Abstract
Background: Patients with a typical depression are more likely to respond to monoamine oxidase inhibitors than to tricyclic antidepressants. They are frequently offered psychotherapy in the absence of controlled tests. There are no prospective, randomized, controlled trials, to our knowledge, of psychotherapy for atypical depression or of cognitive therapy compared with a monoamine oxidase inhibitor. Since there is only 1 placebo-controlled trial of cognitive therapy, this trial fills a gap in the literature on psychotherapy for depression.
Methods: Outpatients with DSM-III-R major depressive disorder and atypical features (N = 108) were treated in a 10-week, double-blind, randomized, controlled trial comparing acute-phase cognitive therapy or clinical management plus either phenelzine sulfate or placebo. Atypical features were defined as reactive mood plus at least 2 additional symptoms: hypersomnia, hyperphagia, leaden paralysis, or lifetime sensitivity to rejection.
Results: With the use of an intention-to-treat strategy, the response rates (21-item Hamilton Rating Scale for Depression score, ≤9) were significantly greater after cognitive therapy (58%) and phenelzine (58%) than after pill placebo (28%). Phenelzine and cognitive therapy also reduced symptoms significantly more than placebo according to contrasts after a repeated-measures analysis of covariance and random regression with the use of the blind evaluator’s final Hamilton Rating Scale for Depression score. The scores between cognitive therapy and phenelzine did not differ significantly. Supplemental analyses of other symptom severity measures confirm the finding.
Conclusions: Cognitive therapy may offer an effective alternative to standard acute-phase treatment with a monoamine oxidase inhibitor for outpatients with major depressive disorder and atypical features.
PATIENTS WITH major depressive disorder (MDD) and atypical features have shown a preferential response to phenelzine sulfate compared with imipramine hydrochloride in placebo-controlled trials,1-7 prompting the recommendation of monoamine oxidase inhibitors (MAOIs) as the standard of care.8 Unfortunately, MAOIs have dietary restrictions, contraindications, and well-known side effects.3,9 Although Reimherr et al10 and Pande et al11 showed that fluoxetine hydrochloride may offer an alternative, many patients cannot or will not take antidepressant medication. Since psychosocial treatments are sought by and prescribed for depressed patients with atypical features, studies of efficacy and effectiveness are needed.
Cognitive therapy is effective for some patients with MDD.12,13 Mercier and colleagues14 showed that cognitive therapy reduced symptoms of depressed outpatients with atypical features. In depressed outpatients who were not subtyped, acute-phase cognitive therapy is more effective than waiting-list controls,15 has not differed significantly from antidepressant medication used alone,12,16-19 and may not differ when combined with pharmacotherapy.12,19,20
We defined atypical features as a sub-type of MDD during which patients have reactive mood and at least 2 of the following 4 symptoms: hyperphagia, hypersomnia, leaden paralysis, or a lifetime history of interpersonal sensitivity to rejection, resulting in functional impairment. This definition is comparable with that used in DSM-IV21 and has heuristic value in selecting patients who respond to phenelzine.
The purpose of this double-blind, placebo-controlled trial of outpatients with MDD and atypical features was to compare acute-phase cognitive therapy with the standard of care, phenelzine. Phenelzine was selected instead of a selective serotonin reuptake inhibitor because there is more support for its efficacy in this patient population.1-7 We hypothesized that cognitive therapy and phenelzine would each reduce depressive symptoms significantly more than placebo and that the active treatments would produce comparable reductions.
PATIENTS AND METHODS
PATIENTS
The protocol was approved by the institutional review board. Subjects (recruited through media, printed announcements, and self or practitioner referrals) underwent triage by telephone. Outpatients (N = 366) with the complaint of depression participated in the Structured Clinical Interview for DSM-III-R (Outpatient Version),22 which uses the DSM-III-R23 criteria for MDD and other disorders. To assess MDD with atypical features, the Atypical Depression Diagnostic Scale (Jonathan W. Stewart, MD, written communication, October 20, 1988, and March 20, 1990) was administered for the initial episode. If the diagnosis was absent at the nadir, symptoms were reassessed at “any other time during the episode.” Criteria (according to the Atypical Depression Diagnostic Scale) for depression with atypical features included (1) maintains reactive mood and (2) shows 2 or more of the following: (a) increased appetite or weight gain; (b) over-sleeping; (c) sensation of leaden paralysis or extreme heaviness of arms or legs, while depressed; and (d) lifetime sensitivity to interpersonal rejection.1,2
Diagnoses were confirmed by a faculty-level diagnostician at a follow-up interview. Entry criteria were (1) DSM-III-R MDD, (2) definite atypical depression, and (3) score of 14 or more on the 21-item Hamilton Rating Scale for Depression (HRSD-21)24 at the initial or follow-up interview. All patients provided a medical history and a physician reviewed laboratory screening.
Patients were excluded if they (1) had a concurrent medical disorder or treatment that might cause depressive symptoms or required medication incompatible with MAOIs; (2) refused to be randomized or to maintain a tyramine-free diet; (3) had other current primary comorbid psychiatric disorders (eg, organic mental disorders, psychotic disorders, schizophrenia, schizoaffective disorders, alcoholism, or drug abuse or dependency in the last 6 months); (4) scored less than 14 on the HRSD-21 at diagnostic evaluation and follow-up, or before randomization (see description of nonspecific treatment below); (5) could not complete questionnaires; (6) represented an imminent suicide risk; or (7) had previously had an adequate trial of MAOIs or cognitive therapy that failed.
