Abstract
Human papillomavirus (HPV), the primary cause of cervical cancer, is also associated with the development of anal cancer. Relatively little is known about the epidemiology of anal HPV infection among healthy females and its relationship to cervical infection. We sought to characterize anal HPV infection in a cohort of adult women in Hawaii. Overall, 27% (372 of 1,378) of women were positive for anal HPV DNA at baseline compared with 29% (692 of 2,372) with cervical HPV DNA. Among women with paired anal and cervical samples, anal infection without accompanying cervical infection was observed in 14% (190 of 1,363). Concurrent anal and cervical HPV infections were observed in 13% (178 of 1,363) of women. Women with cervical HPV infection had >3-fold increased risk of concurrent anal infection. Concurrent anal and cervical HPV infection was most prevalent among the youngest women and steadily decreased through age 50 years. By contrast, the prevalence of anal infection alone remained relatively steady in all age groups. Compared with cervical infections, the overall distribution of HPV genotypes in the anus was more heterogeneous and included a greater proportion of nononcogenic types. A high degree of genotype-specific concordance was observed among concurrent anal and cervical infections, indicating a common source of infection. Nevertheless, the association of anal intercourse with anal HPV infection was limited to those women without accompanying cervical infection. The relationship of anal to cervical infection as described in this study has implications for the development of anal malignancies in women.
Introduction
Human papillomavirus (HPV), the primary cause of cervical cancer, is also associated with the development of anal cancers (1, 2). A high prevalence of HPV infection in the anus has been observed in immunocompromised, HIV-infected men and women (2, 3), but relatively little is known about the epidemiology of anal HPV infection among healthy women and its relationship to cervical infection. Although it is established that HPV infection can be transmitted to women through receptive anal intercourse (1, 4), alternate routes of transmission may be possible.
As part of a longitudinal cohort study of cofactors of persistent HPV infection of the cervix, we sought to characterize contemporaneous HPV infection in anal specimens collected from cohort participants. Our objective was to determine the type-specific presence of anal HPV infection, with and without concurrent cervical infection, and to identify factors associated with anal-cervical HPV status.
Materials and Methods
In 1999, we initiated a multiethnic cohort in Hawaii to identify determinants of persistent HPV infection of the cervix. The study was approved by the Committee on Human Subjects of the University of Hawaii and individual hospital institutional review boards. Written informed consent was obtained from all study subjects. Eligible subjects included women ages ≥18 years who were residents of Oahu. Women with a prior hysterectomy, who were pregnant within the past year, who had blood-clotting disorders, or who could not speak and understand English were ineligible to participate.
A total of 2,392 ethnically diverse women were recruited from five clinic sites in Honolulu, HI, and followed with repeat visits at 4-month intervals. Study sites included an urban medical center-based clinic servicing a largely indigent population, two university-based health clinics, a health maintenance organization, and a hospital-based clinical research center.
Cervical and Anal Specimen Collection
At each visit, trained clinicians obtained exfoliated cervical cell samples for cytology and HPV DNA detection. A Dacron swab and cytology brush were used consecutively to sample the entire ectocervix and endocervix, including the entire transformation zone. The swab and brush were then each placed in separate vials of 1.0 mL buffered medium (Digene Corp., Gaithersburg, MD). The two cervical samples were later combined in the laboratory for HPV DNA testing.
The collection of anal specimens was optional for study subjects. Following the cervical specimen collection, an exfoliated anal cell specimen was obtained using a Dacron swab moistened with sterile water. The swab was inserted −1.5 to 2.0 cm into the anus and rotated 360° clockwise (five times) and counterclockwise (five times). The swab was placed in 1.0 mL medium.
Interviewer-Administered Questionnaire
An interviewer-administered survey was conducted at each study visit. At enrollment, a short survey queried social and demographic information as well as information on tobacco and alcohol use. The survey at the subsequent follow-up visit included questions regarding medical, sexual, and reproductive histories as well as tobacco and alcohol use. The present report focuses on baseline anal and cervical HPV DNA results as well as survey data collected at baseline and the second follow-up visit.
HPV DNA Testing and Genotyping
DNA was extracted from anal and cervical specimens using commercial reagents (Qiagen, Inc., Valencia, CA). The PCR reaction used a modified version of the original degenerate primer system, the PGMY09 and PGMY11 primer pairs with HMB01, which together amplify a 450-bp region of the L1 HPV genome (5). GH20 and PC04 primers were used to coamplify a 268-bp region of the human β-globin gene as an internal control for sample sufficiency.
