Abstract
Regulatory T (Treg) cells maintain dominant control of immune responses to foreign materials and microbes. Appropriate Treg cell suppression of immune responses is essential for the maintenance of efficacious defensive responses and the limitation of collateral tissue damage due to excess inflammation. Allergy and infection are well studied and frequent afflictions in which Treg cells play an essential role. As such, they provide excellent models to communicate the significance and relevance of Treg cells to complementary and alternative medicine (CAM).
Treg Ubiquity and Universality: Relations to Foreign Bodies
The major principles underlying the dynamic immune system, including constituents, interrelationships and feedback mechanisms have been established, with emphasis on regulatory T (Treg) cells (1). The goal of this article is (i) to detail Treg cells, (ii) to delineate Treg cell function in allergy and infection and (iii) to examine the pathological consequences of aberrant Treg cell activity. Allergy and infection are well-studied and frequent afflictions, and as such, they provide excellent models to communicate the significance and relevance of Treg cells to complementary and alternative medicine (CAM).
Considerable inflammation, resulting from allergic hypersensitivity or immune response to infection, has the potential to induce deleterious effects on an individual's tissues and overall well-being. Recent evidence has served to elucidate the mechanism of action and substantiate the usage of a veritable array of traditional herbs, folk medicines and other compounds found in nature, which have been employed to attenuate inflammatory complications. Of interest to practitioners, researchers and patients of CAM modalities are those compounds that maintain powerful immunomodulatory capacity via direct or indirect action on Treg cells (Table 1).
Table 1.
CAM compounds with regulatory capability and utility in Allergy and Infection
| Natural product | Activity | Reference |
|---|---|---|
| Brazilian green propolis | Modulate initial inflammation | (2,3) |
| Lower parasitemia | ||
| Ganoderma lucidum (mycelia extract) | Activate κB DNA binding | (4,5) |
| Increase TNF-α, IL-6, IFN-γ | ||
| Inhibit histamine release | ||
| Phellinus linteus (mycelia extract) | Lower TNF-α, IFN-γ production | (6) |
| Lower IgG levels | ||
| Piper methysticum (kava) | Inhibit TNF-α release | (7) |
| Induce NF-κB transcription | ||
| Allium sativum (garlic) | Increase TH1 cytokine response | (8) |
| Enhance macrophage and NK cell activity | ||
| Increase T cell proliferation | ||
| (−)-epigallocatechin gallate (green tea) | Decrease IL-2, IFN-γ, TNF-α levels | (9) |
| Decrease T cell proliferation | ||
| Probiotics | Induce Tr1 and TH3 induction | (10–14) |
| Lactobacillus casei | Propogates oral tolerance | |
| BGN4 (Bifidobacterium bifidum) | Decreases IgA and IgE production | |
| VSL #3 | Dampens NF-κB proinflammatory signals | |
| Decreases levels of cell degranulation | ||
| Staphyloccocal superantigen B | Treg cell suppressor | (2,15) |
| Ergosterol peroxide (Tricholoma populinum) | Decreases allergic symptoms | (5) |
| ASHMI | Decreases TH2 response and cytokines profile | (16,17) |
| Ganoderma lucidum | Lowers IL-5, IL-13 and IgE levels | |
| Radix Sophora flavescentis | ||
| Radix Glycyrrhiza uralensis | ||
| KRN7000 | Immunostimulantion | (18) |
| Agelus mauritianus derivative | Increases IFN-γ, IL-4, and IL-12 | |
| Enhances NK cell activity |
A cornucopia of herbal medicines has shown clinical effectiveness in the attenuation of allergic and infection-induced inflammatory pathology. Traditional therapeutics for allergic complications includes immunotherapy, antihistamines and glucocorticoids. A newly researched compound, termed anti-asthma herbal medicine intervention (ASHMI), which is an extract of three herbs, has shown effectiveness and benefit over traditional treatment options for asthma. ASHMI mainly downregulates TH2 cell responses, increases lung function through direct modulation of smooth muscle contraction and decreases peripheral blood eosinophils and serum IgE. ASHMI does not induce a state of general immunosuppression like steroids, such as prednisone, which suppress TH1 and TH2 cell responses.
