Abstract
The perceived bitterness intensity for bitter solutions of propylthiouracil (PROP), sucrose octa-acetate (SOA), quinine HCl and caffeine were examined in a genetically informative sample of 392 females and 313 males (mean age of 17.8 ± 3.1 years), including 62 MZ and 131 DZ twin pairs and 237 sib pairs. Broad-sense heritabilities were estimated at 0.72, 0.28, 0.34, and 0.30 for PROP, SOA, quinine, and caffeine, respectively, for perceived intensity measures. Modeling showed 1) a group factor which explained a large amount of the genetic variation in SOA, quinine, and caffeine (22–28% phenotypic variation), 2) a factor responsible for all the genetic variation in PROP (72% phenotypic variation), which only accounted for 1% and 2% of the phenotypic variation in SOA and caffeine, respectively, and 3) a modest specific genetic factor for quinine (12% phenotypic variation). Unique environmental influences for all four compounds were due to a single factor responsible for 7–22% of phenotypic variation. The results suggest that the perception of PROP and the perception of SOA, quinine, and caffeine are influenced by two distinct sets of genes.
Keywords: bitter taste, propylthiouracil, quinine hydrochloride, sucrose octa-acetate, twin
Introduction
The study of genetic influences on bitter taste perception originated from the discovery in the 1930s that some individuals had taste blindness to phenylthiocarbamide (PTC), whereas others found it extremely bitter (Anonymous, 1931). Subsequently, many studies were carried out (Guo and Reed, 2001) on PTC and the structurally related compound propylthiouracil (PROP) to assess this variability and to determine the root causes. Initial family studies (Snyder, 1931) strongly suggested that PTC nontasting was due to a recessive allele in a single gene with early twin studies supporting this (Levit and Soboleva, 1935). On this basis, PTC tasting was used as a zygosity determinant for a time in twin studies (Ardashnikov et al., 1936; Rife, 1938), but it was soon realized that twins could be indisputably monozygotic but discordant for PTC tasting (Dencker et al., 1959). Subsequently, PTC and PROP perception began to be studied as a more complex trait in twin (Kaplan et al., 1967; Martin, 1975) and other studies. Indeed, it has now been established that PTC detection thresh-old is a genetically controlled trait with heritability estimated at ~0.5 (Morton et al., 1981; Drayna et al., 2003), with more recent work showing that 55–85% of variation in PTC detection threshold is due to three single nucleotide polymorphisms (SNPs) (five haplotypes) in the bitter taste receptor gene TAS2R38 (Kim et al., 2003) on chromosome 7.
In contrast, there has been little genetic investigation of human sensitivities to other bitter compounds, notable exceptions being two studies of quinine detection threshold, which estimated the heritability to be 0.55 and 0.85 for a twin and family sample, respectively (Smith and Davies, 1973), and also 0.11 using a twin sample (Krondl et al., 1983). Here, we investigate the extent of genetic influence on the perception of bitter compounds sucrose octa-acetate (SOA), quinine, and caffeine, in addition to PROP, and examine the extent of genetic covariation among measures of sensitivity to these four bitter compounds.
From the large body of work in murine models, it appears that, generally, the perceptions of bitter compounds are moderately heritable and are predominantly controlled by a few loci. In mice and golden hamsters, heritability estimates of the consumption of SOA, quinine, and caffeine range from 0.09 to 0.57, depending on solution concentration (Le Roy et al., 1999; Frank et al., 2004). Early studies into SOA perception of mice suggested monogenic control with complete dominance of the taster phenotype (Whitney et al., 1989). Further study of SOA perception identified a single locus, Soa, with three alleles responsible for taster, nontaster, and intermediate taster phenotypes (Harder et al., 1992) and was localized to a region on chromosome 6 (Capeless et al., 1992; Bachmanov et al., 2001). The intake of quinine HCl is suggested to be controlled by a gene, Qui, with three alleles (Lush, 1984) causing the taster, intermediate taster, and non-taster phenotypes (Bachmanov et al., 1996); however, unlike SOA, quinine perception is considered to be polygenic (Boughter et al., 1992; Harder and Whitney, 1998).
There is also some evidence from the murine work to suggest moderate to strong associations among sensitivities to some bitter compounds and that common loci may be responsible for this. For example, SOA perception has been found to correlate with quinine and PROP but not with caffeine and PTC (Harder et al., 1992; Whitney and Harder, 1994; Boughter and Whitney, 1998). The SOA locus controls bitter perception of SOA (Whitney and Harder, 1994) but also influences perception of quinine (Boughter and Whitney, 1998) and PROP (Harder and Whitney, 1998), providing evidence that the observed phenotypic covariation between sensitivity to these compounds is genetically influenced. We must stress, however, that there are differences between murine and human bitter taste perceptions. For example, unlike in humans, PTC and PROP perceptions are not correlated in mice (Harder et al., 1996; Harder and Whitney, 1998), with the mouse T2R8 bitter taste receptor responding to PROP but not to PTC (Chandrashekar et al., 2000). In conjunction with the knowledge that there are known species-specific differences in bitter taste receptor repertoires (Shi et al., 2003), murine models should be used only as a guide to human studies.
In humans, there is some work demonstrating phenotypic associations between perceptions of various bitter compounds (McBurney et al., 1972; Yokomukai et al. 1993; Delwiche et al., 2001a). Delwiche et al. 2001 examined the correlations between individual bitter taste perceptions to determine the possible number of bitter transduction mechanisms for the tested compounds. Data were analyzed from 26 individuals who rated and ranked the perceived intensity of 11 bitter compounds, with principle components and cluster analyses suggesting four clusters. The first cluster contained denatonium benzoate, tetralone, caffeine, SOA, and quinine; the second contained urea, tryptophan, phenylalanine, and epicatechin; and the third and fourth contained magnesium sulfate and PROP, respectively. On examination of the physical parameters of each compound, it was found that all the compounds in cluster one had at least one methyl group and that three of the four compounds in cluster two contained at least one amine group, suggesting that there could be two separate bitter perception pathways for these types of compounds.
In the present study, we use a large genetically informative twin and sibling sample to examine the extent to which associations among the bitter compounds PROP, SOA, quinine, and caffeine are genetically influenced. Using structural equation modeling, variation of individual variables and covariation among phenotypes were partitioned into genetic and environmental components, establishing the heritability and the extent to which common genetic factors influence variation in multiple bitter taste phenotypes.
Materials and methods
Participants
Participants were a subset of adolescent and young adult twins and their singleton siblings (Wright and Martin, 2004) who have participated in previous studies of the genetics of skin moles (e.g., Aitken et al., 1994; Zhu et al., 1999) and cognition (e.g., Wright et al., 2001; Posthuma et al., 2005). The sample for whom taste sensitivity results were available consisted of 520 females and 409 males (mean age 17.7 ± 3.1 years, range 12–26 years) and included 102 MZ twin pairs, 201 DZ pairs, and 229 sib pairs (twin plus singleton sibling) (Table 1).