Each 50 μL reaction consisted of 1 × PCR II buffer (Perkin-Elmer, Norwalk, CT), 6 mmol/L MgCl2 (Perkin-Elmer), 200 μmol/L each of dATP, dTTP, dGTP, and dCTP (Perkin-Elmer), 7.5 units AmpliTaq Gold (Perkin-Elmer), 50 pmol each of PGMY09 and PGMY11 (Sigma, St. Louis, MO), 10 pmol HMB01 (Sigma), 10 pmol each of GH20 and PC04 (Midland Certified Reagent Co., Midland, TX), sterile H2O, and 5 μL specimen DNA. Positive controls consisted of constructed plasmid DNA containing the entire genome of cloned HPV 16 DNA. Negative controls were void of template. PCR was done in a 96-well format on a Perkin-Elmer 9600 as follows: 95°C for 9 minutes; 40 cycles of 95°C for 30 seconds, 55°C for 1 minute, and 72°C for 1 minute; 72°C for 5 minutes; and 4°C hold.
Amplified specimens were run on 2% precast agarose gels prestained with ethidium bromide (Invitrogen, Carlsbad, CA). Specimens positive for the 450- and 268-bp bands of HPV and (β-globin, respectively, were considered to be positive. Specimens found negative for β-globin on coamplification were reamplified in a single amplification reaction. Those remaining negative for β-globin were excluded from analysis.
The original DNA specimens from HPV-positive specimens were subsequently genotyped using a reverse line blot detection method for 37 different HPV types, including 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, CP6108, and IS39 (6, 7). Genotyping reagents were kindly supplied by Roche Molecular Systems (Pleasonton, CA). HPV PCR was done in reactions identical to that described above with the exception of the use of dUTP (instead of dTTP) and 5′ biotinylated primers. PCR products were denatured and hybridized to a nylon membrane containing the immobilized HPV probes. This genotyping assay also included probes for high and low levels of human β-globin gene. Amplicons hybridized to probes were detected using streptavidin-horseradish peroxidase–mediated color precipitation. HPV-positive specimens that were subsequently found to be negative in the genotyping assay were considered to be unclassified HPV-positive specimens.
Statistical Analyses
Women providing and not providing anal specimens were compared by unconditional multiple logistic regression. Odds ratios (OR) and 95% confidence intervals (95% CI) were computed by exponentiating the coefficients and 95% CI for the individual variables. Age (continuous variable) and race/ethnicity (White, Japanese, Hawaiian, Filipino, and other) were included as covariates.
The associations of risk factors with HPV status were also evaluated by unconditional multiple logistic regression and including age and race/ethnicity as covariates. Evaluation of anal-cervical HPV status was made using women negative for both anal and cervical infection as the reference.
For both analyses, continuous variables, such as lifetime number of sexual partners, were categorized, and indicator variables were created representing the different variable categories. ORs and 95% CIs were computed for indicator variables using the lowest level as the reference. A test for linear trend in the logit of risk was done by comparing twice the difference in log likelihood for models with and without a trend variable based on a χ2 distribution with 1 degree of freedom. The trend variable was assigned the median for the appropriate category.
Results
Cervical specimens were collected from all 2,392 women enrolled in this study. This cohort of healthy adult women represents ~0.7% of the eligible population on the island. Among the participating women, 1,566 (65.5%) agreed to provide anal specimens. Compared with women not providing anal specimens, women providing anal specimens were more likely to be White and more likely to have engaged in anal intercourse (Table 1). There were no differences between the two groups in cervical HPV infection at baseline.
Table 1.
