Abstract
Acute organophosphate (OP) pesticide poisoning causes tens of thousands of deaths each year across the developing world. Standard treatment involves the administration of intravenous atropine and oxime to counter acetylcholinesterase inhibition at the synapse. The usefulness of oximes, such as pralidoxime and obidoxime, has however been challenged over the past 20 years by physicians in many parts of the world who have failed to see benefit in their clinical practice. We have carried out a systematic review to find randomised controlled trials (RCTs) of oximes in OP poisoning. Two RCTs have been published involving 182 patients treated with pralidoxime. The RCTs did not find benefit with pralidoxime and have been used to argue that pralidoxime should not be used in OP poisoning. These physicians must be congratulated for attempting important studies in a difficult environment. However, the studies did not take into account recently clarified issues important for outcome and the published methodology is unclear, therefore such a generalised statement cannot be supported by the published results. There are many reasons why oximes may not be relevant in the overwhelming self-poisoning typical of the tropics. However, we believe that a large RCT is required to compare the current WHO-recommended pralidoxime regimen (>30mg/kg bolus followed by >8mg/kg/hr infusion) with placebo to determine definitively the role of oxime therapy in OP self-poisoning. Such a study will need to be designed with pre-defined subgroup analysis to allow the identification of patient subgroups that may benefit from oximes.
Introduction
Deliberate self-poisoning has reached epidemic proportions in parts of the developing world where the toxicity of available poisons and sparse medical facilities ensure a high fatality rate.1,2 Many deaths are due to organophosphate (OP) pesticides and occur in the young economically active age group.2-5 Fatality rates of 20% are common and the WHO has estimated that 200,000 people die each year from pesticide poisoning6 (although the accuracy of these figures is keenly debated7). Unfortunately, the widespread use of OP pesticides in the developing world’s agricultural communities will make reduction of deaths by primary prevention a difficult task.
OP pesticides inhibit acetylcholinesterase (AChE) at the muscarinic and nicotinic synapses by depositing a phosphoryl group at the enzyme’s active site (reaction 1, figure 1); this results in an accumulation of acetylcholine and uncontrolled activation of cholinergic synapses. Standard therapy involves attempts to reduce absorption with gastric lavage and/or activated charcoal, plus administration of atropine and oxime to counter the effects of absorbed pesticide.8-10 The use of high doses of atropine is well established, the use of oximes more controversial.
Figure 1. Reaction of organophosphate pesticides with acetylcholinesterase.
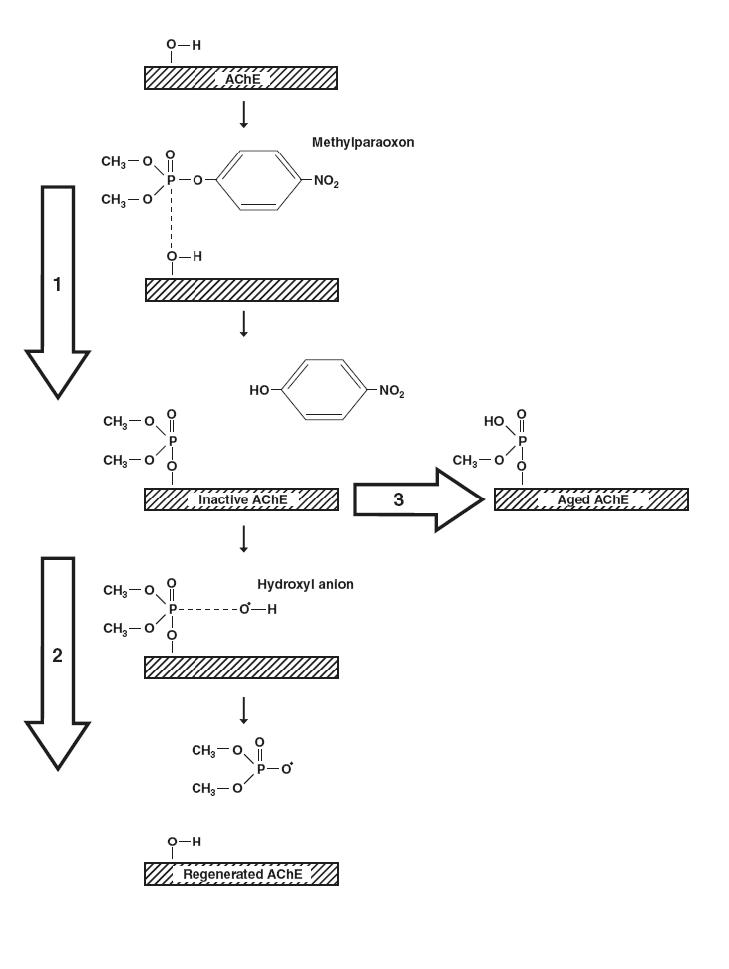
A dimethylphosphorylated organophosphate pesticide (methylparaoxon) inhibits acetylcholinesterase (AChE) by phosphorylating the serine hydroxyl group at the enzyme’s active site (reaction 1). This reaction occurs very quickly. Active AChE is subsequently regenerated by a hydroxyl ion attacking the phosphorylated serine residue, removing the phosphate moiety and releasing active enzyme (reaction 2). This regenerative process, however, is much slower than inhibition, requiring hours to days to occur (spontaneous reactivation t½ ∼ 0.7h for dimethyl and 31h for diethyl compounds). While in the inactive state, the enzyme is prone to ‘ageing’ (reaction 3) in which one alkyl side chain of the phosphoryl moiety is removed non-enzymatically, leaving a hydroxyl group in its place. ‘Aged’ AChE with its negatively charged phosphate can no longer be attacked by a negatively