Table 1.
Number of families before and after data screening and removal of outliers
| Family type | Initial | After data screening | After outliers removed |
|---|---|---|---|
| MZ twin pair | 45 | 34 | 33 |
| MZ twin pair + sib | 57 | 29 | 29 |
| DZ twin pair | 126 | 93 | 92 |
| DZ twin pair + sib | 75 | 40 | 39 |
| one twin + one sib | 23 | 37a | 36 |
The number of one twin + one sib families increases after cleaning as some twin pair + sib families lose one twin during the cleaning procedure.
Zygosity for 93% of the same sex twin pairs had been typed from DNA using a commercial kit (AmpF1STR Profiler Plus Amplification Kit, ABI) that analyzed nine independent highly polymorphic DNA markers plus the amelogenin marker for sex (Wright and Martin, 2004). This gives a probability less than 10−4 that a pair of DZ twins is concordant at all nine markers. Where zygosity had not been established by DNA, it was determined from phenotype (height, hair, and eye color).
General procedure
The taste test battery was administered as part of a mail and phone study that also assessed olfaction, health and well-being, personality, laterality, reading, and spelling (Wright and Martin, 2004). While the taste test took 30–45 min, total testing time for all components of the study was approximately two and a half hours. Participants were contacted by letter and invited to participate; test materials and instructions, including the taste test, were included in a mail pack with the initial contact letter. Shortly after receiving the test pack, a research assistant telephoned the twins/siblings to ascertain whether they had received the pack, if they were willing to participate, and to answer any questions. In addition, reminder calls were made to maximize response rates. Participants returned completed taste test score sheets along with the other test materials and the signed consent form in a reply paid envelope provided. Two movie tickets were sent to those who completed and returned their responses, in appreciation of the time taken to complete the study. The study was approved by the Queensland Institute of Medical Research (QIMR) Human Research Ethics Committee.
Taste test
The tasting battery comprised 10 different solutions, of which five were bitter, four were sweet, and one was neutral (i.e., water). The five bitter-tasting solutions included 2.0 ×10−4 M SOA, 1.81 × 10−4 M quinine HCl (quinine), 0.050 M caffeine, 6.0 × 10−4 M PROP, and 4.99 × 10−6 M denatonium benzoate. The four sweet solutions included 0.60 M glucose, 0.30 M fructose, 8.0 × 10−5 M neohesperidine dihydrochalcone (NHDC), and 1.4 × 10−3 M aspartame. The battery was selected so that responses to a range of structurally diverse compounds could be tested and at concentrations to allow a rough approximation of the average perceived intensity for all solutions. Each solution and the water control were presented twice (i.e., total of 20 solutions).
The solutions were presented in color-coded 2-ml polypropylene microcentrifuge tubes with flip tops (Thomas Scientific) and were numerically labeled to accommodate participants who may be color blind. The flip tops cannot be lost due to their physical attachment to the tube and will not open unless forced by the participant, yet they are easily held in one hand and lids are flipped open with a thumb. The tubes were inserted through small holes cut into quarter-inch flexible antistatic polyethylene foam at 1-inch intervals along an inch wide sheet. This design allowed for the distribution of all test stimuli simultaneously within a single foam strip. The first 10 solutions in the strip contained one presentation of each of the nine compounds plus the water control. These 10 solutions were then presented again but in a different order, with the ordering of the solutions the same for all participants. The order of the 20 solutions was SOA, water, caffeine, glucose, quinine HCl, fructose, NHDC, PROP, aspartame, denatonium benzoate, fructose, glucose, PROP, aspartame, quinine HCl, NHDC, caffeine, water, SOA, and denatonium benzoate. As 20 different colored tubes could not be obtained, 10 were used, one for each different solution. This taste test resists destruction under normal handling conditions and is easily sent by mail. In the present study, the polyethylene strip containing the tubes was loosely rolled, placed in a padded postbag, and mailed by regular post to participants. In addition, a dry PROP strip, which had been soaked in a saturated PROP solution, was administered but was not examined in these analyses given the primary focus was to investigate covariation among different bitter compounds and not covariation between different measures of the same compound.
Detailed written instructions, as well as a summary sheet of the key points that participants could refer to while completing the test, were provided in the mail pack. Briefly, participants were instructed on how to rate the perceived intensity of a solution (and the PROP paper) relative to taste and nontaste sensations. To this end, examples such as the sweetness of cotton candy (fairy floss), the sourness of lemon juice, the bitterness of tonic water, the warmth of lukewarm water, the heat of a hot day, the pain from biting your tongue, and other examples were given. On the score sheet provided, perceived intensity was rated by marking a dash, using a pencil, on a labeled magnitude scale (LMS) (Green et al., 1993) with labels of no sensation, barely detectable, weak, moderate, strong, very strong, and strongest imaginable placed at 0, 2, 7, 20, 40, 61, and 114 mm. The use of this scale minimizes ceiling effects and provides a continuous measure that is desirable for quantitative analysis. The quality of the taste, whether it was salty, sweet, sour, bitter, savory, or stinging, was also indicated. The procedure for administering the taste test was described in five steps: 1) open the tube, swish solution around in mouth for 5 s, and spit out, 2) rate the perceived intensity of the solution, 3) rate the quality of the taste, 4) rinse mouth out four times with tap water, and 5) repeat steps 1–4 for each tube. Participants were also instructed not to complete the taste test if suffering from a cold or flu until they had completely recovered, not to eat or smoke, and drink only water for at least 1 h before the test.
In addition, participants answered questions relating to previous head injury and otitis media (middle ear infection), smoking behavior, and the use of cologne, perfume, or after-shave, as it is conceivable that these could have an effect on taste phenotypes (Bartoshuk et al., 1996). Responses to the question ‘‘Have you ever suffered a head injury?’’ included ‘‘no,’’ ‘‘yes (but not seriously),’’ ‘‘yes (had either a concussion or loss of consciousness),’’ and ‘‘yes (both concussion and loss of consciousness).’’ Similarly, responses to ‘‘Have you ever suffered from a middle ear infection?’’ included ‘‘no,’’ ‘‘yes (but not serious),’’ ‘‘yes (required antibiotics more than once),’’ and ‘‘yes (required tubes in ears).’’ The question ‘‘Do you currently smoke cigarettes?’’ required a ‘‘yes’’ or ‘‘no’’ response and ‘‘How many days a week do you use cologne, perfume, or aftershave?’’ included responses of ‘‘1–2 days a week,’’ ‘‘3–4 days a week,’’ ‘‘5–7 days a week,’’ and ‘‘do not use any.’’