Treatment of infection with antimicrobial drugs poses an array of complications, including resistance and physical ailments, e.g. diarrhea. Allium sativum, extracted from garlic, has the potential to bolster TH1 cell-mediated responses to pathogens, such as Leishmania major. The proposed mechanism includes enhancement of TH1 cytokine response, T lymphocyte proliferation and NK cell activity. This method of antimicrobial treatment offers benefits over pharmaceutical drugs; however, its efficacy and mechanism of action needs further research and elucidation.
CAM benefits greatly from research done to determine a scientific basis for confident treatment decisions, as it lends credibility to CAM modalities and, most importantly, offers efficacious treatment options to a large segment of the population afflicted with allergic and infectious complications. Knowledge of the dynamic relationship between Treg cells and immune system responses to foreign antigens is essential in order to approach Treg cells as a clinical target for the alleviation of complications arising from allergy, asthma, dermatitis and infection.
Treg Subsets: Three of a Kind
The family greatly responsible for immunomodulation consists of two key subsets: naturally arising and peripherally induced Treg cells. Although the ontogenic relationship between the two is not well understood, both subsets have been characterized by distinct differentiation patterns and functions (19).
Naturally Occurring Treg Cells
Naturally occurring Treg (nTreg) cells compose 5–10% of peripheral T cells, maintain a distinct lineage and develop in the thymus. nTreg cells are directed to largely aid in tolerance to self-antigen in the periphery via suppressive actions on both TH1 and TH2 cell-mediated immune responses, with greater specificity for the former (20–24).
nTreg cells inhibitory action is contact dependent. Interaction of CTLA-4 and TGF-β1, expressed on the cell surface, with respective ligands and receptors on target cells, triggers the downregulation of effector cell IL-2Rα receptors and a subsequent decrease in fitness (20,23,25). Alternate mechanisms proposed include the perforin-dependent induction of T cell apoptosis and the reduction of dendritic cell's ability to prime T cells, through direct suppression of cytokine secretion or tryptophan metabolism (2,15,26–28).
Peripherally Induced Treg Cells
The adaptive Treg cell subset includes type 1 Treg (Tr1) cells and T helper 3 (TH3) cells. Tr1 and TH3 cells are implicated in immune responses to foreign antigens in the periphery, alongside nTreg cells (22).
While extrathymic generation of Treg cells is not as well understood as nTreg cell generation, Tr1 and TH3 cells are derived from naïve T cells in an environment supporting suitable antigenic and cytokine stimulation (24,29). Located in peripheral lymphoid tissue, these cells produce a distinct cytokine profile upon TCR-mediated activation (22). Tr1 and TH3 cells mediate immunosuppression through the secretion of IL-10 and TGF-β1 (21,23,30,31). Tr1 and TH3 cells become significant in peripheral self-tolerance when the pool of self-antigen-specific nTreg cells is deficient (31).
Treg Cell Singularity
For the purposes of this review and owing to the fact that definitive comparative research on the subsets is insufficient for precise differentiation, nTreg, Tr1 and TH3 cells will be collectively referred to as Treg cells.
At One with Nature: Modulating Responses of Allergenic Challenge
Aberrant immune responses to environmental allergens are relatively common with wide variations in severity and manifestation. Depending on the allergen size and mode of exposure, an atopic individual may experience afflictions ranging from asthma to seasonal allergic rhinitis to atopic dermatitis. Atopic allergic sensitization involves the overproduction of IgE against environmental allergens, e.g. grass, house dust mites, pollen and animal proteins (30,32). Allergen-specific IgE, on the surface of mast cells and basophils, upon binding to allergen, triggers the release of histamine and mediators resulting in immediate symptomology (33). Often, patients with allergic diseases have a deficient ability to suppress T cell responses to allergen by Treg cells (32,34).
Treg cell's role in the prevention of sensitization to allergens has recently been explored (32). Evidence of significant Treg cell suppressive action on TH2 cell-mediated immune responses supports the notion that their depletion or functional dysregulation may be responsible for atopic pathology (32).
Allergy
Seasonal allergic rhinitis, more commonly known as Hay Fever, involves the deposition of allergens on the nasal mucosa followed by an immediate hypersensitivity reaction. Allergens involved, like grass pollen, are typically too large to enter into the lower airways, rendering asthmatic complications unusual. Treg cells have the capability to reduce or prevent TH2 cell-mediated allergic rhinitis and atopic sensitization disorders (32,34).