charged nucleophile, i.e. OH. or an oximate group, and regeneration is no longer possible. This reaction occurs considerably faster with enzymes that have been inhibited by dimethylated pesticides (t½ ∼ 3.7h) than those inhibited by diethylated pesticides (t½ ∼ 31h). The slower the regenerative process, the greater the quantity of inactive AChE available for ageing. Pralidoxime catalyses the regeneration of active AChE by exerting a nucleophilic attack on the phosphoryl group, transferring it from the enzyme to itself. By speeding up reaction 2, it reduces the quantity of inactive AChE available for ageing. However, because ageing occurs more rapidly with dimethylated pesticides, pralidoxime is only useful before about 12h with dimethylated enzyme. In trials of pralidoxime, the identification of the poison taken, in particular its dimethyl/diethyl status, is therefore essential. (Data from refs. 14,18).
Oximes reactivate acetylcholinesterase by removing the phosphoryl group (reaction 2, figure 1). Pralidoxime is the oxime most often used worldwide and occurs in two common forms: pralidoxime chloride (2-PAM; molecular weight 173; used worldwide) and mesylate (P2S; MW 232; used in the UK).11 The great majority of its effects are on the peripheral nervous system since its lipid solubility is low and entry into the CNS limited. Atropine works at muscarinic synapses, competitively antagonising the accumulated acetylcholine. The main therapeutic effect of pralidoxime is predicted to be recovery of neuromuscular transmission at nicotinic synapses.
In vitro experiments have shown that oximes are effective reactivators of human AChE inhibited by OP compounds.12 In some situations, however, reactivation of inhibited AChE by oximes will likely be absent or limited, for example where there is: i) poor affinity for the particular OP-AChE complex, ii) insufficient dose or duration of treatment, iii) persistence of the OP within the patient and therefore rapid reinhibition of newly reactivated enzyme, and iv) ageing of the inhibited AChE (reaction 3, figure 1; refs.9, 13-15).
In 1961, Sundvall reported that the minimum effective plasma concentration of P2S was 4mg/l in cats poisoned with a quaternary analogue of the nerve agent sarin.16 This result has since been uncritically extrapolated to all oxime and OP interactions. It has now become clear, however, that the degree of reactivation is dependent on the specific identity and concentrations of both oxime and OP.9,12,13
For example, most OP pesticides can be classified as compounds that form either a dimethylphosphoryl- or a diethylphosphoryl-AChE complex. In vitro studies have shown that while seven times as much pralidoxime as obidoxime is required for reactivation of dimethyl-OP inhibited AChE, diethyl-OPs require 20 times more pralidoxime than obidoxime.17 Diethyl compounds both reactivate and age significantly slower than dimethyl compounds.
The ‘ageing’ of inhibited AChE is particularly important since aged enzyme cannot be reactivated by oximes. The therapeutic window for oximes is, therefore, very much determined by the rate of ageing. The half-life of ageing of dimethylphosphorylated and diethlyphosphorylated AChE, as determined in isolated human red cells in vitro, is 3.7 hours and 33 hours, respectively,14,18 and the therapeutic window therefore (taken as four times t½) a maximum of 13 or 132 hours, respectively.11,14 Clinical data of OP-poisoned patients treated with obidoxime illustrates the importance of poison load, time elapsing between poisoning and oxime administration, and influence of the poison type (in particular dimethyl versus diethyl) on the effectiveness of reactivation of erythrocyte AChE in vivo (figure 2).
Figure 2. Influence of poison concentration and delay of obidoxime therapy on the reactivation of erythrocyte AChE.
