Abstract
Interstitial cystitis (IC) is a painful disorder which affects urinary bladder function in cats and humans. We have used patch clamp techniques to examine the possibility that the properties of primary afferent neurons are changed in feline interstitial cystitis (FIC). We measured transient receptor potential vanilloid receptor 1 (TRPV1) responses to capsaicin (CAPS) in dorsal root ganglion (DRG) neurons (L4-S3) from normal cats and cats with FIC. We show that FIC neurons are increased in size and exhibit CAPS responses which are increased in amplitude and desensitize slowly. CAPS responses desensitized seven times slower in FIC neurons. Phorbol 12,13-dibutyrate (PDBu), an activator of PKC, slowed the desensitization of CAPS responses in normal cat bladder and non-bladder neurons, but had no effect in FIC neurons. Bisindolylmaleimide, an inhibitor of PKC, reversed the PDBu effects in normal cat neurons and normalized the desensitization of CAPS responses in FIC neurons. Our data suggest that FIC afferent neurons exhibit abnormal CAPS responses. The latter may be due to enhanced endogenous activities of PKC.
Keywords: Dorsal root ganglia, Capsaicin, Nociception, Transient receptor potential vanilloid receptor 1, Protein kinase C, Phosphorylation, Desensitization, Interstitial cystitis, Diaryl piperazine
Interstitial cystitis (IC) is a chronic pelvic pain syndrome of humans [2,8,14]. A comparable disorder, feline interstitial cystitis (FIC), has been identified in domestic cats[3,4]. Previous studies revealed an increased density of peptidergic afferent nerves in the bladders of FIC cats suggesting that an abnormality of afferent pathways might contribute to the disorder [5,12].
Bladder pain and hyperactivity can be induced by activation of capsaicin (CAPS)-sensitive, unmyelinated (C-type) afferent nerves [7,18,20]. Intravesical administration of CAPS induces bladder pain; whereas desensitization of C-fiber bladder afferents with intravesical administration of resiniferatoxin, an ultrapotent CAPS analog, has been reported to reduce bladder overactivity [18]. The CAPS sensitivity of C-fiber afferent nerves is conferred by the presence of a temperature-acid sensing receptor called the transient receptor potential vanilloid receptor 1 (TRPV1)[6,10]. Protein kinase C phosphorylation of TRPV1 has been reported to sensitize the response of this receptor to agonists [1,22]. Here, we investigated the properties of primary afferent neurons in lumbo-sacral dorsal root ganglia (DRG) from normal cats and cats with FIC [19]. We show that the neurons from FIC cats are increased in size and exhibit slowly desensitizing CAPS-induced currents related to increased PKC phosphorylation of TRPV1. We conclude that abnormal TRPV1 responses of afferent neurons may contribute to bladder pain in FIC.
To identify FIC in cats, we utilized criteria as previously described [4]. Cells were isolated from L4-S3 DRG of cats using methods previously described for primary cultures of DRG neurons of adult rat [15]. In some experiments, the population of DRG neurons that innervate the urinary bladder was labeled by retrograde axonal transport of a fluorescent dye, fast blue (FB, 4% w/v, Polyloy, Gross Umstadt, Germany [21]). In these experiments, dye-labeled primary afferent neurons were identified using an inverted phase-contrast microscope (Nikon, Tokyo, Japan) with fluorescent attachments (UV-1A filter; excitation wavelength, 365 nm).
Gigaohm-seal whole-cell recordings of CAPS-currents were recorded in DRG neurons of normal adult and FIC cats after 2-5 days in culture using whole-cell patch clamp techniques. Processes which appear after 5 days in culture may significantly contribute to a slowly decaying membrane capacitance component (Cm). Neurons from cultures older than 5 days in which slowly decaying component become significant were excluded in our analysis. Patch pipettes were pulled from capillary glass tubes (Accufil 90, Clay-Adams) and fire polished. Immediately before recording, the serum containing media was replaced with phosphate buffered saline. Whole-cell currents were voltage clamped using an Axopatch 200 A (Axon Instruments, Foster City, California) amplifier. Pulse generation, current recording and data analysis used pClamp software (Axon Instruments). Currents were sampled at 500 μs, and filtered at 2 kHz. Capacitive currents and up to 80% of the series resistance were compensated. The decay of TRPV1 currents in response to CAPS and after addition of PDBu or BIM in the presence of CAPS was fitted with single exponentials using pClamp software regression analysis.