Characteristics of study participants by provision of anal specimens, Hawaii female HPV cohort, 1999 to 2004
| Anal specimen
|
|||
|---|---|---|---|
| Not collected (n = 826), n (%) | Collected (n = 1,566), n (%) | Age/race–adjusted OR (95% CI) | |
| Cervix | |||
| HPV− | 546 (66.1) | 1,155 (73.8) | 1.0 (Reference) |
| HPV+ | 280 (33.9) | 411 (26.2) | 1.0 (0.8–1.2) |
| Age (y) | |||
| Mean (SD) | 29.3 (10.2) | 38.3 (13.4) | P < 0.0001 |
| <30 | 519 (62.8) | 514 (32.8) | 1.0 (Reference) |
| 30–39 | 178 (21.6) | 344 (22.0) | 2.0 (1.6–2.5) |
| 40–49 | 77 (9.3) | 345 (22.0) | 4.6 (3.5–6.1) |
| ≥50 | 52 (6.3) | 363 (23.2) | 6.7 (4.9–9.3) Ptrend < 0.0001 |
| Race/ethnicity | |||
| White | 226 (27.4) | 616 (39.3) | 1.0 (Reference) |
| Japanese | 90 (10.9) | 189 (12.1) | 0.6 (0.4–0.8) |
| Hawaiian | 152 (18.4) | 211 (13.5) | 0.5 (0.4–0.7) |
| Filipino | 63 (7.6) | 94 (6.0) | 0.6 (0.4–0.9) |
| Other | 295 (35.7) | 456 (29.1) | 0.7 (0.6–0.9) |
| Marital status | |||
| Single | 495 (59.9) | 586 (37.4) | 1.0 (Reference) |
| Married | 213 (25.8) | 640 (40.9) | 1.2 (1.0–1.5) |
| Divorced/separated | 115 (13.9) | 303 (19.4) | 0.9 (0.7–1.2) |
| Widowed | 3 (0.4) | 37 (2.4) | 2.3 (0.7–8.1) |
| Alcohol drinking* | |||
| Never | 490 (59.5) | 890 (56.9) | 1.0 (Reference) |
| Ever | 333 (40.5) | 674 (43.1) | 1.0 (0.8–1.1) |
| Cigarette smoking† | |||
| Never | 525 (63.8) | 948 (60.6) | 1.0 (Reference) |
| Ever | 298 (36.2) | 617 (39.4) | 1.0 (0.8–1.2) |
| Ever pregnant‡ | |||
| No | 159 (51.3) | 242 (35.9) | 1.0 (Reference) |
| Yes | 151 (48.7) | 432 (64.1) | 1.0 (0.7–1.4) |
| Age (y) at initial sex‡ | |||
| Mean (SD) | 17.1 (3.0) | 17.7 (3.6) | P = 0.46 |
| >16 | 179 (58.1) | 408 (61.8) | 1.0 (Reference) |
| ≤16 | 129 (41.9) | 252 (38.2) | 0.8 (0.6–1.1) |
| Lifetime no. sexual partners | |||
| Mean (SD) | 8.4 (11.4) | 17.0 (62.9) | P = 0.99 |
| 0–2 | 63 (20.6) | 117 (18.1) | 1.0 (Reference) |
| 3–7 | 132 (43.1) | 237 (36.7) | 1.1 (0.7–1.7) |
| >7 | 111 (36.3) | 292 (45.2) | 1.2 (0.8–1.6) Ptrend = 0.08 |
| Anal intercourse | |||
| Never | 273 (88.5) [95.8] | 532 (79) [91.3] | 1.0 (Reference) |
| Ever | 36 (11.6) | 141 (21) | 1.9 (1.3–2.8) |
| Current (past 3 mo) | 12 [4.2] | 51 [8.7] | 3.0 (1.5–6.0) |
Drinking alcohol at least weekly for ≥6 months.
Smoking cigarettes daily for ≥6 months.
Queried at second visit; therefore, smaller numbers reflect only the subset women from baseline completing the second visit.
Among the women enrolled in the study, 2,372 (99.2%) had sufficient cervical specimens measured by the presence of human β-globin. Among the 1,566 women agreeing to provide anal specimens, 1,378 (87.9%) had sufficient specimens. Of the 208 cervical and anal insufficient specimens excluded from analysis, 5 were negative for (β-globin in both cervical and anal specimens, resulting in a total of 1,363 paired, concurrently collected cervical and anal specimens.
Overall, 27% (372 of 1,378) of women were positive for anal HPV DNA compared with 29% (692 of 2,372) who were positive for cervical HPV DNA. Among the 1,363 women with paired anal and cervical HPV specimens, concurrent anal and cervical HPV infection was observed in 13% (178 of 1,363) of women, anal infection without accompanying cervical infection was observed in 14% (190 of 1,363) of women, and cervical infection alone was observed in 14% (191 of 1,363) of women (Fig. 1). Women with a cervical HPV infection had >3-fold increased risk of concurrent anal infection (OR, 3.3; 95% CI, 2.5–4.4, adjusted for age and race/ethnicity).
Figure 1.