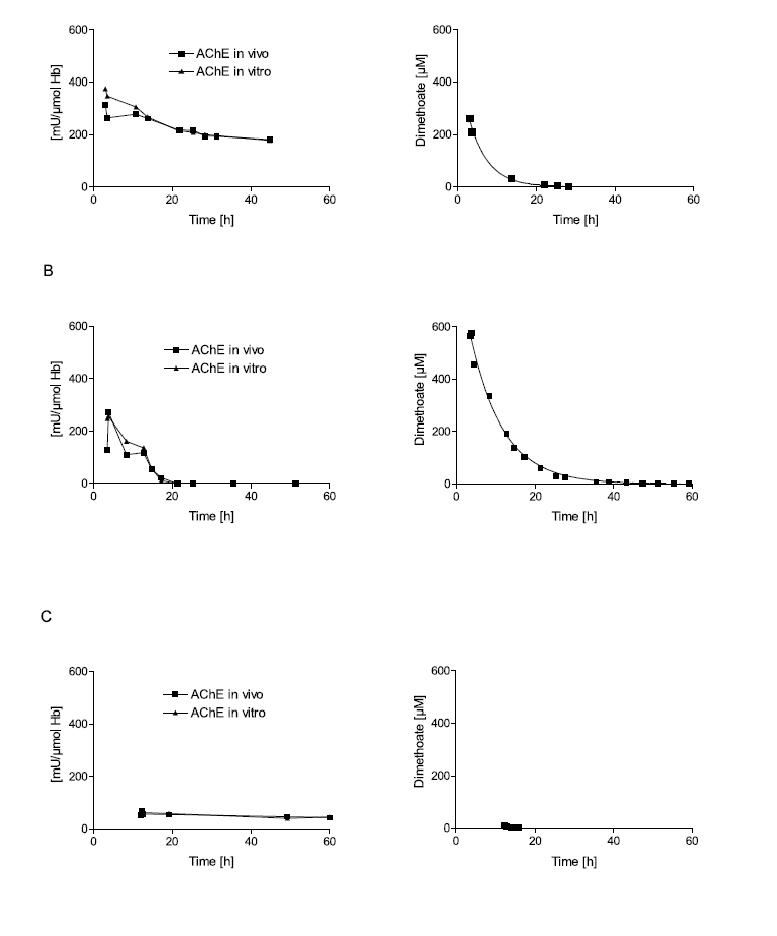
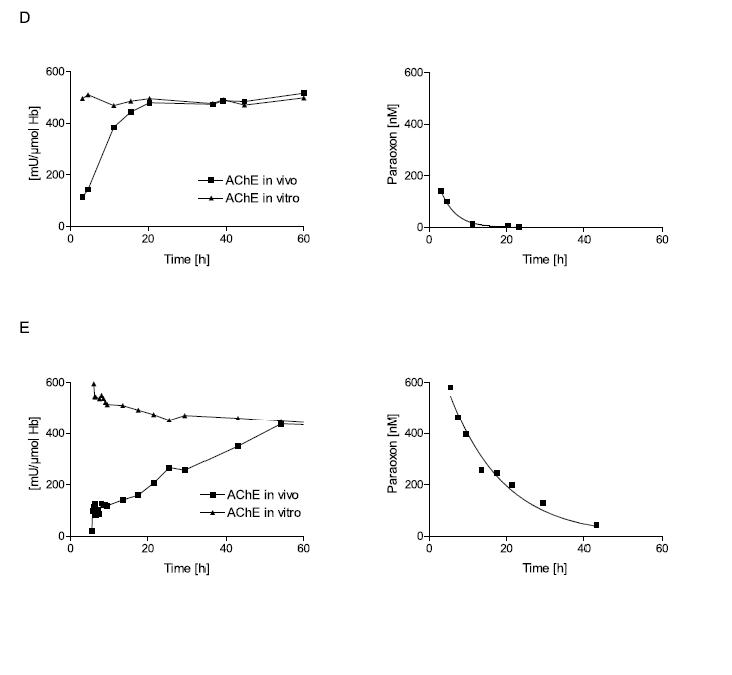
Erythrocyte AChE was determined according to Worek et al.42 and referred to the haemoglobin content of blood (mU/μmol HbFe); the normal value is about 600 mU/μmol Hb. Activity in vivo demonstrates the activity of diluted (1:100) venous blood of the patient; activity in vitro demonstrates its activity after reactivation with a supratherapeutic dose of obidoxime. This value indicates the amount of enzyme that is not aged and therefore potentially reactivateable.15 The poison concentration in plasma was determined by HPLC. All patients were treated with atropine and an IV bolus dose of 250 mg obidoxime followed by continuous infusion at 750 mg/24h for the period indicated by the time scale. This regimen produced plasma concentrations between 10 and 20 μM obidoxime. Obidoxime was started at the first time point shown in the figures; the x-axis indicates time post ingestion. Dimethoate is a dimethylphosphorylated OP, while parathion is a diethylphosphorylated OP. Patient A had ingested a moderate dose of dimethoate 3h before obidoxime administration. This therapy resulted in near complete reactivation of the unaged fraction of AChE. Since the poison concentration was low and declined rapidly, obidoxime was able to keep the enzyme activity at 1/3 of normal. Patient B had ingested a high dose of dimethoate 3.5h before obidoxime administration. While initial reactivation was observed, the enzyme activity gradually fell due to the higher and more persistent dimethoate concentration. Patient C had ingested a small dose of dimethoate about 12h before obidoxime treatment. During this period (3-4 half-lives), some 90% of the enzyme had been aged. Hence, obidoxime did not show any significant effect although the poison concentration was very low at that time. (Note that some spontaneous reactivation of the unaged AChE is always observed when the poison concentration is low.) Patient D had ingested a moderate dose of parathion 3h before administration of the obidoxime regimen. Most of the enzyme was still reactivatable and reactivation in vivo increased steadily while the paraoxon concentration in plasma declined. Patient E had ingested a high dose of parathion 5.5h before obidoxime treatment. Only a small fraction of the AChE was reactivated initially but this fraction increased during the next few days as the paraoxon concentration fell gradually. Due to the slow ageing of the diethyl-phosphorylated enzyme, reactivatability decreased only slowly. The data show that oximes are particularly effective with diethyl-OPs, provided treatment is long enough to counteract re-inhibition. With dimethyl-OPs, oximes have to be given as early as possible and are only effective at moderate poison load (Eyer et al., in preparation).
