upregulation of fractalkine in vascular endothelial cells has been induced by IL-1-β and TNF-α (Garcia et al., 2000; Ahn et al., 2004). Delivery of a dominant-negative form of IκBα in rat aortic endothelial cells significantly reduced the induction of fractalkine by IL-1-β, lipopolysaccharide and TNF-α suggesting a role for NF-κB in fractalkine induction. A similar mechanism of upregulation of fractalkine in the urothelium with bladder inflammation may involve IL-1-β and TNF-α. The opposite may also occur whereby fractalkine may induce proinflammatory cytokine release from the urinary bladder as previously demonstrated in the spinal cord dorsal horn (Johnston et al., 2004).
Alternatively or in combination, interactions with neurotrophic factors expressed in the inflamed urinary bladder may also contribute to fractalkine upregulation. Recent experiments from several laboratories including our own have demonstrated the influence of target organ-neuron interactions in the adult animal (Murray et al., 2004; Steers and de Groat, 1988; Steers et al., 1991b; Steers et al., 1991a; Tuttle et al., 1994a; Tuttle et al., 1994b; Steers et al., 1996; Dupont et al., 2001; Vizzard, 2000a, 2000b, 2001; Yoshimura and de Groat, 1999). It has also been suggested that a common peripheral action of cytokines and chemokines in neuropathic pain may be the induction of cyclooxygenase-2 (Marchand et al., 2005). COX-2 mRNA and protein, prostaglandin D2 and E2 are unregulated in the inflamed urinary bladder after CYP-treatment (Hu et al., 2003). Treatment with a COX-2 specific inhibitor, a tetra-substituted furanone, significantly reduced bladder overactivity (voiding frequency and non-voiding contractions) in CYP-treated rats (Hu et al., 2003). Whether or not fractalkine can affect bladder and sensory function alone or by interaction with neurotrophic factors, cytokines or COX-2 remains to be determined.
In summary, these studies have demonstrated significant changes in fractalkine and fractalkine receptor expression in the urinary bladder after CYP-induced cystitis examined at three time points (acute, intermediate and chronic). This study has demonstrated that inflammation of the urinary bladder induces changes in bladder fractalkine expression and immunoreactivity in the urothelium. Unlike our findings with cytokines (Malley and Vizzard, 2002) but similar to our results with neurotrophic factor expression (Vizzard, 2000a; Murray et al., 2004), fractalkine expression persists during chronic bladder inflammation suggesting that the chronic changes in micturition reflexes and sensory processing associated with cystitis may be influenced by chemokines and neurotrophic factors released in the urinary bladder. Future studies will determine the contribution of fractalkine to lower urinary tract function after cystitis by performing addition or subtraction experiments.
Acknowledgments
R. Yuridullah was supported, in part, by a Neuroscience Summer Undergraduate Research Fellowship from the University of Vermont, Departments of Anatomy and Neurobiology and Neurology. This work was funded by NIH grants DK051369, DK060481, DK065989, NS040796.
References
- Ahn SY, Cho CH, Park KG, Lee HJ, Lee S, Park SK, Lee IK, Koh GY. Tumor necrosis factor-alpha induces fractalkine expression preferentially in arterial endothelial cells and mithramycin A suppresses TNF-alpha-induced fractalkine expression. Am J Pathol. 2004;164:1663–1672. doi: 10.1016/s0002-9440(10)63725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates A beta fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci. 1999;19:859–867. doi: 10.1523/JNEUROSCI.19-02-00859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- Birder LA. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol. 2005a;289:F489–495. doi: 10.1152/ajprenal.00467.2004. [DOI] [PubMed] [Google Scholar]
- Birder LA. Role of the urothelium in urinary bladder dysfunction following spinal cord injury. Prog Brain Res. 2005b;152:135–146. doi: 10.1016/S0079-6123(05)52009-0. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, deGroat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Nat Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT. Role for pituitary adenylate cyclase activating polypeptide (PACAP) in cystitis-induced plasticity of micturition reflexes. Reg Peptides. 2005;130:157. doi: 10.1152/ajpregu.00734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancellor MB, Yoshimura N. Treatment of interstitial cystitis. Urology. 2004;63:85–92. doi: 10.1016/j.urology.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Dattilio A, Vizzard MA. Up-regulation of protease activated receptors in bladder after cyclophosphamide induced cystitis and colocalization with capsaicin receptor (VR1) in bladder nerve fibers. J Urol. 2005;173:635–639. doi: 10.1097/01.ju.0000143191.55468.1d. [DOI] [PubMed] [Google Scholar]
- Dinarello CA (1998) Overview of inflammatory cytokines and their role in pain. In: Cytokines and Pain (Watkins LR, Maier SF, eds), pp 1–20. Boston: Birkhauser Verlag.