Preliminary data screening
Prior to analysis, the data were subjected to a screening protocol. Firstly, participants who scored the perceived intensity of the water tubes stronger than moderate (>20 mm) on the LMS were excluded as this probably indicated that the testing protocol was not followed correctly (i.e., not rinsing between stimuli). Secondly, participants whose total score for the 18 test solutions was below 200 mm or above 1800 mm were removed as these participants were ageusic, hypogeusic, or using the scale in a way that could not be compared to the rest of the population. For those who did not complete all stimuli within the test battery, the average of completed scores was calculated, and if this average, when applied across all solutions, would have placed the individual outside the acceptable ranges, they were also removed. Thirdly, participants were removed if the scores for the two presentations of the same compound differed by more than 80 mm as the results were unreliable and may indicate that these subjects’ ratings were highly influenced by stimulus order. This screening procedure resulted in the removal of 24% of the sample, with the new subsample comprising 705 individuals, 392 females and 313 males (mean age of 17.8 ± 3.1 years), including 63 MZ twin pairs, 135 DZ pairs, and 237 sib pairs (Table 1). It was the overly high scoring of water that resulted in the removal of most of these individuals, which suggests inadequate rinsing between presentations, an unfortunate consequence of the study being performed at home without direct supervision. All genetic modeling used the screened sample.
The focus of the present study was on the four bitter compounds PROP, SOA, quinine, and caffeine. The remaining bitter compound, denatonium benzoate, was not included as it showed a distinctly bimodal distribution that was resistant to transformation and thus violated the assumption of normality required for maximum likelihood (ML) analysis. The four sweet compounds will be analyzed separately.
Statistical analyses
Statistical analyses used structural equation modeling using the statistical package Mx (Neale et al., 2002) and ML estimation procedures. Models were assessed by comparing double the negative log-likelihood (−2LL, the model fit statistic) between nested models as this difference is distributed a symptotically as a χ2. Alternative models can then be assigned a P-value, and if <0.05, the model is considered a significantly worse fit. Data were screened for normality and for outliers in both univariate and multivariate models. Outlying families were detected and excluded by using the %p option in Mx that uses a standardized Mahalanobis distance to compute a z-score for each family, with values outside the −3 to +3 range indicating excessive similarities or differences relative to other families in the sample and model expectations (e.g., excessively different MZ pairs or exceedingly similar DZ pairs relative to other MZ and DZ pairs in the sample).
To establish regularity in sampling and measurement, the homogeneity of means and variances according to birth order (first born, second born), zygosity, and sex were tested using Mx (McGregor et al., 1999). The testing of assumptions allows the mean and variance of each relative type (e.g., first twin of MZ females) to be estimated separately; these estimates are then steadily simplified while observing the change in model fit. Covariates (age, sex, history of head injury, history of otitis media, smoking behavior, and use of cologne, perfume, and aftershave) were modeled as fixed effects (regressions and deviations from the mean) and assessed for significance to establish whether they needed to be included in the multivariate modeling. Equating covariance between MZM and MZF twin pairs and across same sex and opposite sex DZ pairs acts as a preliminary test for sex differences in genetic and/or environmental variance components of the measures. The twin sibling covariance was tested for difference from that of twins by equating it to the DZ correlation. MZ and DZ twin pair covariances were then equated to test whether there was a significant difference between them, indicative of significant genetic influence.
The aim of variance components modeling is to estimate how much of the residual variation in an observed trait is due to genetic and environmental sources while simultaneously accounting for measured fixed effects. Using twins, the proportion of variation due to each of these sources was estimated by taking advantage of the differences in genetic relatedness between MZ (share all genes) and DZ (share half of genes) pairs. These known differences between MZ and DZ pairs allow the estimation of additive genetic (A), non-additive genetic (D), common environment (C), and unique environment (E, includes experimental error) parameters in a variance components model. Due to the fact that the twins in this sample were reared together, the C and D parameters are negatively confounded and, as such, cannot be simultaneously estimated on any one trait (Keller and Coventry, 2005). In choosing whether D parameters should be modeled, the MZ and DZ correlations were examined. If the MZ twin correlation is more than double the DZ twin correlation, it is indicative of nonadditive genetic influence, which can include dominance and epistasis. However, if the MZ twin correlation is less than double the DZ twin correlation, it is indicative of common environmental factors, and C parameters are modeled.
As the perceptions of different bitter-tasting compounds are moderately correlated, multivariate models were specified that utilized the additional information gained from the cross-trait correlations to estimate common sources of variation between these traits in addition to heritability. Twin correlations indicated the importance of C factors for one measure and the importance of D for the other measures, thus a Cholesky decomposition of A, C, D, and E factors was used as a starting point. Based on expectations formed by examining the phenotypic correlations and the factor loadings of the Cholesky models, alternative independent pathway models were assessed. In the first model, it was hypothesized that a common genetic bitter taste factor may underlie the covariation between measures and that the residual genetic variation would be due to phenotype-specific factors. In the second, two group genetic factors and two specific genetic factors were tested. In all models (Cholesky and independent), the covariates were modeled as regressions or deviation effects on the mean. In addition, as there were two measurements for each compound, these were examined in a repeated measures model in which two presentations were treated as observations of the one underlying score. In these models, the two presentations are constrained to be equal with any consistent mean differences between the first and second presentation accounted for by a presentation order effect which was modeled as an additional covariate. All variance components (A, D, C, and E) are then constrained to equally affect each presentation of the same compound as both presentations are equally imperfect measures of the true underlying phenotype. The test unreliability (U) is then estimated from the variance that is not shared between the first and second presentation.
The distributions of the PROP, SOA, quinine, and caffeine scores were slightly positively skewed, so they were transformed to improve normality of the distribution. The square root transformation produced the most normal distribution for all measures as determined by visual inspection of the distributions. Three of the families were identified as bivariate outliers at the assumptions testing stage and two additional families as multivariate outliers. All five families were excluded from analyses. The composition of this final sample is shown in Table 1.
Results
The phenotypic correlations between the perceived intensity scores of the solutions are displayed in Table 2. The pattern of correlations suggested a definite clustering between SOA, quinine, and caffeine (0.34–0.56) as correlations were similar to those between duplicate presentations of the same compound (0.42–0.56). This was in contrast to PROP that had lower correlations (0.12–0.28) with the other compounds and a much higher correlation between presentations (0.80).
Table 2.
Phenotypic correlations among perceived intensity measures for PROP, SOA, quinine, and caffeine and the two presentations (1 and 2) of each compound
| PROP
|
SOA
|
Quinine
|
Caffeine
|
|||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| PROP | ||||||||
| 1 | — | |||||||
| 2 | 0.80 | — | ||||||
| SOA | ||||||||
| 1 | 0.21 | 0.15 | — | |||||
| 2 | 0.23 | 0.26 | 0.42 | — | ||||
| Quinine | ||||||||
| 1 | 0.21 | 0.22 | 0.44 | 0.41 | — | |||
| 2 | 0.12 | 0.17 | 0.35 | 0.46 | 0.56 | — | ||
| Caffeine | ||||||||
| 1 | 0.30 | 0.28 | 0.57 | 0.44 | 0.51 | 0.42 | — | |
| 2 | 0.19 | 0.28 | 0.34 | 0.56 | 0.40 | 0.52 | 0.48 | — |
There were no birth order or zygosity effects for intensity ratings of any of the compounds. Additionally, history of head injury and use of cologne, perfume, and aftershave did not have a significant effect on any of the phenotypes and were not included in further analyses. The remaining fixed effects of age, sex, history of otitis media, and the presentation effect significantly influenced some measures and were included in the multivariate models. The magnitudes of these fixed effects on the square root–transformed measures in the final model are shown in Table 3. There was a consistent negative effect of age on the perceived intensity of all four solutions, demonstrating decreased sensitivity to bitter compounds as children get older. A significant sex effect on the SOA and quinine measures were also indicated, males found SOA more intense and females found quinine more intense. History of otitis media had a consistent positive effect but was only significant for the SOA measure. Also, the second presentation of SOA was, on average, scored higher than the first, and the second presentation of PROP was scored lower. This may reflect the differing order of presentation of the initial and duplicate samples.