The extracellular solution was Dulbecco phosphate buffer (Sigma). The pipette (intracellular) solution contained (mM): KCl, 120; K2HPO4, 10; NaCl, 10; MgCl2, 2; EGTA, 1; HEPES, 10; pH adjusted to 7.4 with HCl. To this solution, Mg-ATP (3 mM), cAMP (0.3 mM) and tris-GTP (0.5 mM) were added just prior to the experiments. Capsaicin (Calbiochem), a TRPV1 antagonist (Neurogen), the phorbol ester, phorbol 12,13-dibutyrate (Research Biochemicals), and the PKC inhibitor bisindolylmaleimide I HCl (Calbiochem) were dissolved in DMSO (100 mM) and used at less than 0.01% of their stock concentration. At these dilutions, DMSO alone had no effect on TRPV1 responses to CAPS. Stock solutions in 10-100 mM were stored at -20°C and diluted in the external recording solution just before experiments. The TRPV1 antagonist (diaryl piperazine, NDT9515223, patent no. NO 02/08221) which was a gift from Neurogen (Connecticut) was shown previously to be a potent and selective TRPV1 inhibitor (Ki = 6 nM for H+ inhibition and 35 nM for CAPS response inhibition [16]). Extracellularly applied drugs were pipetted from stock solutions at 10-100 times the final concentration and rapidly mixed in the recording chamber as described previously [15]. Results are reported as mean ± S.E.M. Statistical analysis used t-test, two-tail, and unequal variance. Data were considered to be statistically different if P < 0.05.
Experiments were conducted in lumbosacral DRG neurons (L4-S3) from 11 normal (n = 81 cells) and 9 FIC cats (n = 86 cells). Only cells that were responsive to capsaicin (CAPS) were analyzed here. Of these, 13 normal cells (3 cats) and 11 FIC cells (5 cats) were bladder neurons retrogradely labeled with FB. Since our initial experiments suggested that TRPV1 responses were similarly changed in labeled and unlabelled FIC neurons from the same cats we subsequently conducted all our studies in unlabeled neurons. CAPS-sensitive neurons from FIC cats, were on the average 28% larger that those from normal cats as determined by membrane capacitance measurements (P < 0.05, 57±5 pF, n = 24, in normal cat neurons; 73 ± 3 pF in FIC cat neurons).
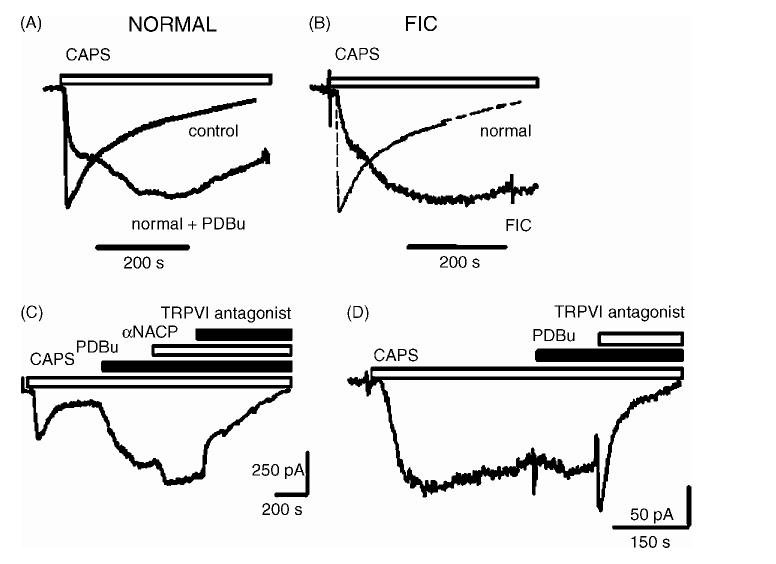
CAPS has been reported to have a half maximal effect at concentrations between 0.5 and 0.75 μM [17]. Application of CAPS at a submaximal concentration (0.5 μM) induced an inward current (50 pA to 3.7 nA in amplitude) in 25 of 41 normal DRG neurons (61%), and 24 of 43 FIC neurons (56%,Fig. 1). The average peak of the CAPS-currents normalized to membrane capacitances were 2.2 larger in FIC neurons (16 ± 2 pA/pF in 22 FIC neurons versus 7.3 0.8 pA/pF in 16 normal cat neurons, P < 0.05). In normal neurons± during prolonged application of CAPS (1-5 min), the currents declined with time due to desensitization (Fig. 1A and B, dotted trace). CAPS responses in FIC cells activated more slowly and desensitized 7.9-fold more slowly than those in normal neurons (Fig. 1B, slower trace, FIC).
