Abstract
We aimed to compare the anti-inflammatory activities of six species of Curcuma drugs using adjuvant arthritis model mice. When orally administered 1 day before the injection of adjuvant, the methanol extract of Curcuma phaeocaulis significantly inhibited paw swelling and the serum haptoglobin concentration in adjuvant arthritis mice. Also when orally administered 1 day after the injection of adjuvant, the methanol extract of Curcuma phaeocaulis significantly inhibited paw swelling. Other Curcuma species (Curcuma longa, Curcuma wenyujin, Curcuma kwangsiensis, Curcuma zedoaria and Curcuma aromatica) had no significant inhibitory effects on adjuvant-induced paw swelling. Cyclooxygenase (COX)-2 activity was significantly inhibited by the methanol extract of C. phaeocaulis. Curcuminoids' (curcumin, bis-demethoxycurcumin and demethoxycurcumin) were rich in C. longa, but less in C. phaeocaulis and C. aromatica, not in C. wenyujin, C. kwangsiensis and C. zedoaria, suggesting that curcuminoids' contents do not relate to inhibition of arthritis swelling. Therefore, C. phaeocaulis may be a useful drug among Curcuma species for acute inflammation, and the active constituents of C. phaeocaulis are not curcuminoids.
Keywords: adjuvant, arthritis, COX-2, Curcuma, haptoglobin
Introduction
Many reports have suggested useful pharmacological properties of Curcuma drugs such as anti-inflammatory (1), anti-tumor (2) and immunological effects (3). Traditionally, Curcuma drugs called ‘Ukon’ and ‘Gajutsu’ in Japanese have been used in Oketsu syndromes (caused by the obstruction of blood circulation) in Chinese medicine (4). Since the pharmacological effects of curcuminoids, especially curcumin, have been investigated, such as radical scavenging (5), the inhibition of nitric oxide (NO) (6,7), anti-inflammation (8), anti-tumor (9), anti-allergy (10) and anti-dementia (11), the usefulness of ‘Ukon’ derived from Curcuma longa has been intensively studied. Pharmacological studies of other Curcuma species were very few, because botanical origins of Curcuma drugs could not be easily identified due to similarity of morphology, and variety of naming derived from used parts and producing areas. At present, four Curcuma drugs are prescribed in Chinese Pharmacopoeia; Yujin (the tubers of Curcuma wenyujin, C. longa, Curcuma kwangsiensis or Curcuma phaeocaulis); Jianghuang (the rhizome of C. longa); Pian-Jianghuang (the rhizome of C. wenyujin) and Ezhu (the rhizomes of C. phaeocaulis, C. kwangsiensis or C. wenyujin). In addition, the rhizomes of Curcuma zedoaria and Curcuma aromatica have been used in Japan as a medicine or supplement. We previously compared five types of Curcuma drugs (rhizomes of C. longa, C. kwangsiensis, C. phaeocaulis, C. wenyujin and C. zedoaria), which were correctly identified by molecular biological analysis (12), on vasomotion in isolated rat aortas, and we found that the methanol extracts of all species had NO-independent relaxation effects (13). However, potencies of these vasomotion effects were not significantly different between species. In this study, we focused on evaluating differences among the anti-inflammatory activities of six Curcuma drugs (C. longa, C. wenyujin, C. phaeocaulis, C. kwangsiensis, C. zedaria and C. aromatica).
Materials and Methods
The six Curcuma drugs were used as shown in Table 1 and Fig. 1, which were correctly identified by the molecular biological method previously reported (12). All drugs were stored in the Museum of Materia Medica, Institute of Natural Medicine, University of Toyama (TMPW), Japan.
Table 1.