Allergen-specific Treg cells are also modulators of immune response to dietary allergens and intimately involved in the development of oral tolerance. Karlsson et al. illustrates Treg cell functionality in an experiment utilizing children with allergy to cow's milk (35). A majority of the allergic children developed oral tolerance to milk following a period of milk restriction. Development of β-lactalbumin-specific Treg cells, in those children who developed tolerance to cow's milk, was found to be responsible for the newfound tolerance. Induction of tolerance to allergen, via antigen-specific Treg cell generation, is noted in individuals encountering pollen, dust mites and other environmental antigens (2,32). This signifies the malleability of immune system response and balance afforded by antigen-specific Treg cells (35,36).
Taken altogether, the response of an individual to allergen encounter is dependent on many factors, including the quantity and activity of antigen-specific Treg and the TH2 effector populations (36).
Asthma
Asthma is a chronic airway inflammatory disease triggered by allergic exposure and hallmarked by airway inflammation, bronchial hyperreactivity, lung eosinophilia and excessive TH2 cytokine production (28,34).
Inappropriate TH2 cell response to inhaled allergens elicits airway inflammation. The complex biosignaling cascade resulting in asthma manifestation is mediated by the over production of IL-4, IL-5, IL-9 and IL-13, which serves to regulate IgE production and to sequester effector cells to the airway. Effector cells that are directed towards airway tissues enhance airway inflammation and hyperresponsiveness through the generation of additional proinflammatory cytokines and autocoids (32,37). In many cases, asthma pathology is due to an excess of TH2 cell quantity or activity, leading to a skewing towards a proinflammatory cytokine profile (28).
Mouse models of airway inflammation allow for the examination of allergen-specific Treg cells activity in vivo. The transfer of ovalbumin (OVA) peptide-specific Treg cells to OVA sensitized mice reduced TH2 type cytokine expression in the lungs, airway hyperreactivity and effector cell recruitment. The ameliorative effects were dependent upon IL-10; however, TH2 cells were the source of IL-10 secretion, rather than Treg cells (34). Treg cells may reduce inflammatory response through a contact-dependent manner, e.g. enhancing the secretion of IL-10 by TH2 cells.
Treg cells aid in the suppression of inflammatory responses to inhaled antigen and are essential for the induction of allergenic tolerance. Administration of inhaled allergen, with prior induction of allergen-specific Treg cells, prevents allergen sensitization and airway inflammation upon later exposure. Thus, immunotherapy offers a powerful function based on the modulation of allergen-specific Treg cell suppression of TH2 responses (32).
Dermatitis
Atopic dermatitis is a chronic inflammatory skin disorder in which TH2 effector cells migrate to the dermis, and under IL-12 conditions, become TH0 or TH1 cells. In atopic individuals, a dysregulation of effector T cells and an impairment of Treg cell suppression are involved in the development of inflammatory pathology (38). While various treatment options exist, including the use of steroids, antihistamines and aggregative factor elimination, an increasing number of patients are finding little relief and are requiring other therapeutic modalities, some of which may be derived from CAM (39).
Throwing Water on the Inflammation: Treg Cell's Delicate Relations to Infectious Pathology
To deal with microorganisms, the body has evolved intricate defense mechanisms. The process of pathogen control involves the recruitment of immune system cells to the site of infection. Necessary components include inflammatory cells, cytotoxic T cells and NK cells. The generation of antigen-specific Treg cells is a crucial regulatory element in the immune response to infection by bacteria, parasites, fungi and viruses, as well as the fostering and maintenance of tolerance to non-pathogenic microbes (21,40). Dysregulation of Treg cell-mediated anti-inflammatory pathways poses potentially great risks to health (41).