Table 3.
Estimates of covariate effects for age, sex, history of otitis media, and order of presentation in the most parsimonious model (from Figure 1)
| Age | Sex | Otitis media | Presentation | |
|---|---|---|---|---|
| PROP | −0.13 (−0.20, −0.06)a | 0.23 (−0.17, 0.63) | 0.11 (−0.08, 0.30) | −0.29 (−0.44, −0.15) |
| SOA | −0.08 (−0.12, −0.04) | 0.30 (0.04, 0.55) | 0.21 (0.07, 0.35) | 0.52 (0.36, 0.69) |
| Quinine | −0.05 (−0.10, −0.00) | −0.28 (−0.56, 0.00) | 0.11 (−0.04, 0.26) | 0.06 (−0.09, 0.21) |
| Caffeine | −0.09 (−0.13, −0.05) | −0.06 (−0.31, 0.20) | 0.12 (−0.02, 0.25) | 0.13 (−0.02, 0.27) |
The regression and deviations were applied to the means of the transformed variables. Nonsignificant estimates italicized and 95% confidence intervals in parentheses.
For example, if respondent is aged 16, transformed PROP score would on average be reduced by 2.08 (−0.13 × 16).
Sibling correlations were shown not to differ from their respective DZ twin correlations, so the composite DZ/sib correlation was used in place of the DZ twin correlation in subsequent modeling. MZ twin correlations were significantly higher than the DZ/sib correlations for each of the bitter taste phenotypes suggesting genetic influence. MZ correlations for SOA, quinine, and caffeine were more than double the DZ correlations, whereas the MZ twin correlations of the PROP measures were less than double the DZ twin correlations. Table 4 shows the mean and standard deviations by sex and MZ and DZ/sib correlations (with 95% confidence intervals).
Table 4.
Means ± SD of untransformed perceived intensities of PROP, SOA, quinine, and caffeine for females and males plus MZ and DZ/sib twin correlations (ML) with 95% confidence intervals
| Females (N) | Males (N) | MZr | DZ/sibra | |
|---|---|---|---|---|
| PROP | ||||
| 1 | 35.1 ± 31.0 (383) | 37.2 ± 31.7 (308) | 0.78 (0.67, 0.85) | 0.56 (0.47, 0.63) |
| 2 | 30.5 ± 28.1 (381) | 34.4 ± 30.0 (305) | 0.68 (0.53, 0.78) | 0.36 (0.28, 0 .44) |
| SOA | ||||
| 1 | 41.3 ± 23.3 (386) | 45.5 ± 25.0 (311) | 0.49 (0.26, 0.64) | 0.13 (0.00, 0.25) |
| 2 | 49.9 ± 23.3 (381) | 52.3 ± 29.1 (304) | 0.29 (0.07, 0.48) | 0.18 (0.06, 0 .30) |
| Quinine | ||||
| 1 | 41.9 ± 25.5 (383) | 38.3 ± 23.7 (310) | 0.46 (0.23, 0.62) | 0.10 (−0.02, 0.23) |
| 2 | 43.0 ± 25.3 (380) | 39.2 ± 25.1 (302) | 0.54 (0.35, 0.68) | 0.16 (0.03, 0.29) |
| Caffeine | ||||
| 1 | 45.9 ± 24.6 (386) | 47.4 ± 26.3 (308) | 0.51 (0.28, 0.66) | 0.14 (0.03, 0.25) |
| 2 | 50.1 ± 27.1 (376) | 47.2 ± 26.9 (304) | 0.45 (0.24, 0.61) | 0.20 (0.08, 0.32) |
The number of participants changes between presentations as not all participants completed the entire test.
The DZ twin correlations could be equated to the sibling correlations to give a single DZ/sib correlation.
Variance components modeling of bitter taste
Given the magnitudes of MZ and DZ twin correlations, an atheoretical Cholesky model was specified in which A, C, E, and U components of variance contributed to PROP and A, D, E, and U components of variance contributed to SOA, quinine, and caffeine. The significance of the C and D parameter estimates was assessed by dropping them from the model. There was no significant worsening of model fit after removal of the PROP-specific C factor (Δ−2LL = 1.17, 1 df, P = 0.28) or any of the three D factors (Δ−2LL = 0, 1 df, P = 1; Δ−2LL = 0, 2 df, P = 1; Δ−2LL = 2.14, 3 df, P = 0.54). However, we note that the estimates for the specific C factor (PROP) and the shared D factor (SOA, quinine, and caffeine) were moderately large and that the confidence intervals were wide indicating low power to detect C and to discriminate A and D factors. All subsequent models only estimated A, E, and U factors.
The final independent pathway model drew on results of the Cholesky solution and included a genetic factor structure that was based on the parameter/factor structure of the AEU Cholesky model. It contained four genetic factors: a group factor loading on SOA, quinine, and caffeine; a second group factor that loaded on PROP, SOA, and caffeine; and two specific factors, one for PROP and one for quinine. This model showed a good fit to the data (Δ−2LL = 2.08, 2 df, P = 0.35) and was progressively simplified by reducing the E factor structure (Δ−2LL = 0, 1 df, P = 1; Δ−2LL = 0, 2 df, P = 1; Δ−2LL = 0.22, 3 df, P = 1) and dropping of the nonsignificant PROP-specific genetic factor (Δ−2LL = 0, 1 df, P = 1). An alternate independent pathway model was also tested that included one common additive genetic factor and specific additive genetic factors for each of the PROP, SOA, quinine, and caffeine measures with E parameters kept as a Cholesky structure. The fit of this model was significantly worse than the Cholesky base model (Δ−2LL = 6.52, 2 df, P = 0.04) and was not examined further.