Curcuma drugs used in this study and the yields of extracts
| Herbal drug name | Abbreviation | Part used | Cultivated area | Yield (%) | TMPW No.a | ||
|---|---|---|---|---|---|---|---|
| Japanese | Chinese | Scientific name | |||||
| Ukon | Jianghuang | Curcuma longa L. | CL | Rhizome | Guangdong, China | 14.0 | 22 295b |
| 14.8 | 19 910c | ||||||
| Henkyouou | Pian-Jianghuang | C. wenyujin Y.H. Chen and C. Ling | CW | Rhizome | Zhejiang, China | 12.1 | 22 292b |
| 12.3 | 19 911c | ||||||
| Gajutsu | Ezhu | C. phaeocaulis Val. | CP | Rhizome | Sichuan, China | 6.5 | 22 297b |
| 9.2 | 20 237c | ||||||
| Gajutsu | Ezhu | C. kwangsiensis S.G. Lee and C.F. Liang | CK | Rhizome | Guangxi, China | 2.0 | 22 471b |
| 1.9 | 19 912c | ||||||
| Gajutsu | — | C. zedoaria Rosc | CZ | Rhizome | Kagoshima, Okinawa, Japan | 8.9 | 22 473b |
| 7.0 | 20 285c | ||||||
| Haruukon | — | C. aromatica | CA | Rhizoma | Okinawa, Japan | 7.5 | 22 312b |
| 8.9 | 20 284c | ||||||
Figure 1.
Morphologies of used Curcuma drugs.
Preparation of Extracts
Methanol extracts were prepared as follows: 400 g of powdered drug was placed in methanol (1 l × 2) for 12 h at room temperature. The combined supernatants were evaporated on a water bath to obtain the methanol extracts. The extracts were dissolved in dimethyl sulfoxide (DMSO) (stock solution) and then suspended in olive oil in animal experiments. The extract was administered orally [500 mg kg−1, 400 µl per mouse (olive oil : stock solution in DMSO =320 µl : 80 µl)].
Induction of Arthritis
The mice were handled in accordance with the Guide for Animal Experiments, University of Toyama. Arthritis was induced in male ddY mice (6 week old; SLC, Shizuoka, Japan) by injecting 50 µg per 50 µl−1 of Complete Freund's adjuvant (CFA) (Sigma, St Louis, USA) into the right-hind footpad. The vehicle injection was 50 µl paraffin oil. In Fig. 2, immediately before and 1 day after injection of CFA, right footpad swelling (length × wide) was measured with slide calipers. In Fig. 3, immediately before, 1 day after and 2 day after the injection of CFA, right footpad swelling (length × wide) was measured with slide calipers. Extracts were administered once 1 day after the adjuvant injection. The change rate of paw swelling was calculated. The paw sizes did not change before and after extract administration.
