Abstract
Study Objective: Data on poisoning with MCPA (4-chloro-2-methylphenoxyacetic acid) is limited to six case reports. Our objective is to describe outcomes from intentional self-poisoning with MCPA in a prospective case series of 181 patients presenting to hospitals in Sri Lanka.
Methods: Patient information was collected by on-site study doctors as part of an ongoing prospective cohort study of poisoned patients. History, clinical details and blood samples were obtained prospectively.
Results: Overall clinical toxicity was minimal in 85% of patients, including mild gastrointestinal symptoms in 44% of patients. More severe clinical signs of chlorophenoxy poisoning reported previously such as rhabdomyolysis, renal dysfunction and coma also occurred, but were uncommon. Eight patients died (4.4%). Most deaths occurred suddenly from cardiorespiratory arrest within 48 hours of poisoning; the pathophysiological mechanism of death was not apparent. The correlation between admission plasma MCPA concentration and clinical markers of severity of toxicity (physical signs, symptoms and elevated creatine kinase) was poor.
Conclusions: Intentional self-poisoning with MCPA generally causes mild toxicity, but cardiorespiratory arrest and death may occur. All patients should receive routine resuscitation and supportive care. It seems reasonable to correct acidosis and maintain an adequate urine output, but there is insufficient evidence to support other specific interventions. Our data do not support a clinical role for measurement of plasma MCPA in the acute management of poisoning and insufficient data were available to fully examine the utility of measured electrolytes and creatine kinase.
Introduction
Background
It is estimated that over 300,000 people die from pesticide poisoning each year in Asia and the Western Pacific.(1) Sri Lanka has a major problem with intentional self-poisoning, with high total and youth suicide rates.(2) The mortality from intentional self poisoning in Sri Lanka is high given ready access to potent pesticides by the largely rural population. Most poisoning deaths in Sri Lanka are due to organophosphorus (OP) pesticides, but deaths from intentional self-poisoning with other pesticides, such as chlorophenoxy herbicides are noted.(3) Toxicity from chlorophenoxy herbicides is also important in developed countries such as the United States where it is one of the most commonly used classes of pesticides.(4)
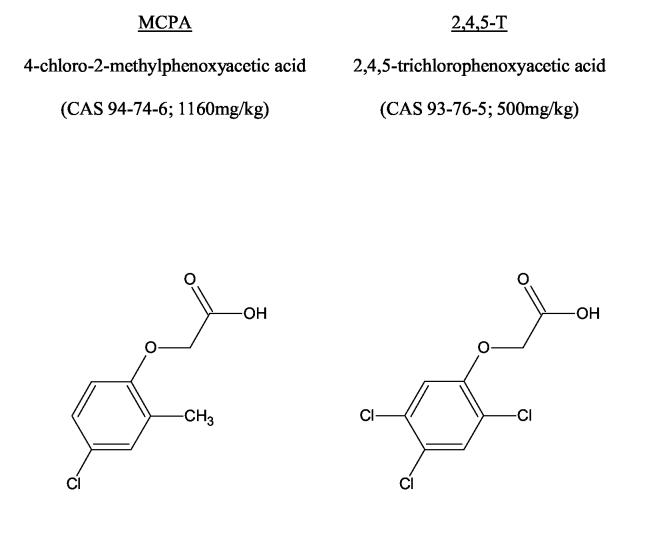
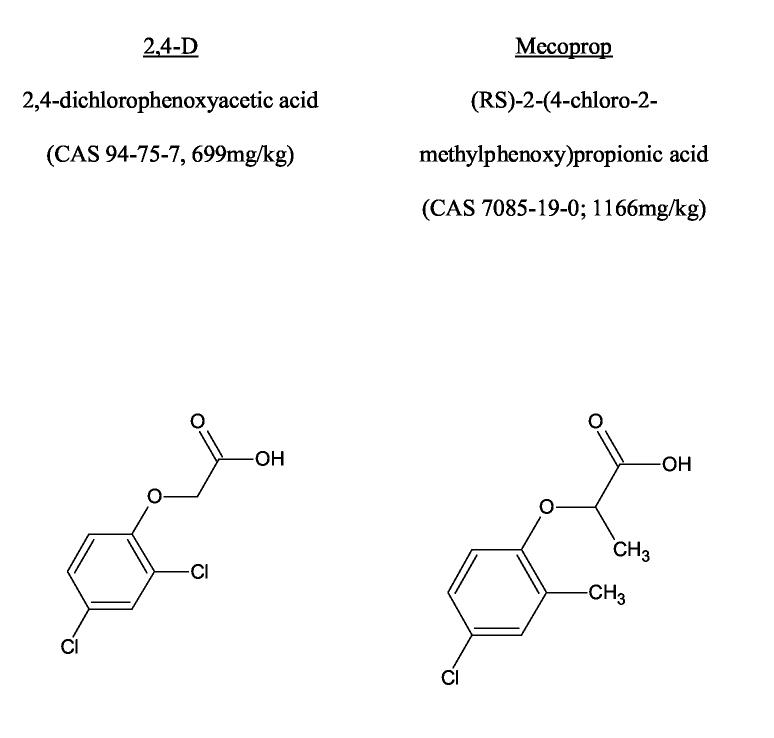
The most common chlorophenoxy herbicides are presented in Figure 1. The human literature on acute chlorophenoxy poisoning is limited to small case series and single reports of severe toxicity.(5) A third of these cases died and many others had clinical features of severe toxicity (Table 1). Most of these were reports of poisoning with 2,4-D. The selection of more serious cases for publication may mean that this does not represent the usual toxicity from chlorophenoxy poisoning nor an accurate estimate of their lethality. This is important given that some chlorophenoxy herbicides appear to have different mechanisms of toxicity and clinical features.(6-8)
Figure 1.
Common name, International Union of Pure and Applied Chemistry (IUPAC) name; Chemical Abstracts Service (CAS) identifier; animal LD50 (rat) and structure of the common chlorophenoxy herbicides.(41;42)
Table 1.