However, clinical experience in the developing world has led to doubt about the clinical relevance of oximes for any form of OP poisoning.19-25 In particular, Senanayake’s group reported no difference in outcome in Sri Lanka when pralidoxime was unavailable in their hospital.23 They argued that pralidoxime was of no clinical benefit and should not be used. Proponents of oximes responded that these physicians were using too low a dose and that a loading dose of at least 30mg/kg followed by an infusion of >8mg/kg/hr was required for clinical benefit (refs. 9, 26, 27 and JA Vale, personal communication).
When these issues were debated in the mid-1990s, no RCTs of oximes in OP poisoning had been published. We have therefore carried out a systematic search for such RCTs to find the evidence for oximes producing clinical benefit in OP poisoned patients.
Methods
We carried out a systematic search for clinical trials by searching Medline, Embase, and Cochrane databases (last checked 01/02/02), cross referencing from other articles, and contacting experts in the field to identify unpublished studies. All articles that were selected with the text words ‘organophosphate’ or ‘oxime’ together with ‘poisoning’ or ‘overdose’ were examined. Articles that could possibly be randomised clinical trials were retrieved to determine if this was the case. The web was also searched using www.google.com and the keywords ‘organophosphate’, ‘oxime’, and ‘trial’ (last checked 01/02/02),
A draft of this manuscript was sent to members of the Christian Medical College (CMC) Vellore group for their comments - these physicians carried out the two published RCTs. We requested that they complete a CONSORT statement to clarify the methodology used in their trials.28 The manuscript was emailed on two occasions and followed up with phone calls. Unfortunately, we did not receive a response to our enquiry. Previous communication with this group indicates that they have recently started recruiting patients to another placebo-controlled RCT of pralidoxime therapy (1g stat vs. placebo). However, no results are yet available and the expected date of completion is unknown.
Results
The initial standard database searches located only two RCTs. More ad-hoc methods such as the review of references cited in recent review articles and web searching of the journal Journal of Association of Physicians of India located two further trials. In total, we found two published RCTs (table 1), and one paper and one meeting abstract describing two further small clinical trials. All identified studies assessed pralidoxime; clinical trials of obidoxime or other oximes have not been reported.
TABLE 1.
| RCT | 1 (Aug 91-Dec 92) | 2 (?Dates) | |||||
|---|---|---|---|---|---|---|---|
| Controls (‘Low-dose’) | Cases (‘High-dose’) | Controls | Cases | ||||
| Number | 36 | 36 | 55 | 55 | |||
| Dose | Bolus: 22/mg/kg dose | No bolus loading dose | Placebo saline only | No bolus loading dose | |||
| (estimated /kg - see legend) | No infusion | Day 1: 5.6mg/kg/hr | Days 1-3: 3.7mg/kg/hr | ||||
| Day 2: 2.8mg/kg/hr | |||||||
| Day 3: 1.8mg/kg/hr | |||||||
| Day 4: 0.9mg/kg/hr | |||||||
| Pesticides | dimethyl OPs | 56% | 61% | Not stated | Not stated | ||
| diethyl Ops | 25% | 22% | |||||
| unknown | 19% | 17% | |||||
| Time from ingestion to start of Rx | <6hrs | 8 | 3 | <5hrs | 14 | 8 | |
| 6-12hrs | 6 | 14 | 6-10hrs | 13 | 15 | ||
| 12-24hrs | 15 | 10 | 11-15hrs | 12 | 9 | ||
| 24-36hrs | 5 | 5 | 16-20hrs | 4 | 9 | ||
| 36-48hrs | 2 | 4 | >21hrs | 10 | 9 | ||
| Pseudocholinesterase levels | 338.9 (260.5) | 441.3 (450.3) | 743.7 (1254) | 283.2 (243) | |||
| Deaths | 5 (14%) | 8 (22%) | 3 (5%) | 16 (29%) | |||
| Ventilated | 17 (47%) | 24 (67%) | 22 (40%) | 37 (67%) | |||
| Intermediate syndrome | 13 (36%) | 20 (56%) | 19 (35%) | 36 (65%) | |||
The two published RCTs of pralidoxime from the Christian Medical College, Vellore. Patients recruited to the trial were on the medical ITU with a history and signs/symptoms suggestive of organophosphate poisoning plus a pseudocholinesterase level <50% of normal; the number of patients excluded was not stated. Randomisation was in blocks of four; methods of concealment to reduce bias were not stated. Patient weight was not stated; to estimate quantities of pralidoxime given to patients as mg/kg, an estimated mean weight of 45kg was used [patients in a poisoning RCT in Sri Lanka had a mean weight of 48.2kg (SD 8.9)43].