Of the 366 patients studied, 287 (78.4%) were diagnosed as having MDD; of the patients with MDD, 242 (66.1% of those studied) were also diagnosed as having atypical depression. One hundred eighty-one (49.5%) were eligible. Thirty-nine (21.5%) refused consent (generally because of scheduling problems or the desire to receive or avoid a study treatment). The nonspecific treatment baseline (designed to identify and exclude patients who respond to early, nonspecific effects) was initiated when patients signed consent (n = 142). Subjects participated in 2 sessions (during 14 days) of nonspecific treatment by watching a videotape on mood disorders and reporting on symptoms from the HRSD-21. At the end of nonspecific treatment, 13 patients had responded (ie, HRSD-21 <14 or no MDD) and were referred.
Final eligibility for randomization was evaluated by confirming that the inclusion criteria were met. One hundred eight (76.0% of the consenting and eligible) patients were randomized (Table 1) under the supervision of the statistician (D.M.), who kept research personnel blind to assignment (phenelzine or placebo) during the study.
TREATMENT PROCEDURES
Cognitive Therapy
Cognitive therapy was conducted as described by Beck et al29 for 10 weeks, in 20 individual sessions held twice weekly. Three experienced male therapists provided cognitive therapy. Two were doctoral-level clinical psychologists and 1 was a psychiatrist. An offsite consultant (see acknowledgments) used the Cognitive Therapy Scale30,31 to evaluate competence and provide feedback to therapists and investigators.
Therapists participated in weekly group supervision. Of 64 total Cognitive Therapy Scale ratings (ie, 2 planned per patient), 9 (14%) received scores of less than 40. The grand mean Cognitive Therapy Scale score across all therapists was 46.1 ± 4.1. The analyses of variance showed no statistically significant differences in the mean Cognitive Therapy Scale scores among therapists (F2= 2.50; P = .09) or across years (F4= 1.40; P = .24).
Phenelzine and Placebo
A treatment manual32 modeled after the National Institute of Mental Health Treatment of Depression Collaborative Research Program33 guided the psychiatrist’s (M.S.) clinical management of phenelzine or placebo. Each patient was introduced to precautions necessary for using phenelzine safely. The 11 sessions spread over 10 weeks involved adjusting medication and recording symptoms, side effects, weight, and blood pressure.
When symptom reduction and monoamine oxidase inhibition of 80% or more were achieved, the patient continued to receive that dose. Compliance was assessed by pill counts and patient diaries. Dosage levels and schedules were changed to optimize response while keeping side effects within the tolerable range and reducing dropouts.
Phenelzine and placebo were identical in appearance. Patients treated in both conditions followed a lowtyramine MAOI diet. The dose (taken once or twice daily) was increased gradually during the 10 weeks to achieve a therapeutic response to a phenelzine sulfate dose of approximately 0.85 mg/kg (<50 kg, 2 tablets; 50-65 kg, 3 tablets; 66-80 kg, 4 tablets; and ≥81 kg, 5 or 6 tablets) or 1 mg/kg in all patients not responding to a lower dose.34 Patients receiving phenelzine sulfate reported taking the following average amounts per day: week 4, 60.1 ± 2.3 mg; week 7, 64.4 ± 2.9 mg; and week 10, 64.0 ± 2.4 mg.
Platelet monoamine oxidase activity was determined twice before beginning medication and inhibition was determined (as described by Orsulak et al35). To protect the double blind, blood was drawn from both patients receiving phenelzine and those receiving placebo, and the laboratory provided plausible fictitious results for the placebo group. Actual levels were received for patients taking phenelzine. A target of greater than 80% inhibition was set. Thirty (83%) of 36 patients treated with phenelzine achieved at least 80% of platelet inhibition for 2 consecutive weeks. The average monoamine oxidase percentage inhibition levels for patients treated with phenelzine were as follows: week 4, 87.6% ± 2.9%; week 8, 89.4% ± 1.7%; and week 10,90.5% ± 0.9%. The percentage of patients with monoamine oxidase inhibition of 80% or more was 91% at week 4, 93% at week 8, and 96% at week 10.
OUTCOME MEASURES
The 5 domains assessed were psychiatric diagnoses and symptom severity (reported herein), and cognitive, interpersonal, and personality functioning (to be reported separately). The blind evaluators collected the following symptom severity measures and scored DSM-III-R criteria for MDD at treatment weeks 4, 7, and 10 or at patient exit.
Symptom severity was measured as follows to compare these data with existing studies. The HRSD-2124 was collected at initial and follow-up evaluations during nonspecific treatment, randomization, each blind evaluation, weekly, and at exit. The 21-item Beck Depression Inventory36 was collected at initial evaluation and weekly. The Clinical Global Impression Scale,37 a 7-point Likert scale assessing overall clinical status, was collected at initial evaluation, randomization, each blind evaluation, and weekly.