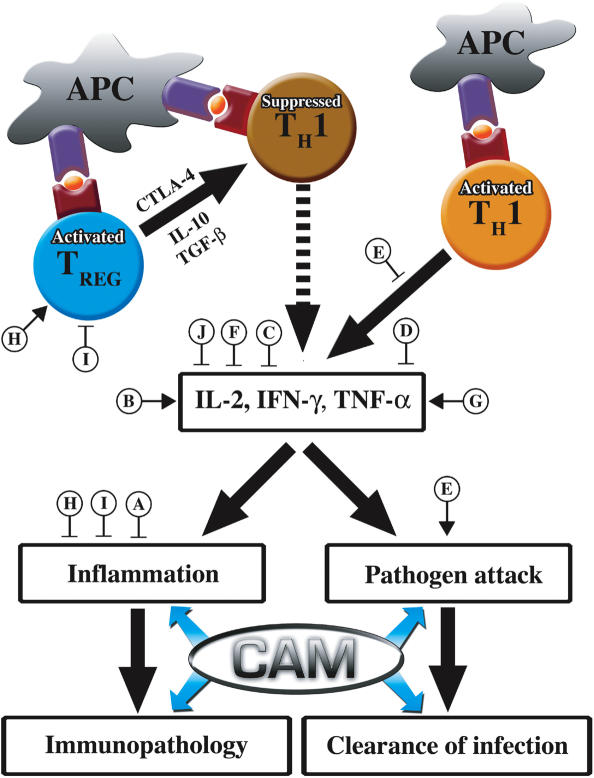
Effective immune response to pathogen is often accompanied by a great deal of inflammation. In excess, this inflammation can cause collateral tissue damage and pathology that necessitates the activation and proliferation of pathogen-specific Treg cells (19,21,42–44; Fig. 1). Conversely, a decrease in defense responsiveness owing to underlying immunosuppression renders the host susceptible to pathogenic infection and subsequent harm. Depending on a number of variables, a reconstitution of immune system responses or Treg cell populations may be necessary in lieu of appropriate antimicrobial or inflammatory therapy.
Figure 1.
Modulation of TH1 cell immune response by Treg cells and CAM. Sites of action throughout the TH1 cell-mediated inflammatory pathway. A, Brazilian green propolis; B, Ganoderma lucidum; C, P. linteus; D, Piper methysticum; E, Allium sativum; F, (−)-epigallocatechin gallate; G, KRN7000; H, probiotics; I, Staphylococcal superantigen B; J, Ergosterol peroxide.
Bacterial Infections
IL-10 secreting Treg cells attenuate bacterial-induced abnormalities caused by massive inflammation. This immunosuppressive cytokine is imperative for dampening excessive inflammation, owing to TH1 responses and increase in TNF-α production, while it is potentially detrimental to pathogen attack, thus making the level of IL-10 in sites of inflammation extremely delicate and specific (44).
A variety of bacterial-induced inflammatory diseases ranging from peritonitis from Escherichia coli, to chronic gastritis from Helicobacter pylori and to chronic hepatitis from Helicobacter hepaticus, reinforce a correlation between IL-10 deficiencies and disease severity. To illustrate this, mice with a deficiency in IL-10, upon exposure to Listeria monocytogenes, have a greater severity of brain lesions, because of increased proinflammatory cytokine production in the brain (44–46). In this instance, either specialized Treg cells or their IL-10 secretions help to limit TH1 cell-mediated inflammation and damage during infection.
Recognition of hazardous microbes, allergens and toxins as pathogenic agents activates the gastrointestinal immune system. Antigen-specific Treg cells, which mediate oral tolerance to commensal microbes, differentiate between harmless inhabitants of the gut and pathogens. A break in the development or maintenance of oral tolerance may result in an astounding array of detrimental inflammatory disorders, including inflammatory bowl disease (IBD) and colitis.
IBD and colitis are conditions in which the immune system of patients reacts excessively to indigenous intestinal bacteria. Treg cell depletion in these disorders effectively breaches tolerance and allows for massive inflammation in the gut. In vivo transfer of Treg cells suppresses disease development, through IL-10, TGF-β and CTLA-4-dependent mechanisms (21).
Colonization of gastric and duodenal mucosa by H. pylori induces strong immune responses involving innate immune system cells as well as H. pylori-specific T and B cells (27). Treg cells dampen the immune response to H. pylori, effectively limiting acute infection-induced pathology, at the cost of bacterial persistence and long-term pathology, i.e. chronic infection (27). Prevention of inflammation via IL-10 and TGF-β may prove useful in the control of H. pylori infection (21).
Interestingly, varieties of bacteria including Mycobacterium tuberculosis, Yersinia entercolitica and Bordetella pertussis induce production of IL-10 by macrophages. This cytokine manipulation efficiently triggers immunosuppression and attenuates bacterial attack, by means of inducing development of IL-10 secreting Treg cells (46,47).