Clinical features following oral exposure to chlorophenoxy herbicides.*
| Gastrointestinal |
| Commonly oral burning, vomiting, abdominal pain, diarrhoea; rarely haemorrhage. |
| Neuromuscular |
| Skeletal muscle effects include muscle weakness, fasciculations, hyper- or hyporeflexia, ataxia, nystagmus, and myotonia which may progress to rhabdomyolysis. Both myopathic and neuropathic changes have been noted on neurophysiology testing. Occasionally prolonged agitation, confusion, coma, miosis, convulsions and nystagmus. |
| Cardiovascular |
| Tachycardia and hypotension |
| Respiratory |
| Hyperventilation with respiratory alkalosis may occur early in the poisoning, or later secondary to metabolic acidosis. CNS depression and respiratory muscle weakness may cause hypoventilation. |
| Other effects |
| Metabolic acidosis, pyrexia, renal failure, and electrolyte changes (particularly hypocalcaemia and hypokalaemia). |
| Death |
| Autopsy has revealed congestion and oedema of lungs, liver, kidneys, adrenals and brain. Early necrosis of the liver and kidneys has also been reported. The mechanism of death has not been confirmed. |
Significant variations in the description of manifestations of chlorophenoxy herbicide toxicity is noted between each individual review.
Importance
MCPA (4-chloro-2-methylphenoxyacetic acid) is a widely used chlorophenoxy herbicide. It is the most important cause of chlorophenoxy poisoning in the North Central Province (NCP) of Sri Lanka.(3) There is limited information on clinical toxicity from MCPA poisoning, with only six single case reports in the literature.(9-14)
Goals of this investigation
We describe the clinical outcomes of MCPA poisoning from data gathered during a large, prospective case series of patients who presented to three Sri Lankan hospitals with a history of intentional self-poisoning.
Methods
Study design
This was a prospective descriptive study of the exposure history and clinical details on consecutive patients admitted to three hospitals in Sri Lanka (Figure 2).
Figure 2.
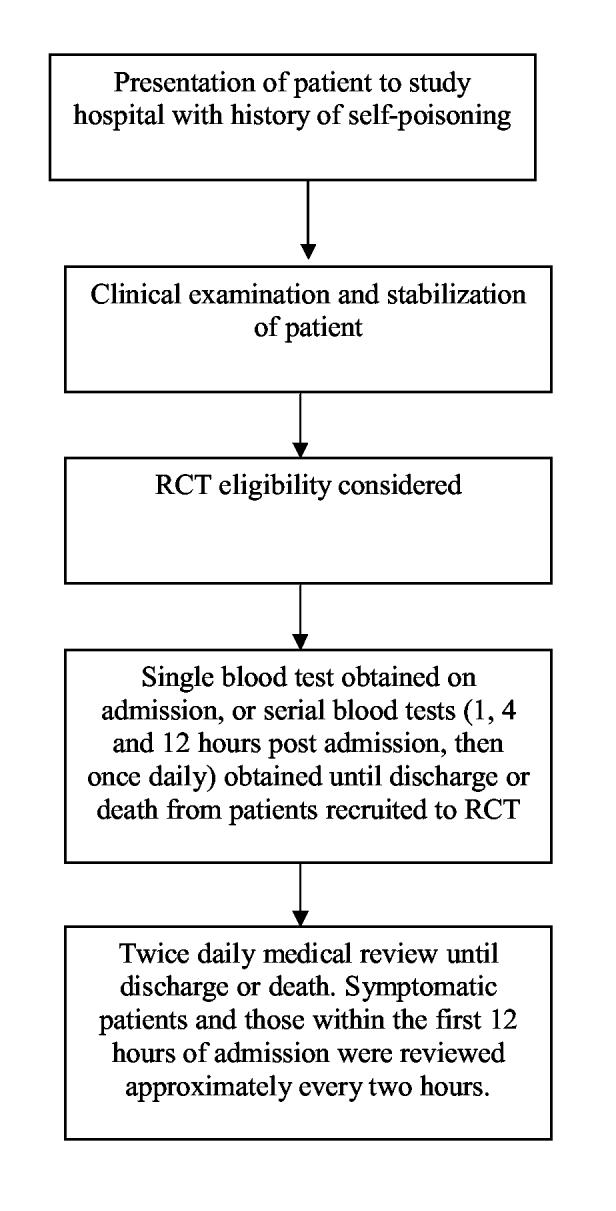
Design of the prospective cohort study of poisoned patients
Setting
Patients presenting to three of the General Hospitals in Sri Lanka between 2nd April 2002 and 27th December 2003 with a history of MCPA poisoning were included. Resources are limited in these hospitals, particularly monitored beds in the ICU and wards and laboratory facilities. Blood tests are not usually obtained in most poisoned patients. All patients were stabilized in the ward and managed with supportive care. Patients with severe poisoning were admitted to ICU if beds were available.
Selection of participants and intervention:
All patients presenting to study hospitals with self poisoning were included. A large subset of these patients were part of a randomised controlled trial (RCT) evaluating the efficacy of activated charcoal (ISRCTN02920054). All patients with a history of acute poisoning were eligible for inclusion in this RCT, except for those who are under the age of 14 years, known to be pregnant or reported ingestion of either hydrocarbons alone or corrosives. Written informed consent was obtained prior to enrolment. Ethics approval was obtained from the University of Colombo, Sri Lanka and Oxford, United Kingdom.
Convenience blood samples were obtained on admission from 91 of the 113 patients recruited to the RCT between 8th May 2002 and 21st September 2003 for quantification of the plasma MCPA concentration using gas chromatography mass spectroscopy. These samples were in addition to those obtained by the treating physician for clinical indications.