STATISTICAL ANALYSES
The hypothesized response rates were 33% for placebo and 64% for both phenelzine and cognitive therapy. The primary dependent variable (identified a priori) was the HRSD-21 score collected at week 10 by an evaluator blind to treatment assignment. Randomization was restricted by stratification. Strata included length of current episode (≤2 years vs >2 years) and marital status at the time of the initial diagnostic evaluation (cohabiting or married vs living without a partner [single, divorced, separated, or widowed]).
Analysis of covariance (ANCOVA) was selected a priori as the primary statistical analysis. A repeated-measures ANCOVA with 3 treatments (cognitive therapy, phenelzine, and placebo) by 3 times (blind evaluation at weeks 4, 7, and 10) was conducted, where the covariates were age at onset and HRSD-21 at randomization. When attrition occurred, the end point was carried forward.
Within secondary analyses, a random coefficients regression (RCR) analysis was used to complement the ANCOVA, because it accommodates attrition of subjects over time by using available data without carrying over end points. The primary parameters estimated by RCR are overall slopes for each treatment supplemented by tests of differences among the slopes. Inclusion of covariates in the RCR models was evaluated by means of backward elimination with a P<.10 criterion for retention.
All analyses were of an intention-to-treat strategy (ie, using the 108 patients randomized). Discrete variables were reported as percentage of frequency (eg, number and percentage), while continuous variables were reported with a mean ± SE. An α level of P≤.05 was used to define significance. Fisher exact tests were 2 tailed, while χ2 tests, analyses of variance, ANCOVAs, and RCR analyses were 1 tailed.
RESULTS
SAMPLE DESCRIPTION
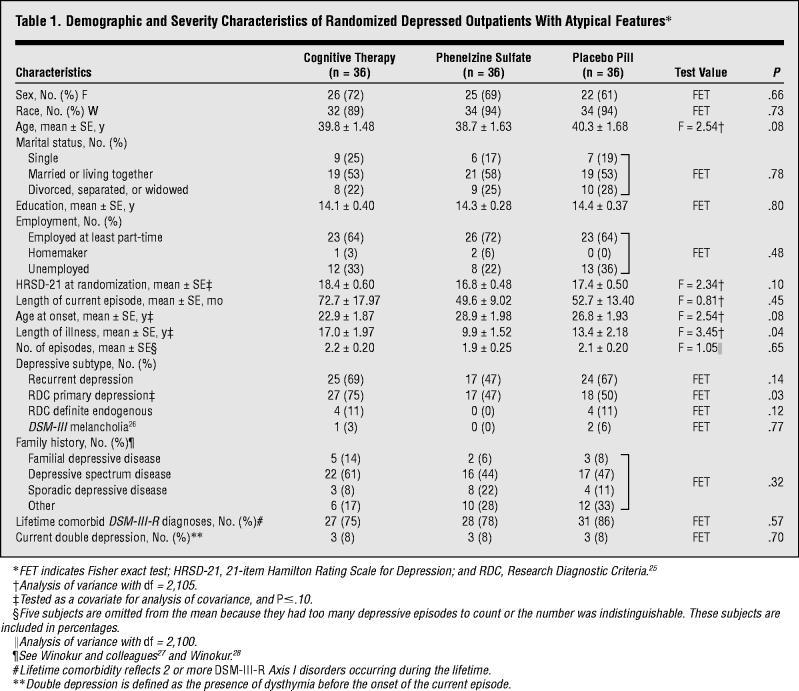
One hundred forty-two outpatients with MDD and atypical features consented to participate in the trial. The subjects who were eligible and ineligible for randomization did not differ significantly on the variables in Table 1.
Table 1.
Demographic and Severity Characteristics of Randomized Depressed Outpatients With Atypical Features
Attrition
Thirty-six patients were randomized to each cell. Attrition differed significantly among the treatment cells (χ22= 22.04; P≤.001). Five patients (14%) dropped out of cognitive therapy, 9 (25%) from phenelzine, and 23 (64%) from placebo. Attrition in the placebo group was significantly greater than in cognitive therapy (χ21 = 18.94; P≤.001) and phenelzine (χ21 = 11.03; P≤.001). The attrition between active treatments did not differ significantly (χ21= 1.42; P = .23). Of patients in cognitive therapy, 1 dropped out before the first session, 2 moved, and 2 found the study procedures unacceptable.
Nine patients randomized to phenelzine did not complete the trial. Of these, the psychiatrist withdrew 3 whose depressive symptoms required alternative treatment. Six dropped out. One dropped out after the first session, 3 found the treatment unacceptable, and 2 found the study unacceptable.
Twenty-three patients randomized to placebo did not complete the trial. The psychiatrist withdrew 4 because their symptoms necessitated alternative treatment and 2 because they were noncompliant with study procedures. Of the 17 patients who withdrew their consent, 2 dropped out before the first session, 14 found the treatment unacceptable, and 1 found the study unacceptable.