Fig. 1.
CAPS responses in DRG neurons from normal and FIC cats. (A) Time course of CAPS response in a neuron from normal cat in response to CAPS alone (0.5 μM, control) and PDBu (0.5 μM) applied immediately after the onset of CAPS response. (B) Time course of CAPS response in a neuron from normal cat (normal, thin dotted lines, same trace as control in (A)) and a neuron from an FIC cat in response to CAPS alone (0.5 μM, FIC). (C) Modulation of CAPS (0.5 μM) response in a normal cat neuron by PDBu (0.5 μM) and α-naphthyl acid phosphate (αNACP, 5 μM) and after TRPV1 antagonist (5 μM). (D) Slow onset, slowly desensitizing response to CAPS (0.5 μM) in a neuron from a cat with FIC. PDBu (0.5 μM) applied after partial desensitization of CAPS response. TRPV1 antagonist inhibited the partially desensitized current.
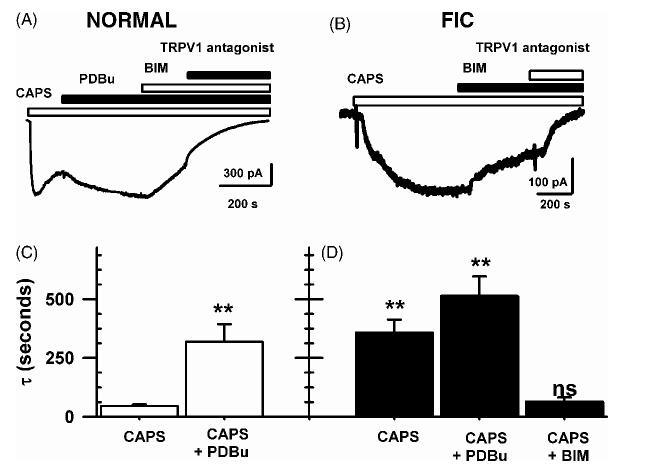
Because protein kinase C (PKC) has been implicated in the modulation of TRPV1 desensitization [9,13,22], we evaluated the role of PKC in the changes in TRPV1 currents in FIC neurons. We examined the interactions between CAPS and agents that activate or inhibit PKC (phorbol 12,13-dibutyrate, PDBu; bisindolylmaleimide, BIM) or inhibit phosphatases (±-naphthyl acid phosphate). In normal neurons (n = 6) and FIC neurons (n = 5) application of PDBu alone had no measurable effects (not shown). However, PDBu (0.5 μM), applied prior to (n = 6, not shown) or together with CAPS (n =8, Fig. 1A) slowed the onset and slowed the desensitization of the CAPS-induced currents (Fig. 1A and C) in normal neurons and increased the current amplitudes (9 ± 4 pA/pF in untreated neurons, versus 22 ± 11 pA/pF in PDBu treated neurons). The PDBu modulated CAPS-currents were similar in time course to CAPS-activated currents seen in FIC neurons in absence of PDBu (Fig. 1B and D). In normal neurons, PDBu also enhanced the partially desensitized CAPS responses (n = 16, Fig. 1C). These PDBu modulated, CAPS-activated currents in normal neurons were further increased in amplitude (50-200%) by α-naphthyl acid phosphate (5 μM, a non-selective phosphatase inhibitor, Fig. 1C). In FIC neurons, PDBu (0.5 μM) slowed the desensitization of CAPS responses in FIC neurons from 358 ± 22 s (n = 22) to 515 ± 83 s (n =8, P = 0.07) without changing current amplitudes (Fig. 1D). This suggests that, in FIC neurons, TRPV1 currents were nearly maximally phosphorylated by PKC. Consistent with this idea, BIM (0.5 μM) accelerated the desensitization of CAPS-activated currents in normal neurons after PDBu (5 μM, Fig. 2A, n = 5) and led to more rapidly desensitizing currents in FIC neurons after CAPS alone (Fig. 2B, n = 7).
Fig. 2.
CAPS-induced TRPV1 responses in normal cat and FIC neurons. (A) BIM (0.5 μM) reversed the PDBu (0.5 μM) enhancement of CAPS-current in a normal cat neuron. TRPV1 antagonist inhibited this current (5 μM). (B) BIM (0.5 μM) enhanced the desensitization rates of the response to CAPS alone in a FIC neuron. (C) Time constants of desensitization after CAPS and after CAPS and PDBu in normal cat neurons. (D) Time constants of desensitization after CAPS, CAPS and PDBu and CAPS and bisindolylmaleimide in FIC neurons. Data in (C) and (D) are the average current decay time constants fitted by single exponentials for each drug treatment and S.E.M.;**P < 0.001 relative to CAPS-current in normal neurons.