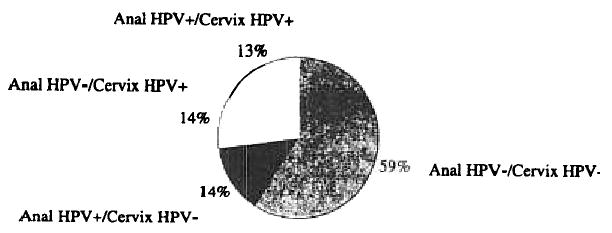
HPV infection in anal-cervical specimen pairs, Hawaii HPV cohort, 1999 to 2004.
Overall, 35 distinct HPV genotypes were detected in both anal and cervical specimens. HPV genotypes tended to be more diverse among anal specimens compared with cervical specimens, whereas oncogenic HPV types were much more common in cervical specimens than in anal specimens. These contrasts were most apparent between infections limited to either the anus or the cervix (Fig. 2). Among women with anal infection alone, 34 genotypes were detected of which oncogenic types comprised 52% and nononcogenic types comprised 48% of infections. The most common genotype among anal HPV+/cervix HPV− specimens was nononcogenic type HPV 84, which was observed in 8% of HPV-positive specimens followed by nononcogenic HPV 62 and oncogenic types HPV 16, 51, and 53, each of which comprised 7% of specimens. HPV genotypes could not be identified in 17% (33 of 191) of anal HPV+/cervix HPV− specimens. Excluding untyped specimens, multiple genotypes were identified in 46% (72 of 158) of these HPV-positive anal specimens with an average of 1.7 genotypes per specimen.
Figure 2.

Distribution of HPV genotypes among HPV-positive women.
Among women with cervical infection alone, 32 genotypes were detected of which oncogenic HPV 16 was the most common, representing 12% of HPV-positive specimens followed by oncogenic HPV types 53 (7%), 58 (6%), and 52 (6%) and nononcogenic type 62 (6%). Oncogenic and nononcogenic HPV types comprised 73% and 28%, respectively, of anal HPV−/cervix HPV+ specimens. HPV genotypes could not be identified in 24% (46 of 190) of these cervical specimens. Excluding untyped specimens, multiple genotypes were identified in 38% (89 of 144) of anal HPV−/cervix HPV+ specimens with an average of 1.6 genotypes per specimen.
A high degree of genotypic concordance was observed among the 178 women with concurrent anal HPV and cervical HPV infection (Table 2). Complete concordance [i.e., identical genotype(s)] was observed in 26% (46 of 178) of anal HPV+/cervix HPV+ specimen pairs. Partial concordance (i.e., agreement in some but not all genotypes) was observed in 53% (95 of 178) of specimen pairs. Completely different genotypes were observed in only 14% (24 of 178) of specimen pairs. There seemed to be no pattern of genotypic concordance or discordance by type (data not shown).
Table 2.
HPV type-specific concordance of concurrent infections of the anus and cervix at baseline, Hawaii HPV cohort. 1999 to 2004
| Anal HPV+/cervical HPV+ pairs | n (%) |
|---|---|
| Identical genotype(s) | |
| Partly identical genotypes | |
| Different genotype(s) | |
| Undetermined genotype in at least one specimen pair | |
| Total |
Notably, genotypes were unclassified in anal and/or cervix specimens in only 7% (13 of 178) of concurrent infections, a much smaller proportion of unidentified specimens than either anal HPV infections alone or cervical infections alone. Multiple genotypes were also more common in concurrently infected women. Infection with multiple HPV types comprised 64% (108 of 170) of HPV-positive cervix specimens and 54% (92 of 171) of HPV-positive anal specimens (excluding untyped specimens). This concurrently infected group averaged 2.1 and 1.9 genotypes per specimen in the cervix and the anus, respectively.
We found significant age differences among women by anal and cervical HPV status (P < 0.0001, race-adjusted; Table 3). HPV-negative (anus and cervix) women were the oldest with a mean age of 40.9 years and women with concurrent anal and cervical infection were the youngest with a mean age of 29.2 years. We observed an inverse dose-response relationship of age with both anal HPV−/cervical HPV+ infection and anal HPV+/cervical HPV+ infection. There was no association of age with anal HPV+/cervical HPV− infection.
Table 3.