RCT 1
The first trial in 72 patients was carried out between August 1991 and December 1992 at the Christian Medical College in Vellore, India.29,30 There was no untreated control group. A 1g bolus of pralidoxime (termed ‘low dose’) was compared with 12g given as a reducing infusion over four days without a loading dose (termed ‘high dose’). This RCT reported an increased mortality rate (22% vs. 14%; OR 1.77, 95% CI 0.52-6.0) and increased requirement for ventilation (67% vs. 47%; OR 2.04, 95% CI 0.78-5.3) amongst patients who received the infusion compared to those who received the bolus dose. The authors argued that ‘high-dose’ pralidoxime was therefore “associated with a worse outcome” and should have “no role in the routine management of patients with OP poisoning”.
RCT 2
The dates of the second trial are not given in either publication.31,32 Following on from RCT 1, it compared ‘high-dose’ pralidoxime (i.e. 12g by continuous infusion without loading dose) with placebo saline infusion in 110 patients. Although the published details are incomplete, it appears that a different dosage regimen to that of RCT 1 was used: 12g over 3 days (estimated 3.7mg/kg/hr for a 45kg patient). The ‘high-dose’ regimen was associated with a significantly higher risk of death (29% vs. 5%; OR 7.1, 95% CI 1.9-26.0) and requirement for ventilation (67% vs. 40%; OR 3.1, 95% CI 1.4-6.7). The authors concluded that 2PAM “has no role in the management of patients with organophosphorus poisoning and .. does more harm than good”.
Trial 3
A trial of pralidoxime was carried out in 37 patients in Tehran, Iran, during the early 1990s.33 Seventeen patients received atropine alone while 17 received 600-800mg pralidoxime every 4-8h for four days in addition to atropine. The pralidoxime dose was based on the patient’s condition. Methods of allocation and concealment are not stated but recent discussion with Prof. Balali-Mood (Mashad, Iran) has indicated that it was not randomised.
All patients presented within 10h of pesticide ingestion. 35% of control patients ingested diethylphosphorylated OPs compared to 29% of intervention patients. Pralidoxime use resulted in no significant reduction in the number of patients requiring ventilation (41% vs. 47%; OR 0.79, 95% CI 0.20-3.0) or the number of patients dying (18% vs. 18%; OR 1.0, 95% CI 0.17-5.8). The authors concluded that atropine alone should be used in the treatment of acute OP poisoning.
Trial 4
A fourth study has been published in abstract format only.34 Twenty OP-poisoned patients were included in this trial of 2 vials or 4 vials of pralidoxime iodide but neither trial design nor results are apparent from the abstract. We have been unable to elicit any response from writing to the author. It seems unlikely that conclusive evidence will result from such a small trial.
Discussion
Physicians from the Christian Medical College, Vellore, have carried out two RCTs of pralidoxime in 182 patients with acute OP poisoning. These studies have since been used to argue that oximes should not be used in acute OP poisoning.35 The authors must be congratulated for attempting important studies in such a difficult environment. However, the studies did not take into account recently clarified issues important for outcome and the published methodology is unclear. Therefore such a generalised statement cannot be justified from the published results and we believe that the evidence for or against the use of oximes is not yet established.
Most importantly, the studies did not evaluate the current WHO-sponsored recommendations for pralidoxime therapy (at least 30mg/kg bolus followed by >8mg/kg/hr infusion). Furthermore, they were published before the widespread adoption of the CONSORT guidelines for the reporting of RCTs28 and it is very difficult to determine the methodology of the trials from the published record.
It is likely that the ‘high-dose’ regimen of pralidoxime used in Vellore did not produce an effective plasma concentration. Pharmacokinetic studies have shown that 1g given over 30 mins to patients with a mean weight of 72kg (SD 8.5) falls below a plasma concentration of 4mg/l within 1.5 hrs.36 The weight of the Indian study participants is not given in either paper; however, an estimated mean weight of 45kg (see table legend) would only increase the effective concentration time by a factor of ∼2. In the group receiving the infusion only, it appears doubtful whether an estimated initial dose of 2.8mg/kg over the first 30 mins would ever give a plasma level above 4mg/l.Furthermore, recent studies have suggested that even 4mg/l may actually be insufficient for many pesticides.