The appropriate balance between inflammation and bacterial destruction so that the body accrues minimal tissue damage while putting up adequate host defense as well as development of tolerance to commensals are essential roles of IL-10 secreting Treg cells. Thus, they pose a promising target for the suppression of extreme inflammation and tissue damage during bacterial exposure or infection. It is important to note that different clinical outcomes may result from Treg cell activation status.
Fungal Infections
Treg cell activation limits inflammatory pathology induced by fungal infection but compromises fungal clearance. Pneumocystis carinii and Candida albicans are used in models to ascertain Treg cell function. Infection of Treg cell deficient mice with P. carinii yields fatal pulmonary inflammation, with damage owing to CD4+ effector T cells. Treg cell infusion prevents inflammation and disease development at the cost of increased pathogen load (19,21,48–50). Similarly, Treg cell depletion renders adequate control of C. albicans infection while allowing for large-scale gastrointestinal inflammation to ensue (21).
Parasitic Infections
IL-10 or TGF-β secreting Treg cells have great utility in the balance between parasite clearance and induced immunopathology, as seen in malarial, Plasmodium chabaudi and L. major infections.
The severity of malarial infection directly correlates to the ratio of TGF-β and IL-10 levels to TNF-α levels, i.e. a greater proportion of suppressive cytokines attenuates severity of infection-induced inflammation. IL-10-deficient mice, infected with P. chabaudi, maintain severe infection and massive inflammatory responses resulting in significant host damage (44,51–53). In the case of L. major infection, removal of Treg cells results in the effective parasite clearance, however bad lesions and a robust TH2 response ensue (19,21,27,54). This substantiates the importance of TGF-β and IL-10 secreting Treg cells in controlling parasitic infection and pathology.
Various parasites that have adapted to Treg cell host-defense mechanisms have the ability to modulate Treg cell cytokine production and activation (55–57). Therefore, the role of Treg cells in inflammatory pathology and pathogen clearance may vary significantly between individual parasitic infectious agents.
Viral Infections
Treg cells reduce the severity of immune-mediated inflammatory lesions in viral-induced diseases through the suppression of pathogenic CD4+ T cell activity and the limitation of inflammatory cell sequestration.
Chronic hepatitis C virus infection results in massive hepatic inflammation and damage. In liver biopsies, there is an inverse correlation between peripheral Treg cells and histological inflammatory score (21). Treg cells, specifically those secreting IL-10, are essential for the attenuation of such organ detriment.
Theiler's virus induces murine encephalomyelitis, a TH1 cell-mediated inflammatory disease of the central nervous system, and provides a model of human multiple sclerosis from which exploration of Treg cell functionality in autoimmune detriment is possible. B cell proliferation and autoantibody production, in some viral infections, plays a major role in the development of viral-induced autoimmunity (51,58). Infected mice may experience acute encephalomyelitis or chronic demyelinating disease depending on the strain. Virus-specific Treg cells sufficiently suppress this aberrant CD4+ TH1 cell-mediated response (59). Treg cell modulation of reactions to self, regardless of causation, holds promising implications for a broad spectrum of deleterious autoimmune diseases.
During chronic viral infections, Treg cells are beneficial to the host by maintaining a balance between efficient viral defense and inflammation, while preventing the induction of autoimmune disorders (56–63). Similar to other pathogens, certain viruses, like HIV, have developed mechanisms that directly affect Treg cell function, resulting in reduced antiviral response and increased viral persistence (62–65).
The Maginot Line: Treg Cell's Blockade of Allergic and Infectious Immune Responses
Innovative research and subsequent elucidation of Treg cell involvement in various atopic and infectious pathologies opens up numerous avenues for ameliorative therapies while describing mechanisms of disease pathogenesis. It is apparent that the Treg cell quantity and activation state are integral and equally important factors in the development and maintenance of inflammatory immunopathology (36).
Treg cell involvement in immune response to pathogens is delicate since Treg cells actively suppress immunopathology during infection, while concomitantly supporting persistence of infection during chronic disease. Aberrant modulation of immune responses by Treg cells may be owing to inappropriate quantity or functionality of Treg cells. Excessive immune suppression results in enhanced pathogen survival, through clearance inhibition, which may lead to long-term persistence, pathogen damage and increased potential for transmission (21). On the other hand, depressed Treg cell activity allows little control of inflammatory responses resulting in collateral tissue damage (21,41). A balance between effector T and Treg cell responses in sites of chronic infection may allow parasite survival in host while maintaining host immune memory and control of the pathogen (44).