Treatment Exposure
Patients treated with cognitive therapy completed an average of 17.4 ± 0.9 sessions (range, 0-20) during an average of 8.8 ± 0.5 weeks (range, 0-11.1). Patients treated with phenelzine completed an average of 9.8 ± 0.4 sessions (range, 1-11) during an average of 8.8 ± 0.5 weeks (range, 0-10.6). Patients treated with placebo completed an average of 6.9 ± 0.6 sessions (range, 0-11) during an average of 5.9 ± 0.6 weeks (range, 0-10.3).
PRIMARY OUTCOME MEASURES AND ANALYSES
The raw data collected for each group at baseline and at blind evaluations (weeks 4, 7, and 10) were reduced to unadjusted means and SEs for the HRSD-21, Clinical Global Impression Scale, and Beck Depression Inventory (Table 2).
Table 2.
Unadjusted Mean ± SE Symptom Severity Scores Before Treatment Through Weeks 4, 7, and 10 (End Point Carried Over)*
| Cognitive Therapy (n = 36) | Phenelzine Sulfate (n = 36) | Pill Placebo (n = 36) | |
|---|---|---|---|
| 21-Item Hamilton Rating Scale for Depression | |||
| Pretreatment | 21.11 ± 0.75 | 20.03 ± 0.60 | 21.22 ± 0.59 |
| Week 0 | 18.36 ± 0.60 | 16.75 ± 0.48 | 17.42 ± 0.50 |
| Week 4 | 15.53 ± 1.15 | 12.64 ± 1.14 | 17.08 ± 0.98 |
| Week 7 | 11.75 ± 1.17 | 8.92 ± 1.13 | 15.03 ± 1.18 |
| Week 10 |
10.25 ± 1.35 |
8.64 ± 1.07 |
14.44 ± 1.26 |
| Beck Depression Inventory | |||
| Pretreatment | 27.58 ± 1.22 | 27.86 ± 1.30 | 28.42 ± 1.17 |
| Week 0 | 25.83 ± 1.19 | 24.86 ± 1.44 | 26.19 ± 1.39 |
| Week 4 | 16.39 ± 1.64 | 16.19 ± 1.56 | 21.33 ± 1.80 |
| Week 7 | 13.00 ± 1.52 | 11.06 ± 1.56 | 19.33 ± 2.02 |
| Week 10 |
11.72 ± 1.62 |
9.67 ± 1.56 |
18.94 ± 2.12 |
| Clinical Global Impression Scale | |||
| Pretreatment | 4.03 ± 0.09 | 4.06 ± 0.04 | 4.06 ± 0.07 |
| Week 0 | 4.03 ± 0.09 | 3.86 ± 0.09 | 3.92 ± 0.10 |
| Week 4 | 3.56 ± 0.23 | 3.36 ± 0.23 | 4.03 ± 0.17 |
| Week 7 | 3.00 ± 0.22 | 2.44 ± 0.24 | 3.64 ± 0.25 |
| Week 10 | 2.47 ± 0.29 | 2.28 ± 0.24 | 3.44 ± 0.27 |
Pretreatment scores occurred before treatment began. Randomization occurred at week 0.
Covariate Selection
Randomization did not achieve complete pretreatment equivalence among cells. Length of illness, Research Diagnostic Criteria primary depression, age at onset, and HRSD-21 score at randomization were identified as possible covariates.
The mean duration of illness (years) for the cognitive therapy cell was significantly greater than for phenelzine, but did not differ from that of the placebo cell. The mean duration of illness for the placebo cell did not differ from that of the phenelzine cell. A rate of Research Diagnostic Criteria primary depression of 75% in the cognitive therapy cell was significantly greater than that in the phenelzine cell, but not greater than that in the placebo cell. There was no difference in primary depression between the phenelzine and placebo cells. The phenelzine cell had the greatest age at onset, while the cognitive therapy cell had the lowest age at onset, and the placebo cell fell in the middle. These cells did not differ significantly. Finally, post hoc comparisons on the HRSD-21 at randomization disclosed no significant differences. All other comparisons among treatments on the variables in Table 1 did not differ significantly.
Because of intercorrelations among the 4 potential covariates, backward elimination was used to select statistically important covariates. Variables that remained significant (P<.10) were age at onset and HRSD-21 score, which were included as covariates in tests of the primary hypothesis.
Repeated-Measures ANCOVA of HRSD-21
With ANCOVA, the main effects for time and treatment were significant (F2,103 = 27.5; P≤.001 and F2,103 = 6.83; P<.01, respectively). The interaction between treatment and time was not significant (F4,103 = 1.12; P = .35). The contrasts for the main effects indicate significant differences when comparing phenelzine with placebo (F1,103 = 11.90; P<.001) and cognitive therapy with placebo (F1,103 = 7.73; P<.01). There was no significant difference between phenelzine and cognitive therapy (F1,103 = 0.37; P = .54). Post hoc pairwise contrasts of the treatment cells showed that at week 4, phenelzine reduced the adjusted mean HRSD-21 scores (13.36 ± 1.06) more than placebo (17.20 ± 1.05) (F1,103 = 6.66; P = .01). At weeks 7 and 10, both cognitive therapy and phenelzine reduced the adjusted mean HRSD-21 score over that of placebo (week 7: F1,103 = 7.29; P<.01 [cognitive therapy vs placebo]; F1,103 = 12.60; P<.001 [phenelzine vs placebo]; week 10: F1,103 = 8.94; P<.01 [cognitive therapy vs placebo]; F1,103 = 9.30; P<.01 [phenelzine vs placebo]). Week 7 adjusted HRSD-21 means were 10.92 ± 1.11 for cognitive therapy, 9.63 ± 1.11 for phenelzine, and15.14 ± 1.09 for placebo. Week 10 adjusted HRSD-21 means were 9.42 ± 1.22 for cognitive therapy, 9.36 ± 1.22 for phenelzine, and 14.56 ± 1.20 for placebo. The HRSD-21 score from week 0 was included as one of the covariates in the adjusted-mean HRSD-21 score for each treatment at weeks 4, 7, and 10 (Figure 1).