An alternative interpretation of this study’s results would therefore be that a loading dose of pralidoxime is required to reach an effective plasma concentration and that a bolus dose alone, while producing an effective concentration for only several hours, offers some benefit.
However, the worse outcome seen in patients who received pralidoxime in RCT 2 suggests that the pralidoxime infusion harms patients. An alternative explanation is that features of the RCT itself led to this result. Neither power calculations nor stopping rules are presented in the published papers. It is not clear why the trial was stopped at 110 patients. Could the differences have been due to chance and might they have equalised out with time?
Sicker patients might also have been randomised to the intervention arm of RCT 2, which had much reduced mean pseudocholinesterase levels at baseline. No information on masking is given and a block size of four was used in both studies. As pointed out by Schulz,37 such a small non-varying block size can often be unravelled: if treatment assignment becomes known after allocation, a sequence can be easily discerned from the pattern of past assignments giving the risk of selection bias, even if concealment has been adequate.
Whether pesticides had dimethyl or diethyl groups was also not controlled for - this is important if only diethylphosphorylated AChEs respond to pralidoxime after 12h.14,38The information is given for RCT 1 (see table 1) but not RCT 2. In this latter study, it would be important to control for both the form of OP ingested and the time post-ingestion that therapy started. Deaths may have occurred in patients ingesting dimethylated compounds who presented after 12hrs when pralidoxime would not be expected to work.
The reports of the two small studies from Iran and north India provide too few details of trial design for any conclusions to be safely drawn.
Conclusion
Detailed observational clinical studies suggest that oximes encourage AChE regeneration.13-15 Animal data consistently show a marked positive effect of oximes on survival.39 Physicians in India, China and Vietnam have recently reported improved outcomes in uncontrolled studies with high doses of pralidoxime (refs. 40, 41, and Drs Pham Due and Nguyen Thi Du, Hanoi, personal communication). However, no clinical trials have formally assessed the efficacy of high dose pralidoxime
Two small RCTs have looked at either lower doses: 1g bolus doses of pralidoxime or 12g given by infusion over 3-4 days. Increased mortality was found with patients receiving the infusion of pralidoxime without a loading dose compared to either placebo or a loading dose alone. The authors of these studies have stated that pralidoxime should not be used for OP poisoning. However, we do not believe that the results of these RCTs can be used to make such a generalized comment and the effectiveness of pralidoxime in our view is still undecided.
Although the studies are methodologically weak, it is quite possible that their conclusions are correct. There are many factors present in self-poisoning cases in South Asia that would reduce the effectiveness of pralidoxime. A study from Sri Lanka showed that around 70% of OP poisoned patients had ingested dimethylated compounds;23 this situation may well be similar in other parts of the tropics and since many patients present more than 12 hours after the poisoning, it may be too late for oximes. In addition, the suicidal dose is often large and the pesticide persistent for several days, resulting in repeated inhibition of any newly reactivated AChE.
We believe that a large high quality RCT comparing the current WHO-recommended regimen with placebo is required to definitively assess the value of pralidoxime in acute OP poisoning. Such a trial may well confirm the Vellore group’s findings.
Randomisation should be stratified according to baseline severity, time to presentation, and class of OP pesticide taken (diethyl or dimethyl), with predefined sub-group hypotheses. Because of the importance of ageing in determining the usefulness of oximes, red blood cell AChE activity and the potential for ex-vivo reactivation will have to be measured for such a study to be fully interpretable. Only after such a study has been completed will it be possible to determine whether OP-poisoned patients benefit from oxime therapy.
Acknowledgements
We thank Martin Wilks, Jan Willems, and the QJM’s editor and reviewer for their critical review of the manuscript. ME is a Wellcome Trust Career Development Fellow in Tropical Clinical Pharmacology and Foulkes Fellow.
Footnotes
Conflict of interest: The authors have recently been funded by the Wellcome Trust, UK, to perform a large randomised controlled trial of pralidoxime in patients with acute OP pesticide poisoning.