The desensitization measured as the time constant of single exponential decay of CAPS-activated currents in FIC neurons after BIM (65 ± 18 s, n =8, Fig. 2D), were comparable to those seen in normal neurons after CAPS alone (46 ± 7s, n = 16, P = 0.16, Fig. 2C). The time constant of single expo-± nential fit to the decay of the CAPS-activated currents (τ) for FB-labeled bladder neurons was 263±97 s (n = 6) in neurons from FIC cats and 34 ± 11 s (n =6, ±P < 0.001) in bladder neurons from normal cats. The time constants for the pooled data in bladder and unlabeled neurons, some of which presumably innervate other visceral and somatic structures, were similarly increased: 358 ± 55 s in FIC (n = 22,Fig. 2D), and 46 ± 7 s in normal neurons (n = 16, Fig. 2C, P < 0.001), suggesting that the change in the CAPS response affected both bladder neurons and non-bladder neurons in cats with FIC.
This study, demonstrated decreased desensitization of vanilloid receptor TRPV1 responses in bladder and unidentified L4-S3 DRG neurons from cats with FIC. The results also indicate that the changes in TRPV1 currents are related to an alteration in PKC-induced phosphorylation of the TRPV1 channel. These findings raise the possibility that the bladder dysfunctions in FIC cats are mediated in part by increased sensitivity of nociceptors in the bladder wall. Our data indicate that the slow desensitization of TRPV1 in FIC neurons is a consequence of increased PKC mediated phosphorylation of the channels. This could be due to an increase in the basally active PKC [22]. This conclusion is based on three observations; first, activation of PKC with PDBu which slows the desensitization of TRPV1 responses to CAPS in normal neurons had no significant effect in FIC neurons (Fig. 1). Secondly, inhibition of PKC with bisindolylmaleimide returned the desensitization rates in FIC neurons to rates comparable to the response to CAPS alone in normal neurons (Fig. 2). Thirdly, the densities of CAPS-activated currents in FIC neurons were twice as large as in normal neurons, consistent with the observation that phosphorylation of TRPV1 by PKC increases CAPS responses in normal neurons (Fig. 1C). Although the mechanism responsible for enhanced basal activity of PKC in FIC is uncertain, the PKC phosphorylation of TRPV1 is likely to have a number of physiological effects, including sensitization of responses to CAPS [1,17,23], heat [17], and protons [17]. Enhanced TRPV1 activity could lead to increased firing of afferent nerves and also increased release of neuropeptides in the bladder wall. It is tempting to speculate that enhanced release of substance P from afferent terminals may account for the plasma extravasation seen in FIC bladders [5]. Substance P is also known to stimulate receptors on afferent neurons to activate PKC which in turn enhances Ca2+ channel currents [15] and may, therefore, also enhance TRPV1 [17]. Therefore, the increased TRPV1 activity in FIC neurons may act to trigger a neurokinin mediated autofeedback mechanism to exacerbate and maintain the electrophysiological abnormalities in FIC neurons.
The sensitization of TRPV1 responses by PKC phosphorylation has been reported to enhance the firing in DRG neurons induced by CAPS [23] and enhance the membrane translocation of TRPV1 receptors in nerve terminals [11]. It would be tempting to speculate that the activation of TRPV1 by normal body temperature would lead to spontaneous firing in CAPS-sensitive DRG afferent nerves. The latter, may contribute to enhanced release of other neurotransmitters and inflammatory mediators from nociceptive neurons. Neuromediators (SP, bradykinin, prostaglandins) may act synergistically at afferent terminals to maintain high levels of nociceptive sensitization in FIC neurons. In addition, our observation that non-bladder FIC neurons exhibit these changes, suggests that the abnormalities in afferent neuron excitability may occur in DRG neurons innervating structures outside the pelvic cavity. This may explain the observation that symptoms of interstitial cystitis extend beyond the bladder, affecting other organs in the body [2,8].
Acknowledgements
This work was supported by grants from NIH to W.C.D. (P01HD39768-03), L.A.B. (DK57284) and CATB (DK47538 and DK64539).