In order to examine for the presence and time course of myotoxicity, serial convenience samples of blood were also obtained in 12 patients for measurement of creatine kinase (CK) activity. These patients presented to one hospital early in the series and were selected only because of the history of MCPA poisoning. CK activity was measured only in the 10 patients who tested positive to MCPA on admission. The medical chart of these patients was manually reviewed to correlate clinical features with plasma concentrations of MCPA and CK activity. Biochemical analyses were conducted by Queensland Health Scientific Services, Australia.
Treatment was determined by the treating physician and their team independent of the research team and irrespective of enrolment in the activated charcoal RCT. Supportive care with IV fluids, respiratory support, and inotropes were given where necessary, however alkalinisation was not used.
Data collection and analysis:
Important demographic details, including the type of poison and time since ingestion were collected on admission. Pre-determined demographic data and details of major clinical outcomes were prospectively recorded by on-site full time trained study doctors for every patient until discharge or death. These were entered directly into a hand-held computer data base at the time of recruitment and with each clinical review and evaluated by a frequency analysis. Other qualitative data were obtained directly from the medical chart for patients who died and those who provided serial blood samples.
Results
A history of MCPA poisoning was reported in 181 patients, (145 of these patients were also recruited to the RCT of charcoal). Time to hospital admission and outcomes of poisoning are listed in Table 2. Ten patients received endotracheal intubation and ventilation for respiratory failure. The incidence of spontaneous vomiting was reviewed at one the study hospitals (accounting for the 63% of all admissions), and was noted to occur in 44% of patients.
Table 2.
Time to presentation and clinical effects of MCPA poisoning.
| N (total = 181) (%) | ||
| Time to presentation | ||
| Unknown | 1(0.5) | |
| <2 hours | 18 (9.9) | |
| 2 to < hours | 61 (33.7) | |
| 4 to <7 hours | 62 (34.2) | |
| 7 to <12 hours | 24 (13.2) | |
| 12 to <24 hours | 9 (5.0) | |
| >24 hours | 6 (3.3) | |
| Glasgow Coma Score (GCS) | On arrival | Lowest during admission |
| 15 | 154 (85.1) | 153 (84.5) |
| 13-14 | 11 (6.1) | 8 (4.4) |
| 10-12 | 9 (5.0) | 9 (5.0) |
| 4-9 | 4 (2.2) | 6 (3.3) |
| 3 | 3 (1.6) | 5 (2.8) |
| Lowest recorded systolic blood pressure during admission (mmHg) | ||
| >120 | 46 (25.4) | |
| 101-120 | 103 (56.9) | |
| 80-100 | 21 (11.6) | |
| <80 | Nil | |
| Not recorded (patient alert & interactive) | 11 (6.1) | |
| Outcome at Discharge | ||
| Alive | 173 (95.6) | |
| Dead | 8 (4.4) | |
| Median time to live discharge, days (range | 2.0 (1-7) | |
Eight patients died (4.4%, Table 3). Seven of the eight patients died within 24 to 48 hours of poisoning from cardiorespiratory arrest of an unclear pathophysiological mechanism. A delay in the time to hospital admission did not appear to contribute to this outcome. One patient (Case 5) had bilateral crepitations and purulent secretions consistent with aspiration pneumonia. The medical chart could not be located for one patient (Case 8). Oliguria or dark-coloured urine was noted in five patients. Test bolus doses of atropine were given to some patients on admission who were initially suspected of being poisoned with anticholinesterase pesticides. This was generally not continued, but may have contributed to symptoms in some patients on admission.
Table 3.
Clinical details of MCPA deaths
| Patient | Age/sex | Time to hospital presentation (hours) | Clinical features at presentation | Treatment | Features prior to death | Time to death (hours) |
|---|---|---|---|---|---|---|
| 1 | 48M | 4 | Coingestion of alcohol.RR 22-26/min, HR 104, BP 120/80 mm Hg, GCS 7/15, vomiting, miosis,epistaxis, oliguria despite fluids | MDAC, intubation,IVF, GL, atropine bolus antibiotics,ICU | HR=160/min, BP normal, then cardiorespiratory arrest 2 hours post admission, failed resuscitation | 6 |
| 2 | 48M | 3 | Coingestion of alcohol RR 18/min, HR 80/min, BP 130/80 mm Hg, GCS 3/15, vomiting, miosis, facial flushing. | SDAC, Intubation,IVF, atropine | BP 70/50 mm Hg, HR 96/min, then bradycardia and CRA, failed resuscitation | 6 |
| 3 | 28F | 1 | HR 90/min, BP 110/70 mm Hg, GCS 15/15, vomiting, oral secretions | IVF, GL | BP 110/70 mm Hg, HR 96/min,GCS 15, temp 40.4oC, B/L crepitation, cardiorespiratory arrest one hour later, failed resuscitation. | 24 |