Relationship of HPV status in paired anal-cervical specimens with selected characteristics, Hawaii female cohort, 1999 to 2004
| OR (95% CI)* |
|||||
|---|---|---|---|---|---|
| Anus HPV+/cervix HPV− (n = 191) | Anus HPV−/cervix HPV+ (n = 190) | Anal HPV+/cervix HPV+ (n = 178) | P | ||
| Age (y) | |||||
| Mean (SD) | 40.9 (13.6) | 38.7 (13.4) | 34.9 (12.2) | 29.2 (10.2) | <0.0001 |
| <30 | 1.0 (Reference) | ||||
| 30–39 | 1.1 (0.7–1.7) | 0.8 (0.5–1.2) | 0.4 (0.3–0.6) | ||
| 40–49 | 0.7 (0.5–1.2) | 0.4 (0.3–0.7) | 0.1 (0.1–0.2) | ||
| ≥50 | 0.7 (0.5–1.1)
Ptrend = 0.06 |
0.3 (0.2–0.5)
Ptrend < 0.0001 |
0.1 (0.04–0.2)
Ptrend < 0.0001 |
||
| Race/ethnicity | |||||
| Caucasian | 1.0 (Reference) | ||||
| Japanese | 0.3 (0.2–0.6) | 0.8 (0.5–1.4) | 0.6 (0.3–1.2) | ||
| Filipino | 0.6 (0.3–1.1) | 0.7 (0.3–1.4) | 0.5 (0.2–1.1) | ||
| Hawaiian | 0.5 (0.3–0.9) | 0.8 (0.5–1.4) | 0.5 (0.3–1.0) | ||
| Other | 0.9 (0.6–1.3) | 0.9 (0.6–1.4) | 0.7 (0.5–1.1) | ||
| Alcohol drinking† | |||||
| Never | 1.0 (Reference) | ||||
| Ever | 1.5 (1.0–2.1) | 1.6 (1.1–2.2) | 1.3 (0.9–1.9) | ||
| Current | 1.4 (0.9–2.1) | 1.3 (0.9–2.0) | 1.4 (0.9–2.1) | ||
| Cigarette‡ | |||||
| Never | 1.0 (Reference) | ||||
| Ever | 1.2 (0.9–1.7) | 1.1 (0.8–1.6) | 1.1 (0.8–1.6) | ||
| Current | 1.4 (0.9–2.3) | 1.2 (0.8–2.0) | 1.7 (1.0–2.3) | ||
| History of Chlamydia§ | |||||
| No | 1.0 (Reference) | ||||
| Yes | 1.2 (0.6–2.7) | 1.7 (0.9–3.2) | 2.6 (1.3–5.1) | ||
| Ever pregnant§ | |||||
| No | 1.0 (Reference) | ||||
| Yes | 1.3 (0.7–2.4) | 0.8 (0.5–1.3) | 0.5 (0.3–0.9) | ||
| Age (y) at initial sex§ | |||||
| Mean (SD) | 18.3 (3.6) | 17.2 (3.1) | 17.7 (3.0) | 17.4 (4.8) | 0.02 |
| >16 | 1.0 (Reference) | ||||
| ≤16 | 1.2 (0.8–1.7) | 3.3 (2.3–4.7) | 2.9 (2.0–4.9) | ||
| Lifetime no. sexual partners§ | |||||
| Mean (SD) | 15.9 (66.3) | 14.7 (25.3) | 14.4 (26.0) | 17.2 (53.8) | 1.00 |
| 0–2 | 1.0 (Reference) | ||||
| 3–7 | 1.6 (1.0–2.9) | 4.8 (3.1–7.5) | 8.5 (5.2–14.0) | ||
| >7 | 2.0 (1.3–3.0)
Ptrend = 0.001 |
4.1 (2.7–6.2)
Ptrend < 0.0001 |
5.8 (3.6–9.4)
Ptrend < 0.0001 |
||
| Anal intercourse§ | |||||
| Never | 1.0 (Reference) | ||||
| Ever | 1.2 (0.6–2.3) | 1.3 (0.7–2.2) | 1.7 (1.0–3.1) | ||
| Current (past 3 mo) | 1.4 (0.5–3.7) | 1.3 (0.6–2.9) | 1.6 (0.7–3.6) | ||
NOTE: Frequency by category: age, <30 years 202, 56, 77, 115; 30 to 39 years 164, 50, 48, 37; 40 to 49 years 206, 40, 35, 14; ≥50 years 233, 45, 30, 11; race, Caucasian 298, 90 74 78; Japanese 115, 10, 19, 14; Hawaiian 121, 20, 28, 20; Filipino 56, 10, 11, 10; other 213, 61, 58, 55; alcohol consumption, never 489, 94, 96, 97; ever 313, 96, 95, 80; current 162, 49, 46, 47; smoking, never 512, 113, 118, 113; ever 290, 77, 73, 65; current 94, 31, 29, 35; pregnant, never 72, 22, 52, 71; ever 187, 53, 72, 48, history of Chlamydia, no 224, 60, 99, 87; yes 28, 11, 24, 29; age of initial sex, ≤16 years 80, 30, 43, 56; >16 years 173, 45, 77, 61; lifetime no. sexual partners, 0–2 68, 6, 17, 8; 3–7 77, 24, 47, 57; >16 173, 45, 77, 61; anal intercourse, never 214, 59, 94, 82; ever 44, 14, 31, 38; current 18, 7, 12, 14.