References
- [1].Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., IV Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc. Natl. Acad. Sci. U.S.A. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Buffington CAT. Comorbidity of interstitial cystitis with other un-explained clinical conditions. J. Urol. 2004;172:1242–1248. doi: 10.1097/01.ju.0000137953.49304.6c. [DOI] [PubMed] [Google Scholar]
- [3].Buffington CAT, Chew DJ, DiBartola SP. Lower urinary tract diseases in cats. In: Slatter D, editor. Textbook of Small Animal Surgery. W.B. Saunders; Philadelphia: 2001. third ed. [Google Scholar]
- [4].Buffington CA, Chew DJ, Woodworth BE. Feline interstitial cystitis. J. Am. Vet. Med. Assoc. 1999;215:682–687. [PubMed] [Google Scholar]
- [5].Buffington CA, Wolfe SA., Jr. High affinity binding sites for [3H]substance P in urinary bladders of cats with interstitial cystitis. J. Urol. 1998;160:605–611. [PubMed] [Google Scholar]
- [6].Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- [7].Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J. Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- [8].Erickson DR, Morgan KC, Ordille S, Keay SK, Xie SX. Nonbladder related symptoms in patients with interstitial cystitis. J. Urol. 2001;166:557–561. [PubMed] [Google Scholar]
- [9].Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J. Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cells. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- [11].Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J. Biol. Chem. 2004;279:25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- [12].Pang X, Marchand J, Sant GR, Kream RM, Theoharides TC. Increased number of substance P positive nerve-fibers in interstitial cystitis. Br. J. Urol. 1995;75:744–750. doi: 10.1111/j.1464-410x.1995.tb07384.x. [DOI] [PubMed] [Google Scholar]
- [13].Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–989. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- [14].Sant GR. Interstitial Cystitis. Lippincott-Raven; Philadelphia: 1997. p. 284. [Google Scholar]
- [15].Sculptoreanu A, de Groat WC. Protein kinase C is involved in neurokinin receptor modulation of Ca2+ channels in dorsal root ganglion neurons of the adult rat. J. Neurophysiol. 2003;90:21–31. doi: 10.1152/jn.00108.2003. [DOI] [PubMed] [Google Scholar]
- [16].Valenzano KJ, Grant ER, Wu G, Hachicha M, Schmid L, Tafesse L, Sun Q, Rotshteyn Y, Francis J, Limberis J, Malik S, Whittemore ER, Hodges D. N-(4-Tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties. I. In vitro characterization and pharmacokinetic properties. J. Pharmacol. Exp. Ther. 2003;306:377–386. doi: 10.1124/jpet.102.045674. [DOI] [PubMed] [Google Scholar]
- [17].Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor TRPV1 by capsaicin, protons, heat and anandamide. J. Physiol. (Lond.) 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Watanabe T, Yokoyama T, Sasaki K, Nozaki K, Ozawa H, Kumon H. Intravesical resiniferatoxin for patients with neurogenic detrusor overactivity. Int. J. Urol. 2004;11:200–205. doi: 10.1111/j.1442-2042.2003.00782.x. [DOI] [PubMed] [Google Scholar]
- [19].Westropp JL, Buffington CAT. In vivo models of interstitial cystitis. J. Urol. 2002;167:694–702. doi: 10.1016/S0022-5347(01)69129-8. [DOI] [PubMed] [Google Scholar]
- [20].Yoshimura N, Smith CP, Chancellor MB, de Groat WC. Pharmacologic and potential biologic interventions to restore bladder function after spinal cord injury. Curr. Opin. Neurol. 2000;13:677–681. doi: 10.1097/00019052-200012000-00011. [DOI] [PubMed] [Google Scholar]
- [21].Yoshimura N, White G, Weight FF, de Groat WC. Patch clamp recordings from subpopulations of autonomic and afferent neurons identified by axonal tracing techniques. J. Auton. Nerv. Syst. 1994;49:85–92. doi: 10.1016/0165-1838(94)90024-8. [DOI] [PubMed] [Google Scholar]
- [22].Zhou Y, Li GD, Zhao ZQ. State-dependent phosphorylation of epsilon-isozyme of protein kinase C in adult rat dorsal root ganglia after inflammation and nerve injury. J. Neurochem. 2003;85:571–580. doi: 10.1046/j.1471-4159.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- [23].Zhou Y, Zhou ZS, Zhao ZQ. PKC regulates capsaicin-induced currents of dorsal root ganglion neurons in rats. Neuropharmacology. 2001;41:601–608. doi: 10.1016/s0028-3908(01)00106-x. [DOI] [PubMed] [Google Scholar]