Figure 1.
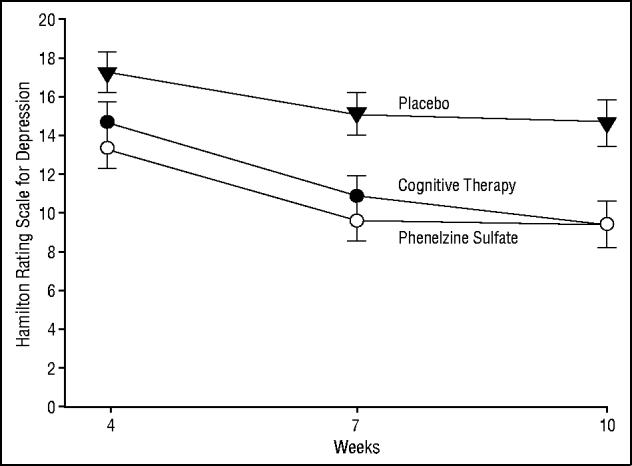
Analysis of covariance of 21-item Hamilton Rating Scale for Depression from the blind evaluator for weeks 4, 7, and 10. At week 10 the active treatments reduced symptoms significantly more than placebo (phenelzine sulfate [F1,103 = 9.30; P<.01] and cognitive therapy [F1,103 = 8.94; P<.01], where 36 patients were randomized to each group).
SECONDARY OUTCOME MEASURES AND ANALYSES
Response Rates
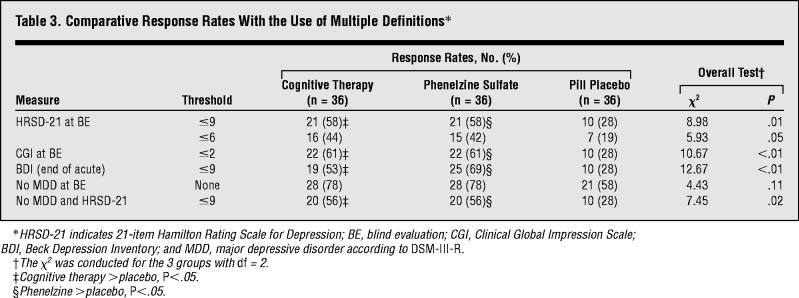
Most definitions of positive response rates for the active treatments (which used end points from each measure and were unadjusted for the influence of the covariates) were greater than 50% (Table 3). Analyses by χ2 showed significant differences in response rates when positive response was defined at blind evaluation as follows: HRSD-21 score of 9 or less (χ22 = 8.98; P = .01), Clinical Global Impression Scale score of 2 or less (χ22 = 10.67; P<.01), and no MDD with an HRSD-21 score of 9 or less (χ22= 7.45; P = .02). Post hoc comparisons on these definitions of response showed that cognitive therapy and phenelzine produced higher response rates than placebo, and that cognitive therapy and phenelzine did not differ. With the use of traditional α levels, response rates did not differ between the 3 groups when a positive response was defined as an HRSD-21 score of 6 or less (χ22 = 5.93; P = .05) and no MDD (χ22 = 4.43; P = .11).
Table 3.
Comparative Response Rates With the Use of Multiple Definitions
Random Regression Modeling
In the RCR analysis, only the HRSD-21 score at randomization was retained through the backward elimination steps. To linearize change in HRSD-21 scores over the 10 weeks, the time scale was transformed by using the natural logarithm of day of randomization +1; thus, slope estimates approximate change in HRSD-21 score per unit change in the log of day after baseline. Significantly different slopes over time were found among the 3 treatments (treatment × time interaction, F2,161 = 4.25; P<.02). There is a significantly larger negative slope for the phenelzine cell (F1,161 = 7.56; P<.01) compared with placebo, and for the larger cognitive therapy slope compared with placebo (F1,161 = 5.29; P<.03). The slopes for the 2 active treatments did not differ significantly (F1,161 = 0.23; P = .63). Slopes estimates (per natural logarithm of day in treatment) for the 3 treatments (with SEs) are −0.78 ± 0.33 for placebo, −1.83 ± 0.31 for cognitive therapy, and −2.03 ± 0.31 for phenelzine. The estimated time course of HRSD-21 scores for each of the 3 cells across the 10 weeks of acute-phase treatment was based on a subject with an average baseline HRSD-21 score of 17.5 (Figure 2).
Figure 2.