The final model is shown as a path diagram in Figure 1 with the estimated fixed effects of the covariates shown in Table 3. The model comprised a group genetic factor (A1) that explained 27%, 22%, and 28% of variation in SOA, quinine, and caffeine, respectively; a second group factor (A2) explaining 72% of the variation in PROP, 1% of variation in SOA, and 2% of the variation in caffeine; and a specific genetic factor (A3) that explained 12% of variation in quinine. Test unreliability (U) explained a further 21% of variation in PROP and 59%, 45%, and 50% of variation in the SOA, quinine, and caffeine, respectively. This is a significant issue that can greatly decrease the estimations of heritability. The remaining variance was attributed to a general unique environmental factor (E1) that was responsible for 7%, 14%, 22%, and 19% of variation in the PROP, SOA, quinine, and caffeine phenotypes, respectively. Heritability was calculated by summing the square of the genetic path coefficients leading to each phenotype and was 0.72, 0.28, 0.34, and 0.30 for the PROP, SOA, quinine, and caffeine perceived intensity measures, respectively.
Figure 1.
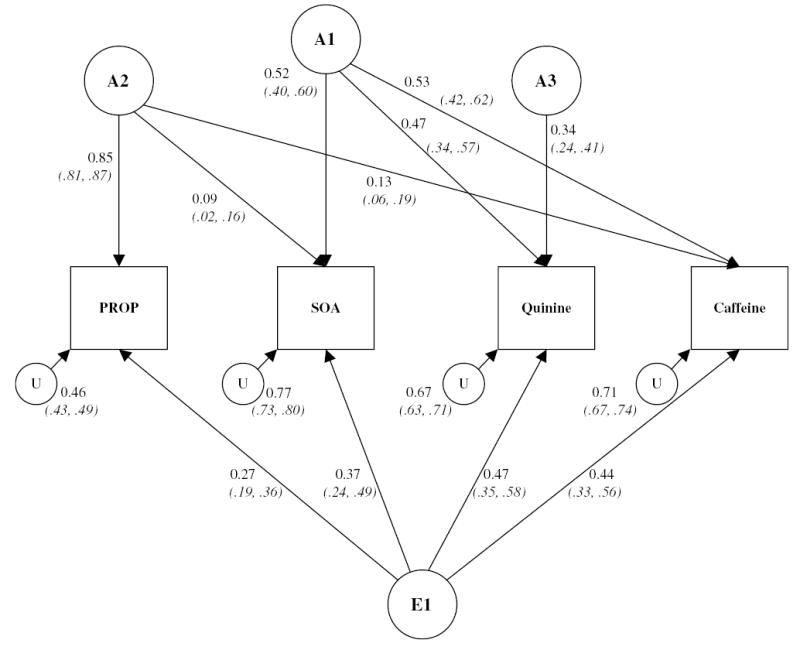
Standardized path diagram depicting the additive genetic (A) and unique environmental (E) variation in perceived intensity of PROP, SOA, quinine, and caffeine solutions and covariance between measures. U represents the test unreliability of each compound. Standardized path coefficients (which should be squared to get percentage of variance accounted for) are shown, with 95% confidence intervals in parentheses.
Discussion
This is the first study to examine the heritability of the perceived intensity of four structurally diverse bitter compounds, PROP, SOA, quinine HCl, and caffeine. A high heritability was found for PROP (0.72) with more modest heritabilities estimated for SOA, quinine, and caffeine (0.28, 0.34, and 0.30, respectively). Multivariate modeling indicated that no general (common) genetic factor influenced all four compounds. A common genetic group factor was responsible for the majority of the genetic variation in SOA, quinine, and caffeine, accounting for 27%, 22%, and 28% of the phenotypic variation, respectively. This is consistent with Delwiche et al. 2001 who found a common factor for individual differences to these compounds. A genetic factor that was responsible for all the genetic variation in PROP only accounted for 1% and 2% of variation in SOA and caffeine. A large genetic factor is expected as Bufe et al. 2005 had found that the TAS2R38 gene haplotypes accounted for most of the perceptual variability to PROP. There was also a specific genetic factor responsible for a modest 12% of variation in the perceived intensity of quinine. In contrast, a single common factor explained all the unique environmental variance for all four bitter taste phenotypes, accounting for 7–22% of the variation, and unreliability explained the remainder of the variance (21–59%). This is consistent with others (Lawless, 1979; Delwiche et al., 2001a) who suggest a factor that impacted perception of all bitters; that is, the gain of the bitter taste system appears to be manipulated in all bitter compounds across the board.
The heritability of the perceived intensity of suprathreshold PROP solution used in this study is higher, but comparable to heritability estimates from detection threshold measures of the related compound PTC, for which heritability has been estimated at 0.5 (Drayna et al., 2003) and 0.55 (Morton et al., 1981), and a genetic association study which has shown 55–85% of variation in PTC is attributable to three SNPs in the bitter taste receptor TAS2R38 (Kim et al., 2003). Our higher heritability estimate may be due to a number of factors. Firstly, the relationship between PTC intensity ratings and TAS2R38 genotype is orderly, but the genotype relationship to PROP is much less so, especially at high PROP concentrations (Bufe et al., 2005). This observation, in conjunction with several other lines of evidence, suggests that additional genotype and/or environmental effects influence PROP intensity ratings. Secondly, detection threshold has been shown not always to predict or be associated with suprathreshold response (e.g., Bartoshuk, 1978, 2000; Mojet et al., 2005) and, therefore, these measures may represent distinct phenotypes with different proportions of genetic and environmental influences. Thirdly, the evidence from mice and the golden hamster demonstrates that heritability estimates vary with the concentration of the compound used in the measure, suggesting that either the magnitude of the genetic effect changes or additional genetic effects arise as the concentrations are altered. Finally, the heritability estimate for PROP obtained in this study could be slightly inflated (~10%) by the potential common environmental influence, which was found to be nonsignificant in preliminary Cholesky models due to the low power to detect C and D parameters.
Heritability estimates of SOA, quinine, and caffeine (0.28, 0.34, and 0.30, respectively), while lower than PROP, are reasonable when compared to those from murine studies. For example, in mice, the heritability of SOA consumption at two different concentrations was estimated at 0.27 and 0.40 (Le Roy et al., 1999), and for two concentrations of SOA intake in golden hamsters, the narrow sense heritability has been estimated at 0.08 and 0.57 (Frank et al., 2004). Similarly, for quinine and caffeine perception, heritability is concentration dependent in the golden hamster, with heritabilities of 0.11 and 0.31 for quinine and 0.41 and 0.55 for caffeine (Frank et al., 2004). Moreover, while our heritability estimate of 0.34 for quinine is lower than the estimates 0.55 and 0.85 for a quinine detection threshold obtained from twin and family samples, respectively (Smith and Davies, 1973), this early study had a relatively small sample (N = 134 individuals) and large standard errors. Additionally, as perceived intensity measures do not necessarily correspond with detection threshold measures (e.g., Bartoshuk, 1978, 2000; Mojet et al., 2005), we can expect heritability differences to be there between these two quinine perception phenotypes. Our heritability estimates would likely be higher if methods were employed to increase test–retest reliability with SOA, quinine, and caffeine. For example, using the same battery in the laboratory under supervision and ensuring proper rinsing with deionized water increase the test–retest reliability of the SOA and caffeine measures from 0.42 and 0.48, respectively, to approximately 0.63 for both (unpublished data).