- Dinarello CAD. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest. 1997;112:321S–329S. doi: 10.1378/chest.112.6_supplement.321s. [DOI] [PubMed] [Google Scholar]
- Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75:125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- Driscoll A, Teichman JMH. How do patients with interstitial cystitis present? J Urol. 2001;166:2118–2120. [PubMed] [Google Scholar]
- Dupont MC, Spitsbergen JM, Kim KB, Tuttle JB, Steers WD. Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol. 2001;166:1111–1118. [PubMed] [Google Scholar]
- Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, Patel DD. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GE, Xia Y, Chen S, Wang Y, Ye RD, Harrison JK, Bacon KB, Zerwes HG, Feng L. NF-kappaB-dependent fractalkine induction in rat aortic endothelial cells stimulated by IL-1beta, TNF-alpha, and LPS. J Leukoc Biol. 2000;67:577–584. doi: 10.1002/jlb.67.4.577. [DOI] [PubMed] [Google Scholar]
- Haskell CA, Cleary MD, Charo IF. Unique role of the chemokine domain of fractalkine in cell capture. Kinetics of receptor dissociation correlate with cell adhesion. J Biol Chem. 2000;275:34183–34189. doi: 10.1074/jbc.M005731200. [DOI] [PubMed] [Google Scholar]
- Ho N, Koziol JA, Parsons CL (1997) Epidemiology of interstitial cystitis. In: Interstitial Cystitis (Sant GR, ed), pp 9–16. Philadelphia: Lippincott-Raven Publishers.
- Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr C. 2003;284:R574–R585. doi: 10.1152/ajpregu.00465.2002. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia. 2002;37:314–327. [PubMed] [Google Scholar]
- Johansson SL, Ogawa K, Fall M (1997) The pathology of interstitial cystitis. In: Interstitial Cystitis (Sant GR, ed), pp 143–152. Philadelphia: Lippincott-Raven Publishers.
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindia JA, McGowan E, Jochnowitz N, Abbadie C. Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain. J Pain. 2005;6:434–438. doi: 10.1016/j.jpain.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337:362–367. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics. 2002;9:5–13. doi: 10.1152/physiolgenomics.00117.2001. [DOI] [PubMed] [Google Scholar]
- Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1 beta promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O'Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic Ganglia and bladder. J Urol. 2004;172:2434–2439. doi: 10.1097/01.ju.0000143549.29867.4e. [DOI] [PubMed] [Google Scholar]
- Nagarsekar A, Hasday JD, Singh IS. CXC chemokines: A new family of heat-shock proteins? Immunol Invest. 2005;34:381–398. doi: 10.1081/imm-200067648. [DOI] [PubMed] [Google Scholar]
- Petrone RL, Agha AH, Roy JB, Hurst RE. Urodynamic findings in patients with interstitial cystitis. J Urol. 1995;153:290A. [Google Scholar]
- Raychaudhuri S, Jiang W-Y, Farber E. Cellular localization of fractalkine at sites of inflammation: antigen-presenting cells in psoriasis express high levels of fractalkine. Br J Dermatol. 2001:1105–1113. doi: 10.1046/j.1365-2133.2001.04219.x. [DOI] [PubMed] [Google Scholar]
- Sant G, Hanno PM. Interstitial cystitis: current issues and controversies in diagnosis. Urology. 2001;57:82. doi: 10.1016/s0090-4295(01)01131-1. [DOI] [PubMed] [Google Scholar]
- Steers WD, de Groat WC. Effect of bladder outlet obstruction on micturition reflex pathways in the rat. J Urol. 1988;140:864–871. doi: 10.1016/s0022-5347(17)41846-5. [DOI] [PubMed] [Google Scholar]
- Steers WD, Creedon DJ, Tuttle JB. Immunity to nerve growth factor prevents afferent plasticity following urinary bladder hypertrophy. J Urol. 1996;155:379–385. [PubMed] [Google Scholar]
- Steers WD, Kolbeck S, Creedon D, Tuttle JB. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J Clin Invest. 1991a;88:1709–1715. doi: 10.1172/JCI115488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steers WD, Ciambotti J, Etzel B, Erdman S, de Groat WC. Alterations in afferent pathways from the urinary bladder of the rat in response to partial urethral obstruction. J Comp Neurol. 1991b;310:1–10. doi: 10.1002/cne.903100309. [DOI] [PubMed] [Google Scholar]
- Sung MJ, Kim W, Ahn SY, Cho CH, Koh GY, Moon SO, Hoon Kim D, Lee S, Kang KP, Jang KY, Park SK (2005) Protective Effect of {alpha}-Lipoic Acid in Lipopolysaccharide-Induced Endothelial Fractalkine Expression. Circ Res. ahead of print. [DOI] [PubMed]
- Tuttle JB, Mackey T, Steers WD. NGF, bFGF and CNTF increase survival of major pelvic ganglion neurons cultured from the adult rat. Neurosci Lett. 1994a;173:94–98. doi: 10.1016/0304-3940(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Tuttle JB, Steers WD, Albo M, Nataluk E. Neural input regulates tissue NGF and growth of the adult rat urinary bladder. J Auton Nerv Syst. 1994b;49:147–158. doi: 10.1016/0165-1838(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000a;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000b;420:335–348. [PubMed] [Google Scholar]
- Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21:125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Boyle MM. Increased expression of growth-associated protein (GAP-43) in lower urinary tract pathways following cyclophosphamide (CYP)-induced cystitis. Brain Res. 1999;844:174–187. doi: 10.1016/s0006-8993(99)01936-8. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF (2005) Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. [DOI] [PubMed]
- Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45:389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Imaizumi T, Hatakeyama M, Tamo W, Kimura D, Kumagai M, Yoshida H, Satoh K. Effect of hypoxia on the expression of fractalkine in human endothelial cells. Tohoku J Exp Med. 2003;200:187–194. doi: 10.1620/tjem.200.187. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder following chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvarova K, Vizzard MA. Distribution and fate of cocaine- and amphetamine-regulated transcript peptide (CARTp)-expressing cells in rat urinary bladder: a developmental study. J Comp Neurol. 2005;489:501–517. doi: 10.1002/cne.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]