Random regression analysis of 21-item Hamilton Rating Scale for Depression (HRSD-21) from the blind evaluator for weeks 4, 7, and 10. Slopes from cognitive therapy and phenelzine sulfate did not differ and were each significantly greater than placebo (F1,161 = 5.29; P<.03 and F1,161 = 7.56; P<.01, respectively, where 36 patients were randomized to each group).
Adverse Effects
There were no serious or persistent adverse events. From 38 potential side effects, 23 symptoms were reported by 3 or more patients and rated by the psychiatrist as “due to the study” and “moderate.” Incidence density ratios (ie, [frequency of symptoms with phenelzine/treatment weeks]/[frequency of symptoms with placebo/treatment weeks]) were computed. Weakness or fatigue, drowsiness or sedation, insomnia, dry mouth, dizziness, and increased appetite were significantly more likely to be reported by patients treated with phenelzine than those treated with placebo (χ21 test; P<.01, Bonferroni correction). More patients treated with phenelzine reported marked side effects (33/36 [92%]) than did patients treated with placebo (19/36 [53%]).
COMMENT
The results of this placebo-controlled, randomized trial indicate that both cognitive therapy and phenelzine are effective treatments for patients with MDD and atypical features. Effects of cognitive therapy and phenelzine were comparable on all outcome measures.
The implication is that cognitive therapy is an effective acute-phase alternative to MAOIs for patients with MDD and atypical depression. This trial has design features relevant to evaluating the efficacy of cognitive therapy and phenelzine for depression. It is only the second randomized trial of cognitive therapy, to our knowledge, to include a pill placebo plus clinical management. The first was the National Institute of Mental Health Treatment of Depression Collaborative Research Program.17 Both studies used an evaluator blind to treatment assignment to assess efficacy, which is infrequent in studies of cognitive therapy. In both studies, therapists were monitored longitudinally. Unlike the National Institute of Mental Health collaborative study, in this study (1) supervision occurred weekly in a group format and was not limited to occasions when competency fell below a set criterion and (2) patients received cognitive therapy twice a week during 10 weeks. This study is also the first independent, prospective replication, to our knowledge, that phenelzine reduces symptoms of MDD and atypical features more than pill placebo.2,7 In the current trial, MAOI levels after administration of phenelzine documented the adequacy of the standard treatment comparison.
The trial has limitations. First, although attrition in this trial was comparable with that of others,8 it was significantly greater for patients treated with placebo than with cognitive therapy or phenelzine. This attrition was largely initiated by the patients, reflecting their right to withdraw, and the ineffectiveness and unacceptability of placebo relative to well-publicized effective alternatives. Random regression analysis and analyses of covariance produced comparable results, suggesting that the differential efficacy of the active treatments relative to placebo in this intention-to-treat sample was not caused by a carryover bias.
Second, the generalizability of the findings requires investigation. The patients represent those who were willing to undergo randomization. The modal patient was a white woman approaching midlife with a moderate level of depression and atypical features. The cognitive therapists were experienced, and weekly supervision likely maintained their adherence and competence. The psychopharmacologist was similarly experienced and used MAOI levels to aid dosing. To produce greater levels of response, remission, and recovery, both practicing cognitive therapists and psychopharmacologists might increase the frequency of cognitive therapy sessions or the dose of phenelzine and would likely increase the duration of treatment. We are conducting a follow-up pilot study to examine how the patients who responded to each treatment fared during 24 months when treatments were continued or discontinued for 8 more months.
Results from the acute phase suggest that cognitive therapy is comparable with pharmacotherapy (ie, more than half of the patients who begin respond). If these findings are replicated, patients with atypical depression will benefit from greater choices. These findings highlight the potential significance of additional randomized controlled trials to evaluate the efficacy of other promising short-term psychotherapies (eg, interpersonal psychotherapy, marital therapy, and behavior therapy), compared with standard pharmacotherapy for patients with atypical depression.
In conclusion, the results of this randomized controlled clinical trial suggest that cognitive therapy, when provided twice weekly by experienced and competent therapists, reduces symptoms more than placebo and as much as phenelzine in outpatients diagnosed as having MDD and atypical features. Acute-phase cognitive therapy appears to be a safe and effective alternative to standard acute-phase treatment with MAOIs for outpatients with atypical depression.
Footnotes
Presented in part at the 150th Annual Meeting of the American Psychiatric Association, May 21, 1997, San Diego, Calif, and at the meeting of the Association for the Advancement of the Behavior Therapy, Miami, Fla, November 15, 1997.