Figure 2.
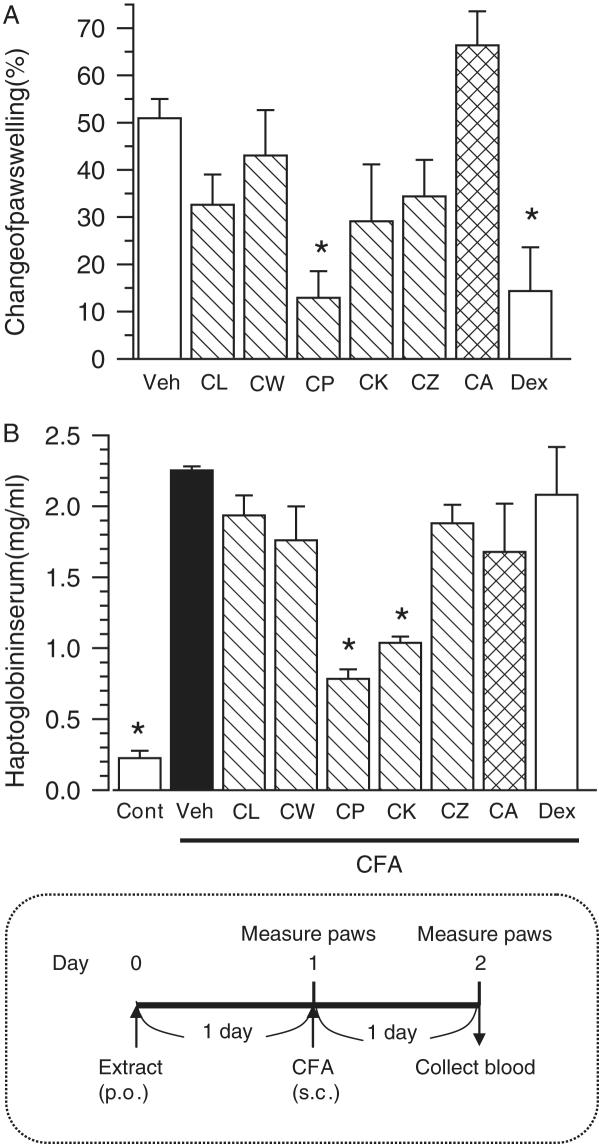
Effects of pretreatment with methanol extracts of Curcuma drugs on paw swelling and increase in serum haptoglobin induced by adjuvant injection. Extracts of Curcuma drugs (500 mg kg−1, hatched columns), dexamethasone (5 mg kg−1) or were administered once 1 day before the adjuvant injection. Arthritis was induced by the injection of Complete Freund's adjuvant (CFA) into the right-hind footpad. Paraffin oil was injected in control mice. (A) Immediately before, and 1 day after injection of the adjuvant, swelling of the right footpad was measured with slide calipers. Change rate of the swelling of Day 2 compared with Day 1 was calculated. (B) After footpad measurement, serum was collected. The concentration of haptoglobin in serum was measured. The time schedule of treatments is shown in the bottom. The values represent the means and SEM of 5 mice. *P < 0.05 when compared with Veh.
Figure 3.
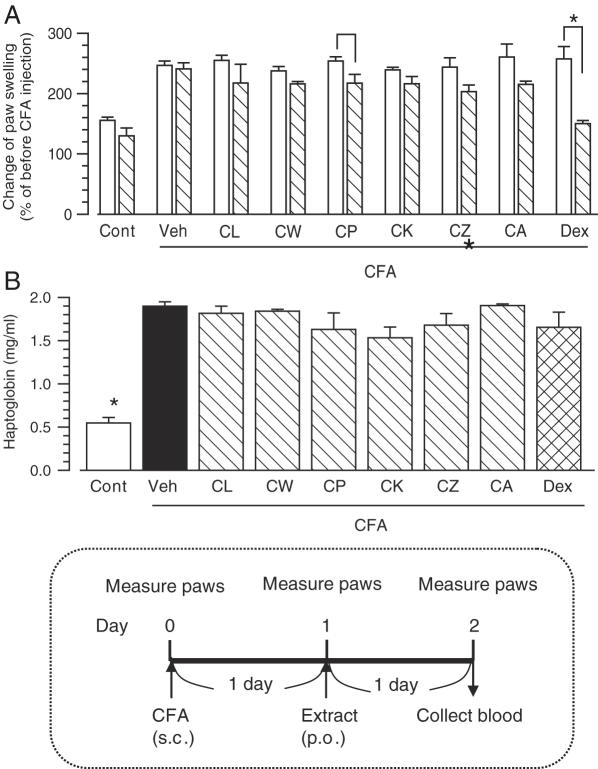
Post-treatment with methanol extracts of Curcuma drugs and its effect on paw swelling and increase in serum haptoglobin induced by adjuvant injection. Extracts of Curcuma drugs (500 mg kg−1), dexamethasone (5 mg kg−1) or 20% DMSO in olive oil (Veh) were administered once 1 day after the adjuvant injection. Arthritis was induced by the injection of Complete Freund's adjuvant (CFA) into the right-hind footpad. Paraffin oil was injected in control mice. (A) Immediately before, 1 day after and 2 days after injection of the adjuvant, swelling of the right footpad was measured with slide calipers. Change rates of the swelling of Day 1 (open columns) and Day 2 (hatched columns) compared with Day 0 were calculated. (B) After footpad measurement, serum was collected at Day 2. The concentration of haptoglobin in serum was measured. The time schedule of treatments is shown in the bottom. The values represent the means and SEM of five mice. *P < 0.05 when compared between before and after extract treatment in (A); *P < 0.05 when compared with Veh in (B).