| 4 | 37M | 5 | Coingestion of alcohol HR 80/min, BP 130/80 mm Hg,GCS 10/15, severe haematemesis, aspiration with bilateral crepitations, excess oral secretions, epistaxis (?trauma) 10 hours: febrile, HR 120/min, miosis, BP 100/70 mm Hg, GCS 6/15, dark urine | SDAC, ETT, IVF,antibiotics,furosemide | 20 hours: RR 30/min, HR 140/min, GCS 6/15, oliguria, Urea 65, serum electrolytes normal 24 hours: RR 40/min, SpO2 92%, HR 140/min GCS 5/15. 27 hours:cardiorespiratory arrest, resuscitated, ‘laboured breathing’,SpO2∼80% despite high flow oxygen, HR 180/min, BP 90/60 mmHg, febrile, oral bleeding. Urea | 30 |
| 115, SE normal. NaHCO3 bolus,furosemide and inotropes commenced 29 hours: multiple cardiorespiratory arrests with non-sustained resuscitation. | ||||||
| 5 | 28F | ? | Day 1 HR 80/min, BP 100/70 mmHg, GCS 11/15, nausea, vomiting, diarrhoea,abdominal pain | MDAC, GL, IVF,furosemide,antibiotics,inotropes,NaHCO3, atropine | Day 2: Laboured breathing,widespread crepitations, BP 95/70 mm Hg, HR 64/min, oliguria.Later in day: Respiratory arrest, “yellow secretions” from ETT, HR | <48 |
| GCS 11/15, vomiting, diarrhoea. B/L crepitations and rhonchi, oral secretions,cyanosis | bolus | 30/min, thencardiorespiratory arrest, failed resuscitation. | ||||
| 6 | 50M | 4 | HR 110/min, BP 120/80 mm Hg, GCS 15/15, oral secretions, fasciculations and diaphoresis. Later that day became confused and agitated | MDAC, GL, IVF,furosemide,atropine bolus. | 20 hours: cardiorespiratory arrest, 21 hours: asystolic arrest, failed resuscitation. | 20 |
| 7 | 45M | 10 | Co-ingestion of alcohol HR 80/min BP 100/40 mm Hg, GCS 3/15, oral secretions, BUN 92 | ETT, IVF, atropine bolus, GL Day 2: NaHCO3 | Day 2: RR 28/min, tidal volume 500 mL, HR 120/min, GCS 8/15,Temp 101.4°F, ‘dark urine’ 2000hrs: RR 40/min, HR 120/min, BP 70/30 mm Hg, GCS 6/15 2300hrs: CRA, failed resuscitation | <48 |
| 8* | 49F | 4 | Asymptomatic, GCS 15/15, systolic BP 130mmHg | SDAC | Cardiorespiratory arrest | 30 |
HR = Heart rate BP=Blood pressure RR = Respiratory rate GCS = Glasgow Coma Score
SDAC = single-dose activated charcoal MDAC = multi-dose activated charcoal
GL = gastric lavage
NaHCO3 = sodium bicarbonate IVF = intravenous fluids
B/L = bilateral SpO2 = Oxygen saturation (pulse oximetry)
ICU = Intensive Care Unit
BUN = blood urea nitrogen
patient́s notes were not able to be obtained for retrospective review.
Of the admission bloods available for analysis, MCPA was not detected in 11/91 patients (detection limit 0.05mg/kg plasma), consistent with minimal or no exposure to MCPA. One of these patients presented with a Glasgow Coma Score (GCS) of 15/15 six hours post-ingestion, but soon developed seizures and abdominal pain. The cause of these symptoms is unknown, but most likely represents poisoning with a substance other than MCPA, for example fipronil. The patient was discharged without complication four days later. All other patients with an undetectable MCPA level developed no signs of poisoning.
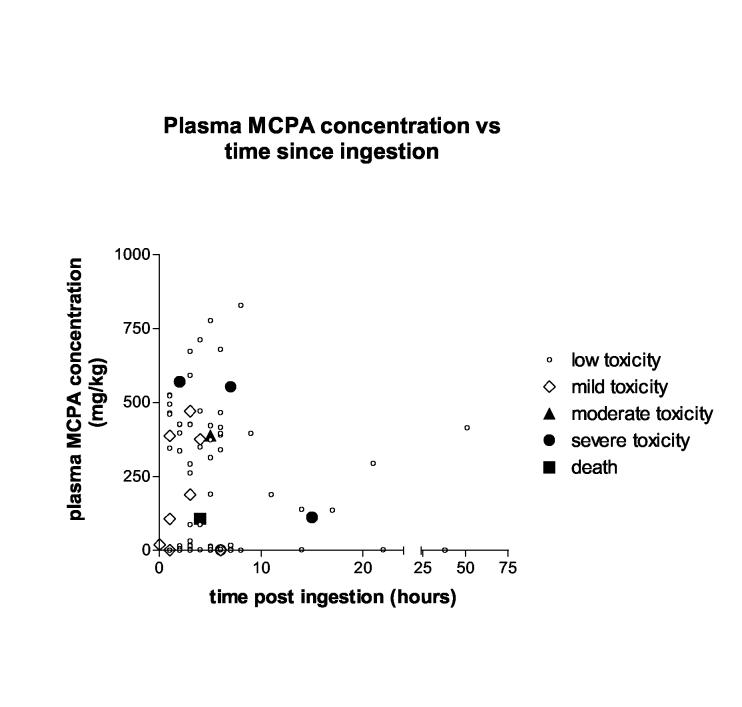
The relationship between admission plasma MCPA concentration, time since ingestion and severity of toxicity appears poor (Figure 3), although only one death (Case 6) was captured in this cohort, given the difficulty in obtaining consent from patients with an altered level of consciousness on presentation. Although CK activity tended to be increased in patients with higher admission MCPA concentrations (>300mg/kg), the correlation appears poor (Table 4 and Figure 4). With the exception of Cases B and C, CK activity was only marginally elevated in these patients. Case B presented with GCS 10/15 and profuse vomiting. Myalgias and weakness developed the following day and resolved within 48 hours. Case C presented with mild haematemesis, diarrhoea, abdominal pain and anuria despite fluid loading. Urine output returned by the next day and despite some ‘throat pain’, the gastrointestinal symptoms had settled. He complained of generalised myalgias, but appeared otherwise well and was discharged within 50 hours. No other investigations were ordered during this admission. The CK increased until discharge (Figure 4), which together with the myalgias, may be consistent with ongoing skeletal muscle toxicity.
Figure 3.
Admission plasma MCPA concentration, time since poisoning, and severity of toxicity during admission. Low toxicity GCS = 15, no other signs Mild toxicity GCS = 13-14, no other signs Moderate toxicity GCS = 11-12, no other signs Severe toxicity GCS <11, hypotension (SBP<90mmHg despite fluid resuscitation) or other complications of severe toxicity such as respiratory failure, renal dysfunction or seizures.
Table 4.