SOA and quinine perceptions in mice are predominantly controlled by single loci (Soa and Qui) each with three alleles behaving dominantly (taster allele dominant over intermediate taster allele which is in turn dominant over nontaster allele) (Bachmanov et al., 1996; Harder et al., 1996). In conjunction with the knowledge that human PROP and PTC detection threshold is predominantly controlled by dominance of the taster haplotype in the bitter taste receptor TAS2R38, it would be reasonable to hypothesize that dominant genetic factors could influence other human bitter taste perception measures. Observing that MZ twin correlations were more than double that of DZ twin pairs for SOA, quinine, and caffeine measures would tend to support dominant genes, but the PROP twin correlations suggested additive genetic influences and common environment as more likely than dominance. Our preliminary Cholesky models did estimate moderate dominance effects on SOA, quinine, and caffeine sensitivities, but these estimates were not significant at P = 0.05. Rather than suggesting that dominant genetic factors do not significantly affect these perception measures, the results simply highlight that very large sample sizes are needed to discriminate additive and dominant genetic parameters in twins. Therefore, the heritabilities reported should be interpreted as broad-sense genetic heritabilities encompassing the sum of additive and dominant acting genetic factors.
The pattern of phenotypic correlations was consistent with the observations of others (McBurney et al., 1972; Yokomukai et al., 1993; Delwiche et al., 2001a). There was a definite clustering for SOA, quinine, and caffeine perception (0.34–0.56), and all three of these compounds correlated to a lesser degree with PROP (0.12–0.28). Modeling demonstrated that a large proportion of this phenotypic covariation between SOA, quinine, and caffeine was due to a group genetic factor (22–28% phenotypic variation; 65–96% genetic variance), suggesting that variation in the perception of these three compounds is influenced by common genes. A common genetic factor is not unexpected as in mice it has been shown that a single locus, Soa, can affect the perception of multiple bitter compounds (Whitney and Harder, 1994; Boughter and Whitney, 1998; Harder and Whitney, 1998), and as such, there could be a similar situation in humans. Also, given the suggestion by Delwiche et al. 2001 that the covariation between sensitivities of SOA, quinine, and caffeine is due to the presence of methyl groups, it may be that the group genetic factor could include genes that influence the pathway involved in the detection of methyl-containing compounds.
While variation in the perceived intensity of SOA, quinine, and caffeine is predominantly controlled by the same set of genetic factors, there was a modest genetic influence for quinine that accounted for 12% of variation, suggesting that quinine perception is influenced by additional genetic factors, that is, an additional pathway that affects quinine perception only. A quinine-specific genetic factor is reasonable, considering that quinine perception in mice has been suggested to be under control of at least three distinct loci (Boughter and Whitney, 1998), a quinine-specific locus Qui (Bachmanov et al., 1996), the Soa locus that affects multiple bitter tastes, and an additional unidentified locus.
Modeling also indicated that all the genetic variation in PROP (72% phenotypic variation) was accounted for by a single factor, which only contributed 1% and 2% to variation in SOA and caffeine, respectively. Considering that a single gene, TAS2R38, accounts for 55–85% of the phenotypic variation in the detection threshold of the related compound PTC (Kim et al., 2003) and that Bufe et al. 2005 has shown that TAS2R38 haplotypes influence both PTC and PROP perception, we suggest that the TAS2R38 gene could be included in, and responsible for, a large component of the PROP genetic factor. If this is the case, we suggest that the TAS2R38 gene would also have a small effect on the variation in SOA and caffeine perception.
It has been suggested by others that there is a general genetic factor that affects variation in the perception of all bitter compounds (Olson et al., 1989). This would tend to be supported by the observation that larger numbers of fungiform papillae and higher taste pore densities of these papillae can result in more intense bitter perceptions (Miller and Reedy, 1990; Delwiche et al., 2001b), although it was noted that differences in papillae number and taste pore densities could adequately predict differences within but not between individuals (Delwiche et al., 2001b). However, we found no evidence to support a general bitter taste factor across all four of our perceived intensity measures, with the above pattern of group factors providing the best fitting model. This does not preclude the existence of a general bitter taste factor, but these results would suggest that the influence of this factor, at least on perceived intensity measures, would be quite small compared with other group and specific genetic factors.
Unique environmental factors specific to each compound were entirely due to measurement error or unreliability (U). An environmental common factor affected the perception of all four compounds and accounted for 7%, 14%, 22%, and 19% of variation in PROP, SOA, quinine, and caffeine, respectively. This factor is systemic to all four compounds but unique to individuals and represents either, genuine environmental differences, for example, diet and state of health, or correlated experimental error, for example, not rinsing between solutions.
Test unreliability accounted for 21% of the variation in PROP and 45–59% of the variation in SOA, quinine, and caffeine phenotypes, with no obvious reason for observing better reliability in the PROP measure compared with the other three compounds. These unreliability estimates are in line with past work that has found the test–retest correlation of suprathreshold PROP intensity to be 0.69 (Tepper et al., 2001) and only 0.54 for detection threshold measures of SOA collected under supervision (Boughter and Whitney, 1993). However, in our test, the unreliability estimate could include variation from other sources such as sensory adaptation, that is, participants’ bitter taste perception could change throughout the test, and unspecified ordering effects, that is, the first time a solution is presented it may follow a bitter compound and the second time a sweet. True unreliability in the present study may be due to lack of compliance because the tests were sent in the mail and completed at home. For example, if participants do not rinse thoroughly between each presentation, this can cause contrast or bleed-through effects across the different test solutions. The unreliability puts a ceiling on heritability estimates, and therefore, the heritability for the perceived intensity of these bitter compounds may in fact be higher.
The finding of a negative effect of age (−0.05 to −0.13) on the perception of bitter taste (significant for all but quinine) is in line with previous studies that have reported decreasing sensitivity with age for the perception of PTC (Schiffman et al., 1994; Guo and Reed, 2001; Mojet et al., 2003), quinine (Cowart et al., 1994), caffeine (Hyde and Feller, 1981), as well as other compounds (Mojet et al., 2003). The age effect may be particularly pronounced in this adolescent and young adult twin sample when taste preferences of many are changing from childish (e.g., sugar) to adult (e.g., beer and olives). In addition to an age effect, history of otitis media significantly increased the perceived bitterness of SOA, and there was a nonsignificant trend observed with the other three compounds. A mechanism for this increase was proposed (Bartoshuk et al., 1996) in which the response of the chorda tympani to otitis media infection results in the increase of the numbers of taste buds per fungiform papillae. The more the taste buds, the more the taste receptor cells, and consequently, the more bitter a compound may be perceived. Sex differences found for the adolescent sample are mostly in line with other studies (Smith and Davies, 1973; Hyde and Feller, 1981; Boughter and Whitney, 1993) except for SOA, with males finding the transformed score of SOA 0.3 more intense, contrasting with a previous report suggesting no sex difference in detection thresholds of SOA (Boughter and Whitney, 1993).