Catherine Judd, PA-C, MS, Steven Krebaum, MS, Melinda Down, PhD, Larry Alder, PA, Monica Basco, PhD, Jeanette Doyle, MA, Charlene McSwain, BSN, Jeanne Nash, RN, and Mustafa Husain, MD, provided clinical research support. Gratitude is expressed to the cadre of cognitive therapists at The University of Texas Southwestern Medical Center, Dallas, including G. Gregory Eaves, PhD, Paul Silver, PhD, and Rodger Kobes, MD, PhD. Julie Lowe, BA, Joe Webster, BS, Brad Witte, BS, Monica Lopez, BS, Dennis Strickland, RPh, and Joe Al-corn, RPh, provided research support. Michelle White, BS, Edna Christian, MA, and Sheria Oswalt, BA, assisted with manuscript preparation. Paul J. Orsulak, PhD, supervised monoamine oxidase assays. Brian F. Shaw, PhD, provided consultation and rated cognitive therapy. Janet Smith, MS, Qin-chang Cheng, PhD, and Janaki Ramanan, PhD, provided programming support, and Barbara Foster, PhD, gave statistical support. Armand Loranger, PhD, and Jonathon W. Stewart, MD, provided consultation on this research. A. John Rush, MD (Betty Jo Hay Distinguished Chair in Mental Health and Rosewood Corporation Chair in Biomedical Science) gave advice. Myron Weiner, MD (professor and vice chairman for clinical services, Aradine S. Ard Chair in Brain Science, and Dorothy L. and John P. Harbin Chair in Alzheimer’s Disease Research) commented on an earlier draft of this report. We also acknowledge the careful and thoughtful comments of the anonymous reviewers. Kenneth Z. Altshuler, MD (Stanton Sharp Professor and Chairman) provided administrative support.
This research was supported in part by grants MH-45043 and MH-38238 from the National Institute of Mental Health, Bethesda, Md (Dr Jarrett). Study medication was donated by Parke-Davis Co, Morris Plains, NJ.
REFERENCES
- 1.Liebowitz MR, Quitkin FM, Stewart JW, McGrath PJ, Harrison W, Rabkin JG, Tricamo E, Markowitz JS, Klein DF. Phenelzine vs imipramine in atypical depression: a preliminary report. Arch Gen Psychiatry. 1984;41:669–677. doi: 10.1001/archpsyc.1984.01790180039005. [DOI] [PubMed] [Google Scholar]
- 2.Liebowitz MR, Quitkin FM, Stewart JW, McGrath PJ, Harrison WM, Markowitz JS, Rabkin JG, Tricamo E, Goetz DM, Klein DF. Antidepressant specificity in atypical depression. Arch Gen Psychiatry. 1988;45:129–137. doi: 10.1001/archpsyc.1988.01800260037004. [DOI] [PubMed] [Google Scholar]
- 3.Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12:185–219. doi: 10.1016/0893-133X(94)00058-8. [DOI] [PubMed] [Google Scholar]
- 4.McGrath PJ, Stewart JW, Nunes EV, Ocepek-Welikson K, Rabkin JG, Quitkin FM, Klein DF. A double-blind crossover trial of imipramine and phenelzine for outpatients with treatment-refractory depression. Am J Psychiatry. 1993;150:118–123. doi: 10.1176/ajp.150.1.118. [DOI] [PubMed] [Google Scholar]
- 5.Quitkin FM, McGrath PJ, Stewart JW, Harrison WM, Tricamo E, Wager SG, Ocepek-Welikson K, Nunes E, Rabkin JG, Klein DF. Atypical depression, panic attacks, and response to imipramine and phenelzine: a replication. Arch Gen Psychiatry. 1990;47:935–941. doi: 10.1001/archpsyc.1990.01810220051006. [DOI] [PubMed] [Google Scholar]
- 6.Quitkin FM, Harrison W, Stewart JW, McGrath PJ, Tricamo E, Ocepek-Welikson K, Rabkin JG, Wager SG, Nunes E, Klein DF. Response to phenelzine and imipramine in placebo nonresponders with atypical depression: a new application of the crossover design. Arch Gen Psychiatry. 1991;48:319–323. doi: 10.1001/archpsyc.1991.01810280035005. [DOI] [PubMed] [Google Scholar]
- 7.Quitkin FM, Stewart JW, McGrath PJ, Tricamo E, Rabkin JG, Ocepek-Welikson IK, Nunes E, Harrison W, Klein DF. Columbia atypical depression: a subgroup of depressives with better response to MAOI than to tricyclic antidepressants or placebo. Br J Psychiatry. 1993;163(suppl 21):30–34. [PubMed] [Google Scholar]
- 8.Clinical Practice Guideline Number 5: Depression in Primary Care, 2: Treatment of Major Depression. Agency for Health Care Policy and Research, US Dept of Health and Human Services; Rockville, Md: 1993. AHCPR publication 93-0551. [Google Scholar]
- 9.Kasper S, Lepine JP, Mendlewicz J, Montgomery SA, Rush AJ. Efficacy, safety, and indications for tricyclics and newer antidepressants. Depression. 1995;2:127–137. [Google Scholar]
- 10.Reimherr FW, Wood DR, Byerley B, Brainard J, Grosser BI. Characteristics of responders to fluoxetine. Psychopharmacol Bull. 1984;20:70–72. [PubMed] [Google Scholar]
- 11.Pande AC, Birkett M, Fechner-Bates S, Haskett RF, Greden JF. Fluoxetine versus phenelzine in atypical depression. Biol Psychiatry. 1996;40:1017–1020. doi: 10.1016/0006-3223(95)00628-1. [DOI] [PubMed] [Google Scholar]