Admission MCPA, peak creatine kinase (CK) activity and severity of toxicity in patients providing serial blood samples.♯
| Case (age,sex) | Admission plasma MCPA (mg/kg) | Peak CK activity (U/L)* | Severity of toxicity§ |
| Admission MCPA concentration >300mg/kg | |||
| A(27,M) | 672 | 402 | Low |
| B (26,F) | 569 | 942 | Severe |
| C (19,M) | 591 | 4770 | Moderate |
| D (39,M) | 387 | 338] | Mild |
| E (25,F) | 415 | 493 | Mild |
| F (27,M) | 340 | NE | Low |
| Admission MCPA concentration <20mg/kg | |||
| G (21,M) | 16 | 393 | Low |
| H (25,M) | 17 | 272 | Low |
| I (38,F) | 0.8 | NE | Low |
| J (16,M) | <0.05 | NE | Low |
Reference range: <200U/L for males and <160U/L for females; NE represents ‘not elevated’. Changes in CK activity during the admission are shown in Figure 4.
Based on data collected by study doctors and individual chart review: GCS = 15, no other signs, minimal gastrointestinal (GI) symptoms GCS = 13-14, no other signs, mild to moderate GI symptoms or myalgias, GCS = 11-12, no other signs, severe GI symptoms or myalgias GCS <11, hypotension (SBP<90mmHg despite fluid resuscitation) or other complications of severe toxicity such as respiratory failure, renal dysfunction or seizures.
Serial blood samples were obtained in 12 patients. The CK activity was not determined in two of these patients because MCPA was undetectable
Figure 4.
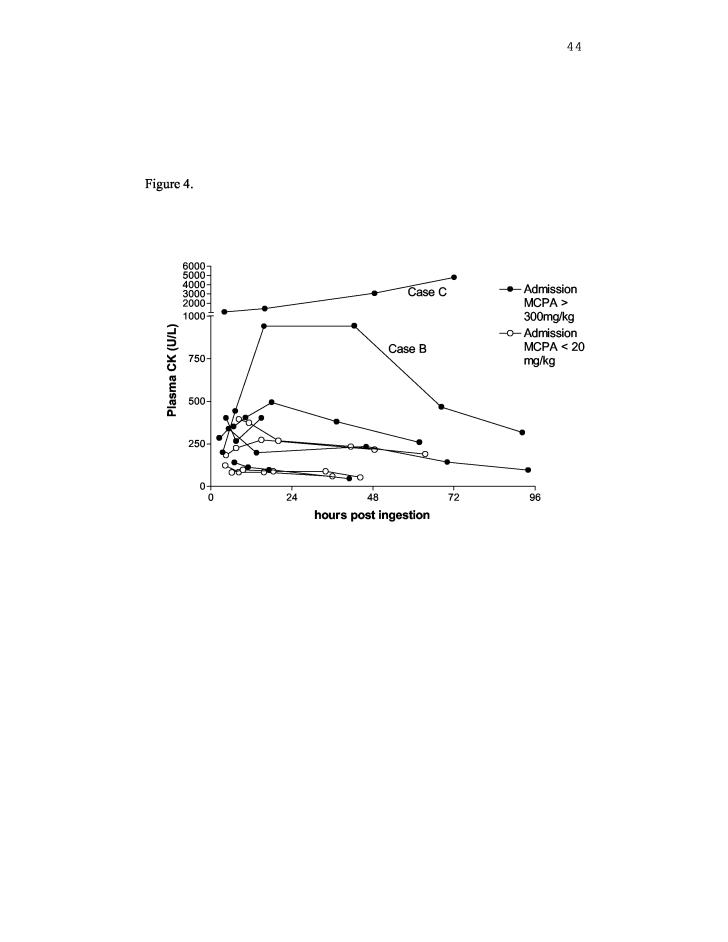
Creatine Kinase (CK) activity,, time since admission and admission plasma MCPA concentration in patients providing serial blood samples
Limitations
This study was performed in secondary referral hospitals in rural areas of Sri Lanka. They have very limited resources and laboratory tests are not routinely performed. Monitored beds in the ICU or wards are not routinely available. The patient-doctor and patient-nurse ratios are high, so data on clinical features exceeding those obtained for the purpose of our study are limited. Most deaths occurred in unmonitored beds in the absence of blood tests, which limits our ability to determine the mechanism of death.. Further, as blood tests were only taken on those who consented to enrolment in the RCT, patients who were most unwell were less likely to have MCPA blood concentrations measured.
Discussion
This is the first published prospective case series of major outcomes from chlorophenoxy self-poisoning. Mild gastrointestinal symptoms were noted, but overall, toxicity from MCPA poisoning was low and mortality was less than other common pesticide poisonings presenting to the same hospitals. Although animal data suggest that other chlorophenoxy herbicides are more toxic than MCPA (Figure 1), it seems unlikely that a third of poisonings with other chlorophenoxy herbicides will be fatal. The case series and single reports that this outcome was based on were probably published because of their severity or unusual presentation, representing a reporting bias.(5)
Clinical toxicity
Variable clinical features have previously been reported for MCPA poisoning, but all are consistent with those described in our patients (Table 1). We found that 85% of patients had minimal toxicity (despite a large reported exposure in some) and mortality was 4.4%. This in-hospital death rate is higher than that noted from medications, but lower than that of other pesticides including the herbicides glyphosate (5%) and paraquat (60%) and organophosphorus insecticides (11%).(15) Blood levels confirmed ingestion of MCPA in 88% of samples tested and there was only one patient where the combination of laboratory investigations and clinical features suggested that MCPA was not responsible for the clinical presentation despite a history of ingestion. Therefore these data should be an accurate reflection of the clinical course from acute intentional self poisoning with MCPA.
Other clinical features of MCPA poisoning were not systematically recorded in each patient, but abdominal pain, myalgia, fasciculations, throat pain and miosis were noted by study doctors in some patients.