Measurement of Haptoglobin
After measuring the footpads, blood (1 ml) was collected from the vena cava and added to a serum collection tube. After centrifugation (3500 r.p.m., 20 min), the supernatant was transferred into a new tube as a serum sample (400 µl). The concentration of haptoglobin in serum was measured using PHASE RANGE (Tridelta Development Limited, Maynooth, Ireland).
Measurement of COX-1 and COX-2
Cyclooxygenase (COX) activity was measured using a Colorimetric COX inhibitor assay kit (Cayman, Ann Arbor, USA). The methanol extracts or indomethacin were dissolved in DMSO and added to the enzyme reaction mixture.
Quantifying Curcuminoid Content of Curcuma Drugs
Standard curcumin, bis-demethoxycurcumin and demethoxycurcumin were isolated and identified as pure compounds by spectra data in our laboratory (13). Each standard curcuminoid (1 mg) was accurately weighed and dissolved in methanol at a concentration of 0.2 mg ml−1. To draw calibration curves, a series of standard solutions were prepared from the stock solution and filtered through a 0.2 µm Millipore filter (Advantec, Tokyo, Japan). Dried Curcuma drugs were pulverized and powdered. Methanol extracts of 20 mg were accurately weighed and dissolved in methanol at a concentration of 0.4 mg ml−1. After ultrasonication for 30 min, supernatants were obtained by centrifugation at 2500 r.p.m. for 10 min. The supernatants were transferred into volumetric flasks, and methanol was added to obtain a final volume of 100 ml. After filtration through a 0.2 µm Milllipore filter (Advantec), 5 µl was injected into the HPLC system for analysis. The JASCO HPLC system (Jasco, Tokyo, Japan) is composed of a PU-1580 intelligent HPLC pump, a DG-1580-53 3-line degasser, a LG-1580-02 ternary gradient unit, a CO-1565 intelligent column oven, an AS-2057 plus intelligent sampler and an MD-1510 diode array detector. Comparative analysis was carried out using a Mightysil RP-18GP (15 mm, 250 mm × 4.6 mm i.d.) with a column temperature of 40°C. The mobile phase was acetonitrile:water:acetic acid = 45 : 55 : 1. The flow rate was 1.0 ml min−1 and the detection wavelength was 410 nm. The chromatographic data were collected and processed using BORWIN-PDA APPLICATION and BORWIN CHROMATOGRAPHY Software (version 1.5, Jasco). The values of retention time of curcumin, bis-demethoxycurcumin and demethoxycurcumin were 15.44, 11.75 and 13.48 min, respectively. The standard curves of curcuminoids were made using a dose of 2–100 µg ml−1.
Statistical Analysis
Statistical comparisons were carried out using one-way analysis of variance followed by Dunnett's post hoc test or paired t-test. Values of P < 0.05 were considered significant. The means of the data are presented together with the SEM.
Results
We previously investigated the time-course of the paw edema and confirmed that swelling peaked at 24 h post-injection under our experimental conditions. In Fig. 2, methanol extracts (500 mg kg−1) were orally administered 1 day before the adjuvant injection. Treatment with the C. phaeocaulis (CP) extract significantly reduced the paw edema (Fig. 2A). Although treatments with extracts of C. longa (CL), C. kwangsiensis (CK) and C. zedoaria (CZ) tended to reduce slightly the paw swelling, treatment with C. wenyujin (CW) and C. aromatica (CA) did not. A steroidal anti-inflammatory drug, dexamethasone, was used as a reference. At a dose of 5 mg kg−1, dexamethasone significantly inhibited paw swelling. Although indomethacin (5 and 10 mg kg−1), a non-steroidal anti-inflammatory drug, was also used in this experiment, several mice died after severe body weight loss. Extract-treated groups showed no adverse effects. After measuring the paw swelling, serum haptoglobin was detected as an acute inflammation marker (14). The concentration of serum haptoglobin intensively increased (10-fold the control) by adjuvant injection (Fig. 2B). Serum haptoglobin was significantly reduced in a CP extract-treated group (inhibition rate: 72.5%) and CK extract-treated one (inhibition rate: 59.9%); however, no significant reduction was seen in other Curcuma drug extract-treated groups. Dexamethasone (5 mg kg−1) did not reduce serum haptoglobin.