References
- 1.Murray CJL, Lopez AD. Harvard School of Public Health; Cambridge, MA: 1996. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries and risk factors in 1990 and projected to 2020 [Volume 1 of 10 in the Global Burden of Disease and Injury Series] [Google Scholar]
- 2.Eddleston M, Sheriff MHR, Hawton K. Deliberate self-harm in Sri Lanka: an overlooked tragedy in the developing world. BMJ. 1998;317:133–135. doi: 10.1136/bmj.317.7151.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Hoek W, Konradsen F, Athukorala K, Wanigadewa T. Pesticide poisoning: a major health problem in Sri Lanka. Soc Sci Med. 1998;46:495–504. doi: 10.1016/s0277-9536(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Wig N, Chaudhary D, Sood N, Sharma B. Changing pattern of acute poisoning in adults: experience of a large northwest Indian hospital. J Assoc Phys India. 1997;45:194–197. [Google Scholar]
- 5.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. Q J Med. 2000;93:715–731. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- 6.WHO in collaboration with UNEP . Public health impact of pesticides used in agriculture. WHO; Geneva: 1990. [Google Scholar]
- 7.Karalliedde L, Eddleston M, Murray V. The global picture of organophosphate insecticide poisoning. Organophosphates and health. In: Karalliedde L, Feldman F, Henry J, Marrs T, editors. Imperial Press; London: 2001. [Google Scholar]
- 8.Ballantyne B, Marrs TC. Overview of the biological and clinical aspects of organophosphates and carbamates. In: Ballantyne B, Marrs TC, editors. Clinical and experimental toxicology of organophosphates and carbamates. Butterworth Heinemann; Oxford: 1992. pp. 3–14. [Google Scholar]
- 9.Johnson MK, Jacobsen D, Meredith TJ, et al. Evaluation of antidotes for poisoning by organophosphorus pesticides. Emerg Med. 2000;12:22–37. [Google Scholar]
- 10.Eddleston M, Singh S, Buckley N. Acute organophosphate poisoning. Clinical Evidence. 2002;7 in press. [PubMed] [Google Scholar]
- 11.Bismuth C, Inns RH, Marrs TC. Efficacy, toxicity and clinical uses of oximes in anticholinesterase poisoning. In: Ballantyne B, Marrs TC, editors. Butterworth Heinemann; Clinical and experimental toxicology of organophosphates and carbamates: Oxford: 1992. pp. 555–577. [Google Scholar]
- 12.Worek F, Kirchner T, Backer M, Szinicz L. Reactivation by various oximes of human erythrocyte acetylcholinesterase inhibited by different organophosphorus compounds. Arch Toxicol. 1996;70:497–503. doi: 10.1007/s002040050304. [DOI] [PubMed] [Google Scholar]
- 13.Willems JL, de Bisschop JP, Verstraete AG, et al. Cholinesterase reactivation in organophosphorus poisoned patients depends on the plasma concentrations of the oxime pralidoxime methylsulphate and of the organophosphate. Arch Toxicol. 1993;67:79–84. doi: 10.1007/BF01973675. [DOI] [PubMed] [Google Scholar]
- 14.Worek F, Backer M, Thiermann H, et al. Reappraisal of indications and limitations of oxime therapy in organophosphate poisoning. Hum Exp Toxicol. 1997;16:466–472. doi: 10.1177/096032719701600808. [DOI] [PubMed] [Google Scholar]
- 15.Thiermann H, Mast U, Klimmek R, et al. Cholinesterase status, pharmacokinetics and laboratory findings during obidoxime therapy in organophosphate poisoned patients. Hum Exp Toxicol. 1997;16:473–480. doi: 10.1177/096032719701600809. [DOI] [PubMed] [Google Scholar]
- 16.Sundwall A. Minimum concentrations of N-methylpyridinium-2-aldoxime methane sulphonate (P2S) which reverse neuromuscular block. Biochem Pharmacol. 1961;8:413–417. doi: 10.1016/0006-2952(61)90059-4. [DOI] [PubMed] [Google Scholar]
- 17.Thiermann H, Szinicz L, Eyer F, et al. Modern strategies in therapy of organophosphate poisoning. Toxicol Lett. 1999;107:233–239. doi: 10.1016/s0378-4274(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 18.Worek F, Diepold C, Eyer P. Dimethylphosphoryl-inhibited human cholinesterases: inhibition, reactivation and ageing kinetics. Arch Toxicol. 1999;73:7–14. doi: 10.1007/s002040050580. [DOI] [PubMed] [Google Scholar]
- 19.Nalin DR. Epidemic of suicide by malathion poisoning in Guyana. Report of 264 cases. Trop Geog Med. 1973;25:8–14. [PubMed] [Google Scholar]
- 20.Ganendran A, Balabaskaran S. Pralidoxime as an insignificant reactivator in severe anticholinesterase (organophosphate insecticide) poisoning. SEAsian. J Trop Med Publ Hlth. 1976;7:543–550. [PubMed] [Google Scholar]
- 21.du Toit PW, Muller FO, Vantonder WM, Ungerer MJ. Experience with intensive care management of organophosphate insecticide poisoning. SAMJ. 1981;60:227–229. [PubMed] [Google Scholar]