Seven of eight deaths were due to a cardiorespiratory arrest of an unclear aetiology. Speculation of factors leading to death in these patients is restricted by the very limited availability of laboratory tests. Oliguria and discoloured urine was noted in five of these patients, which could indicate renal toxicity and be an important predictor of severe toxicity or death.
Mechanism of death
Uncoupling of oxidative phosphorylation may be an important component in the cause of death in patients with large chlorophenoxy herbicide exposures. Chlorophenoxy herbicides uncouple oxidative phosphorylation in vitro through an unclear mechanism of action.(16;17)
Uncoupling describes the process whereby oxygen consumption and heat production increase out of proportion to the generation of adenosine triphosphate.(18) It may be caused by extrinsic factors such as chemicals or drugs which disrupt mitochondrial function.(19) Initially this leads to an increase in mitochondrial respiration,(20;21) until the declining levels of ATP are insufficient for essential cellular functions including active transport pumps such as Na-K ATPase. This leads to loss of cellular ionic and volume regulation, and if ATP is not supplied promptly, the effect is irreversible and cell death occurs.(19)
Clinical features consistent with marked uncoupling of oxidative phosphorylation have been reported in cases of human chlorophenoxy herbicide poisoning. Tachypnoea with respiratory alkalosis have been noted in cases of severe poisoning, some of whom died (5;13)(D Roberts and P. Piyasena, unpublished observations), which may be consistent with increased mitochondrial respiration from mild uncoupling. More severe poisoning may be characterised by severe hypoxia, metabolic acidosis, hyperventilation, hyperkalaemia, fever, elevated creatine kinase, tachycardia, generalised muscle rigidity, hypotension, and cardiac asystole.(5;7;22;23) Some of these features were noted in patients who died in our series (Table 3, patients 3,4,7, and perhaps 8). However, these data were not in the dataset collected prospectively under the protocol.
Correlation between plasma pesticide concentrations and toxicity
The relationship between plasma chlorophenoxy herbicide level and toxicity is not clear. A depressed level of consciousness has been reported with plasma chlorophenoxy concentrations from 80 mg/L to over 1000 mg/L,(24) while concentrations more than 500 mg/L may be associated with severe toxicity.(25) But death occurred in two patients with a blood MCPA level of 180 mg/L and 230 mg/L.(11;13) A patient survived severe MCPA poisoning (hypotension and limb myotonia) despite a blood concentration of 546 mg/L two hours after ingestion. The muscle effects resolved after a number of days when the MCPA level was less than 100mg/L.(9)
The discordance between admission MCPA levels and the peak toxicity observed during hospital admission is noted in Figure 3. In contrast, concentration dependent uncoupling of oxidative phosphorylation has been demonstrated with rat mitochondria in vitro.(16) The poor correlation between clinical toxicity and plasma concentrations may reflect poor correlation between plasma (measured) and intracellular (mitochondrial) concentrations. Mechanisms which alter the distribution of MCPA between these two spaces includes MCPA-induced damage to cell membranes which increases penetration of the herbicide through cell membranes.(5;26-30) In rats, high plasma concentrations of MCPA damage cell membranes and induce toxicity, but the correlation between plasma levels, membrane damage and toxicity is poor.(29;30)
Another potential mechanism is variation in blood pH, which alters protein binding and tissue distribution. Acidosis increases the proportion of non-ionised MCPA (pKa 3.07), which is lipophilic, and therefore readily crosses the cell membrane, increasing the intracellular concentration. This has been observed in in vitro studies and is similar to that observed for aspirin. (23;31) Similarly, an alkaline plasma pH is expected to decrease tissue binding and increase plasma levels.(25;32;33) Therefore, the distribution of MCPA between plasma and intracellular spaces is likely to vary with plasma pH.
Routine measurement of plasma MCPA levels in patients with intentional self poisoning does not appear useful. Instead, quantification of markers of intracellular toxicity, such as CK from skeletal muscle, may be useful to monitor the effects of MCPA poisoning. Of the patients who had serial blood samples, only two patients (Cases B and C) had significant toxicity (Table 4, Figure 4). Myalgias were reported by these two patients, particularly in Case C where the CK was 1030 on admission and increased until discharge. In most patients, peak CK activity was delayed (Figure 4).
Management of patients with MCPA poisoning
All patients should receive routine resuscitation and supportive care. There is insufficient evidence to describe other specific managements of acute MCPA poisoning. It would be reasonable to correct acidosis based on the likely adverse effect of acidosis on MCPA distribution kinetics leading to increased intracellular concentrations.
All significant poisonings (ie symptomatic oral ingestions) should be treated cautiously, preferably in an ICU or other unit with continuous monitoring for 24 to 48 hours. In our series, initial mild toxicity as indicated by normal vital signs and level of consciousness on admission did not preclude subsequent severe toxicity and death. However, we observed that no patients who were completely asymptomatic for the first six hours went on to a fatal outcome. Late deterioration in a reasonably well patient has also been reported with poisoning with MCPP (a related chlorophenoxy herbicide). In this case severe metabolic derangement and death occurred in a patient who was fully conscious prior to an asystolic arrest.(7)
Our data suggest measurement of plasma MCPA confirms exposure but does not provide further information on the severity of poisoning. Confirmation of MCPA exposure may not be necessary in patients presenting with intentional MCPA self-poisoning given that the history was accurate in 88% of patients in our series. We had insufficient data to suggest whether electrolytes, blood gases or CK may be better indicators of the severity of poisoning.
An adequate urine output (>1mL/kg) should be ensured in all patients. Urinary alkalinisation (pH >7.5) and an adequate urine output limits reabsorption and promotes renal excretion.(23;33-35) It might be reasonable to consider these measures in patients who are symptomatic, particularly if they have signs of toxicity. A randomised controlled trial is required to determine the efficacy of this treatment.