In conclusion, this study has examined the perceived intensity phenotypes of four structurally diverse bitter compounds, PROP, SOA, quinine HCl, and caffeine and established that a substantial amount of variation in these traits is due to genetic factors. It was also shown that a substantial amount of the observed phenotypic covariation between SOA, quinine, and caffeine perception was due to shared genetic influences and that the genetic influences on PROP perception had very little influence on the perception of the other three bitter compounds. This suggests that there are two distinct pathways responsible for the perception of these four compounds, one for PROP and another for the perception of SOA, quinine, and caffeine.
Acknowledgments
We thank Kirsten J. Mascioli and Christopher Tharp from the Monell Chemical Senses Center for manufacturing the taste tests and from the QIMR, Alison Mackenzie, Romana Leisser, and Kim Eldridge for project coordination and data entry, David Smyth and Daniel Parkes for computer support, and Michelle Luciano for advice on the analysis. We also thank the twins and their families for providing their data. This work was supported by National Institute of Health, grants DC02995 to P.A.S.B. and DC004698 to D.R.R., and Australian NHMRC (241944).
References
- Aitken JF, Green A, Eldridge A, Green L, Pfitzner J, Battistutta D, Martin NG. Comparability of naevus counts between and within examiners, and comparison with computer image analysis. Br J Cancer. 1994;69:487–491. doi: 10.1038/bjc.1994.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Six in ten ‘tasteblind’ to bitter chemical. Sci News Lett. 1931;9:249. [Google Scholar]
- Ardashnikov SN, Lichtenstein EA, Martynova RP, Soboleva GV, Postnikova EN. The diagnosis of zygosity in twins. J Hered. 1936;27:465–468. [Google Scholar]
- Bachmanov AA, Li X, Li S, Neira M, Beauchamp GK, Azen EA. High-resolution genetic mapping of the sucrose octaacetate taste aversion (Soa) locus on mouse chromosome 6. Mamm Genome. 2001;12:695–699. doi: 10.1007/s00335-001-2061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet. 1996;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM. The psychophysics of taste. Am J Clin Nutr. 1978;31:1068–1077. doi: 10.1093/ajcn/31.6.1068. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM. Comparing sensory experiences across individuals: recent psychophysical advances illuminate genetic variation in taste perception. Chem Senses. 2000;25:447–460. doi: 10.1093/chemse/25.4.447. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Reed D, Williams A. Supertasting, earaches and head injury: genetic and pathology alter out taste. Neurosci Biobehav Rev. 1996;20:79–87. doi: 10.1016/0149-7634(95)00042-d. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Harder DB, Capeless CG, Whitney G. Polygenic determination of quinine sensitivity among mice. Chem Senses. 1992;17:427–434. [Google Scholar]
- Boughter JD, Jr, Whitney G. Human taste thresholds for sucrose octaacetate. Chem Senses. 1993;18:445–448. [Google Scholar]
- Boughter JD, Jr, Whitney G. Behavioural specificity of the bitter taste gene Soa. Physiol Behav. 1998;63:101–108. doi: 10.1016/s0031-9384(97)00398-3. [DOI] [PubMed] [Google Scholar]
- Bufe B, Breslin PAS, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim U, Drayna D, Meyerhof W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capeless CG, Whitney G, Azen EA. Chromosome mapping of Soa, a gene influencing gustatory sensitivity to sucrose octaacetate in mice. Behav Genet. 1992;22:655–663. doi: 10.1007/BF01066636. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJP. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Cowart BJ, Yokomukai Y, Beauchamp GK. Bitter taste in aging: compound-specific decline in sensitivity. Physiol Behav. 1994;56:1237–1241. doi: 10.1016/0031-9384(94)90371-9. [DOI] [PubMed] [Google Scholar]
- Delwiche JF, Buletic Z, Breslin PAS. Covariation in individuals’ sensitivities to bitter compounds: evidence supporting multiple receptor/transduction mechanisms. Percept Psychophys. 2001a;63:761–776. doi: 10.3758/bf03194436. [DOI] [PubMed] [Google Scholar]
- Delwiche JF, Buletic Z, Breslin PAS. Relationship of papillae number to bitter intensity of quinine and PROP within and between individuals. Physiol Behav. 2001b;74:329–337. doi: 10.1016/s0031-9384(01)00568-6. [DOI] [PubMed] [Google Scholar]
- Dencker SJ, Hauge M, Kaij L. An investigation of the PTC taste character in monochorionic twin pairs. Acta Genet Stat Med. 1959;9:236–244. [PubMed] [Google Scholar]
- Drayna D, Coon H, Kim U, Elsner T, Cromer K, Otterud B, Baird L, Peiffer AP, Leppert M. Genetic analysis of a complex trait in the Utah genetic reference project: a major locus for PTC taste ability on chromosome 7q and a secondary locus on chromosome on chromosome 16. Hum Genet. 2003;112:567–572. doi: 10.1007/s00439-003-0911-y. [DOI] [PubMed] [Google Scholar]
- Frank ME, Wada Y, Makino J, Mizutani M, Umezawa H, Katsuie Y, Hettinger TP, Blizard DA. Variation in intake of sweet and bitter solutions by inbred strains of golden hamsters. Behav Genet. 2004;34:465–476. doi: 10.1023/B:BEGE.0000023651.99481.d5. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore M. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993;18:683–702. [Google Scholar]
- Guo SW, Reed DR. The genetics of phenylthiocarbamide perception. Ann Hum Biol. 2001;28:111–142. doi: 10.1080/03014460151056310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder DB, Boughter JD, Whitney G. PTC-avoidance polymorphism and other bitter-avoidance differences among mice in long-term preference tests. Chem Senses. 1996;21:612. [Google Scholar]
- Harder DB, Capeless CG, Maggio JC, Boughter JD, Jr, Gannon KS, Whitney G, Azen EA. Intermediate sucrose octa-acetate sensitivity suggests a third allele at mouse bitter taste locus Soa and Soa-Rua identity. Chem Senses. 1992;17:391–401. [Google Scholar]
- Harder DB, Whitney G. A common polygenic basis for quinine and PROP avoidance in mice. Chem Senses. 1998;23:327–332. doi: 10.1093/chemse/23.3.327. [DOI] [PubMed] [Google Scholar]
- Hyde RJ, Feller RP. Age and sex effects on taste of sucrose, NaCl, citric acid and caffeine. Neurobiol Aging. 1981;2:315–318. doi: 10.1016/0197-4580(81)90041-5. [DOI] [PubMed] [Google Scholar]