- 12.Hollon SD, DeRubeis RJ, Evans MD, Wiemer MJ, Garvey MJ, Grove WM, Tuason VB. Cognitive therapy and pharmacotherapy for depression: singly and in combination. Arch Gen Psychiatry. 1992;49:774–781. doi: 10.1001/archpsyc.1992.01820100018004. [DOI] [PubMed] [Google Scholar]
- 13.Jarrett RB, Rush AJ. Short-term psychotherapy of depressive disorders: current status and future directions. Psychiatry. 1994;57:115–132. doi: 10.1080/00332747.1994.11024675. [DOI] [PubMed] [Google Scholar]
- 14.Mercier MA, Stewart JW, Quitkin FM. A pilot sequential study of cognitive therapy and pharmacotherapy of atypical depression. J Clin Psychiatry. 1992;53:166–170. [PubMed] [Google Scholar]
- 15.Robinson LA, Berman JS, Neimeyer RA. Psychotherapy for the treatment of depression: a comprehensive review of controlled outcome research. Psychol Bull. 1990;108:30–49. doi: 10.1037/0033-2909.108.1.30. [DOI] [PubMed] [Google Scholar]
- 16.Blackburn IM, Bishop S, Glen AIM, Whalley LJ, Christie J. The efficacy of cognitive therapy in depression: a treatment trial using cognitive therapy and pharmacotherapy, each alone and in combination. Br J Psychiatry. 1981;139:181–189. doi: 10.1192/bjp.139.3.181. [DOI] [PubMed] [Google Scholar]
- 17.Elkin I, Shea MT, Watkins JT, Imber SD, Sotsky SM, Collins JF, Glass DR, Pilkonis PA, Leber WR, Docherty JP, Fiester SJ, Parloff MB. National Institute of Mental Health Treatment of Depression Collaborative Research Program: general effectiveness of treatments. Arch Gen Psychiatry. 1989;46:971–982. doi: 10.1001/archpsyc.1989.01810110013002. [DOI] [PubMed] [Google Scholar]
- 18.McKnight DL, Nelson-Gray RO, Barnhill J. Dexamethasone suppression test and response to cognitive therapy and antidepressant medication. Behav Ther. 1992;23:99–111. [Google Scholar]
- 19.Murphy GE, Simons AD, Wetzel RD, Lustman PJ. Cognitive therapy and pharmacotherapy: singly and together in the treatment of depression. Arch Gen Psychiatry. 1984;41:33–41. doi: 10.1001/archpsyc.1984.01790120037006. [DOI] [PubMed] [Google Scholar]
- 20.Beutler LE, Scogin F, Kirkish P, Schretlen D, Corbishley A, Hamblin D, Meredith K, Potter R, Bamford CR, Levenson AI. Group cognitive therapy and alprazolam in the treatment of depression in older adults. J Consult Clin Psychol. 1987;55:550–556. doi: 10.1037/0022-006X.55.4.550. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 22.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R. New York State Psychiatric Institute, Biometrics Research Department; New York, NY: 1989. [Google Scholar]
- 23.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Revised Third Edition American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;12:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Third Edition American Psychiatric Association; Washington, DC: 1980. [Google Scholar]
- 27.Winokur G, Behar D, Van Valkenberg C, Lowry M. Is a familial definition of depression both feasible and valid? J Nerv Ment Dis. 1978;166:764–768. doi: 10.1097/00005053-197811000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Winokur G. Familial (genetic) subtypes of pure depressive disease. Am J Psychiatry. 1979;136:911–913. doi: 10.1176/ajp.136.7.911. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Rush AJ, Shaw B, Emery G. Cognitive Therapy of Depression. Guilford Press; New York, NY: 1979. [Google Scholar]
- 30.Vallis TM, Shaw BF, Dobson KD. The Cognitive Therapy Scale: psychometric properties. J Consult Clin Psychol. 1986;54:381–385. doi: 10.1037//0022-006x.54.3.381. [DOI] [PubMed] [Google Scholar]
- 31.Young J, Beck AT. Cognitive Therapy Scale: Rating Manual. Center for Cognitive Therapy; Philadelphia, Pa: 1980. [Google Scholar]
- 32.Schaffer M, Kobes R, Krebaum S, Basco MR. Atypical Depression: Treatment in the Medication Clinic. University of Texas Southwestern Medical Center; Dallas, Tex: 1990. [Google Scholar]
- 33.Fawcett J, Epstein P, Feister S, Elkin I, Autry J. Clinical management–imipramine/placebo administration manual: NIMH Treatment of Depression Collaboration Research Program. Psychopharmacol Bull. 1987;23:309–324. [PubMed] [Google Scholar]
- 34.Robinson DS, Nies A, Ravaris CL, Ives JO, Bartlett D. Clinical pharmacology of phenelzine. Arch Gen Psychiatry. 1978;35:629–635. doi: 10.1001/archpsyc.1978.01770290111010. [DOI] [PubMed] [Google Scholar]
- 35.Orsulak PJ, Wittman PD, Akers LC. Evaluation of and HPLC assay for monitoring monoamine oxidase (MAO) inhibition in human platelets and plasma [abstract] Clin Chem. 1991;37:999. [Google Scholar]
- 36.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 37.Guy W.ECDEU Assessment Manual for Psychopharmacology 1976Dept of Health and Human Services; Washington, DC: Publication ADM 76-338 [Google Scholar]