Pathogens are evolving self-serving strategies to increase survival potential, through the establishment of favorable conditions for Treg cell priming, recruitment and survival (21). Certain pathogens and their products, e.g. Staphylococcal superantigen B, HTLV-1 and HIV, directly target and modulate Treg cell function (2). Evolved pathogen mechanisms for Treg cells manipulation underscores the power and utility that Treg cells hold and presents another means for medical treatment of related diseases, e.g. implementation of means to effectively antagonize pathways in which pathogens directly modulate Treg cell functioning.
Increased research is necessary in order to determine Treg cell functioning in relation to individual allergic and pathogen induced disease states. This will afford CAM researchers insight into the appropriate means of approaching a variety of human disorders with respect to Treg cells.
Treg cell's essential role in the management of allergy and infection has been detailed using specific allergens or pathogens as examples. Harmony between regulatory and effector arms of the immune system is a necessity for good health. Treg cell intricacy and specificity to individual allergens or pathogens impels further research and highlights Treg cells overall importance to human health and CAM. The conceptual framework laid down is consistent with various disease states, including autoimmunity and tumor pathogenesis, which will be a futile subject.
Conflict of Interest
Aristo Vodjani is co-owner of Immunosciences Lab. Inc. He declares no conflict of interest.
References
- 1.Vojdani A, Erde J. Regulatory T cells, a potent immunoregulatory target for CAM researchers: the ultimate antagonist (I) Evid Based Complement Altern Med. 2006;3:25–30. doi: 10.1093/ecam/nek022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatila TA. Role of regulatory T cells in human diseases. J Allergy Clin Immunol. 2005;116:949–59. doi: 10.1016/j.jaci.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 3.Shimazawa M, Chikamatsu S, Morimoto N, Mishima S, Nagai H, Hara H. Neuroprotection by Brazilian green propolis against in vitro and in vivo ischemic neuronal damage. Evid Based Complement Alternat Med. 2005;2:201–7. doi: 10.1093/ecam/neh078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo MC, Weng CY, Ha CL, Wu MJ. Ganoderma lucidum mycelia enhance innate immunity by activating NF-κB. J Ethnopharmacol. 2006;103:217–22. doi: 10.1016/j.jep.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Lindequist U, Niedermeyer TH, Julich WD. The pharmacological potential of mushrooms. Evid Based Complement Alternat Med. 2005;2:285–99. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inagaki N, Shibata T, Itoh T, Suzuki T, Tanaka H, Nakamura T, et al. Inhibition of IgE-dependent mouse triphasic cutaneous reaction by a boiling water fraction separated from mycelium of Phellinus linteus. Evid Based Complement Alternat Med. 2005;2:369–74. doi: 10.1093/ecam/neh105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folmer F, Blasius R, Morceau F, Tabudravu J, Dicato M, Jaspars M, Diederich M. Inhibition of TNFalpha-induced activation of nuclear factor κB by kava (Piper methysticum) derivatives. Biochem Pharmacol. 2006;71((8)):1206–18. doi: 10.1016/j.bcp.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Ghazanfari T, Hassan ZM, Khamesipour A. Enhancement of peritoneal macrophage phagocytic activity against Leishmania major by garlic (Allium sativum) treatment. J Ethnopharmacol. 2006;103:333–7. doi: 10.1016/j.jep.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Watson JL, Vicario M, Wang A, Moreto M, McKay DM. Immune cell activation and subsequent epithelial dysfunction by Staphylococcus enterotoxin B is attenuated by the green tea polyphenol (−)-epigallocatechin gallate. Cell Immunol. 2005;237:7–16. doi: 10.1016/j.cellimm.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–7. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 11.Rautava S, Kalliomaki M, Isolauri E. New therapeutic strategy for combating the increasing burden of allergic disease: Probiotics-A Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota (NAMI) Research Group report. J Allergy Clin Immunol. 2005;116:31–7. doi: 10.1016/j.jaci.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Kwack K, Kim DY, Ji GE. Oral probiotic bacterial administration suppressed allergic responses in an ovalbumin-induced allergy mouse model. FEMS Immunol Med Microbiol. 2005;45:259–67. doi: 10.1016/j.femsim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Tien MT, Girardin SE, Regnault B, Le Bourhis L, Dillies MA, Coppee JY, et al. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176:1228–37. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- 14.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-β-bearing regulatory cells. J Immunol. 2005;174:3237–46. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 15.Goleva E, Cardona ID, Ou LS, Leung DY. Factors that regulate naturally occurring T regulatory cell-mediated suppression. J Allergy Clin Immunol. 2005;116:1094–100. doi: 10.1016/j.jaci.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 16.Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, et al. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol. 2005;116:517–24. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Engler RJ. Alternative and complementary medicine: a source of improved therapies for asthma? A challenge for redefining the specialty? J Allergy Clin Immunol. 2000;106:627–9. doi: 10.1067/mai.2000.110504. [DOI] [PubMed] [Google Scholar]
- 18.Haefner B. Drugs from the deep: marine natural products as drug candidates. Drug Discov Today. 2003;8:536–44. doi: 10.1016/s1359-6446(03)02713-2. [DOI] [PubMed] [Google Scholar]
- 19.Fehervari Z, Sakaguchi S. Regulatory T cells. In: Lotze MT, Thompson AW, editors. Measuring Immunity. Oxford: Elsevier; 2005. pp. 322–35. [Google Scholar]
- 20.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–17. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 22.Chattopadhyay S, Mehrotra S, Chhabra A, Hegde U, Mukherji B, Chakraborty NG. Effect of CD4+CD25+ and CD4+CD25− T regulatory cells on the generation of cytolytic T cell response to a self but human tumor-associated epitope in vitro. J Immunol. 2006;176:984–90. doi: 10.4049/jimmunol.176.2.984. [DOI] [PubMed] [Google Scholar]
- 23.Maggi E, Cosmi L, Liotta F, Romagnani P, Romagnani S, Annunziato F. Thymic regulatory T cells. Autoimmun Rev. 2005;4:579–86. doi: 10.1016/j.autrev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Chiappelli F. The molecular immunology of mucositis: implications for evidence-based research in alternative and complementary palliative treatments. Evid Based Complement Alternat Med. 2005;2:489–94. doi: 10.1093/ecam/neh129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montagnoli C, Fallarino F, Gaziano R, Bozza S, Bellocchio S, Zelante T, et al. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J Immunol. 2006;176:1712–23. doi: 10.4049/jimmunol.176.3.1712. [DOI] [PubMed] [Google Scholar]
- 26.Bousso P. Lymph node choreography: Treg cells join the dance. Nat Immunol. 2006;7:11–3. doi: 10.1038/ni0106-11. [DOI] [PubMed] [Google Scholar]
- 27.Lundgren A, Stromberg E, Sjoling A, Lindholm C, Enarsson K, Edebo A, et al. Mucosal FOXP3-expressing CD4+CD25 high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun. 2005;73:523–31. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seroogy CM, Gern JE. The role of T regulatory cells in asthma. J Allergy Clin Immunol. 2005;116:996–9. doi: 10.1016/j.jaci.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25−CD4+ precursors. J Immunol. 2005;175:6107–16. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 30.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, et al. Tolerance induced by inhaled antigen involves CD4+ T cells expressing membrane-bound TGF-β and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–62. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson DS, Larche M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004;114:1389–97. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellavite P, Conforti A, Pontarollo F, Ortolani R. Immunology and homeopathy. 2. Cells of the immune system and inflammation. Evid Based Complement Altern Med. 2006;3:13–24. doi: 10.1093/ecam/nek018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–47. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. J Exp Med. 2004;199:1679–88. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maizels RM. Infections and allergy—helminths, hygiene and host immune regulation. Curr Opin Immunol. 2005;17:656–61. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Bochner BS, Busse WW. Allergy and asthma. J Allergy Clin Immunol. 2005;115:953–9. doi: 10.1016/j.jaci.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 38.Verhagen J, Akdis M, Traidl-Hoffmann C, Schmid-Grendelmeier P, Hijnen D, Knol EF, et al. Absence of T-regulatory cell expression and function in atopic dermatitis skin. J Allergy Clin Immunol. 2006;117:176–83. doi: 10.1016/j.jaci.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi H, Takahashi K, Mizuno N, Kutsuna H, Ishii M. An alternative approach to atopic dermatitis: part I—case-series presentation. Evid Based Complement Altern Med. 2004;1:49–62. doi: 10.1093/ecam/neh015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kingston H, Mills G. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–55. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 41.Haddad PS, Azar GA, Groom S, Boivin M. Natural health products, modulation of immune function and prevention of chronic diseases. Evid Based Complement Alternat Med. 2005;2:513–20. doi: 10.1093/ecam/neh125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–11. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muthukuru M, Jotwani R, Cutler CW. Oral mucosal endotoxin tolerance induction in chronic periodontitis. Infect Immun. 2005;73:687–94. doi: 10.1128/IAI.73.2.687-694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–8. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sewnath ME, Olszyna DP, Birjmohun R, ten Kate FJ, Gouma DJ, van Der Poll T. IL-10-deficient mice demonstrate multiple organ failure and increased mortality during Escherichia coli peritonitis despite an accelerated bacterial clearance. J Immunol. 2001;166:6323–31. doi: 10.4049/jimmunol.166.10.6323. [DOI] [PubMed] [Google Scholar]
- 46.Kullberg MC, Rothfuchs AG, Jankovic D, Caspar P, Wynn TA, Gorelick PL, et al. Helicobacter hepaticus-induced colitis in interleukin 10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect Immun. 2001;69:4232–41. doi: 10.1128/IAI.69.7.4232-4241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGuirk P, McCann C, Mills KHG. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin-10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–31. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–91. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Montagnoli C, Bacci A, Bozza S, Gaziano R, Mosci P, Sharpe AH, et al. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J Immunol. 2002;169:6298–308. doi: 10.4049/jimmunol.169.11.6298. [DOI] [PubMed] [Google Scholar]
- 50.Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–8. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 51.May J, Lell B, Luty AJ, Meyer CG, Kremsner PG. Plasma interleukin-10 tumor necrosis factor TNF-α ratio is associated TNF promoter variants and predicts malarial complications. J Infect Dis. 2000;182:1570–3. doi: 10.1086/315857. [DOI] [PubMed] [Google Scholar]
- 52.Othoro C, Lal AA, Nahlen B, Koech D, Orago AS, Udhayakumar V. A low interleukin-10 tumor necrosis factor TNF-α ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis. 1999;179:279–82. doi: 10.1086/314548. [DOI] [PubMed] [Google Scholar]
- 53.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun. 1999;67:4435–42. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–7. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Omer FM, Riley EM. Transforming growth factor-β production is inversely correlated with severity of murine malaria infection. J Exp Med. 1998;188:39–48. doi: 10.1084/jem.188.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Omer FM, de Souza JB, Riley EM. Differential induction of TGF-β regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J Immunol. 2003;171:5430–6. doi: 10.4049/jimmunol.171.10.5430. [DOI] [PubMed] [Google Scholar]
- 57.Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–66. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 58.Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, et al. Hepatic expansion of a virus-specific regulatory CD8+ T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–72. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haynes LM, Vanderlugt CL, Dal Canto MC, Melvold RW, Miller SD. CD8+ T cells from Theiler's virus-resistant BALB/cByJ mice downregulate pathogenic virus-specific CD4+ T cells. J Neuroimmunol. 2000;106:43–52. doi: 10.1016/s0165-5728(00)00212-5. [DOI] [PubMed] [Google Scholar]
- 60.Suvas S, Kumaguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suvas S, Azkur AK, Kim BS, Kumaguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–32. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 62.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated in vivo in persistent HCV infection. Hepatology. 2003;38:1437–48. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 63.Kinter AL, Hennessey M, Bell A, Kern S, Lin Y, Daucher M, et al. CD25+CD4+ regulatory T cells from the peripheral of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200:331–43. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD25+CD4+ regulatory T cells control T cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–9. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwashiro M, Messer RJ, Peterson KE, Stromnes IM, Sugie T, Hasenkrug KJ. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc Natl Acad Sci USA. 2004;98:9226–30. doi: 10.1073/pnas.151174198. [DOI] [PMC free article] [PubMed] [Google Scholar]