Adjusted for age and race/ethnicity.
Drinking alcohol at least weekly for ≥6 months.
Smoking cigarettes daily for ≥6 months.
Queried at second visit; therefore, numbers reflect the subset of women from baseline completing the second visit.
Alcohol consumption (weekly for ≥6 months) was positively associated with the risk of cervical infection alone, although no association of current alcohol intake with risk was observed among any other group. A history of Chlamydia infection and nulliparity were associated with concurrent anal and cervical infection. Early age at initial sexual intercourse was a risk factor for both cervical infection alone and concurrent anal-cervical infection but not for anal HPV infection alone. A positive association of lifetime number of sexual partners was consistent among all three groups (cervical HPV infection alone, concurrent anal and cervical infection, and anal infection alone), although the magnitude of the association was attenuated among those with anal HPV infection.
A history of anal intercourse was associated with anal infection alone (OR, 1.7; 95% CI, 1.0–3.1) but was not associated with either cervical infection alone or concurrent anal-cervical infection. Recent anal intercourse (within the past 3 months), however, was not associated with anal infection alone for any group.
We further explored the relationship of age by anal-cervical HPV status (Fig. 3). Concurrent anal and cervical HPV infection was most prevalent among the youngest women and steadily decreased through age 50 years. The prevalence of cervical HPV infection without accompanying anal infection also showed an overall although less dramatic decrease with age. In contrast, the prevalence of anal infection alone remained steady at all age groups.
Figure 3.

HPV DNA detection by age group.
Conclusion
HPV, the primary cause of cervical cancer, is also associated with the development of anal cancers (1, 2). The relationship of anal to cervical infection as described in our findings has implications for the development of anal malignancies in women. The incidence of anal cancer has increased among both men and women in the United States over the past 2 decades, and rates have been comparable among sexes in recent years (8, 9). A recent study detected HPV in up to 90% of anal tumor samples from both men and women (1).
The histology of the anus shares important parallels with the cervix, including a transitional area of the epithelium, analogous to the cervical transformation zone, where columnar and squamous epithelium meet (2). Furthermore, there is evidence that HPV-induced malignancies of the anus share a natural history similar to that of the cervix beginning with viral infection and progressing to dysplastic lesions and, finally, invasive cancer (10).
Our results suggest that anal HPV infection is a common infection among healthy, sexually active females with prevalence comparable with cervical infection. It also suggests that anal and cervical HPV infections are strongly correlated. Women with cervical infection had >3-fold increased risk of contemporaneous anal infection and 13% of women were infected at both anatomic sites. The multifocal nature of HPV-related disease has been shown in previous studies whereby it is not uncommon for anal and cervical squamous intraepithelial lesions to occur concurrently or consecutively (11, 12).
A high degree of genotype-specific concordance was observed among concurrent infections, indicating a common source of infection, such as vaginal and anal intercourse with the same infected partner or partners. Nevertheless, anal intercourse was not associated with HPV infection among concurrently infected women. Recent studies have observed a lack of association between anal intercourse and anal HPV infection among women (13). This suggests alternate routes of transmission, including sexual and nonsexual means. Moscicki et al. speculated that HPV shed into vaginal discharge might be spread to the anus given the close proximity of the vagina and anus (13). There is evidence that HPV can be transmitted to the genitals through nonpenetrative sexual contact involving the fingers or mouth of partners (14).