- Kaplan AR, Fisher R, Karras A, Griffin F, Powell W, Marsters RW, Glanville EV. Taste thresholds in twins and siblings. Acta Genet Med Gemellol. 1967;16:229–243. doi: 10.1017/s112096230001307x. [DOI] [PubMed] [Google Scholar]
- Keller MC, Coventry WL. Quantifying and addressing parameter indeterminacy in the classical twin design. Twin Res Hum Genet. 2005;8:201–213. doi: 10.1375/1832427054253068. [DOI] [PubMed] [Google Scholar]
- Kim U, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste senstivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- Krondl M, Coleman P, Wade J, Milner J. A twin study examining the genetic influence on food selection. Hum Nutr Appl Nutr. 1983;37A:189–198. [PubMed] [Google Scholar]
- Lawless HT. The taste of creatine and creatinine. Chem Senses Flavour. 1979;4:249–258. [Google Scholar]
- Le Roy I, Pager J, Roubertoux PL. Genetic dissection of gustatory sensitivity to bitterness (sucrose octaacetate) in mice. CR Acad Sci III–Vie. 1999;322:831–836. doi: 10.1016/s0764-4469(00)86647-0. [DOI] [PubMed] [Google Scholar]
- Levit SG, Soboleva GV. Comparative intrapair correlations of fraternal twins and siblings. J Hered. 1935;30:389–396. [Google Scholar]
- Lush IE. The genetics of tasting in mice. III Quinine. Genet Res. 1984;44:151–160. doi: 10.1017/s0016672300026355. [DOI] [PubMed] [Google Scholar]
- Martin NG. Phenylthiocarbamide tasting in a sample of twins. Ann Hum Genet. 1975;38:321–326. doi: 10.1111/j.1469-1809.1975.tb00616.x. [DOI] [PubMed] [Google Scholar]
- McBurney DH, Smith DV, Shick TR. Gustatory cross adaptation: sourness and bitterness. Percept Psychophys. 1972;11:228–232. [Google Scholar]
- McGregor B, Pfitzner J, Zhu G, Grace M, Eldridge A, Pearson J, Mayne C, Aitken JF, Green AC, Martin NG. Genetic and environmental contributions to size, color, shape, and other characteristics of melanocytic naevi in a sample of adolescent twins. Genet Epidemiol. 1999;16:40–53. doi: 10.1002/(SICI)1098-2272(1999)16:1<40::AID-GEPI4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Miller IJ, Reedy FE. Variations in human taste bud density and taste intensity perception. Physiol Behav. 1990;47:1213–1219. doi: 10.1016/0031-9384(90)90374-d. [DOI] [PubMed] [Google Scholar]
- Mojet J, Christ-Hazelhof E, Heidema J. Taste perception with age: pleasantness and its relationships with threshold sensitivity and supra-threshold intensity of five taste qualities. Food Qual Prefer. 2005;16:413–423. [Google Scholar]
- Mojet J, Heidema J, Christ-Hazelhof E. Taste perception with age: generic or specific losses in supra-threshold intensities of five taste qualities? Chem Senses. 2003;28:397–413. doi: 10.1093/chemse/28.5.397. [DOI] [PubMed] [Google Scholar]
- Morton CC, Cantor RM, Corey LA, Nance WE. A genetic analysis of taste threshold for phenylthiocarbamide. Acta Genet Med Gemellol. 1981;30:51–57. doi: 10.1017/s0001566000006619. [DOI] [PubMed] [Google Scholar]
- Neale, M.C., Boker, S.M., Xie, G. and Maes, H.H. (2002) Mx: Statistical Modeling, 6th edn, Department of Psychiatry, Richmond, VA.
- Olson JM, Boehnke M, Neiswanger K, Roche AF, Siervogel RM. Alternative genetic models for the inheritance of the phenylthiocarbamide taste deficiency. Genet Epidemiol. 1989;6:423–434. doi: 10.1002/gepi.1370060305. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Luciano M, de Geus EJC, Wright MJ, Slagboom PE, Montgomery GW, Boomsma DI, Martin NG. A genomewide scan for intelligence identifies quantitative trait loci on 2q and 6p. Am J Hum Genet. 2005;77:318–326. doi: 10.1086/432647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rife DC. Contributions of the 1937 National Twins‘ Convention to research. J Hered. 1938;29:83–90. [Google Scholar]
- Schiffman SS, Gatlin LA, Frey AE, Heiman SA, Stagner WC, Cooper DC. Taste perception of bitter compounds in young and elderly persons: relation to lipophilicity of bitter compounds. Neurobiol Aging. 1994;15:743–750. doi: 10.1016/0197-4580(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Shi P, Zhang J, Yang H, Zhang Y. Adaptive diversification of bitter taste receptor genes in mammalian evolution. Mol Biol Evol. 2003;20:805–814. doi: 10.1093/molbev/msg083. [DOI] [PubMed] [Google Scholar]
- Smith SE, Davies PDO. Quinine taste thresholds: a family study and a twin study. Ann Hum Genet. 1973;37:227–232. doi: 10.1111/j.1469-1809.1973.tb01830.x. [DOI] [PubMed] [Google Scholar]
- Snyder LH. Inherited taste deficiency. Science. 1931;74:151–152. doi: 10.1126/science.74.1910.151. [DOI] [PubMed] [Google Scholar]
- Tepper BJ, Christensen CM, Cao J. Development of brief methods to classify individuals by PROP taster status. Physiol Behav. 2001;73:571–577. doi: 10.1016/s0031-9384(01)00500-5. [DOI] [PubMed] [Google Scholar]
- Whitney G, Harder DB. Genetics of bitter perception in mice. Physiol Behav. 1994:1141–1147. doi: 10.1016/0031-9384(94)90358-1. [DOI] [PubMed] [Google Scholar]
- Whitney G, Harder DB, Gannon KS. The B6. SW bilineal congenic sucrose octaacetate (SOA)-taster mice. Behav Genet. 1989;19:409–416. doi: 10.1007/BF01066167. [DOI] [PubMed] [Google Scholar]
- Wright MJ, De Geus E, Ando J, Luciano M, Posthuma D, Ona Y, Hansell NK, Van Baal C, Hiraishi K, Hasegawa T, Smith G, Geffen G, Geffen L, Kanba S, Miyake A, Martin NG, Boomsma D. Genetics of cognition: outline of a collaborative twin study. Twin Res. 2001;4:48–56. doi: 10.1375/1369052012146. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Martin NG. Brisbane adolescent twin study: outline of study methods and research projects. Aust J Psychol. 2004;56:65–78. [Google Scholar]
- Yokomukai Y, Cowart BJ, Beauchamp GK. Individual differences in sensitivity to bitter tasting substances. Chem Senses. 1993;18:669–681. [Google Scholar]
- Zhu G, Duffy DL, Eldridge A, Grace M, Mayne C, O’Gorman L, Aitken JF, Neale MC, Hayward NK, Green AC, Martin NG. A major quantitative-trait locus for mole density is linked to the familial melanoma gene CDKN2A: a maximum-likelihood combined linkage and association analysis in twins and their sibs. Am J Hum Genet. 1999;65:483–492. doi: 10.1086/302494. [DOI] [PMC free article] [PubMed] [Google Scholar]