Paw Edema Significantly Reduced by C. phaeocaulis
In Fig. 3, methanol extracts (500 mg kg−1) were orally administered 1 day after the adjuvant injection. Treatment with the C. phaeocaulis (CP) extract significantly reduced paw edema (Fig. 3A). Although treatments with extracts of CL, CW, CK, CZ and CA tended to reduce the paw swelling, these were not significant. Dexamethasone (5 mg kg−1) significantly inhibited paw swelling. Serum haptoglobin was slightly reduced in CP and CK extracts-treated groups, but not significantly. Dexamethasone (5 mg kg−1) also did not reduce serum haptoglobin significantly either.
Curcuma Extracts Showed Inhibitory Activity on Enzymatic Activities In Vitro
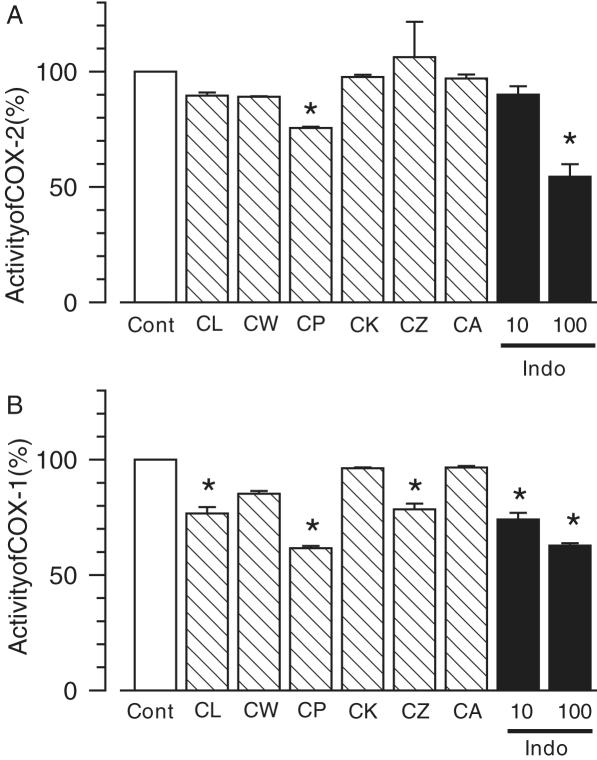
The inhibitory effects of Curcuma extracts on in vitro enzymatic activities were measured against COX-2 and COX-1 (Fig. 4). The methanol extract of CP (500 µg/ml) significantly inhibited COX-2 activity (inhibition rate: 24.4%). However, CL, CW, CK, CZ and CA extracts demonstrated no significant inhibition. For COX-1, CP, CL and CZ extracts showed inhibitory activity, and the inhibition rate with the CP extract was the most remarkable (inhibition rate: 38.4%). Indomethacin, a COX inhibitor, inhibited COX-2 (inhibition rate: 45.5% inhibition at 100 µM) and COX-1 (inhibition rate: 37.2% at 100 µM) activities dose dependently. The inhibitory efficacy of COX-2 with the methanol extract of CP was weaker than that of 100 µM indomethacin.
Figure 4.
Inhibitory activities of methanol extracts of Curcuma drugs on COX-2 and COX-1 activities. The methanol extracts of Curcuma drugs (500 µg ml−1, hatched columns), indomethacin (10 and 100 µM, closed columns), or the vehicle (0.1% DMSO, open columns) were added to the enzyme reaction mixture. COX-2 (A) and COX-1 (B) activities were measured using the Colorimetric COX inhibitor assay kit. The values represented the means and SEM. n = 3. *P < 0.05 when compared with Veh.