Cross-contamination of specimens during the collection process was not likely. Cervical and anal samples were taken separately using different swabs/brushes and placed into medium in separate collection vials that were each immediately capped after specimen collection. Furthermore, swabs from participants’ back as well as from the examination table were routinely taken as clinical and environmental controls to monitor possible HPV contamination during the collection process. HPV DNA was not detected in any clinical or environmental control specimens.
The 14% of women with anal HPV infection alone may represent a unique group. Indeed, these women were older and less likely to be Japanese and Hawaiian. Unlike the other two groups (cervical infection alone and concurrent anal and cervical infection) where risk of infection decreased with age, age was not associated with anal HPV infection alone. The sharp contrast of age with anal and cervical HPV status may imply differences in sexual practices by age and/or differences in immune surveillance. A history of ever engaging in anal intercourse was associated with increased risk of HPV only among these women with infection limited to the anus and no association was observed for recent anal intercourse. With the exception of anal intercourse, associations with sexual risk factors were either absent or attenuated among this group compared with other groups.
Our results suggest that different HPV genotypes may have different tropism to the anus compared with the cervix. The overall distribution varied considerably in the anus compared with the cervix. Anal genotypes were more heterogeneous with a higher proportion of nononcogenic types. The predominance of nononcogenic types in the anus compared with the cervix may explain the relatively low incidence of anal cancers compared with cervical cancers.
Multiple genotypes were more common in both the anus and the cervix of women with concurrent anal-cervical infections compared with women with infections limited to either site alone. This observation together with the high level of genotypic concordance may indicate that women with concurrent infection represent a group that, due to immune function or other factors, are more susceptible to infections with different HPV types as well as multifocal infections affecting more than one anatomic area.
The proportion of unidentified genotypes in infections limited to either the anus or the cervix was substantially higher than in concurrent anal-cervical infections. A possible explanation is that concurrent infections represent infections of higher viral levels and are therefore more likely to be transmitted between the cervix and the anus (either through sexual or nonsexual means) and also more successfully genotyped through the PCR hybridization–based assay.
A large number of anal specimens relative to cervical specimens were excluded from analysis based on the negative β-globin results. The proportions of β-globin-negative anal and cervix specimens we observed are very similar to that reported in a previous study of concurrently collected anal and cervical specimens in women (15). The ability to detect human β-globin, which resides in nuclear DNA, is facilitated in cervical specimens, which are collected from the largely mucus-lined cervical epithelium. By contrast, anal specimens come from the nonmucus, keratinized epithelium of the anus. Others have reported difficulty in obtaining nuclear DNA markers from highly keratinized cells, which may have a substantial proportion of anucleated cells (16).
Our ability to evaluate the relationship of anal intercourse and anal HPV infection was limited by sampling biases. Information on sexual practices was limited to those women who returned for the second visit when the comprehensive survey was administered. Furthermore, women agreeing to provide anal specimens were different from women those who did not with respect to age, race/ethnicity, and sexual practices. Asian and Hawaiian populations were less likely to consent to provide anal specimens. This may be attributed to cultural issues surrounding comfort level in undergoing the anal specimen collection process. Younger women were also less likely to provide anal specimens.
Differential exposure bias (i.e., bias in the proportion of women having engaged in anal intercourse) is likely to have masked the true relationship of anal intercourse with anal HPV infection. Women who reported never engaging in anal intercourse were also less likely to provide anal specimens. This may be attributed to embarrassment or discomfort in undergoing anal sampling for these women. Alternatively, women not engaging in anal intercourse may not have perceived a risk for anal HPV infection and therefore declined anal sampling. Women who engaged in anal intercourse, conversely, may have perceived a risk for anal HPV and therefore been more likely to provide an anal sample.
Female anal and cervical cancer incidences differ in their age distribution with anal cancers occurring at a later age than cervical cancers. In the United States from 1998 to 2002, the average age of diagnosis of invasive anal cancer among women was 63.2 years compared with 50.5 years among women with invasive cervical cancer (10).
This is consistent with the older age distribution we observed among anal HPV+/cervix HPV− women. There are several possible explanations. Women may either acquire HPV at a later age due to initiation of anal intercourse at a later age than vaginal intercourse. Alternatively, anal HPV may be acquired at ages similar to cervical HPV but may be a more persistent infection in the anus, such that its prevalence remains steady among older women. Given that persistence of oncogenic types is the most important risk factor for the development of cervical cancer (17), longitudinal evaluation of anal HPV infection will be important. In future analyses, we will explore the duration of anal HPV infection over time and the relationship between the duration of infection in the anus relative to the cervix.