- 22.Delilkan AE, Namazie M, Ong G. Organophosphate poisoning: a Malaysian experience of one hundred cases. Med J Malaysia. 1984;39:229–233. [PubMed] [Google Scholar]
- 23.de Silva HJ, Wijewickrema R, Senanayake N. Does pralidoxime affect outcome of management in acute organophosphate poisoning. Lancet. 1992;339:1136–1138. doi: 10.1016/0140-6736(92)90733-j. [DOI] [PubMed] [Google Scholar]
- 24.Singh S, Batra YK, Singh SM, Wig N, Sharma BK. Is atropine alone sufficient in acute severe organophosphate poisoning? Experience of a northwest Indian hospital. Int J Clin Pharmacol Therap. 1995;33:628–630. [PubMed] [Google Scholar]
- 25.Sungur M, Guven M. Intensive care management of organophosphate insecticide poisoning. Crit Care. 2001;5:211–215. doi: 10.1186/cc1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson MK, Vale JA, Marrs TC, Meredith TJ. Pralidoxime for organophosphorus poisoning [letter] Lancet. 1992;340:64. doi: 10.1016/0140-6736(92)92487-z. [DOI] [PubMed] [Google Scholar]
- 27.Vale JA. Rationale for oxime therapy: pralidoxime as an antidote in organophosphate insecticide poisoning. Hum Exp Toxicol. 1996;15:77. [Google Scholar]
- 28.Moher D, Schulz KF, Altman DG. for the CONSORT Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–1194. [PubMed] [Google Scholar]
- 29.Samuel J, Thomas K, Jeyaseelan L, Peter JV, Cherian AM. Incidence of intermediate syndrome in organophosphorus poisoning. J Assoc Physic India. 1995;43:321–323. [PubMed] [Google Scholar]
- 30.Samuel J, Peter JV, Thomas K, Jeyaseelan L, Cherian AM. Evaluation of two treatment regimens of pralidoxime (1gm single bolus dose vs. 12gm infusion) in the management of organophosphorus poisoning. J Assoc Physic India. 1996;44:529–531. [PubMed] [Google Scholar]
- 31.Cherian AM, Jeyaseelan L, Peter JV, et al. INCLEN Monograph series on Critical International Health Issues. 7 1997. Effectiveness of pralidoxime in the treatment of organophosphorus poisoning - a randomised, double-blind, placebo-controlled clinical trial. [Google Scholar]
- 32.Cherian AM, Peter JV, Samuel J, et al. Effectiveness of P2AM (PAM-pralidoxime) in the treatment of organophosphorus poisoning. A randomised, double-blind, placebo-controlled trial. J Assoc Physic India. 1997;45:22–24. [Google Scholar]
- 33.Abdollahi M, Jafari A, Jalali N, et al. A new approach to the efficacy of oximes in the management of acute organophosphate poisoning. Iranian J Med Sci. 1995;20:105–109. [Google Scholar]
- 33.Dadan R. A clinical trial of PAM (pralidoxime iodide) in organophosphate poisoning in District Ballia (part of Eastern UP) 1999. www.japi.org/freepapers18ppoison.htm. www.japi.org/freepapers18ppoison.htm Abstract:
- 35.Peter JV, Cherian AM. Organic insecticides. Anaes Intens Care. 2000;28:11–21. doi: 10.1177/0310057X0002800102. [DOI] [PubMed] [Google Scholar]
- 36.Medicis JJ, Stork CM, Howland MA, Hoffman RS, Goldfrank LR. Pharmacokinetics following a loading plus a continuous infusion of pralidoxime compared with the traditional short infusion regimen in human volunteers. Clin Toxicol. 1996;34:289–295. doi: 10.3109/15563659609013791. [DOI] [PubMed] [Google Scholar]
- 37.Schulz KF. Subverting randomization in controlled trials. JAMA. 1995;274:1456–1458. [PubMed] [Google Scholar]
- 38.Hansen ME, Wilson BW. Oxime reactivation of RBC acetylcholinesterases for biomonitoring. Arch Environ Contam Toxicol. 1999;37:283–289. doi: 10.1007/s002449900516. [DOI] [PubMed] [Google Scholar]
- 39.Thompson DF, Thompson GD, Greenwood RB, Trammel HL. Therapeutic dosing of pralidoxime chloride. Drug Intell Clin Pharmacol. 1987;21:590–593. doi: 10.1177/1060028087021007-804. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Chaudhry D, Behera D, Gupta D, Jindal SK. Aggressive atropinisation and continuous pralidoxime (2-PAM) infusion in patients with severe organophosphate poisoning: experience of a northwest Indian hospital. Human Exp Toxicol. 2001;20:15–18. doi: 10.1191/096032701671437581. [DOI] [PubMed] [Google Scholar]
- 41.Zheng G, Song S, Li M. Comparison of effects between concentrated-dose and non-concentrated-dose pralidoxime chloride on respiratory muscle paralysis in acute organophosphorus pesticide poisoning. Zhonghua Nei Ke Za Zhi. 2000;39:655–7. [PubMed] [Google Scholar]
- 42.Worek F, Mast U, Kiderlen D, Diepold C, Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta. 1999;288:73–90. doi: 10.1016/s0009-8981(99)00144-8. [DOI] [PubMed] [Google Scholar]
- 43.Eddleston M, Rajapakse S, Rajakanthan, et al. Anti-digoxin Fab fragments in cardiotoxicity induced by ingestion of yellow oleander: a randomised controlled trial. Lancet. 2000;355:967–972. doi: 10.1016/s0140-6736(00)90014-x. [DOI] [PubMed] [Google Scholar]