Testing of Curcuma Samples from China and Japan Confirmed That Curcumin was Rich in CL
The content of three curcuminoids in the methanol extracts of Curcuma drugs was quantified as shown in (Table 2). In CP and CA, a very small amount of curcumin was contained in the methanol extract, in addition to bis-demethoxycurcumin and demethoxycurcumin were hardly detected. None of the three curcumnoids was present in CW, CK and CZ, whereas large amounts of curcuminoids were detected in CL. In particular, curcumin concentrations were high in the CL methanol extract. We tested many Curcuma samples from China and Japan, and confirmed that curcumin was rich in CL (3.9–12.3% in the methanol extract), but not in CP, CW, CK, CZ and CA.
Table 2.
Curcuminoid contents in the methanol extracts
| Contents (% in the methanol extract) | |||
|---|---|---|---|
| Curcumin | Bisdemethoxy-curcumin | Demethoxy-curcumin | |
| C. longa | 12.30 | 4.86 | 3.62 |
| C. wenyujin | ND | ND | ND |
| C. phaeocaulis | 0.89 | ND | t |
| C. kwangsiensis | ND | ND | ND |
| C. zedoaria | ND | ND | ND |
| C. aromatica | 0.11 | ND | ND |
ND, Not detected; t, trace.
Discussion
The methanol extract of CP significantly reduced paw swelling (Fig. 2A) and the expression of an inflammation marker, haptoglobin in serum (Fig. 2B), of mice when it was administered orally 1 day before the adjuvant injection. Also in case of treatment with the methanol extracts 1 day after the adjuvant injection when the paw was maximally swollen, CP significantly reduced paw swelling (Fig. 3A). However, treatment with the methanol extracts of CL, CW, CZ and CA had no clear effects on inflammation. Therefore, CP is to be expected the most effective in reducing arthritis swelling among Curcuma drugs.
The methanol extract of CP had inhibitory activity on inflammation-related enzymes, COX-2 (Fig. 4A). A selective COX-2 inhibitor, SC-58125, rapidly reverses paw edema, the level of PGE2, the expression of COX-2, and serum IL-6 and paw IL-6 levels in arthritis rats induced by CFA (15). Upregulated COX-2 also enhances PG production, and inflammation progresses further (15). Therefore, the inhibitory effect of CP on COX-2 activity may be useful to prevent inflammation. Both CP and indomethacin were not selective inhibitors of COX-2 (Fig. 4). Some indomethacin-treated mice died (data not shown). It is known that indomethacin treatment has a high risk of several adverse effects (gastrointestinal, hepatic and kidney disorders), which may be caused mainly by the inhibitory effect of COX-1. Considering that CP had anti-inflammatory effects on arthritic mice (Figs 1 and 2) with no adverse effects in spite of its COX-1 inhibitory action; some constituents which relieve the adverse effects may be contained in the methanol extract of CP. Dexamethasone did not inhibit the serum haptoglobin concentration (Figs 1 and 2). Each class of anti-inflammatory drugs has a specific effect on the regulation of acute phase proteins (16), and dexamethasone is known not to reduce the haptoglobin expression (17).
In an arthritis animal model, IL-1β (18) and TNF-α (15) are increased by inflammatory stimulation, and IL-1β induces COX-2 transcription in inflammatory cells (19). At the same time, increased IL-1β and TNF-α in inflammation upregulate the IL-6 expression (20), and IL-6 stimulation in the liver induces the translocation of activated STAT3 molecules to nuclei (21,22). Transcription of the haptoglobin gene is enhanced through multiple IL-6 response elements, and haptoglobin increases in the liver and serum (23). Since the methanol extract of CP reduced serum haptoglobin in arthritis, it may inhibit anywhere in the cytokine pathway. We will investigate whether the expression levels of these cytokines in serum, and the COX-2 level in paw tissue, are changed by treatment with CP extract.