Patients with severe toxicity require prompt and effective plasma and urinary alkalinisation, and possibly haemodialysis if facilities are available.(5;35) While there is limited information to support alkalinisation, it is rarely associated with adverse effects when administered carefully and with close observation.(35;36) Signs consistent with uncoupling of oxidative phosphorylation are likely to be associated with a poor outcome.
A better description of the clinical, biochemical, histopathological and histochemical features of severe toxicity and death is required to confirm the mechanism of death in patients. This may help to guide clinical management.
Conclusion
We found that toxicity from MCPA poisoning is low compared to some other agrochemicals, with 85% of patients having minimal clinical toxicity; but toxicity is still significant with a mortality of 4.4%. Further research is required to identify early markers of poor outcome and to determine if alkalinisation or other interventions can improve the prognosis in this group.
Acknowledgements
Study doctors who assisted with data and sample collection, and hospital doctors for their cooperation with the study. Manel Abeyawardene, Vincent Alberts Brock Jones, and in particular Mary Hodge from Queensland Health Pathology and Scientific Services for assistance with analyses. The Medical Superintendent of Anuradhapura General Hospital for permission to access medical records, and Medical Records staff who located these records. Jason and Alison Roberts for assistance with locating some of the older references.
Reference List
- 1.Eddleston M, Phillips MR. Self poisoning with pesticides. Br.Med.J. 2004;328:42–4. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, editor. World Health Report 2002. World Health Organization; Geneva: 2002. Annex Table 2 Deaths by cause, sex and mortality stratum in WHO Regions, estimates for 2001. [Google Scholar]
- 3.Roberts D, Karunarathna A, Buckley NA, Manuweera G, Sheriff MHR, Eddleston M. Influence of Pesticide Regulation and Integrated Pest Management on Acute Poisoning Deaths in Sri Lanka. Bulletin of the World Health Organization. 2003;81(10):708–17. [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson D, Kiely T, Grube A. Pesticides industry sales and usage -1998 and 1999 market estimates. Environmental Protection Agency; Washington: 2002. [Google Scholar]
- 5.Bradberry SM, Watt BE, Proudfoot AT, Vale JA. Mechanisms of toxicity, clinical features, and management of acute chlorophenoxy herbicide poisoning: a review. J.Toxicol.Clin.Toxicol. 2000;38(2):111–22. doi: 10.1081/clt-100100925. [DOI] [PubMed] [Google Scholar]
- 6.Aromataris EC, Astill D.St.J., Rychkov GY, Bryant SH, Bretag AH, Roberts ML. Modulation of the gating of ClC-1 by S-(7) 2-(4-chlorophenoxy) propionic acid. Br.J.Pharmacol. 1999;126:1375–82. doi: 10.1038/sj.bjp.0702459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickey W, McAleer JJA, Callender ME. Delayed sudden death after ingestion of MCPP and ioxynil:an unusual presentation of hormonal weedkiller intoxication. Postgrad Med J. 1988;64:681–2. doi: 10.1136/pgmj.64.755.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liantonio A, Accardi A, Carbonara G, Fracchiolla G, Loiodice F, Tortorella P, et al. Molecular requisites for drug binding to muscle CLC-1 and renal CLC-K channel revealed by the use of phenoxy-alkyl derivatives of 2-(p-chlorophenoxy)propionic acid. Molecular Pharmacology. 2002;62(2):265–71. doi: 10.1124/mol.62.2.265. [DOI] [PubMed] [Google Scholar]
- 9.Schmoldt A, Iwersen S, Schluter W. Massive ingestion of the herbicide 2-methyl-4-chlorophenoxyacetic acid. J.Toxicol.Clin.Toxicol. 1997;35(4):405–8. doi: 10.3109/15563659709043374. [DOI] [PubMed] [Google Scholar]
- 10.Geldmacher von- Mallinckrodt M, Lautenbach L. Zwei todliche Vergiftungen (Suicid) mit chlorierten Phenoxyessigsauren(2,4-D und MCPA) [2 cases of fatal poisoning (suicide) with chlorinated phenoxyacetic acids (2,4-D and MCPA)] Arch Toxikol. 1966;21:261–78. [PubMed] [Google Scholar]
- 11.Johnson HR, Koumides O. A further case of MCPA poisoning. Br.Med.J. 1965:629–30. doi: 10.1136/bmj.2.5462.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones DIR, Knight AG, Smith AJ. Attempted suicide with herbicide containing MCPA. Arch Environ Health. 1967;14:363–6. doi: 10.1080/00039896.1967.10664747. [DOI] [PubMed] [Google Scholar]
- 13.Popham RD, Davies DM. A case of M.C.P.A. poisoning. BMJ. 1964;1:677–8. doi: 10.1136/bmj.1.5384.677-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyne BA. Chemically induced or inherited myotonia? Emerg.Med. 1993;5:149–244. [Google Scholar]
- 15.Senarathna L, Mohammad F, Eddleston M. Correlation of human toxicity case fatality ratio (CFR) for pesticides with animal LD50 [abstract]. The Asia Pacific Association of Medical Toxicology 4th International Conference, Philippines.2004. p. 26. [Google Scholar]
- 16.Zychlinski L, Zolnierowicz S. Comparison of uncoupling activities of chlorophenoxyherbicides in rat liver mitochondria. Toxicol.Lett. 1990;52:25–34. doi: 10.1016/0378-4274(90)90162-f. [DOI] [PubMed] [Google Scholar]