Acknowledgments
We thank the following individuals and organizations for their assistance with this study: Clara Richards, April Hallback, Arlene McCafferty, Xuemei Zhu, and the staff of the University of Hawaii Cancer Research Center of Hawaii; Dora Irvine and the staff of the Kaiser Permanente Hawaii Medical Systems; Cathy Cramer Bertram, Marge Bernice, and the staff of the Queen’s Medical Center; Louise Medina, Momi Breault, Emily Fritz, and the staff of the University of Hawaii Clinical Research Center; Jamie Boyd and the staff of the University of Hawaii Leeward Community College Health Center; Gwen Barros, Sue Maury, and the staff of the University of Hawaii University Health Services; and Janet Komegay (Roche Molecular Systems). Reagents for the HPV PGMY-LB assay were kindly supplied by Roche Molecular Systems.
Footnotes
Grant support: National Cancer Institute grant CA077318-05 and Research Centers in Minority Institutions award P20 RR11091 from the National Center for Research Resources, NIH.
References
- 1.Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–80. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 2.Palefsky JM. Human papillomavirus and anogenital neoplasia in human immunodeficiency virus-positive men and women. J Natl Cancer Inst Monogr. 1998;23:15–20. doi: 10.1093/oxfordjournals.jncimonographs.a024166. [DOI] [PubMed] [Google Scholar]
- 3.Williams AB, Darragh TM, Vranizan K, et al. Anal and cervical human papillomavirus infection and risk of anal and cervical epithelial abnormalities in human immunodeficiency virus infection. Obstet Gynecol. 1994;83:205–11. [PubMed] [Google Scholar]
- 4.Holly EA, Ralston ML, Darragh TM, et al. Prevalence and risk factors for anal squamous intraepithelial lesions in women. J Natl Cancer Inst. 2001;93:843–9. doi: 10.1093/jnci/93.11.843. [DOI] [PubMed] [Google Scholar]
- 5.Gravitt P, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gravitt PE, Peyton CL, Apple RJ, et al. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peyton CL, Gravitt PE, Hunt WC, et al. Determinants of genital human papillomavirus detection in a US population. J Infect Dis. 2001;183:1554–64. doi: 10.1086/320696. [DOI] [PubMed] [Google Scholar]
- 8.Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer. 2004;101:281–8. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 9.Surveillance, Epidemiology, and End Results Program (SEER), National Cancer Institute, Public Use Data; 2004 Nov.
- 10.Palefsky JM, Holly EA, Gonzales J, et al. Detection of human papillomavirus DNA in anal intraepithelial neoplasia and anal cancer. Cancer Res. 1991;51:1014–9. [PubMed] [Google Scholar]
- 11.Moscicki AB, Hills NK, Shiboski S, et al. Risk factors for abnormal anal cytology in young heterosexual women. Cancer Epidemiol Biomarkers Prev. 1999;8:173–8. [PubMed] [Google Scholar]
- 12.Scholefield JH, Hickson WG, Smith JH, et al. Anal intraepithelial neoplasia: a part of a multifocal disease process. Lancet. 1992;340:1271–3. doi: 10.1016/0140-6736(92)92961-e. [DOI] [PubMed] [Google Scholar]
- 13.Moscicki AB, Durako SJ, Houser J, et al. Human papillomavirus infection and abnormal cytology of the anus in HIV-infected and uninfected adolescents. AIDS. 2003;17:311–20. doi: 10.1097/00002030-200302140-00004. [DOI] [PubMed] [Google Scholar]
- 14.Winer RL, Lee S-K, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–26. doi: 10.1093/aje/kwf180. Erratum in: Am J Epidemiol 2003;157:858. [DOI] [PubMed] [Google Scholar]
- 15.Canadas MP, Bosch FX, Junquera ML, et al. Concordance of prevalence of human papillomavirus DNA in anogenital and oral infections in high-risk populations. J Clin Microbiol. 2004;42:1330–2. doi: 10.1128/JCM.42.3.1330-1332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryan JT, Brown DR. Transmission of Human papillomavirus type 11 infection by desquamated cornified cells. Virology. 2001;281:35–42. doi: 10.1006/viro.2000.0777. [DOI] [PubMed] [Google Scholar]
- 17.Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high-risk human papillomavirus (HPV) as an indication of high-grade squamous intraepithelial lesions in young women: population-based follow-up study. BMJ. 2002;325:572–6. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]