The pharmacological activities of curcumin have been studied intensively from many viewpoints, suggesting that curcumin inhibits LPS-induced NO production (5) and iNOS expression (6), COX-2 expression and activation (24), and expression of several inflammatory markers. In addition, curcumin is cytotoxic in several cell types (25,26), not only in cancer cells (27,28). Hepatotoxicity was reported in animal experiments using curcumin-containing Curcuma drugs (29,30), and although curcumin has many useful pharmacological and possible therapeutic activities, its safety should be considered carefully. However, CP contains very low amounts of curcuminoids (Table 2), but showed anti-inflammatory activity (Figs 1 and 2), suggesting that some active constituents other than curcuminoids may exist in CP. Since a recent study showed that significant amounts of furanodienone and curcumenol were contained in CP (31), these compounds may be candidates for active principles. As very little basic pharmacological research or chemical analyses of CP have been performed, the usefulness of CP should be investigated in future studies.
Acknowledgments
We particularly thank Mrs K. Hayashi for her technical support. This work was partially supported by Tamura Foundation, and Grant-in-Aid for Scientific Research (B), No. 14406030 in 2002–04 and No. 17406004 in 2005–07, from Japan Society for the Promotion of Science, and by the 21st Century COE Program from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.Ahmed S, Anuntiyo J, Charles J, Malemud CJ, Haqqi TM. Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis. Evid Based Complement Altern Med. 2005;2:301–8. doi: 10.1093/ecam/neh117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozaki Y. Antiinflammatory effect of Curcuma xanthorrhiza Roxb., and its active principles. Chem Pharm Bull. 1990;38:1045–8. doi: 10.1248/cpb.38.1045. [DOI] [PubMed] [Google Scholar]
- 3.Gonda R, Tomoda M, Ohara N, Takada K. Arabinogalactan core structure and immunological activities of ukonan C, an acidic polysaccharide from the rhizome of Curcuma longa. Biol Pharm Bull. 1993;16:235–8. doi: 10.1248/bpb.16.235. [DOI] [PubMed] [Google Scholar]
- 4.Li SZ. ‘Ben-cao gang-mu’. In: Jiangxi , editor. House, Beijing: The People's Health Pub.; 1977. pp. 880–5. [Google Scholar]
- 5.Sreejayan N, Rao MN. Free radical scavenging activity of curcuminoids. Arzneimittelforschung. 1996;46:169–71. [PubMed] [Google Scholar]
- 6.Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IκB kinase and NFκB activation in macrophages. Biochem Pharmacol. 2000;60:1665–76. doi: 10.1016/s0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- 7.Onoda M, Inano H. Effect of curcumin on the production of nitric oxide by cultured rat mammary gland. Nitric Oxide. 2000;4:505–15. doi: 10.1006/niox.2000.0305. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee M, Tripathi LM, Srivastava VM, Puri A, Shukla R. Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat. Immunopharmacol Immunotoxicol. 2003;25:213–24. doi: 10.1081/iph-120020471. [DOI] [PubMed] [Google Scholar]
- 9.Khar A, Ali AM, Pardhasaradhi BV, Begum Z, Anjum R. Antitumor activity of curcumin is mediated through the induction of apoptosis in AK-5 tumor cells. FEBS lett. 1999;19:165–8. doi: 10.1016/s0014-5793(99)00114-3. [DOI] [PubMed] [Google Scholar]
- 10.Ram A, Das M, Ghosh B. Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biol Pharm Bull. 2003;26:1021–4. doi: 10.1248/bpb.26.1021. [DOI] [PubMed] [Google Scholar]
- 11.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–7. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki Y, Fushimi H, Cao H, Cai SQ, Komatsu K. Sequence analysis of Chinese and Japanese Curcuma drugs on the 18S rRNA gene and trnK gene and the application of amplification-refractory mutation system analysis for their authentication. Biol Pharm Bull. 2002;25:1593–9. doi: 10.1248/bpb.25.1593. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki Y, Goto H, Tohda C, Hatanaka F, Shibahara N, Shimada Y, et al. Effects of Curcuma drugs on vasomotion in isolated rat aorta. Biol Pharm Bull. 2003;26:1135–43. doi: 10.1248/bpb.26.1135. [DOI] [PubMed] [Google Scholar]