- 17.Brody TM. Effect of certain plant growth substances on oxidative phosphorylation in rat liver mitochondria. Proc.Soc.Exp.Biol.Med. 1952;80:533–6. doi: 10.3181/00379727-80-19681. [DOI] [PubMed] [Google Scholar]
- 18.Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochimica et Biophysica Acta. 2003;1604:77–94. doi: 10.1016/s0005-2728(03)00027-6. [DOI] [PubMed] [Google Scholar]
- 19.Gregus Z, Klaassen CD. Mechanisms of toxicity. In: Klaassen CD, editor. Casarett and Doull’s Toxicology - The basic science of poisons. 5th ed. McGraw-Hill; New York: 1996. pp. 35–74. [Google Scholar]
- 20.Brody TM. Action of sodium salicylate and related compounds on tissue metabolism in vitro. J Pharmacol Exp Ther. 1956;117(1):39–51. [PubMed] [Google Scholar]
- 21.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955;217:383–93. [PubMed] [Google Scholar]
- 22.O’Reilly JF. Prolonged coma anddelayed peripheral neuropathy after ingestion of phenoxyacetic acid weedkillers. Postgrad.Med.J. 1984;60:76–7. doi: 10.1136/pgmj.60.699.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yip L. Salicylates. In: Dart RC, editor. Medical Toxicology. 3 ed. Lippincott Williams Wilkins; Philadelphia: 2004. pp. 739–49. [Google Scholar]
- 24.Chlorophenoxy herbicides . Recognition and management of pesticide poisonings. In: Reigart JR, Roberts JR, editors. 5 ed. United States Environmental Protection Agency; Washington: 1999. pp. 94–9. [Google Scholar]
- 25.Flanagan RJ, Meredith TJ, Ruprah M, Onyon LJ, Liddle A. Alkaline diuresis for acute poisoning with chlorophenoxy herbicides and ioxynil. Lancet. 1990;335(8687):454–8. doi: 10.1016/0140-6736(90)90677-w. [DOI] [PubMed] [Google Scholar]
- 26.Bukowska B, Goszczynska K, Duda W. Effect of 4-chloro-2-methylphenoxyacetic acid and 2,4-dimethylphenol on human erythrocytes. Pesticide Biochemistry and Physiology. 2003;77:92–8. [Google Scholar]
- 27.Elo H, Ylitalo P. Substantial increase in levels of chlorophenoxyacetica acids in the CNS of rats as a result of severe intoxication. Acta Pharmacol Et Toxicol. 1977;41:280–4. doi: 10.1111/j.1600-0773.1977.tb02149.x. [DOI] [PubMed] [Google Scholar]
- 28.Elo HA, Ylitalo P, Kyotilla J, Hervonen H. Increase in the penetration of tracer compounds into the rat brain during 2-methyl-4-chlorophenoxyacetic acid (MCPA) intoxication. Acta Pharmacol Et Toxicol. 1982;50:104–7. doi: 10.1111/j.1600-0773.1982.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 29.Hervonen H, Elo HA, Ylitalo P. Blood-brain barrier damage by 2-methyl-4-chlorophenoxyacetic acid herbicide in rats. Toxicol.Appl.Pharmacol. 1982;65:23–31. doi: 10.1016/0041-008x(82)90358-1. [DOI] [PubMed] [Google Scholar]
- 30.Elo HA, Ylitalo P. Distribution of 2-methyl-4-chlorophenoxyacetic acid and 2,4-dichlorophenoxyacetic acid in male rats: Evidence for the involvement of the central nervous system in their toxicity. Toxicol.Appl.Pharmacol. 1979;51:439–46. doi: 10.1016/0041-008x(79)90368-5. [DOI] [PubMed] [Google Scholar]
- 31.Cabral MG, Viegas CA, Teixeira MC, Sa-Correia I. Toxicity of chlorinated phenoxyacetic acid herbicides in the experimental eukaryotic model Saccharomyces cerevisiae: role of pH and of growth phase and size of the yeast cell population. Chemosphere. 2003;51:47–54. doi: 10.1016/s0045-6535(02)00614-8. [DOI] [PubMed] [Google Scholar]
- 32.Arnold EK, Beasley VR. The pharmacokinetics of chlorinated phenoxy acid herbicides: a literature review. Vet Hum Toxicol. 1989;31(2):121–5. [PubMed] [Google Scholar]
- 33.Rowland M, Tozer TN. Clinical Pharmacokinetics - Concepts and Applications. 2nded Lea & Febiger; Philadelphia: 1989. [Google Scholar]
- 34.Braunlich H, Bernhardt H, Bernhardt I. Renal handling of 2-Methyl-4-Chlorophenoxyacetic acid (MCPA) in rats. Journal of Applied Toxicology. 1989;9(4):255–8. doi: 10.1002/jat.2550090409. [DOI] [PubMed] [Google Scholar]
- 35.Proudfoot AT, Krenzelok EP, Vale JA. AACT/EAPCCT position paper on urinary alkalinisation. J.Toxicol.Clin.Toxicol. 2004;42(1):1–26. doi: 10.1081/clt-120028740. [DOI] [PubMed] [Google Scholar]
- 36.Liebelt EL. Sodium bicarbonate. In: Dart EC, et al., editors. Medical Toxicology. 3 ed. Philadelphia; Lippincott Williams Wilkins: 2004. pp. 257–61. [Google Scholar]
- 37.Singh S, Yadav S, Sharma N, Malhotra P, Bambery P. Fatal 2,4-D (ethyl ester) ingestion. JAPI. 2003;51:609–10. [PubMed] [Google Scholar]
- 38.Bronstein AC. Herbicides. In: Dart EC, editor. Medical Toxicology. 3 ed. Lippincott Williams Wilkins; Philadelphia: 2004. pp. 1515–29. [Google Scholar]
- 39.O’Malley M. Chlorophenoxy herbicides. In: Olsen KM, editor. Poisoning and drug overdose. 4 ed. McGraw-Hill Companies; San Francisco: 2004. pp. 164–5. [Google Scholar]
- 40.Henry J, Wiseman H. Management of poisoning - A handbook for healthcare workers. WHO International Programme on Chemical Safety; Geneva: 1997. [Google Scholar]
- 41.Farm chemicals handbook ’99. Meister Publishing Company; Willoughby: 1999. [Google Scholar]
- 42.IPCS . World Health Organization; Geneva: 2001. WHO recommended classification of pesticides by hazard and guidelines to classification 2000-2001. [Google Scholar]