- 14.Giffen PS, Turton J, Andrews CM, Barrett P, Clarke CJ, Fung KW, et al. Markers of experimental acute inflammation in the Wistar Han rat with particular reference to haptoglobin and C-reactive protein. Arch Toxicol. 2003;77:392–402. doi: 10.1007/s00204-003-0458-7. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996;97:2672–9. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.do Nascimento CO, Hunter L, Trayhurn P. Regulation of haptoglobin gene expression in 3T3-L1 adipocytes by cytokines, catecholamines, and PPARγ. Biochem Biophys Res Commun. 2004;313:702–8. doi: 10.1016/j.bbrc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Lewis EJ, Bishop J, Cashin CH. Automated quantification of rat plasma acute phase reactants in experimental inflammation. J Pharmacol Methods. 1989;21:183–94. doi: 10.1016/0160-5402(89)90053-3. [DOI] [PubMed] [Google Scholar]
- 18.Silva JC, Rocha MF, Lima AA, Brito GA, de Menezes DB, Rao VS. Effects of pentoxifylline and nabumetone on the serum levels of IL-1β and TNFα in rats with adjuvant arthritis. Inflamm Res. 2000;49:14–19. doi: 10.1007/PL00000198. [DOI] [PubMed] [Google Scholar]
- 19.Crofford LJ, Tan B, McCarthy CJ, Hla T. Involvement of nuclear factor kappa B in the regulation of cyclooxygenase-2 expression by interleukin-1 in rheumatoid synoviocytes. Arthritis Rheum. 1997;40:226–36. doi: 10.1002/art.1780400207. [DOI] [PubMed] [Google Scholar]
- 20.Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83:585–92. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4:S233–42. doi: 10.1186/ar565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;347:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Kinzie E, Berger FG, Lim SK, Baumann H. Haptoglobin, an inflammation-inducible plasma protein. Redox Rep. 2001;6:379–85. doi: 10.1179/135100001101536580. [DOI] [PubMed] [Google Scholar]
- 24.Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, et al. Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25:1671–9. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- 25.Donatus IA, Sardjoko, Vermeulen NP. Cytotoxic and cytoprotective activities of curcumin. Effects on paracetamol-induced cytotoxicity, lipid peroxidation and glutathione depletion in rat hepatocytes. Biochem Pharmacol. 1990;39:1869–75. doi: 10.1016/0006-2952(90)90603-i. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda H, Ninomiya K, Morikawa T, Yoshikawa M. Inhibitory effect and action mechanism of sesquiterpenes from Zedoariae Rhizoma on D-galactosamine/lipopolysaccharide-induced liver injury. Bioorg Med Chem Lett. 1998;8:339–44. doi: 10.1016/s0960-894x(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 27.Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199–208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 28.Dorai T, Cao YC, Dorai B, Buttyan R, Katz AE. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293–303. doi: 10.1002/pros.1074. [DOI] [PubMed] [Google Scholar]
- 29.Deshpande SS, Lalitha VS, Ingle AD, Raste AS, Gadre SG, Maru GB. Subchronic oral toxicity of turmeric and ethanolic turmeric extract in female mice and rats. Toxicol Lett. 1998;95:183–93. doi: 10.1016/s0378-4274(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 30.Kandarkar SV, Sawant SS, Ingle AD, Deshpande SS, Maru GB. Subchronic oral hepatotoxicity of turmeric in mice-histopathological and ultrastructural studies. Indian J Exp Biol. 1998;36:675–9. [PubMed] [Google Scholar]
- 31.Yang EQ, Li SP, Chen Y, Lao SC, Wang YT, Dong TTX, Tsim KWK. Identification and quantitation of eleven sesquiterpenes in three species of Curcuma rhizomes by pressurized liquid extraction and gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2005;39:552–8. doi: 10.1016/j.jpba.2005.05.001. [DOI] [PubMed] [Google Scholar]